Abstract
Background
Although tobacco exposure remains the most important risk factor of tumorigenesis of small cell lung cancer (SCLC), its prognostic value has failed to reach a consensus until now. Accordingly, we conducted a meta‐analysis to investigate the prognostic value of pretreatment smoking status (smokers vs. never‐smokers) in SCLC.
Methods
The four databases PubMed, Medline, Embase, and Cochrane library were searched to identify the relevant literature from the inception dates to 24 June 2020. The primary outcome was overall survival (OS), and the secondary endpoint was progression‐free survival (PFS). The hazard ratios (HRs) with 95% confidence intervals (CIs) were extracted to assess the relationship between pretreatment smoking status and patient survival. Sensitivity analysis was performed to assess the stability of the pooled results. Begg's funnel plot and Egger's test were applied to detect the publication bias. All statistical analyses were performed using RevMan V.5.3 and STATA version 15.0 software.
Results
A total of 27 studies involving 12 047 patients with SCLC (9137 smokers and 2910 never‐smokers) were included in this meta‐analysis. The results showed that smoking history was closely related to poorer survival outcome (OS: HR = 1.17, 95% CI: 1.12–1.23, P < 0.00001; I 2 = 0%; PFS: HR = 1.20, 95% CI: 1.06–1.35, P = 0.004; I 2 = 0%).
Conclusions
Smoking history should be considered as an independent poor prognostic factor for patients with SCLC. More large‐scale prospective studies are warranted to testify the prognostic value of pretreatment smoking status.
Keywords: Meta‐analysis, prognosis, small cell lung cancer, smoking
We conducted a meta‐analysis of 27 studies involving 12047 patients to investigate the prognostic value of pretreatment smoking status for small cell lung cancer (SCLC), which demonstrated that smoking history was closely related to poorer survival outcome. Smoking history should be considered as an independent poor prognostic factor for patients with SCLC.
Introduction
According to GLOBOCAN 2018, lung cancer ranks as the leading cause of death worldwide. 1 Small cell lung cancer (SCLC) is one of the most aggressive and lethal type which accounts for approximately 15% of patients diagnosed with lung cancer. 2 In the era of precision medicine, the ability to precisely evaluate prognosis is of significant assistance in order to improve survival outcome of patients with SCLC. For decades, numerous retrospective studies and some meta‐analyses have been conducted to investigate poor prognostic factors of SCLC, factors like distant metastases (extensive stage) 3 and tumor biomarkers (eg, neuron‐specific enolase [NSE]) 4 have all been proposed as poor prognostic indicators in SCLC patients.
The tumorigenesis of lung cancer, especially SCLC, is strongly linked to tobacco exposure. Based on large‐population‐based epidemiological data, 97.5% of 4782 patients with SCLC were found to be current or ever smokers. 5 Furthermore, smoking history is a popular item to be extracted in studies for prognostic analysis of SCLC, but results are contradictory and no consensus has been reached until now. Herein, we conducted a systemic review and meta‐analysis to evaluate the prognostic value of pretreatment smoking status for patients with SCLC.
Methods
Search strategy
The four databases PubMed, Medline, Embase, and Cochrane library were searched to identify the relevant literature from the inception dates to 24 June 2020 based on the combination of two search words: “small cell lung cancer NOT non‐small cell lung cancer” and “prognosis OR prognostic factors”. The range was then greatly narrowed by screening studies under the inclusion criteria of containing smoking status and its association with overall survival (OS) and/or progression‐free survival (PFS). Furthermore, the reference lists of every relevant article were checked with an expectation that additional articles would be highlighted for inclusion. The protocol of this meta‐analysis was open on PROSPERO, the International Prospective Register of Systematic Reviews (CRD42020194028).
Inclusion and exclusion criteria
The inclusion criteria were as follows: (i) studies that enrolled patients who were histologically or cytologically confirmed to have SCLC (both limited‐stage or extensive‐stage were allowed); (ii) contained pretreatment smoking status of all patients; (iii) contained evaluation of the association between with smoking status and OS and/or PFS, the hazard ratio (HR) and 95% confidence interval (95% CI) or P‐value were available; and (iv) the language of the document was English.
