Abstract
Purpose
To report treatment outcomes of ab interno canaloplasty using the Visco360 and Omni system devices as a standalone procedure or combined with cataract surgery in patients with open-angle glaucoma (OAG).
Design
Retrospective, single-center, consecutive case series.
Study Patients
Eighty-nine eyes of 64 patients aged 43 to 91 with open-angle glaucoma treated with ab interno canaloplasty between January 2018 and September 2019. Eyes with previous incisional glaucoma surgery and eyes with less than 90 degrees of viscodilation were excluded.
Intervention
Patients underwent ab interno canaloplasty as a stand-alone procedure or in conjunction with cataract surgery. Ab interno canaloplasty was performed with either the Visco360 or Omni System devices (Sight Sciences, Menlo Park, CA). Treatment consisted of viscodilation without trabeculotomy.
Main Outcome Measures
Primary outcome measures were mean IOP and mean number of glaucoma medications. Additional analysis included the impact of degrees of treatment on treatment outcomes.
Results
Preoperative mean IOP was 24.5 ± 8.3; the number of preoperative glaucoma medications was 2.5 ± 1.3. At 18 months postoperative, the mean IOP was reduced 36% to 15.8 ± 2.5 (P<0.001) and glaucoma medications were reduced 32% to 1.7 ± 1.5 (P<0.05). Higher preoperative IOP was significantly correlated with increased IOP lowering. Reduction of mean IOP and medications were not significantly different between standalone ab interno canaloplasty vs cataract surgery/ab interno canaloplasty. Reduction of mean IOP and medications were not significantly different between patients with 180 degrees of treatment vs 360 degrees of treatment.
Conclusion
Ab interno canaloplasty reduces IOP and glaucoma medication use in patients with OAG whether as a standalone surgery or in combination with cataract surgery.
Keywords: ab interno, canaloplasty, MIGS, open angle glaucoma, Visco360, Omni, glaucoma surgery, viscodilation
Precis
In patients with open-angle glaucoma, ab interno canaloplasty lowers intraocular pressure and medication usage. The outcomes at 18-months are similar in patients undergoing standalone ab interno canaloplasty vs ab interno canaloplasty combined with cataract surgery. Treating 360 degrees did not improve outcomes significantly compared to 180 degrees.
Introduction
The primary treatment for glaucoma is to lower the intraocular pressure (IOP), whether through medications, laser procedures, or surgical procedures.1 Ab interno canaloplasty is a surgical procedure intended to lower the IOP in patients with glaucoma. This surgery involves an internal approach to viscodilation of Schlemm’s canal through a clear corneal incision.2,3 The Visco360 and Omni System devices (Sight Sciences, Menlo Park, CA) are inserted through a clear corneal incision into the anterior chamber. The tip of the device is used to penetrate the trabecular meshwork and create an opening into Schlemm’s canal. The surgeon can then advance a microcatheter from the device through the opening of the meshwork into Schlemm’s canal and circumnavigate the canal up to 180 degrees in each direction. As the catheter is retracted into the device, viscoelastic is expressed from the tip of the catheter to viscodilate Schlemm’s canal. The device is entirely removed from the eye at the conclusion of the surgery.
