Abstract
Purpose
Dexamethasone and other corticosteroids are administered intravitreally to treat a variety of retinal diseases. As a side effect, they can alter intraocular pressure (IOP). The purpose of this study is to describe the incidence, severity, and management of ocular hypertension following the administration of multiple intravitreal injections of dexamethasone implants.
Materials and Methods
A total of 78 eyes of 78 subjects (males 62%; females 38%; mean age 67 ± 13 years SD) received a total of 152 intravitreal injections of 0.7 mg dexamethasone implants over 4 years. Indications included retinal vein occlusion (87%), diabetic macular edema (9%), wet-type age-related macular degeneration (4%). Ocular hypertension was defined as intraocular pressure above 23 mmHg or any pressure increase of 10 mmHg or more from baseline values. IOP was measured by applanation tonometry before the injection (T0), as well as one week (T1), one month (T2), and three months (T3) afterwards.
Results
Five percent (4/78) of subjects developed ocular hypertension after the 1st injection. On the second and third rounds, additional 7.2% (3/42) and 4.2% (1/24) of subjects developed the same side effect. Among the 8 subjects who received a fourth injection, none was found with OHT. Pressure elevations were detected at T2 and T3. In all patients, topical medical therapy was sufficient to lower the IOP below threshold. Mean pressure variations following the first injection as compared to previous recorded values were +0.97 mmHg (T1), +0.92 mmHg (T2), and −0.41 mmHg (T3) (p < 0.05). Mean pressure variations following the second injection were +0.54 mmHg (T1), +0.23 mmHg (T2) and −0.66 mmHg (T3) (p < 0.05).
Conclusion
Ocular hypertension is a recognized side effect of intravitreal dexamethasone. Some patients develop it right after the first injection, while others develop it subsequently, on the 2nd or 3rd round. This side effect becomes most apparent 30–90 days following the implantation procedure and responds well to topical pressure-lowering medications.
Keywords: intravitreal dexamethasone implant, Ozurdex injection, hypertension, side effect, complications, retinal vein occlusion
Introduction
Intravitreal corticosteroids are routinely prescribed in clinical practice. They can be used as first-line medication or when other treatments fail. Common indications include refractory macular edema resistant to anti-VEGF therapy,1–6 retinal vein occlusion (RVO),7–9 and chronic non-infectious uveitis,10 among others.
Dexamethasone is one of the corticosteroids available for intravitreal use. It is injected in the form of a biodegradable implant that slowly releases 0.35 mg or 0.7 mg of active drug into the vitreous over a period of about 6 months.11,12 However, this route of administration is associated with some side effects that include cataract formation and intraocular pressure (IOP) spikes. Ocular hypertension (OHT) can occur immediately after the injection due to volume expansion, or weeks to months later due to the steroid effects on aqueous drainage.13 Identifying and treating this side effect is important, as elevated IOP constitutes an important risk factor for primary open-angle glaucoma (POAG) incidence and progression.14
The effects of single intravitreal injections of dexamethasone implants on ocular pressure spikes have already been described in literature. The MEAD study showed that as many as 41.5% of patients receiving the 0.7 mg implant and 37.6% of patients receiving the 0.35 mg implant required pressure-lowering medications.15 The SAFODEX study showed similar results, with as many as 31% of eyes ultimately requiring pressure-lowering medications.16 In both studies, topical medications were generally effective in treating pressure spikes and incisional glaucoma surgery was performed in less than 1% of cases. In other studies, the incidence of this side effect was much lower.3,7,9,16–25 This probably depends on the threshold value set to define ocular hypertension and the population characteristics.26 Variables that increase the risk of secondary OHT include: pre-existing glaucoma, higher baseline IOP, younger age, previous episode of ocular hypertension following a steroid injection, uveitis, and higher steroid dose usage.27,28
In clinical practice, however, macular edema does not always resolve with a single injection and retreatment is often necessary. The effects of repeated injections on IOP have not been studied extensively. For this reason, authors provide additional data on the relationship between repeated intravitreal injections of dexamethasone implants and ocular pressure.