The exclusion criteria were as follows: (i) reviews, meta‐analyses, case reports, and conference reports; (ii) duplications; (iii) studies without analysis of prognostic value of pretreatment smoking status SCLC; and (iv) studies where necessary effect data was unable to be obtained from the text.
Data extraction and quality assessment
Data including first author, publication year, number of patients, numbers of smokers and non‐smokers, country, ethnicity, median age and range, percentage of limited‐disease and extensive‐disease SCLC, percentage of male and female patients, treatments (chemotherapy, radiotherapy, surgery), types of survival analysis (univariate or multivariant) and outcome (in particular, HRs and their 95% CIs for OS and PFS, P‐value). The primary outcome was OS, and the secondary endpoint was PFS. In general, patients with a documented smoking history (current or former smokers) were classified as smokers, and those without any documented smoking history were classified as never‐smokers.
Quality assessment was performed by the Newcastle‐Ottawa Scale (NOS), consisting of three domains: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). Two independent reviewers evaluated the risk of bias of each study, and disagreement was resolved by discussion or consultation with an independent third reviewer. A study was considered to be of high quality if its NOS score was six or higher.
Statistical analysis
The primary outcome was overall survival (OS), and the secondary endpoint was progression‐free survival (PFS). The effect sizes namely HR and 95% CI or P‐value of the dichotomous variable (“smokers” or “never‐smokers” of pretreatment smoking status) were extracted from each study and pooled to assess the prognostic value of pretreatment smoking status for SCLC. Cochran's Q test and Higgins' I 2 statistic were performed to evaluate the heterogeneity of the included studies based on I 2 and P‐values. If I 2 was ≤50% and P‐value was >0.10, the heterogeneity in different included studies was acceptable and we chose a fixed‐effects model to combine all studies, otherwise a random‐effects model was used. We also conducted sensitivity analysis to assess the influence of each study on the overall analysis and final result. Publication bias was assessed by Begg's funnel plot and Egger's linear regression. When the combined HR was >1, the range of 95% CI did not cross 1, and for two‐tailed P‐values <0.05, the result was considered statistically significant and served as an adverse prognostic factor. All statistical analyses were performed using the Review Manager software (RevMan V.5.3, Cochrane Collaboration, London, UK) and Stata/SE version 15.0 for Windows (Stata Corporation, College Station, TX, USA).
Results
Study research
A total of 8137 records were retrieved from four databases (1352 from PubMed, 4158 from Medline, 2420 from Embase, and 2017 from the Cochrane library). After removing duplicated publications and screening titles or abstracts, 241 full text relevant articles were reviewed for eligibility, and 27 studies met the inclusion criteria and were finally included in our analysis. The process of identification for eligible articles is shown in Fig 1.
Figure 1.
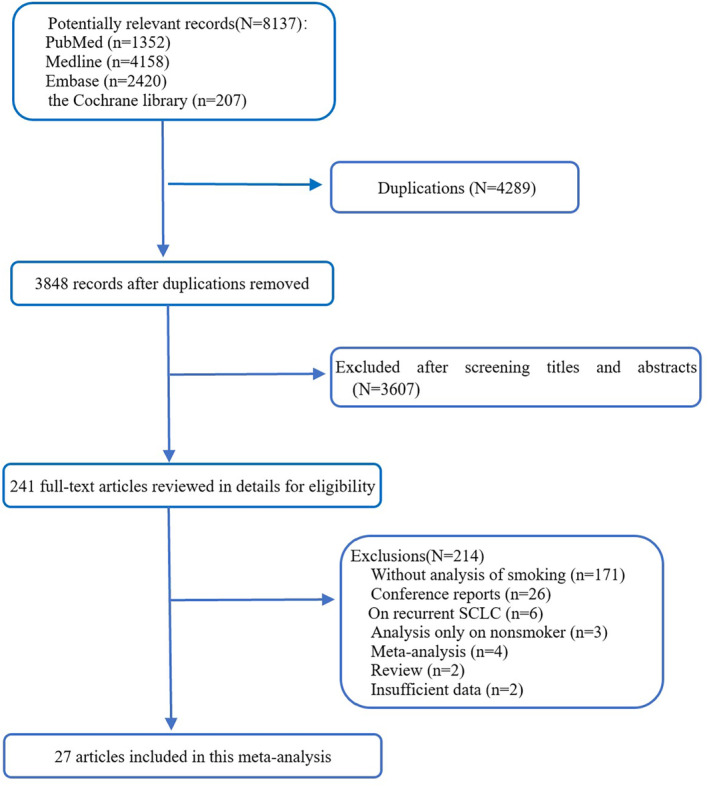
Flow diagram of included studies.