Trabeculectomy and aqueous shunt surgery are traditional surgical options for glaucoma. These options involve disruption of the conjunctiva and creation of a new outflow channel whereby aqueous filters out of the eye, bypassing the natural outflow pathway including the trabecular meshwork and Schlemm’s canal. Trabeculectomy and aqueous shunt surgery result in the creation of a subconjunctival bleb. Numerous short-term and delayed postoperative complications may result from the creation of this subconjunctival bleb and from bypassing the normal outflow pathway of the eye.4
Ab interno canaloplasty is one of a variety of surgical procedures described as minimally invasive glaucoma surgery (MIGS).5 These surgeries differ from traditional glaucoma surgery in that the conjunctiva is not disturbed and no bleb is created. In addition, efforts are made to improve or rehabilitate the function of the normal outflow pathway rather than creating a new outflow pathway.5,6 Ab interno canaloplasty is unique amongst MIGS procedures in that no devices or hardware are implanted in the eye and no significant amount of tissue damage occurs. Many of the MIGS procedures attempt to increase the function of the outflow pathway of the eye by implanting devices into the trabecular meshwork and/or Schlemm’s canal or by cutting or removing part of the trabecular meshwork. Ab interno canaloplasty involves only a small perforation of the trabecular meshwork followed by cannulation and viscodilation of Schlemm’s canal.7
Previously published studies show results for ab externo canaloplasty8–18 and ABiC (ab interno canaloplasty)19 using the iTrack microcatheter, or for ab interno canaloplasty with the Visco360 device.20,21 The technique used in these studies involved 360-degree circumnavigation of Schlemm’s canal. The Visco360 and Omni System devices offer the surgeon the opportunity to titrate the extent of treatment. Schlemm’s canal may be cannulated up to 180 degrees (or less) in each direction from the point of insertion. The purpose of the present study was to retrospectively review the effect of ab interno canaloplasty on both IOP and number of glaucoma medications and in addition to compare outcomes for varying degrees of cannulation and viscodilation.
Methods
This study is a retrospective review of patient data from consecutive patients with open-angle glaucoma treated following normal clinical standard of care. Prior to surgical intervention, patients received comprehensive clinical examinations including evaluation and assessment of the health of the optic nerve and retina by examination and diagnostic testing, assessment of visual fields, measurement of visual acuity, measurement of intraocular pressure, gonioscopic evaluation, and measurement of the corneal thickness. In certain patients, additional lowering of the intraocular pressure or alternative treatment to achieve intraocular pressure lowering was deemed necessary, whether due to evidence of progressive deterioration of the optic nerve, visual field loss, or intolerance of medications. For patients who elected ab interno canaloplasty surgery to achieve lowered intraocular pressure or as an alternate means for control of intraocular pressure, the patient provided written informed consent prior to surgery. The research adhered to the tenets of the Declaration of Helsinki. The study was reviewed and approved by the Western IRB. Waiver of patient consent for this research study was granted due to the retrospective, non-interventional nature of the study. Research data included in the study were non-identifying and adhered to the Health Insurance Portability and Accountability Act. All surgeries were performed by the same surgeon at a single surgery center.
Study Patients
Participants included patients undergoing ab interno canaloplasty as a standalone surgery or in combination with cataract surgery using the Visco360 or Omni System devices. All surgeries were performed by a single surgeon (MT) between January 2018 and September 2019. Patients were adults ages 43 to 91 with a diagnosis of mild to moderate open-angle glaucoma. Exclusion criteria included patients with pseudoexfoliative glaucoma, pigmentary glaucoma, glaucoma associated with ocular trauma, glaucoma associated with ocular inflammation, previous incisional glaucoma surgery and eyes with less than 90 degrees of viscodilation. Both eyes from a patient were included if both met eligibility criteria.
Standard practice protocol was followed including a comprehensive ophthalmic examination prior to surgery assessing ocular history, medication use, visual acuity, intraocular pressure, gonioscopy, slit-lamp biomicroscopy, and dilated fundus examination. Glaucoma medications were continued after surgery and tapered post-operatively when appropriate.
