Abstract
Purpose
To characterize the natural course of diabetic retinopathy (DR) in contemporary clinical practice.
Patients and Methods
This was a retrospective analysis of US claims data collected between January 1, 2006, and April 30, 2017. Patients aged ≥18 years with continuous medical and prescription insurance coverage for 18 months before DR diagnosis (index date) and for a follow-up period of 5 years were included (N=14,490). The time and risk of progressing to severe nonproliferative DR (NPDR) or proliferative DR (PDR) and of developing diabetic macular edema (DME) were evaluated over 5 years in patients stratified by DR severity at initial diagnosis.
Results
The estimated probability of progressing to severe NPDR or PDR within 5 years of diagnosis was 17.6% for patients with moderate NPDR versus 5.8% for mild NPDR. The probability of developing DME within 5 years was 62.6%, 44.6%, and 28.4% for patients diagnosed with severe NPDR, moderate NPDR, and PDR, respectively, versus 15.6% for mild NPDR. Among those observed to progress, median time to severe NPDR or PDR was approximately 2.0 years in patients with moderate NPDR, whereas median time to DME was approximately 0.5 years in patients with severe NPDR, 1.3 years in moderate NPDR, and 1.6 years in PDR. Relative to mild NPDR, adjusted hazard ratios (95% confidence interval) for progression to severe NPDR or PDR within 5 years were 3.12 (2.61–3.72) in patients with moderate NPDR, and for incident DME were 5.92 (5.13–6.82), 3.54 (3.22–3.91), and 1.96 (1.80–2.14) in patients with severe NPDR, moderate NPDR, and PDR, respectively.
Conclusion
The risk of DR progression and DME over 5 years was highest among patients diagnosed with moderate and severe NPDR, respectively. Our findings reinforce the importance of close monitoring for these patients to avoid unobserved disease progression toward PDR and/or DME.
Keywords: diabetic macular edema, diabetic retinopathy, disease progression, real-world evidence
Introduction
Diabetic retinopathy (DR), the most common microvascular complication of diabetes, is a leading cause of adult-onset blindness worldwide.1–3 DR is observable via retinal examination and regular screening is recommended for those with diabetes;4,5 however, it may also be present in individuals who are unaware of their diabetes status.2
The burden of DR is largely attributed to its progression from mild and asymptomatic to severe and vision-threatening. In earlier stages of nonproliferative DR (NPDR), damage to the retinal microvasculature occurs as a consequence of chronic hyperglycemia and manifests as microaneurysms, intraretinal hemorrhages, and the development of diabetic macular edema (DME). Without appropriate treatment, the resulting retinal ischemia promotes neovascularization characteristic of proliferative DR (PDR), leading to vitreous hemorrhage, retinal detachment, neovascular glaucoma, and severe vision loss.4 This progression is accompanied by worsening vision-related quality of life and increasing functional burden.6,7
Clinical guidelines, including the American Academy of Ophthalmology (AAO) Preferred Practice Pattern® and American Diabetes Association (ADA) Position Statement, recommend regular monitoring for patients with DR to track disease progression and identify those who require referral and/or treatment.4,5 Recommended follow-up intervals are dependent on DR severity and the presence of DME, and have been shortened in updated AAO guidance to promote closer monitoring of patients with more advanced disease.4 Eye examinations are recommended every 1–2 years for patients with diabetes and no evidence of DR and then annually for those with mild NPDR. Current ADA and AAO guidelines recommend follow-up intervals of 6–9 months and 6–12 months for patients with moderate NPDR, respectively, decreasing to every 3–6 months and 3–4 months for those with severe NPDR. ADA guidelines recommend that patients with PDR are monitored every 3 months, whereas the AAO recommends follow-up every 2–4 months, depending on the presence of early or high-risk PDR. In addition to closer monitoring, patients with severe NPDR and PDR are typically indicated for treatment, with panretinal laser photocoagulation and intravitreal anti-vascular endothelial growth factor (VEGF) therapy representing the preferred treatment strategies in clinical guidance. For patients across all stages of DR, more frequent monitoring and earlier treatment are advised when concurrent DME, particularly center-involved DME, is present.4,5
Our current understanding of DR progression is based largely on data collected from epidemiological studies. The landmark Early Treatment Diabetic Retinopathy Study (ETDRS) and the Diabetes Control and Complications Trial were 2 of the first studies to characterize the natural history of DR.8,9 Data from the control arms of clinical trials evaluating treatments for DME and DR have also contributed to our knowledge of DR progression.10–12 Rates of progression in more recent studies have generally been lower than those reported in earlier natural history studies, likely due to increased awareness of DR and its risk factors, as well as advances in the management of diabetes.13,14
Although clinical trials have provided valuable insights, DR progression observed under trial conditions may not reflect the experiences of patients and physicians in current clinical practice. The generalizability of trial data may be limited by highly selective patient populations, relatively short follow-up periods, close monitoring, and strict treatment protocols. For example, the 12-step ETDRS Diabetic Retinopathy Severity Scale (DRSS) is typically used to grade DR severity and monitor disease progression in clinical trials due to its ability to detect small anatomical changes between frequently collected retinal images.8 In clinical practice, DR severity is determined by physician assessment using available resources and is broadly categorized as mild, moderate, or severe NPDR or as PDR, according to International Classification of Diseases (ICD) diagnosis codes.