Study characteristics
In total, 12 047 patients with SCLC (9137 smokers and 2910 never‐smokers) in 27 studies were included in this meta‐analysis. All studies were retrospective and had been published between 2009 and 2020 with a NOS score of six or higher. Most of the enrolled studies (26/27) were from an Asian population, and among them 24 studies were from China. [Correction added on 13 October 2020, after first online publication: the preceding sentence has been amended.] Pretreatment smoking status were analyzed by both univariate and multivariate analysis in 12 studies, and the multivariate results extracted, while in the remaining 15 studies only univariate outcomes were available, the results of which were extracted. Effect sizes of correlation between pretreatment smoking status and OS were reported in all 27 studies, and those between smoking status and PFS were available in five studies. Other characteristics of the incorporated literatures are presented in details in Table 1.
Table 1.
Characteristics of all included studies in the meta‐analysis
| First author | Year | Study design | Country | E | N | Smokers (%) | LD‐SCLC (%) | Male (%) | Age (mean, range) | Treatment | Outcome | Analysis type | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. 6 | 2020 | R | China | A | 219 | 81.74% | 0% | 87.90% | 60.5 | C, R | O | U | 7 |
| Yilmaz et al. 7 | 2020 | R | Japan | A | 216 | 56.94% | 27.30% | 85.20% | NA | C, R | O | U | 7 |
| Xu et al. 8 | 2020 | R | China | A | 136 | 61.03% | 47.80% | 76.50% | 61.6 | C, R | O | U | 6 |
| Wu et al. 9 | 2020 | R | China | A | 146 | 73.97% | 40.40% | 78.10% | 57 (19–74) | C, R | O | U | 7 |
| Wang et al. 10 | 2020 | R | China | A | 653 | 62.48% | 58.50% | 64.60% | NA | C, R | O | M | 8 |
| Wang et al. 11 | 2019 | R | China | A | 228 | 79.39% | 50% | 69.70% | 58 (39–71) | C, R | O | U | 7 |
| Li et al. 12 | 2019 | R | China | A | 122 | 54.92% | 100% | 68.90% | 58 | C, R | O, P | U | 7 |
| Liu et al..13 | 2018 | R | China | A | 303 | 77.23% | 37.30% | 90.10% | 63 | C, R | O, P | M | 9 |
| Jin et al. 14 | 2018 | R | China | A | 1156 | 57.96% | 61.90% | 64.40% | 57 (23–85) | C, R | O | M | 8 |
| Guo et al. 15 | 2018 | R | China | A | 128 | 61.72% | 52% | 55.50% | 62 (30–83 | C, R | O | U | 7 |
| Fan et al. 16 | 2018 | R | China | A | 120 | 72.50% | 39.20% | 71.70% | 63.2 | C, R | O | U | 7 |
| Hong et al. 17 | 2018 | R | China | A | 936 | 66.03% | 59.10% | 69.30% | NA | C, R | O, P | M | 8 |
| Pan et al. 18 | 2017 | R | China | A | 275 | 70.18% | 54% | 87% | 62 (33–86) | C, R | O | M | 8 |