Surgical Technique
When ab interno canaloplasty was performed in combination with cataract surgery, the cataract surgery was performed following standard cataract surgery protocol and was performed prior to the ab interno canaloplasty. Otherwise, the technique was the same for all cases. For all cases, topical preservative-free lidocaine was placed on the surface of the eye and the anterior chamber was first deepened with viscoelastic (Provisc, Alcon, Fort Worth, TX). The head was tilted away from the surgeon and the microscope was tilted towards the surgeon to facilitate gonioscopic visualization. A goniolens (Ocular Hill Open Access Surgical Gonio-Left Hand, Ocular Instruments, Bellevue, WA) was placed on the eye with viscoelastic coupling fluid and the nasal trabecular meshwork was visualized. The Visco360 or Omni System device was inserted through the temporal clear corneal incision and advanced towards the nasal trabecular meshwork. A small goniotomy was created with the tip of the device and the cannula was inserted through the goniotomy into Schlemm’s canal. The microcatheter was then advanced counterclockwise 180 degrees or until an obstruction was reached. The microcatheter was then retracted and a controlled amount of viscoelastic (Healon/Healon GV) was expressed from the tip of the microcatheter yielding viscodilation of the canal. This procedure was repeated in the opposite direction for a total of up to 360 degrees of treatment. The anterior chamber was irrigated by coaxial irrigation/aspiration to remove the viscoelastic, the anterior chamber was pressurized to a physiologic pressure, and 0.15 mL of preservative-free Dexamethasone-Moxifloxacin (Imprimis, San Diego, CA) was injected into the anterior chamber. The eye was taped closed after surgery and a protective shield was placed over the eye until the follow-up examination the next day. Topical ketorolac 0.4% was prescribed three times daily for one month. For patients who also underwent cataract surgery, topical prednisolone acetate 1% was prescribed three times daily for one week in addition to the ketorolac.
Standard follow-up visits were typically scheduled for 1 day, 1 week, 1, 3, 6, 12, and 18 months post-operatively. At each follow-up visit the visual acuity, intraocular pressure, glaucoma medication usage, and slit-lamp examination were assessed and recorded. Any significant ocular adverse events for the surgical eye were also recorded.
Outcome Measures
The primary outcome was the difference in mean IOP and number of glaucoma medications from baseline to 1 week, 1, 3, 6, 12, and 18 months after surgery. Additional analysis included comparison of IOP and medication reduction between canaloplasty combined with cataract surgery vs standalone surgery, and comparison of IOP and medication outcomes between eyes treated 180 degrees vs 360 degrees.
Statistical Analysis
This was a retrospective, consecutive series from a single practice. Sample size was determined by the number of available cases and not by statistical power calculations. Means and standard deviations were calculated based on the total available eyes for each time point. Paired t-tests were used to evaluate the differences between baseline and each post-operative timepoint for IOP and medication usage. The level of statistical significance was set at P≤ 0.05. The study was completed before all patients were able to reach 18 months follow up after surgery.
Results
Demographics
Demographics are summarized in Table 1. All patients had been diagnosed with mild to moderate open-angle glaucoma. Of the 89 eyes (64 patients), 72 eyes underwent ab interno canaloplasty combined with cataract surgery and 17 eyes had standalone ab interno canaloplasty surgery.
Table 1.