To address a paucity of data to describe DR progression in contemporary clinical practice, we conducted a retrospective analysis of US claims data to characterize the natural course of DR in the 5 years following initial diagnosis. In particular, we sought to estimate the time and risk of DR worsening in patients newly diagnosed with mild or moderate NPDR, and of developing DME in patients diagnosed across all stages of DR.
Patients and Methods
Design and Setting
This was a 5-year retrospective analysis of data extracted from the IBM® MarketScan® Commercial Claims and Encounters (Commercial) and Medicare Supplemental and Coordination of Benefits (Medicare Supplemental) databases between January 1, 2006, and April 30, 2017 (Figure S1). These databases contain deidentified inpatient, outpatient, and pharmacy claims data, and are compliant with the Health Insurance Portability and Accountability Act.15 Clinical diagnoses within claims data were identified by ICD Ninth/Tenth Revision, Clinical Modification diagnosis codes, whereas evidence of treatment was based on Healthcare Common Procedure Coding System and Current Procedural Terminology codes (Tables S1 and S2, respectively). As a retrospective analysis of deidentified claims data, this study did not require institutional review board approval and written informed consent.
Participants
Analyses included treatment-naïve adults (≥18 years) with ≥1 inpatient or ≥2 outpatient claims indicating newly diagnosed DR between July 1, 2007, and April 30, 2012. The index date for each patient was defined as the date of first DR diagnosis, with a lookback period of 18 months before index, designated the baseline period. Patients were required to have ≥1 claim indicating DR severity (ie, mild, moderate, or severe NPDR or PDR) and have continuous medical and prescription insurance during the baseline period and for ≥5 years after the index date (Figure S1). In the present analysis, follow-up data were collected from eligible patients for 5 years post index, through April 30, 2017.
Patients with evidence of anti-VEGF therapy, laser photocoagulation, intravitreal steroid treatment, or retinal surgery (ie, vitrectomy and retinal detachment) during the baseline period were excluded from this study. Patients were also ineligible if they had a prior diagnosis of DR, DME, age-related macular degeneration, retinal vein occlusion, myopic choroidal neovascularization, or vitreous hemorrhage recorded during the baseline period. Moreover, patients were excluded if DR severity could not be determined at any time during follow-up (eg, claims indicating background/unspecified DR or claims indicating multiple DR stages on the same day).
Outcomes
Two sets of analyses were conducted to estimate the time and risk of (1) progressing to severe NPDR or PDR in treatment-naïve patients with mild or moderate NPDR, and (2) developing DME in all treatment-naïve patients stratified by DR severity at index. Outcomes were measured over 5 years of follow-up, and patients were right-censored in each analysis using criteria described below. Due to database coding limitations, we were unable to determine the laterality of the affected eye(s); however, it was assumed that the most severe DR stage present was coded at each visit and that progression during follow-up had occurred in the same eye. Patterns of DR regression (eg, DR severity improvement due to treatment, improved diabetes management, and/or initial misdiagnosis of DR) were not investigated in this study.
Statistical Analysis
Baseline patient demographics (age, sex, US Census Bureau geographic region, and health plan type) and clinical characteristics (Diabetes Severity Complication Index16 and Elixhauser Comorbidity Index17) were summarized using descriptive statistics. Differences in baseline characteristics between DR severity subgroups were evaluated using chi-squared, Wilcoxon rank-sum, Kruskal–Wallis, or analysis of variance tests as appropriate, with statistical significance defined as P≤0.05.