Materials and Methods
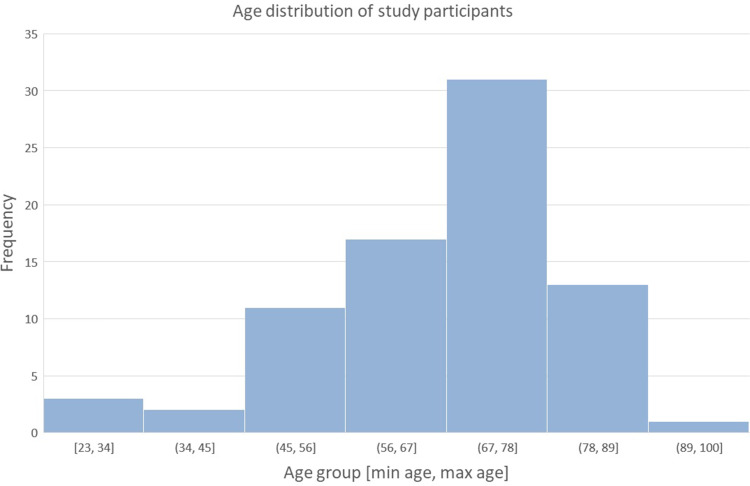
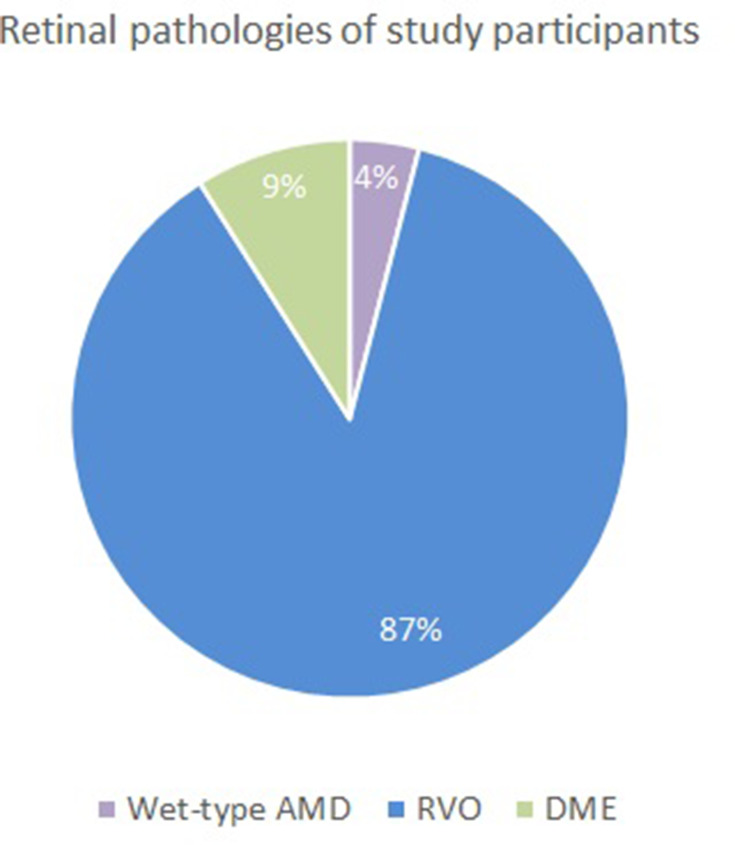
In this retrospective study, 78 eyes of 78 subjects (males 62%; females 38%; mean age 67 years ± 13 SD as depicted in Figure 1) affected by variable degrees of macular edema caused by retinal vein occlusion (87%), diabetic macular edema (9%), and wet-type age-related macular degeneration (wet-AMD) (4%) (Figure 2). In wet-AMD subjects, macular edema was unresponsive to anti-VEGF injections. All patients had within-range IOP upon enrollment. Two patients (2.56%) were taking pressure-lowering medications for early-stage POAG.
Figure 1.
Age distribution of participants. Mean age 67 ± 13 years SD.
Figure 2.
Retinal pathologies of enrolled subjects. Retinal vein occlusion (RVO) in 87% of subjects. Diabetic macular edema (DME) in 9% of subjects. Wet-type age-related macular degeneration (Wet-type AMD) that was refractory to anti-VEGF therapy in 4% of subjects.