Demographics
| Age | |
|---|---|
| Mean | 72.1 |
| Range | 43–91 |
| Sex | |
| Male | n = 41 |
| Female | n = 23 |
| Eye Treated | |
| Left (OS) | n = 45 |
| Right (OD) | n = 44 |
| Lens Status | |
| Phakic | n = 72 |
| Pseudophakic | n = 17 |
| Degrees Treated | |
| 360° | n = 54 |
| 180° | n = 22 |
| All Others | n = 13 |
Primary Outcomes
Intraocular Pressure
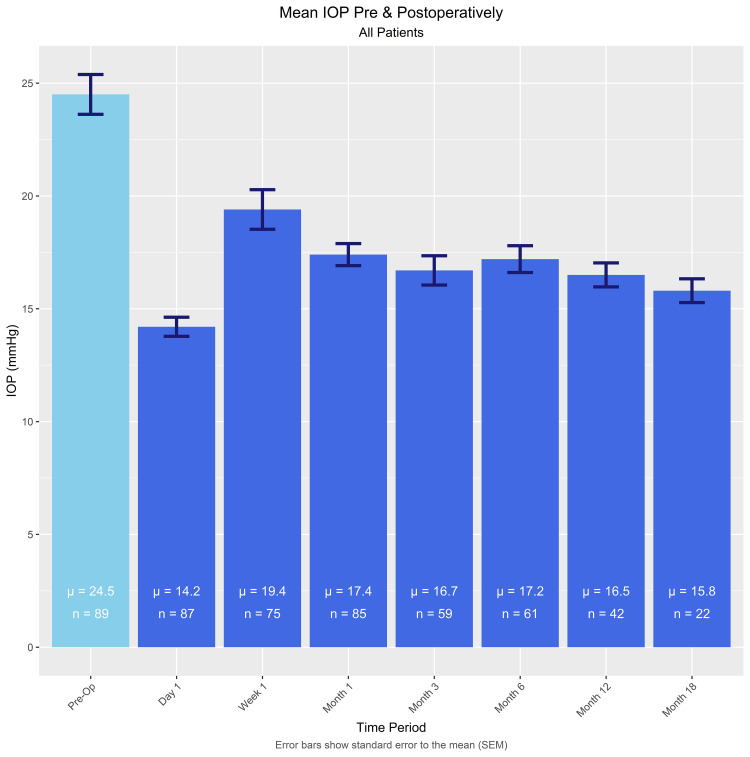
Mean intraocular pressure at baseline and post op day 1, post op week 1, and 1, 3, 6, 12, and 18 months post op are presented in Figure 1. The mean baseline IOP of 24.5 ± 8.3 mmHg reduced significantly to 16.5 ± 3.4 (p<0.0005) at 12 months and 15.8 ± 2.5 (p<0.0005) at 18 months.
Figure 1.
Intraocular pressure at baseline and out to 18 months postoperative.
Glaucoma Medications
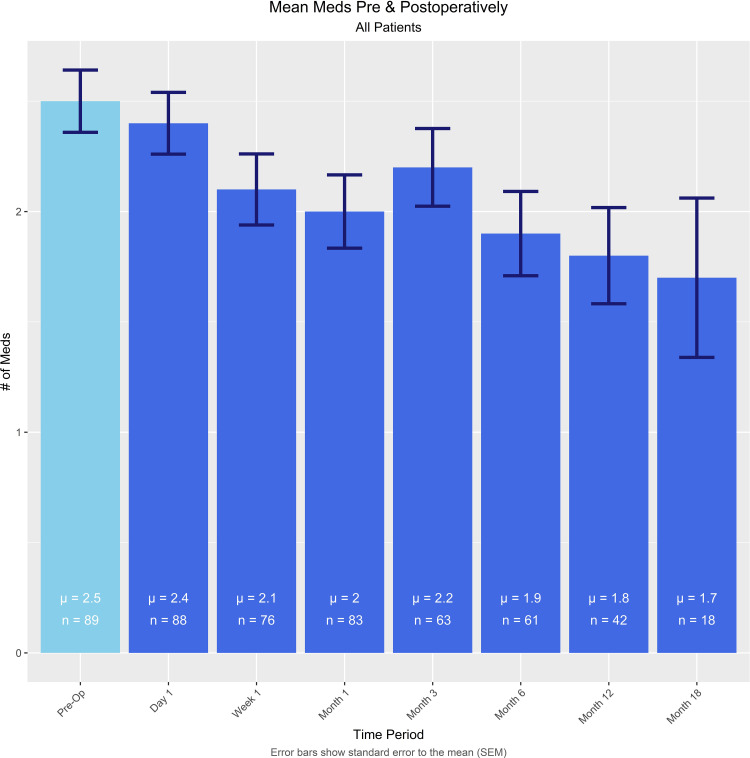
Mean number of glaucoma medications at baseline and post op day 1, post op week 1, and 1, 3, 6, 12, and 18 months post op are presented in Figure 2. Mean baseline medications were reduced significantly from 2.5 ± 1.3 to 1.8 ± 1.4 (p<0.0005) at 12 months and 1.7 ± 1.5 (p<0.05) at 18 months.