| Jiang et al. 19 | 2017 | R | China | A | 107 | 87.85% | 39.30% | 78.50% | 63 | C, R | O | U | 7 |
| Deng et al. 20 | 2017 | R | China | A | 320 | 67.19% | 38.10% | 74.70% | 58 (24–81) | C, R | O, P | U | 7 |
| Chen et al. 21 | 2016 | R | China | A | 393 | 71.76% | 39.90% | 81.90% | 57 | C, R | O, P | U | 7 |
| Cai et al. 22 | 2016 | R | China | A | 144 | 68.06% | 40.50% | 88.50% | 60 (25–80) | C, R | O | M | 8 |
| Xie et al. 23 | 2015 | R | China | A | 383 | 73.11% | 100% | 47.50% | 66.7 | C, R | O | M | 7 |
| Sun et al. 24 | 2015 | R | China | A | 391 | 87.21% | 46.30% | 85.40% | 65 | C, R | O | M | 8 |
| Liu et al. 25 | 2015 | R | China | A | 247 | 67.21% | 52.20% | 81.40% | 70.7 (65–83) | C, R | O | U | 7 |
| Hong et al. 26 | 2015 | R | China | A | 919 | 61.70% | 60.10% | 69.10% | NA | C, R | O | M | 7 |
| Hong et al. 27 | 2015 | R | China | A | 724 | 82.87% | 55.40% | 86.60% | 59 (19–86) | C, R | O | M | 7 |
| Liu et al. 28 | 2013 | R | China | A | 485 | 67.84% | 44.50% | 74.00% | NA | C, R | O | U | 7 |
| Wu et al..29 | 2012 | R | China | A | 200 | 77.50% | 0% | 62.40% | NA | C, R | O | U | 6 |
| Chen et al. 30 | 2010 | R | China | A | 264 | 97.35% | 100% | N | 65 | C, R | O | M | 8 |
| Arinc et al. 31 | 2010 | R | Turkey | A | 200 | 92.00% | 55.80% | 91.40% | 57 (35–78) | C, R | O | U | 7 |
| Ou et al. 5 | 2009 | R | multiple countries | C, A | 2632 | 96.47% | 0% | 53.40% | 68 | C, R, S | O | M | 8 |
A, Asian; C, Caucasian; E, ethnicity; M, multivariate analysis; NA, not available; NOS, Newcastle‐Ottawa Scale; O, overall survival; P, progression‐free survival.; R, retrospective study; U, univariate analysis.[Correction added on 13 October 2020, after first online publication: in Arinc et al. 2010, Ethnicity has been updated from ‘C’ to ‘A’. In Ou et al. 2009, Country and Ethnicity have been updated from ‘China’ to ‘multiple countries’ and ‘C’ to ‘C, A’.]
Correlation between pretreatment smoking status and survival of SCLC
All 27 studies provided data on effect size of pretreatment smoking status for OS of patients with SCLC. As presented in Fig 2a, smoking history predicted a poor OS outcome of SCLC with a HR of 1.17 (95% CI: 1.12–1.23, P < 0.00001; I 2 = 0%).
Figure 2.
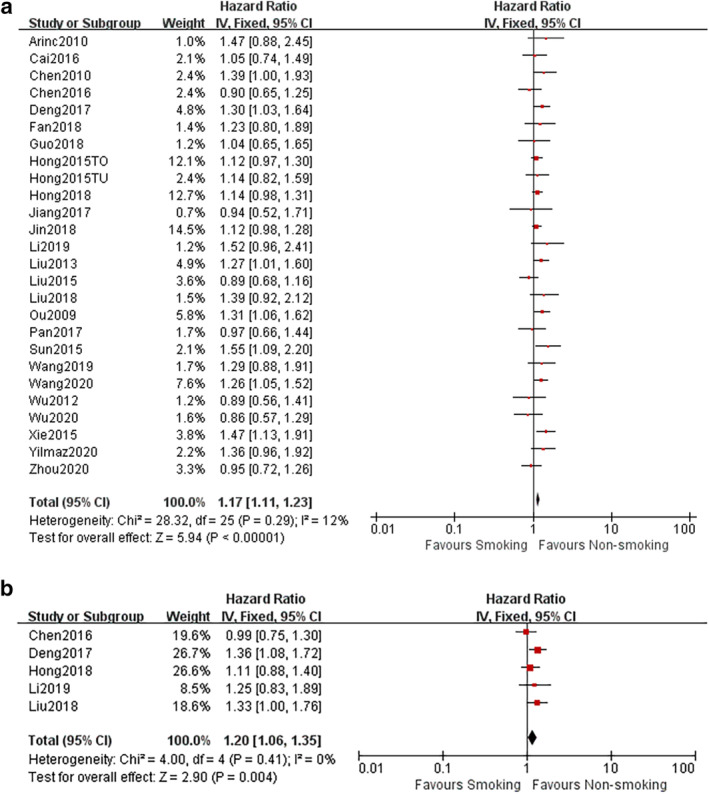
(a) Forest plots of HRs for the association of pretreatment smoking status with overall survival (OS) of patients with SCLC. (b) Forest plots of HRs for the association of pretreatment smoking status with progression‐free survival (PFS) outcome of patients with SCLC. [Correction added on 2 October, after first online publication: figure 2a has been amended.]