Two Kaplan-Meier analyses were performed to estimate the 5-year probability of (1) DR progression (ie, first incidence of severe NPDR or PDR) in patients diagnosed with mild or moderate NPDR, and (2) DME development in all patients stratified by DR severity at index. For both analyses, data were censored at the first record of treatment with anti-VEGF agents, laser photocoagulation, or intravitreal steroids or at the end of the follow-up period, whichever occurred first. Analyses describing DR progression were additionally censored at the first incidence of DME during follow-up. Kaplan-Meier product-limit estimation was used to calculate the probability of being progression or DME free over 5 years; the complement was then taken to express the likelihood of DR progression or DME development over this period. The proportion of patients observed to exhibit DR progression and/or develop DME over 5 years, as well as the median time to these events among those who progressed, were also calculated.
In addition, separate Cox proportional hazard models were fit to compare the risk of (1) DR progression over 5 years between patients with mild and moderate NPDR, and (2) DME development over 5 years among all DR severity subgroups. Follow-up data in each model were censored as described above, and both models were adjusted for baseline patient demographics, clinical characteristics, and other covariates by fitting five-knot restricted cubic splines for all continuous variables. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) describing the relative risks of DR progression and DME development were calculated using mild NPDR as the reference category.
Results
Study Population
In total, 341,832 patients amassed in the MarketScan databases had ≥1 diagnosis code indicating DR between July 1, 2007, and April 30, 2012. Most patients (n=302,082; 88.4%) were excluded due to a lack of continuous insurance coverage during the 18-month baseline and 5-year follow-up periods. After all exclusions, 14,490 (4.2%) treatment-naïve patients with newly diagnosed DR were included in the final analysis cohort (Figure S2). Of these, 6878 (47.5%) patients were diagnosed with mild NPDR at index, 1761 (12.2%) had moderate NPDR, 439 (3.0%) had severe NPDR, and 5412 (37.3%) had PDR. Baseline characteristics were generally balanced across DR severity subgroups and were accounted for in Cox proportional hazard models and adjusted HR calculations (Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics of Treatment-Naïve Patients Newly Diagnosed with Diabetic Retinopathy
| Variables | Mild NPDR (n=6878) | Moderate NPDR (n=1761) | Severe NPDR (n=439) | PDR (n=5412) | P-value |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 62.2 (12.3) | 59.9 (11.8) | 57.9 (11.1) | 58.6 (12.0) | <0.0001 |
| Male, n (%) | 3801 (55.3) | 1017 (57.8) | 244 (55.6) | 2894 (53.5) | 0.014 |
| Health plan type, n (%) | |||||
| HMO | 866 (12.6) | 240 (13.6) | 57 (13.0) | 742 (13.7) | 0.292 |
| PPO | 3267 (47.5) | 876 (49.7) | 210 (47.8) | 2706 (50.0) | 0.036 |
| Other | 2745 (39.9) | 645 (36.6) | 172 (39.2) | 1964 (36.3) | <0.001 |
| Region, n (%) | |||||
| Northeast | 985 (14.3) | 252 (14.3) | 58 (13.2) | 765 (14.1) | 0.926 |
| South | 2410 (35.0) | 712 (40.4) | 183 (41.7) | 2107 (38.9) | <0.001 |
| North Central | 2497 (36.3) | 544 (30.9) | 123 (28.0) | 1686 (31.2) | <0.001 |
| West | 975 (14.2) | 253 (14.4) | 75 (17.1) | 850 (15.7) | 0.054 |
| Unknown | 11 (0.2) | 0 | 0 | 4 (0.1) | 0.247 |
| Clinical characteristics, mean (SD) | |||||
| DCSI score | 1.2 (1.6) | 1.4 (1.7) | 1.4 (1.8) | 1.7 (2.0) | <0.0001 |
| ECI score | 1.6 (1.8) | 1.6 (1.8) | 1.7 (1.9) | 1.7 (2.0) | 0.895 |
Abbreviations: DCSI, Diabetic Complications and Severity Index; ECI, Elixhauser Comorbidity Index; HMO, health maintenance organization; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PPO, preferred provider organization; SD, standard deviation.