Figure 2.
Glaucoma medication usage at baseline and out to 18 months postoperative.
Additional Analysis
Ab Interno Canaloplasty Combined with Cataract vs Standalone
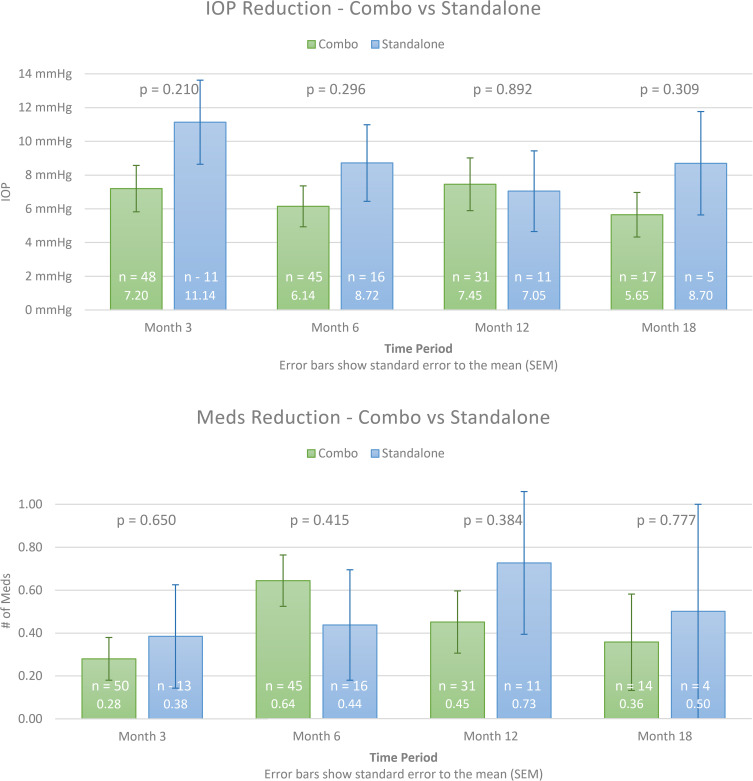
Mean reduction in IOP and mean change in the number of medications from baseline were compared between ab interno canaloplasty combined with cataract vs standalone ab interno canaloplasty at 3, 6, 12, and 18 months after surgery. There were no statistically significant differences between groups for either of these variables at any time point (Figure 3).
Figure 3.
Comparison of standalone ab interno canaloplasty vs combined with cataract surgery. IOP and glaucoma medication usage at baseline and out to 18 months postoperative.
180 vs 360-Degree Treatment
In all of the reported cases, the intent at the beginning of surgery was to cannulate 360 degrees of Schlemm’s canal. When performing ab interno canaloplasty with the Visco360 and Omni Surgical System, the surgeon does not visualize the tip of the catheter as it circumnavigates Schlemm’s Canal. It is assumed that when the catheter is fully extended in either direction it has traversed 180 degrees of the canal. Unless an obstruction is present, the catheter can typically be advanced forward without noticeable resistance. However, should the catheter encounter significant resistance while being advanced, the surgeon's preference was to retract the catheter and avoid further cannulation in that direction. For these cases, the degrees of treatment was approximated as best possible.
There were some instances when cannulation was possible in one direction but not possible in the other direction, either due to blood obscuring the view of Schlemm’s canal or inability to cannulate the canal in that direction. When a patient had only 180 degrees of treatment, they had full cannulation in one direction and no treatment in the opposite direction.
As seen in Figure 4, there was no significant difference in either IOP reduction or reduction of glaucoma medications when comparing 180 degrees of treatment to 360 degrees of treatment.
Figure 4.