Furthermore, pooled data of five studies were available to analyze the impact of smoking history to PFS of SCLC, which again identified the passive prognostic value of pretreatment smoking status (HR = 1.20, 95% CI: 1.06–1.35, P = 0.004; I 2 = 0%) (Fig 2b).
Sensitivity analysis
We conducted a sensitivity analysis to assess the influence of each study on the overall analysis and final results, which showed that any of the studies could be removed without exerting a significant impact on the combined HRs (Fig 3). These findings indicate the favorable stability of our pooled results.
Figure 3.
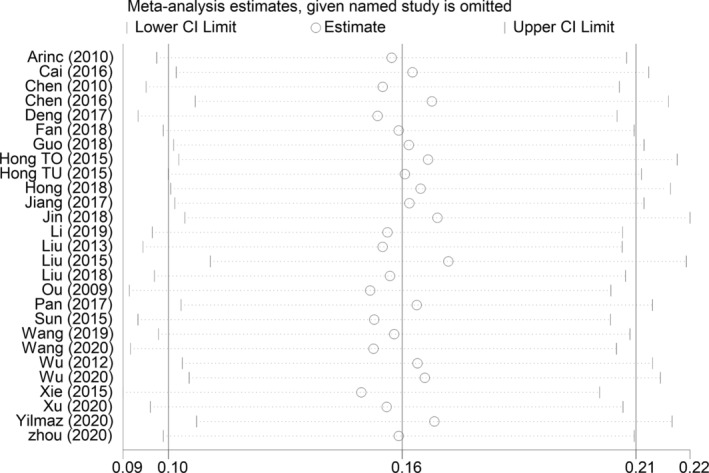
Sensitivity analysis of the included studies.
Publication bias
Publication bias were evaluated in the Begg's funnel plot and Egger's linear regression test, both of which similarly identified no significant publication bias existed in this meta‐analysis: the Begg's funnel plot was symmetrical with a P‐value of 0.868for OS and 0.806 for PFS, respectively (Fig 4a); and in the Egger's test, P‐value was 0.812for OS and 0.966 for PFS, respectively (Fig 4b).
Figure 4.
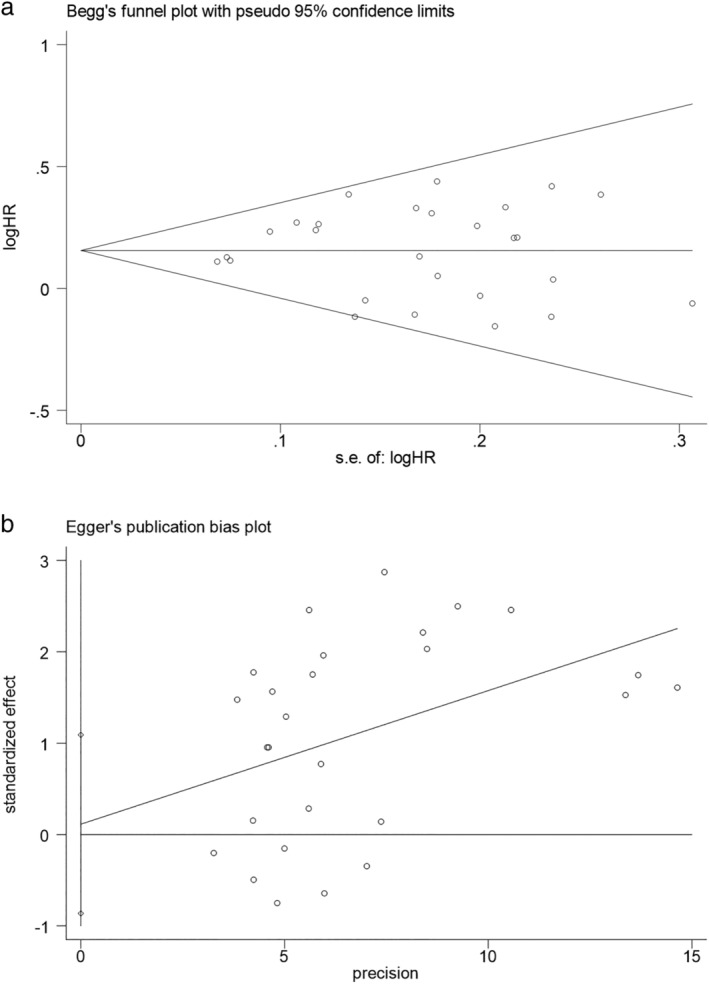
(a) Publication bias of included studies: Begg's funnel plot of OS. (b) Publication bias of included studies: Egger's test of overall survival (OS).