Ethical Approval
This study protocol was approved by “La Sapienza, University of Rome”, Ethics Board (Protocol No. 1076/14).
Inclusion Criteria
Age > 18;
Macular edema caused by retinal vein occlusion, wet-AMD, or diabetic retinopathy;
BCVA < 45 letters ETDRS, equivalent to a LogMAR value between 1 and 0.2;
Central macular thickness on OCT ≥ 285 µm;
Treatment of naïve patients;
Exclusion Criteria
Previous laser treatments or intravitreal procedures;
Cataract;
Glaucoma;
Epiretinal membrane visible on OCT;
Any ocular surgery performed within the previous 6 months;
Pregnancy;
Informed Consent
All subjects signed written consent.
First Assessment and Follow-Ups
The following evaluations were performed upon enrollment:
BCVA measurement on a decimal scale, as well as on ETDRS and logMAR scales;
Intraocular pressure measurement with Goldmann applanation tonometer;
Slit-lamp biomicroscopy of both anterior and posterior segments;
Macular thickness evaluation with Heidelberg Spectralis SD-OCT, fast macula protocol;
Patients were re-evaluated one week (T1), one month (T2) and three months (T3) after each injection in the same fashion.
Implantation Procedure
The implant was injected in the inferotemporal vitreous cavity through the 22-gauge needle of the disposable device. Topical antibiotic/steroid medication was given for 3 days after the implant procedure.
Definition of Ocular Hypertension
Ocular hypertension was defined as intraocular pressure above 23 mmHg or any pressure increase of 10 mmHg or more from baseline values.
Macular Thickness Evaluation with SD-OCT
Macular scans were obtained using the fast macula protocol on the Heidelberg Spectralis SD-OCT. Automatic real time (ART) mean value of 9, which acquires 25 horizontal lines (6 x 6 mm area), each consisting of 1024 A scans per line. The central retinal thickness (CRT) was defined as the distance between the inner limiting membrane to the outer border of the retinal pigment epithelium via the automatic segmentation algorithms.
Statistical Analysis
Data was collected using Microsoft Excel spreadsheets (Microsoft Excel 2010). Statistical analysis was performed using SPSS (IBM SPSS Version 20, IBM, New York, NY, USA). Statistical correlation was determined using the Friedmann test. A P-value < 0.05 was considered significant.
Results
A total of 152 intravitreal injections were performed on 78 eyes. Forty-four (56%) of these subjects received a second intravitreal injection. Twenty-four (31%) received a third injection. Only 8 (10%) received a fourth injection. Repeat injections were performed at least 3 months apart.
Ocular hypertension was defined as >23 mmHg or an increase of 10 mmHg or more from baseline. All patients had within-range IOP upon enrollment. Two patients (2.56%) were taking pressure-lowering medications for POAG.
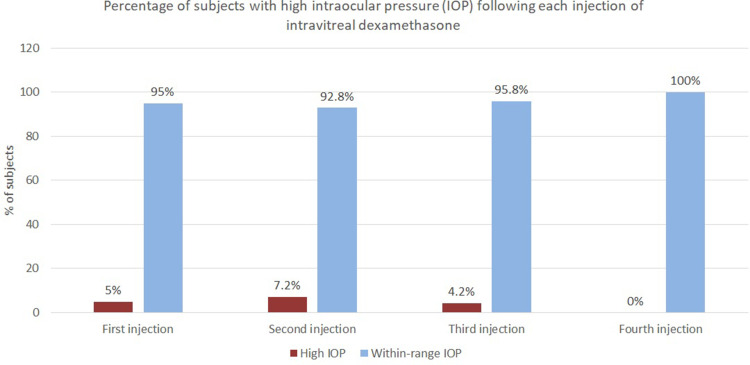
As shown in Figure 3, around 5% (4 out of 78), 7.2% (3 out of 42), and 4.2% (1 out of 24) of patients developed ocular hypertension after, respectively, the first, second, and third injection. The greatest pressure spikes were found 4–12 weeks following each procedure. The highest spike was 38 mmHg recorded one month after the first injection. This represented an increase of 24 mmHg from baseline. This was from a 23-year-old male diagnosed with BRVO, with the following IOP values: 14 mmHg at baseline (T0), 16 mmHg at T1, 38 mmHg at T2, and 26 mmHg at T3. This patient was prescribed hypotensive therapy at time T2.