Comparison of 180 vs 360 degrees ab interno canaloplasty treatment. IOP and glaucoma medication usage at baseline and out to 18 months postoperative.
Predicting Surgical Outcomes
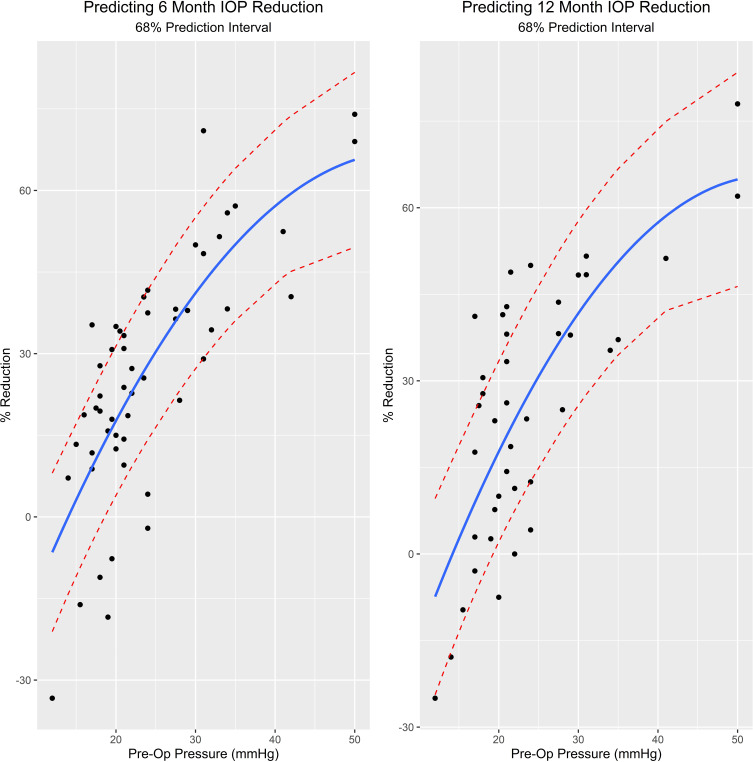
There was a statistically significant (6 months r2 = 0.5954, p < 0.001, 12 months r2 = 0.5332, p < 0.001) positive correlation between the preoperative IOP and the amount of IOP lowering with surgery. Figure 5 shows a trendline and prediction interval that may assist with estimating potential treatment outcomes with ab interno canaloplasty.
Figure 5.
Predicting 12-month IOP reduction after ab interno canaloplasty. Correlation between preoperative IOP and IOP reduction at 12 months postoperative.
Safety – Adverse Events
Mild intraoperative bleeding at the surgery site with subsequent microhyphema was anticipated and was not considered an adverse event for this surgery. There were no clinically significant hyphemas.
Six eyes subsequently required glaucoma filtration surgery due to elevated IOP during the time period of this study. They were included in the study up until the time they underwent additional surgery. Not all 89 eyes had 18 months of follow-up by the end of the study and it is possible that the number of eyes requiring subsequent surgical intervention within 18 months of surgery would be higher than this number. Of note, these six patients had a mean baseline IOP of 29.4 mmHg, approximately 5 mmHg higher than the overall cohort. Mean medication usage was also higher, 3.5 vs 2.5 medications.
Discussion
Ab interno canaloplasty, with or without cataract surgery, lowered the baseline IOP by nearly one-third at 12 and 18 months out from surgery. Medication use was reduced by an average of nearly one medication per eye at 12 and 18 months out from surgery.
Previous studies have shown that cataract surgery alone results in an average IOP reduction of 1.4 mmHg in glaucoma patients at 3 years out from surgery.22 However, this study did not show that cataract surgery conferred any significant additional IOP lowering benefit when combined with ab interno canaloplasty. In fact, the reduction in IOP was numerically greater in the standalone group compared to the combined group. While this difference did not reach statistical significance due to the relatively small number of standalone patients, it may argue against a contribution to the overall IOP-lowering attributable to cataract extraction for these patients. As the study was not randomized the influence of confounding variables cannot be ruled out and this result should be considered accordingly. In addition, the small sample size of this study may not have been powered adequately to show any difference.