Discussion
Our meta‐analysis investigated the clinicopathological characteristics and prognostic value of pretreatment smoking status in SCLC patients. In the 27 included studies, 75.8% of 12 047 patients with SCLC were current or former smokers, and 24.2% were never‐smokers. The results suggested that smoking history was inversely proportional to patient survival (OS: HR = 1.17, 95% CI: 1.12–1.23, P < 0.00001; I 2 = 0%; PFS: HR = 1.20, 95% CI: 1.06–1.35, P = 0.004; I 2 = 0%).
Although smoking history remains the most important risk factor of tumorigenesis of SCLC, 24.2% of 12 047 patients with SCLC were non‐smokers in our meta‐analysis, the frequency of which seems higher than those reported in previous studies. One recognized etiological factor of SCLC in non‐smokers is radon exposure. 32 The gene landscapes of smoker and never‐smoker patients with SCLC differs, and never‐smoker patients were revealed to possess a higher frequency of EGFR, MET, and SMAD4 mutations, 33 but whether the differential mutation profile is a consequence of a diverse pathological mechanism for disease onset and whether potentially actionable oncogenic drivers exist are still unknown. In terms of clinical characteristics, several studies proposed that never‐smoker SCLC was related to a female‐gender predisposition. 33 , 34 In the study by Cardo et al. the authors also found that SCLC patients with a smoking history presented with a higher incidence of brain metastasis, which might partly explain the poorer survival of smoker SCLC patients. 33 Overall, the mechanism behind the prognostic difference between smokers and never‐smokers in SCLC still needs further investigation.
Our meta‐analysis results were limited in that most of our included retrospective studies failed to match the basic characteristics between cohorts of smokers and never‐smokers. Some studies presented multivariate analysis results of smoking status which enabled us to reduce the impact of other unmatched factors on evaluation of prognostic value of smoking status, while in other studies only univariate analysis results were available. However, the reliability of our results has been strongly supported by favorable data under analysis of both sensitivity and publication bias. The reason for negative results reported in some studies may be due to the limited number of non‐smokers along with interference of other factors.
In conclusion, smoking history should be considered as an independent poor prognostic factor for patients with SCLC. More large‐scale prospective studies are warranted to testify the prognostic value of pretreatment smoking status in patients with SCLC.
Disclosure
The authors declare that there are no conflicts of interest.
Acknowledgment
This work was financially supported by China National Key Technology Support Program (2014BAI09B01).