Figure 3.
Percentage of subjects with high versus normal IOP after each injection.
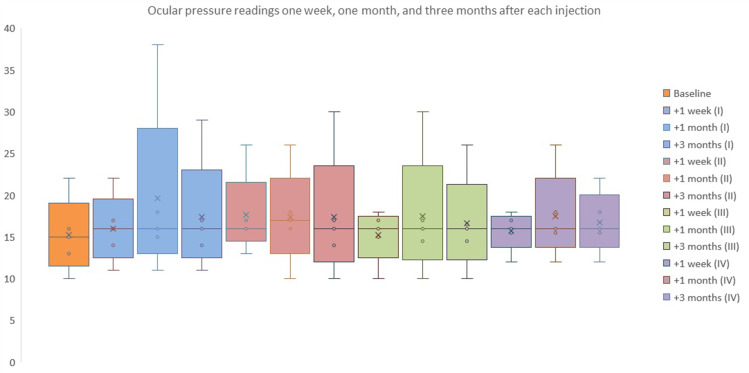
Figure 4 shows mean, median, interquartile ranges at baseline and T1, T2, and T3 after each injection. Mean pressure changes after the first injection as compared to previous recorded value: +0.97 mmHg, +0.92 mmHg, and −0.41 mmHg on T1, T2, and T3, respectively (p < 0.05). Mean pressure changes after the second injection as compared to previous recorded value: +0.54 mmHg, +0.23 mmHg and −0.66 mmHg (p < 0.05).
Figure 4.
Median values and quartiles range at baseline (T0) and one week (T1), one month (T2), and three months (T3) after each injection.
Subjects who developed ocular hypertension (8 out of 78, 10%) were prescribed pressure-lowering drops and all of them responded successfully to medical therapy. The hypotensive drops used were either of the following: timolol, brimonidine and timolol, tafluprost, or bimatoprost. Choices were made on a case-by-case basis depending on the severity of ocular hypertension, as well as the patient’s preferences and underlying systemic comorbidities. Subjects with ocular hypertension were kept on pressure-lowering medications if retreatment was indicated. This explains a decreasing percentage of subjects found with ocular hypertension on subsequent injections, as shown in Figure 3. No glaucoma surgery or laser procedure was performed. IOP spikes did not affect visual acuity or visual fields. No RNFL thickness changes were noted on follow-up OCT RNFL exams.
The two subjects with POAG showed a mild IOP increase. Even if their IOP values remained below the chosen threshold of 23 mmHg, hypotensive therapy in these patients was stepped up to maintain their ocular pressure values similar to baseline.
Discussion
Previous studies have shown that common side effects of intravitreal dexamethasone implants include intraocular hypertension, cataract formation, and more rarely, anterior dislocation.29 The incidence of ocular hypertension varies significantly from study to study, from 6% to almost 50%.3,7,9,16–25 This difference in incidence probably depends on several factors, which include patients’ characteristics, their pre-existing comorbidities, as well as the pressure threshold set to define ocular hypertension. In our study, the cumulative percentage of subjects who developed ocular hypertension after single or multiple dexamethasone injections was 10% (8 out of 78). RVO was the main indication (87%). Table 1 shows a comparison with previous studies. In general, IOP elevation is transient and reaches peak values around 30–60 days following the injection.23 Topical medical therapy is almost always sufficient.15,16,30 Return to baseline values is expected within six months.26
Table 1.