Six of the 89 eyes required more invasive glaucoma surgery during this study due to persistently elevated IOP. However, these patients had higher mean baseline IOP and were using more glaucoma medications on average. For a surgery that attempts to rehabilitate the failing internal drainage system of the trabecular meshwork and Schlemm’s canal, it may be that eyes with higher baseline IOP and medications are more damaged and less able to be rehabilitated. This is currently unknown. However, elevated baseline IOP does not always predict a poor response to treatment. Of the seven eyes with baseline IOP > 40 mmHg, only one required more invasive glaucoma surgery. The mean baseline IOP for these seven eyes was 45.3 mmHg and they were using an average of 3.1 medications. Twelve months out from surgery, the mean IOP for these eyes was 16.7 mmHg and they were using an average of 2 medications. The rate of secondary surgical intervention for IOP control observed in this series over 18 months (6.7%) is comparable to that reported for other MIGS trials including trabecular bypass stents (4.7–8.1%).23–25 It is important to note that not all eyes had completed 18 months of follow-up by the end of this study and the rate of secondary surgical intervention of these patients once they have all reached 18 months after surgery may be higher than 6.7%. Study limitations include the relatively small number of standalone surgery eyes. Nevertheless, the standard deviation at 18 months for this group was similar to that for the overall group providing some confidence in the point estimate for the mean. With 72 eyes in the combined with cataract group, the results are reasonably robust. This study is also limited by the weaknesses inherent to retrospective studies including selection bias and variable follow-up. To mitigate against this potential bias, all eligible patients were included with minimal exclusion criteria. Both eyes from a given patient were included in the analysis when each eye met the eligibility criteria therefore complete independence of all variates was not satisfied. The consequence of this is potential underestimation of variability in the results; however, the measured standard deviation for the IOP at baseline is greater than generally seen in published studies (eg, 3 to 4 mmHg) arguing against such bias. Nevertheless, this potential bias should be considered when interpreting the results. In our view, the increased number of surgical cases and the consequent greater likelihood of revealing infrequent safety issues more than balances this weakness. The study does not attempt to explain how ab interno canaloplasty reduces IOP. There was no further investigation to understand why some eyes were not able to receive a full circumnavigation of 360 degrees.
Disclosure
Dr. Traynor and Mr Hughes received research grant support from Sight Sciences for this project. The authors report no other potential conflicts of interest for this work.
References
- 1.Allingham RR, Damji K, Freedman S, et al. Shields’ Textbook of Glaucoma. 5th Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 2.Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm’s canal for open angle glaucoma: ab-interno vs ab-externo canaloplasty with a tensioning suture. Clin Ophthalmol. 2018;12:2493–2498. doi: 10.2147/OPTH.S178962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd. 2018;32:223–227. doi: 10.1007/s00717-018-0416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gedde SJ, Singh K, Schiffman JC, Feuer WJ. The tube versus trabeculectomy study: interpretation of results and application to clinical practice. Curr Opin Ophthalmol. 2012;23(2):118–126. doi: 10.1097/ICU.0b013e32834ff2d1 [DOI] [PubMed] [Google Scholar]