Progression to Severe NPDR or PDR
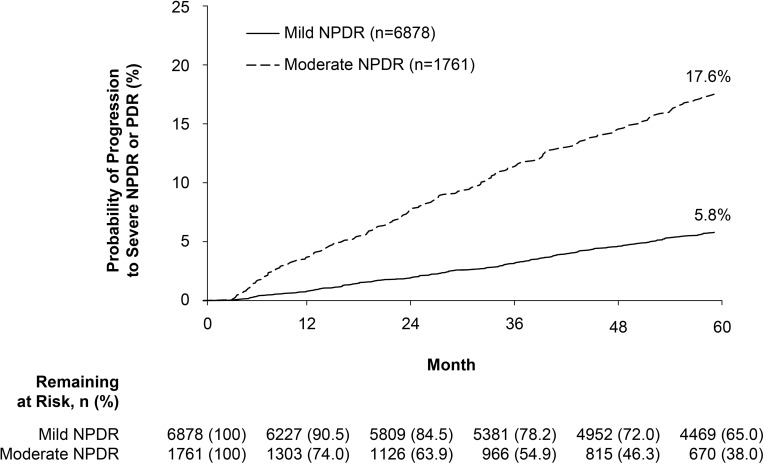
Progression to severe NPDR or PDR within 5 years of diagnosis was approximately 3-fold more likely among patients with moderate versus mild NPDR at index (Figure 1). Using Kaplan-Meier product-limit estimation, the probability of progressing to severe NPDR or PDR over 5 years was 17.6% among patients with moderate NPDR at diagnosis versus 5.8% in patients diagnosed with mild NPDR. Among those who were observed to progress within 5 years (mild NPDR, 328 [4.8%] patients; moderate NPDR, 204 [11.6%] patients), median time to first incidence of DR progression was 982 days (≈2.7 years) and 734.5 days (≈2.0 years) for patients diagnosed with mild and moderate NPDR, respectively.
Figure 1.
Progression to severe NPDR or PDR over 5 years.
Note: Patients with mild or moderate NPDR were censored at the first incidence of diabetic macular edema, or first record of treatment with anti-vascular endothelial growth factor agents, laser photocoagulation, or intravitreal steroids.
Abbreviations: NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Development of DME
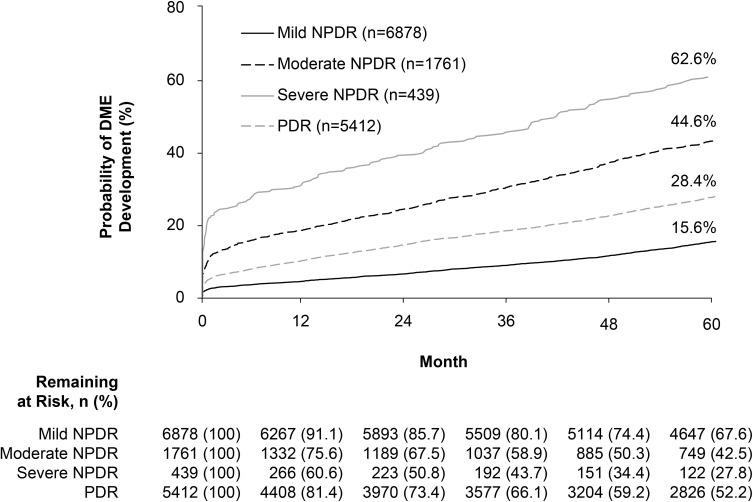
Development of DME in the 5 years following initial DR diagnosis was 3- to 4-fold more likely in those with moderate or severe NPDR than those with mild NPDR, and was almost twice as likely in those with PDR at index (Figure 2). The estimated probability of developing DME over 5 years was 62.6%, 44.6%, and 28.4% among patients with severe NPDR, moderate NPDR, and PDR at diagnosis, respectively, compared with 15.6% in patients diagnosed with mild NPDR. Among those observed to develop DME within 5 years, the median time to first incidence of DME was shortest in patients diagnosed with severe NPDR (248 [56.5%] patients; 180.5 days [≈0.5 years]), followed by those with moderate NPDR (715 [40.6%] patients; 465 days [≈1.3 years]) and PDR (1335 [24.7%] patients; 581 days [≈1.6 years]), and was longest in those with mild NPDR at index (970 [14.1%] patients; 851 days [≈2.3 years]).
Figure 2.
Development of DME over 5 years, by diabetic retinopathy severity at diagnosis.
Note: Patients were censored at the first record of treatment with anti-vascular endothelial growth factor agents, laser photocoagulation, or intravitreal steroids.