Comparison with Previous Studies
| Study | Number of Eyes Treated | Indications | Definition of OHT | Percentage of Participants Who Developed OHT |
|---|---|---|---|---|
| Chin et al (2017)33 | 59 | BRVO, CRVO, uveitis, DME, CME | IOP ≥ 30 mmHg or an increase of ≥ 10 mmHg from baseline | 26.9% |
| Malclès et al (2017)16 | 421 | RVO, DME, CME, uveitis | IOP ≥ 25 mmHg or an increase of ≥ 10 mmHg from baseline | 28.5% |
| Choi et al (2019)23 | 540 | RVO, DME, CME, uveitis | IOP ≥ 25 mmHg or an increase of ≥ 10 mmHg from baseline | 12.6% |
| Mayer et al (2013)21 | 64 | BRVO, CRVO | Increase of 5 mmHg from baseline | 39.5% |
| Bahadorani et al (2018)32 | 183 | BRVO, CRO, DME, uveitis | IOP ≥ 23 mmHg | 31% |
| Haller et al (2010)31 | 841 | BRVO, CRVO | IOP ≥ 25 mmHg | 16% |
| Hemarat et al (2018)34 | 260 | DME, BRVO, CRVO, uveitis, others | IOP ≥ 25 mmHg | 26.2% |
| Sudhalkar et al (2018)35 | 378 | DME, BRVO, CRVO, CME | IOP ≥ 25 mmHg or an increase of ≥ 10 mmHg from baseline | 23.27% |
| Bakri et al (2016)17 | 31 | BRVO, CRVO | IOP ≥22 mmHg | 45% |
| Reid et al (2015)19 | 61 | BRVO, CRVO | IOP ≥ 25 mmHg | 12% |
| This study | 78 | BRVO, CRVO, DME, wet-AMD | IOP ≥ 23 mmHg or an increase of ≥ 10 mmHg from baseline | 10% |
Abbreviations: OHT, ocular hypertension; IOP, intraocular pressure; BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; DME, diabetic macular edema; Wet-AMD, wet-type age-related macular degeneration; CME, cystoid macular edema.
In patients receiving multiple injections, some studies showed no correlation between the number of injections and the risk of developing ocular hypertension,19,31 while one study found an increased risk for IOP spikes below 30 mmHg.32 Our study showed a higher mean IOP after the second injection. This increase was low, but statistically significant (P <0.05). In addition, among patients who did not experience IOP spikes after the first injection, some did after the second or third implantation. Pressure spikes responded well to topical medical therapy in all cases.
Key Messages
1. What is already known about this subject?
Steroids injected in the vitreous body of the eye can increase intraocular pressure in susceptible subjects. In some patients, this effect becomes apparent after multiple injections.
2. What are the new findings?
Subjects who did not develop ocular pressure spikes following the first injection are still at risk for ocular hypertension on subsequent injections.
3. How might these results change the focus of research or clinical practice?
Subjects who receive intravitreal dexamethasone should be always regularly monitored for ocular pressure spikes, regardless of their pre-injection ocular pressure or response to their first injection. With dexamethasone implants, the greatest pressure spikes can be generally expected one month following the injection.
Pressure spikes that develop on subsequent injections can be effectively managed with topical pressure-lowering medications.
Disclosure
The authors have no financial disclosure or conflicts of interest, direct or indirect, to declare, and they had full access to all data in the study.
References
- 1.Khan Z, Kuriakose RK, Khan M, Chin EK, Almeida DRP. Efficacy of the intravitreal sustained-release dexamethasone implant for diabetic macular edema refractory to anti-vascular endothelial growth factor therapy: meta-analysis and clinical implications. Ophthalmic Surg Lasers Imaging Retina. 2017;48:160–166. doi: 10.3928/23258160-20170130-10 [DOI] [PubMed] [Google Scholar]
- 2.Pacella F, Romano MR, Turchetti P, et al. An eighteen-month follow-up study on the effects of Intravitreal Dexamethasone Implant in diabetic macular edema refractory to anti-VEGF therapy. Int J Ophthalmol. 2016;9:1427–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacella F, Ferraresi AF, Turchetti P, et al. Intravitreal injection of ozurdex(®) implant in patients with persistent diabetic macular edema, with six-month follow-up. Ophthalmol Eye Dis. 2016;8:11–16. doi: 10.4137/OED.S38028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacella E, La Torre G, Impallara D, et al. Efficacy and safety of the intravitreal treatment of diabetic macular edema with pegaptanib: a 12-month follow-up. Clin Ter. 2013;164:e121–6. [DOI] [PubMed] [Google Scholar]