- 5.Dickerson JE, Brown RH. Circumferential canal surgery: a brief history. Curr Opin Ophthalmol. 2020;31(2):139–146. doi: 10.1097/ICU.0000000000000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: A systematic review and meta-analysis. PLoS One. 2017;12:e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yook E, Vinod K, Panarelli JF. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(2):147–154. doi: 10.1097/ICU.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 8.Vastardis I, Fili S, Gatzioufas Z, Kohlhaas M. Ab externo canaloplasty results and efficacy: a retrospective cohort study with a 12-month follow-up. Eye Vis. 2019;6:9. doi: 10.1186/s40662-019-0134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg. 2009;35(5):814–824. doi: 10.1016/j.jcrs.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 10.Voykov B, Blumenstock G, Leitritz MA, et al. Treatment efficacy and safety of canaloplasty for open-angle glaucoma after 5 years. Clin Exp Ophthalmol. 2015;43(8):768–771. doi: 10.1111/ceo.12549 [DOI] [PubMed] [Google Scholar]
- 11.Cameron B, Field M, Ball S, Kearney J. Circumferential viscodilation of Schlemm’s canal with a flexible microcannula during non-penetrating glaucoma surgery. Digit J Ophthalmol. 2006;12(1). [Google Scholar]
- 12.Khaimi MA. Canaloplasty using itrack 250 microcatheter with suture tensioning on Schlemm’s canal. Middle East Afr J Ophthalmol. 2009;16(3):127–129. doi: 10.4103/0974-9233.56224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grieshaber MC, Pienaar A, Olivier J, et al. Canaloplasty for primary open-angle glaucoma: long-term outcome. Br J Ophthalmol. 2010;94(11):1478–1482. doi: 10.1136/bjo.2009.163170 [DOI] [PubMed] [Google Scholar]
- 14.Bull H, von Wolff K, Körber N, et al. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: european study results. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1537–1545. doi: 10.1007/s00417-011-1728-3 [DOI] [PubMed] [Google Scholar]
- 15.Lewis RA, Wolff K, Tetz M, et al. Canaloplasty: three-year results of circumferential viscodilation and tensioning of Schlemm canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37(4):682–690. doi: 10.1016/j.jcrs.2010.10.055 [DOI] [PubMed] [Google Scholar]
- 16.Koerber N. Canaloplasty in One Eye Compared With Viscocanalostomy in the Contralateral Eye in Patients With Bilateral Open-angle Glaucoma. J Glaucoma. 2012;21:129–134. [DOI] [PubMed] [Google Scholar]
- 17.Brusini P. Canaloplasty in Open-Angle Glaucoma Surgery: A Four-Year Follow-Up. Sci World J. 2014;2014:1–7. doi: 10.1155/2014/469609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riva I, Brusini P, Oddone F, et al. Canaloplasty in the Treatment of Open-Angle Glaucoma: A Review of Patient Selection and Outcomes. Adv Ther. 2019;36(1):31–43. doi: 10.1007/s12325-018-0842-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaimi MA. Canaloplasty: A minimally invasive and maximally effective glaucoma treatment. J Ophthalmol. 2015;2015:485065. doi: 10.1155/2015/485065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ondrejka S, Körber N. 360°ab-interno Schlemm’s canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol. 2019;13:1235–1246. doi: 10.2147/OPTH.S203917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tracer N, Dickerson JE, Radcliffe NM. Circumferential viscodilation ab interno combined with phacoemulsification for treatment of open-angle glaucoma: 12-month outcomes. Clin Ophthalmol. 2020;14:1357–1364. doi: 10.2147/OPTH.S252965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shingleton BJ, Pasternack JJ, Hung JW, O’Donoghue MW. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15:494–498. doi: 10.1097/01.ijg.0000212294.31411.92 [DOI] [PubMed] [Google Scholar]
- 23.Gonnermann J, Bertelmann E, Pahlitzsch M, et al. Contralateral eye comparison study in MICS & MIGS: trabectome® vs. iStent Inject® Graefes Arch Clin Exp Ophthalmol. 2017;255(2):359–365. doi: 10.1007/s00417-016-3514-8 [DOI] [PubMed] [Google Scholar]
- 24.Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Clinical evaluation of a trabecular microbypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767–1773. doi: 10.2147/OPTH.S114306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhann TJ. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41(12):2664–2671. doi: 10.1016/j.jcrs.2015.06.032 [DOI] [PubMed] [Google Scholar]