Abbreviations: DME, diabetic macular edema; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Risk of DR Progression or Development of DME
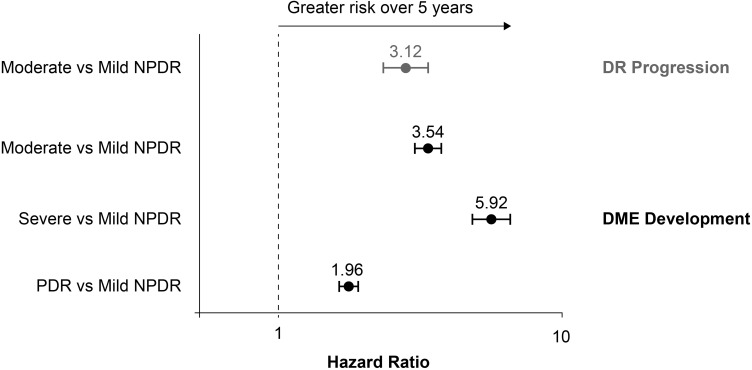
Figure 3 presents adjusted HRs comparing the risk of progression to severe NPDR or PDR over 5 years between patients diagnosed with mild and moderate NPDR, and of DME development over 5 years between all DR severity subgroups. Estimated risks of DR progression and DME development within 5 years were approximately 3-fold higher in patients with moderate versus mild NPDR at index (adjusted HR [95% CI], 3.12 [2.61–3.72] and 3.54 [3.22–3.91], respectively). Relative to mild NPDR, the risk of developing DME over 5 years was almost 6-fold higher in patients with an initial diagnosis of severe NPDR (5.92 [5.13–6.82]) and almost 2-fold higher in those diagnosed with PDR (1.96 [1.80–2.14]).
Figure 3.
Risks of DR progression and DME development over 5 years.
Notes: Gray, estimated risk of progressing to severe NPDR or PDR within 5 years of initial DR diagnosis; black, estimated risk of developing DME within 5 years of initial DR diagnosis. Cox proportional hazard models were adjusted for baseline patient demographics, clinical characteristics, and other covariates by fitting five-knot restricted cubic splines for all continuous variables.
Abbreviations: DME, diabetic macular edema; DR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Discussion
In this retrospective analysis of 14,490 patients in US clinical practice, the risk of progression to severe NPDR or PDR within 5 years was approximately 3-fold greater in patients diagnosed with moderate versus mild NPDR. Among those observed to progress, median time to severe NPDR or PDR was also shorter in patients with moderate NPDR than in those with mild NPDR at diagnosis. Similarly, patients with an initial diagnosis of moderate or severe NPDR were more likely to develop DME within 5 years and did so earlier than patients diagnosed with mild NPDR or PDR. These data demonstrate the rapidity of DR progression in patients with moderate to severe NPDR and highlight the potential for close monitoring to avoid unobserved disease progression and associated vision loss.
The estimated likelihood of progression to severe NPDR or PDR within 5 years of diagnosis was 17.6% in patients with moderate NPDR versus 5.8% in those with mild NPDR. Our results are broadly consistent with other studies that have explored DR progression and its risk factors; however, direct comparisons are hampered by differences in study populations, the inclusion of treated and untreated patients, measures of DR severity, definitions of disease progression, and study outcomes. For example, a previous claims database analysis estimated that the likelihood of progressing from NPDR to PDR within 5 years of diagnosis was 38% in patients with factors that place them at high risk of progression (eg, increased glycosylated hemoglobin) versus 5% in lower-risk individuals.18 The ETDRS demonstrated the relationship between DR severity and the rates of progression to PDR over 5 years,8 whereas the Los Angeles Latino Eye Study found that 39% of patients progressed by ≥2 ETDRS-DRSS steps and 5.3% progressed from NPDR to PDR over a 4-year period.19 Similarly, the UK Progression of Diabetes Study found that 29% of patients with any stage of DR progressed by ≥2 ETDRS-DRSS steps over 6 years of follow-up.20 In the Wisconsin Epidemiologic Study of Diabetic Retinopathy, ≥2-step DR progression was observed in 34–41% of patients and PDR developed in 7–11% over 4 years in the 1980s,21,22 although annualized rates have decreased in more recently diagnosed cohorts.13
We similarly found that the risk of developing DME within 5 years was greatest among patients with an initial diagnosis of moderate or severe NPDR compared with mild NPDR. Interestingly, the estimated likelihood of developing DME was lower in those with PDR than with moderate or severe NPDR. We observed that a higher proportion of patients diagnosed with PDR was censored from our DME analyses over 5 years (23% vs 16–18% across NPDR subgroups), suggesting that these patients may have received prompt intervention in line with clinical guidance4,5 and were censored before potentially developing DME. Alternatively, PDR may be considered an end stage in diabetic eye disease, and physicians coding disease severity in billing statements may not have proactively updated PDR diagnosis codes to include DME after it developed.