- 5.Pacella E, Pacella F, La Torre G, et al. Testing the effectiveness of intravitreal ranibizumab during 12 months of follow-up in venous occlusion treatment. Clin Ter. 2012;163:e413–22. [PubMed] [Google Scholar]
- 6.Bianchi E, Scarinci F, Ripandelli G, et al. Retinal pigment epithelium, age-related macular degeneration and neurotrophic keratouveitis. Int J Mol Med. 2013;31:232–242. doi: 10.3892/ijmm.2012.1164 [DOI] [PubMed] [Google Scholar]
- 7.Garweg JG, Zandi S. Retinal vein occlusion and the use of a dexamethasone intravitreal implant (Ozurdex®) in its treatment. Graefes Arch Clin Exp Ophthalmol. 2016;254:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn P, Fekrat S. Best practices for treatment of retinal vein occlusion. Curr Opin Ophthalmol. 2012;23:175–181. doi: 10.1097/ICU.0b013e3283524148 [DOI] [PubMed] [Google Scholar]
- 9.Pacella F, La Torre G, Basili S, et al. Comparison between “early” or “late” intravitreal injection of dexamethasone implant in branch (BRVO) or central (CRVO) retinal vein occlusion: six-months follow-up. Cutan Ocul Toxicol. 2017;36:224–230. doi: 10.1080/15569527.2016.1254648 [DOI] [PubMed] [Google Scholar]
- 10.Whitcup SM, Robinson MR. Development of a dexamethasone intravitreal implant for the treatment of noninfectious posterior segment uveitis. Ann N Y Acad Sci. 2015;1358:1–12. doi: 10.1111/nyas.12824 [DOI] [PubMed] [Google Scholar]
- 11.Haghjou N, Soheilian M, Abdekhodaie MJ. Sustained release intraocular drug delivery devices for treatment of uveitis. J Ophthalmic Vis Res. 2011;6:317–329. [PMC free article] [PubMed] [Google Scholar]
- 12.Chang-Lin J-E, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–86. doi: 10.1167/iovs.10-5285 [DOI] [PubMed] [Google Scholar]
- 13.Taylor SRJ, Isa H, Joshi L, Lightman S. New developments in corticosteroid therapy for uveitis. Ophthalmologica. 2010;224(Suppl 1):46–53. doi: 10.1159/000318021 [DOI] [PubMed] [Google Scholar]
- 14.Actis AG, Versino E, Brogliatti B, Rolle T. Risk factors for Primary Open Angle Glaucoma (POAG) progression: a study ruled in Torino. Open Ophthalmol J. 2016;10:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 16.Malclès A, Dot C, Voirin N, et al. Safety of intravitreal dexamethasone implant (OZURDEX): the SAFODEX study incidence and risk factors of ocular hypertension. Retina. 2017;37:1352–1359. [DOI] [PubMed] [Google Scholar]
- 17.Bakri SJ, Omar AF, Iezzi R, Kapoor KG. Evaluation of multiple dexamethasone intravitreal implants in patients with macular edema associated with retinal vein occlusion. Retina. 2016;36:552–557. doi: 10.1097/IAE.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 18.Proença Pina J, Turki K, Labreuche J, Duhamel A, Tran THC. Efficacy and safety in retinal vein occlusion treated with at least three consecutive intravitreal dexamethasone implants. J Ophthalmol. 2016;2016:6016491. doi: 10.1155/2016/6016491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid GA, Sahota DS, Sarhan M. Observed complications from dexamethasone intravitreal implant for the treatment of macular edema in retinal vein occlusion over 3 treatment rounds. Retina. 2015;35:1647–1655. doi: 10.1097/IAE.0000000000000524 [DOI] [PubMed] [Google Scholar]
- 20.Tservakis I, Koutsandrea C, Papaconstantinou D, Paraskevopoulos T, Georgalas I. Safety and efficacy of dexamethasone intravitreal implant (Ozurdex) for the treatment of persistent macular edema secondary to retinal vein occlusion in eyes previously treated with anti-vascular endothelial growth factors. Curr Drug Saf. 2015;10:145–151. doi: 10.2174/1574886309666140805142245 [DOI] [PubMed] [Google Scholar]
- 21.Mayer WJ, Wolf A, Kernt M, et al. Twelve-month experience with Ozurdex for the treatment of macular edema associated with retinal vein occlusion. Eye. 2013;27:816–822. doi: 10.1038/eye.2013.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra SK, Gupta A, Patyal S, et al. Intravitreal dexamethasone implant versus triamcinolone acetonide for macular oedema of central retinal vein occlusion: quantifying efficacy and safety. Int J Retina Vitreous. 2018;4:13. doi: 10.1186/s40942-018-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi W, Park SE, Kang HG, et al. Intraocular pressure change after injection of intravitreal dexamethasone (Ozurdex) implant in Korean patients. Br J Ophthalmol. 2019;103(10):1380–1387. doi: 10.1136/bjophthalmol-2018-312958 [DOI] [PubMed] [Google Scholar]
- 24.Pacella E, Vestri AR, Muscella R, et al. Preliminary results of an intravitreal dexamethasone implant (Ozurdex®) in patients with persistent diabetic macular edema. Clin Ophthalmol. 2013;7:1423–1428. doi: 10.2147/OPTH.S48364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korobelnik J-F, Kodjikian L, Delcourt C, et al. Two-year, prospective, multicenter study of the use of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in the clinical setting in France. Graefes Arch Clin Exp Ophthalmol. 2016;254:2307–2318. doi: 10.1007/s00417-016-3394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacella F, Turchetti P, Santamaria V, et al. Differential activity and clinical utility of latanoprost in glaucoma and ocular hypertension. Clin Ophthalmol. 2012;6:811–815. doi: 10.2147/OPTH.S13777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacella E, Pacella F, Cavallotti C, Librando A, Feher J, Pecori-Giraldi J. The combination latanoprost-timolol versus twice daily 0.50% timolol administration either associated or not with latanoprost: efficacy and tolerability in the primary open-angle glaucoma. Eur Rev Med Pharmacol Sci. 2010;14:477–480. [PubMed] [Google Scholar]
- 28.Feher J, Kovacs I, Pacella E, Keresz S, Spagnardi N, Balacco Gabrieli C. Pigment epithelium-derived factor (PEDF) attenuated capsaicin-induced neurotrophic keratouveitis. Invest Ophthalmol Vis Sci. 2009;50:5173–5180. doi: 10.1167/iovs.08-1852 [DOI] [PubMed] [Google Scholar]
- 29.Pacella F, Agostinelli E, Carlesimo SC, et al. Management of anterior chamber dislocation of a dexamethasone intravitreal implant: a case report. J Med Case Rep. 2016;10:282. doi: 10.1186/s13256-016-1077-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J Pharmacol Sci. 2018;138:219–232. doi: 10.1016/j.jphs.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 31.Haller JA, Bandello F, Jr BR, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146.e3. doi: 10.1016/j.ophtha.2010.03.032 [DOI] [PubMed] [Google Scholar]
- 32.Bahadorani S, Krambeer C, Wannamaker K, et al. The effects of repeated Ozurdex injections on ocular hypertension. Clin Ophthalmol. 2018;12:639–642. doi: 10.2147/OPTH.S148990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin EK, Almeida DRP, Velez G, et al. Ocular hypertension after intravitreal dexamethasone (Ozurdex) sustained-release implant. Retina. 2017;37(7):1345–1351. doi: 10.1097/IAE.0000000000001364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemarat K, Kemmer JD, Porco TC, Eaton AM, Khurana RN, Stewart JM. Secondary ocular hypertension and the risk of glaucoma surgery after dexamethasone intravitreal implant in routine clinical practice. Ophthalmic Surg Lasers Imaging Retina. 2018;49(9):680–685. doi: 10.3928/23258160-20180831-05 [DOI] [PubMed] [Google Scholar]
- 35.Sudhalkar A, Kodjikian L, Chhablani J, Bhojwani D, Vasavada A. Intraocular dexamethasone implant position in situ and ocular hypertension. Retina. 2018;38(12):2343–2349. doi: 10.1097/IAE.0000000000001883 [DOI] [PubMed] [Google Scholar]