Overall, our results broadly validate previous studies of DR progression, but in a contemporary cohort receiving routine care in US clinical practice. The risks of DR progression and DME over 5 years were highest among patients diagnosed with moderate and severe NPDR, respectively. Among those observed to progress, the median time to severe NPDR or PDR was approximately 2.0 years in patients diagnosed with moderate NPDR, whereas the median time to DME was approximately 0.5 years in patients with severe NPDR and 1.3 years in those diagnosed with moderate NPDR. These results highlight the potential for rapid disease progression among patients with moderate and severe NPDR and support clinical guidance that recommends follow-up examinations every 6–12 months and 3–6 months, respectively.4,5 Although our data suggest that patients with moderate and severe NPDR should be closely monitored to avoid unobserved disease progression, further research is needed to confirm whether earlier intervention might also be beneficial for these patients. Intravitreal anti-VEGF therapy has been associated with high rates of DR improvement among patients with moderately severe to severe NPDR (ETDRS-DRSS score 47–53) in post hoc analyses of the landmark RIDE/RISE trial and in the ongoing PANORAMA trial;11,23,24 however, it remains to be seen whether such data may inform a shift toward earlier treatment in clinical practice.
Data analyzed in this study were collected from a large commercial payor database; therefore, we expect our results to be more representative of current clinical practice than those observed under trial conditions. However, it should be noted that our analyses are contingent upon the accurate and appropriate coding of diagnoses and treatments by healthcare providers, and that patients with DR who did not seek medical care were unable to be examined. Moreover, the distribution of patients across DR stages in our study was based on a newly diagnosed cohort and may not necessarily reflect that of the general diabetes population or other epidemiological evaluations.14,25
It is important to acknowledge other limitations of this study. There is the potential for coding errors within insurance claims data, as well as a limited availability of relevant health information at the individual patient level. As noted earlier, we were unable to determine the laterality of the studied eye and follow patient eyes separately, which may have provided additional insights. In contrast to clinical trials, there were no fixed time points for monitoring DR progression in this study. Although it is possible that the index date for each patient may not have accurately reflected their first diagnosis of DR, it is unlikely that a prior DR diagnosis would not have been followed up during the 18-month baseline period. A key limitation of our study is the broad manner in which DR is classified in clinical practice (ie, mild, moderate, or severe NPDR or PDR) as opposed to the carefully standardized manner in which DR severity is graded in many prospective studies (ie, 12-step ETDRS-DRSS).8,14
Conclusions
This retrospective analysis of claims data from US clinical practice found that patients diagnosed with moderate and severe NPDR were at greatest risk of DR progression and developing DME within 5 years, respectively. Our findings reinforce the importance of close monitoring for these patients, as advocated in current clinical guidance, to avoid unobserved progression toward vision-threatening levels of DR; namely, the development of PDR and/or DME.
Funding Statement
Financial support was provided by Genentech, Inc., a member of the Roche Group (South San Francisco, CA). Third-party writing assistance, provided by Karina Hamilton-Peel, PhD, CMPP, of Envision Pharma Group, was funded by Genentech, Inc. The sponsor participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
AAO, American Academy of Ophthalmology; ADA, American Diabetes Association; CI, confidence interval; DME, diabetic macular edema; DR, diabetic retinopathy; DRSS, Diabetic Retinopathy Severity Scale; ETDRS, Early Treatment Diabetic Retinopathy Study; HR, hazard ratio; ICD, International Classification of Diseases; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; VEGF, vascular endothelial growth factor.
Disclosure
Andrew Moshfeghi has served as a consultant for Alimera, Allegro, Allergan, Genentech, Inc., Graybug, Novartis, OptiSTENT, Regeneron, Valeant, and Visunex; has served as an investigator for Genentech, Inc., Regeneron, and REGENXBIO Inc.; is a stockholder for OptiSTENT, Pr3vent, and Visunex; has received research funding from Allegro, Genentech, Inc., Novartis, and Regeneron; reports grants and personal fees from Genentech, Inc. during the conduct of the study; reports consulting work and research grants from Genentech, Inc., Novartis, and Regeneron during the conduct of the study; and reports consulting work for and a public equity interest in Ocular Therapeutix during the conduct of the study. Vincent Garmo is an employee of Genentech, Inc.; reports personal fees from and stock in Genentech, Inc. during the conduct of the study; and reports personal fees from and stock in Genentech, Inc. outside the submitted work. Daniel Sheinson and Ibrahim Abbass are employees of Genentech, Inc. Avanti Ghanekar was an employee of Genentech, Inc. during the conduct of the study (current employee of REGENXBIO Inc., Rockville, MD); and reports stock in Genentech, Inc. during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- 1.Klein BEK. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179–183. doi: 10.1080/09286580701396720 [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al.; Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339–e349. doi: 10.1016/S2214-109X(13)70113-X [DOI] [PubMed] [Google Scholar]
- 4.Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127(1):P66–P145. doi: 10.1016/j.ophtha.2019.09.025 [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi: 10.2337/dc16-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis JR, Doan QV, Gleeson M, et al. Vision-related functional burden of diabetic retinopathy across severity levels in the United States. JAMA Ophthalmol. 2017;135(9):926–932. doi: 10.1001/jamaophthalmol.2017.2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazhar K, Varma R, Choudhury F, McKean-Cowdin R, Shtir CJ, Azen SP; Los Angeles Latino Eye Study Group. Severity of diabetic retinopathy and health-related quality of life: the Los Angeles Latino Eye Study. Ophthalmology. 2011;118(4):649–655. doi: 10.1016/j.ophtha.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Ophthalmology. 1991;98(5 suppl):823–833. doi: 10.1016/S0161-6420(13)38014-2 [DOI] [PubMed] [Google Scholar]
- 9.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401 [DOI] [PubMed] [Google Scholar]
- 10.Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130(9):1145–1152. doi: 10.1001/archophthalmol.2012.1043 [DOI] [PubMed] [Google Scholar]
- 11.Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2(10):997–1009. doi: 10.1016/j.oret.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P, McAllister I, Larsen M, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmol Retina. 2018;2(10):988–996. doi: 10.1016/j.oret.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII: the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115(11):1859–1868. doi: 10.1016/j.ophtha.2008.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TY, Mwamburi M, Klein R, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. 2009;32(12):2307–2313. doi: 10.2337/dc09-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IBM Watson Health. IBM MarketScan Research Databases for life sciences researchers. Available from: https://www.ibm.com/downloads/cas/OWZWJ0QO. Accessed October10, 2019.
- 16.Glasheen WP, Renda A, Dong Y. Diabetes Complications Severity Index (DCSI)—update and ICD-10 translation. J Diabetes Complications. 2017;31(6):1007–1013. doi: 10.1016/j.jdiacomp.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 18.Nwanyanwu KH, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36(6):1562–1568. doi: 10.2337/dc12-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varma R, Choudhury F, Klein R, Chung J, Torres M, Azen SP; Los Angeles Latino Eye Study Group. Four-year incidence and progression of diabetic retinopathy and macular edema: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149(5):752–761.e1–3. doi: 10.1016/j.ajo.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratton IM, Kohner EM, Aldington SJ, et al.; UKPDS Group. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594 [DOI] [PubMed] [Google Scholar]
- 21.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IX. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1989;107(2):237–243. doi: 10.1001/archopht.1989.01070010243030 [DOI] [PubMed] [Google Scholar]
- 22.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. X. Four-year incidence and progression of diabetic retinopathy when age at diagnosis is 30 years or more. Arch Ophthalmol. 1989;107(2):244–249. doi: 10.1001/archopht.1989.01070010250031 [DOI] [PubMed] [Google Scholar]
- 23.Wykoff CC. Intravitreal aflibercept for moderately severe to severe non-proliferative diabetic retinopathy (NPDR): the phase 3 PANORAMA study. Presented at: Angiogenesis, Exudation, and Degeneration 2019; February 19, 2019; Miami, FL. [Google Scholar]
- 24.Wykoff CC. A phase 3, double masked, randomized study of the efficacy and safety of aflibercept in patients with moderately severe to severe NPDR: week 100 results. Presented at: Angiogenesis, Exudation, and Degeneration 2020; February 8, 2020; Miami, FL. [Google Scholar]
- 25.Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]