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (6): 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Gaspar LE, McNamara EJ, Gay EG et al Small‐cell lung cancer: Prognostic factors and changing treatment over 15 years. Clin Lung Cancer 2012; 13 (2): 115–22. [DOI] [PubMed] [Google Scholar]
- 3. Nicholson AG, Chansky K, Crowley J et al The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11 (3): 300–11. [DOI] [PubMed] [Google Scholar]
- 4. Tian Z, Liang C, Zhang Z et al Prognostic value of neuron‐specific enolase for small cell lung cancer: A systematic review and meta‐analysis. World J Surg Oncol 2020; 18 (1): 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. S‐HI O, Ziogas A, Zell JA. Prognostic factors for survival in extensive stage small cell lung cancer (ED‐SCLC): The importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol 2009; 4 (1): 37–43. [DOI] [PubMed] [Google Scholar]
- 6. Zhou S, Wang H, Jiang W, Yu Q, Zeng A. Prognostic value of pretreatment albumin‐to‐alkaline phosphatase ratio in extensive‐disease small‐cell lung cancer: A retrospective cohort study. Cancer Manage Res 2020; 12: 2015–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yılmaz A, Tekin SB, Bilici M, Yılmaz H. The significance of controlling nutritional status (CONUT) score as a novel prognostic parameter in small cell lung cancer. Lung 2020; 198: 695–704. [DOI] [PubMed] [Google Scholar]
- 8. X‐q X, W‐q D, Wang D‐Y et al Chinese medicine treatment prolonged survival in small cell lung cancer patients: A clinical observation. Chin J Integr Med 2020. [DOI] [PubMed] [Google Scholar]
- 9. Wu F, Yang S, Tang X, Liu W, Chen H, Gao H. Prognostic value of baseline hemoglobin‐to‐red blood cell distribution width ratio in small cell lung cancer: A retrospective analysis. Thorac Cancer 2020; 11 (4): 888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang C, Jin S, Xu S, Cao S. High systemic immune‐inflammation index (SII) represents an unfavorable prognostic factor for small cell lung cancer treated with etoposide and platinum‐based chemotherapy. Lung 2020; 198 (2): 405–14. [DOI] [PubMed] [Google Scholar]
- 11. Wang D, Guo D, Shi F et al The predictive effect of the systemic immune‐inflammation index for patients with small‐cell lung cancer. Future Oncol 2019; 15 (29): 3367–79. [DOI] [PubMed] [Google Scholar]
- 12. Li X, Li B, Zeng H et al Prognostic value of dynamic albumin‐to‐alkaline phosphatase ratio in limited stage small‐cell lung cancer. Future Oncol 2019; 15 (9): 995–1006. [DOI] [PubMed] [Google Scholar]
- 13. Liu X, Jiang T, Li W et al Characterization of never‐smoking and its association with clinical outcomes in Chinese patients with small‐cell lung cancer. Lung Cancer 2018; 115: 109–15. [DOI] [PubMed] [Google Scholar]
- 14. Jin S, Cao S, Xu S, Wang C, Meng Q, Yu Y. Clinical impact of pretreatment prognostic nutritional index (PNI) in small cell lung cancer patients treated with platinum‐based chemotherapy. Clin Respir J 2018; 12 (9): 2433–40. [DOI] [PubMed] [Google Scholar]
- 15. Guo D, Jing W, Zhu H et al Clinical value of carcinoembryonic antigen for predicting the incidence of brain metastases and survival in small cell lung cancer patients treated with prophylactic cranial irradiation. Cancer Manage Res 2018; 10: 3199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan S, Guan Y, Zhao G, An G. Association between plasma fibrinogen and survival in patients with small‐cell lung carcinoma. Thorac Cancer 2018; 9 (1): 146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong X, Xu Q, Yang Z et al The value of prognostic factors in Chinese patients with small cell lung cancer: A retrospective study of 999 patients. Clin Respir J 2018; 12 (2): 433–47. [DOI] [PubMed] [Google Scholar]
- 18. Pan H, Shi X, Xiao D et al Nomogram prediction for the survival of the patients with small cell lung cancer. J Thorac Dis 2017; 9 (3): 507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang X, Mei X, Wu H, Chen X. D‐dimer level is related to the prognosis of patients with small cell lung cancer. Ann Transl Med 2017; 5 (20): 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng M, Ma X, Liang X, Zhu C, Wang M. Are pretreatment neutrophil‐lymphocyte ratio and plateletlymphocyte ratio useful in predicting the outcomes of patients with small‐cell lung cancer? Oncotarget 2017; 8 (23): 37200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen Y, Yu H, Wu C et al Prognostic value of plasma D‐dimer levels in patients with small‐cell lung cancer. Biomed Pharmacother 2016; 81: 210–7. [DOI] [PubMed] [Google Scholar]
- 22. Cai Q, Luo HL, Gao XC et al Clinical features and prognostic factors of small cell lung cancer: A retrospective study in 148 patients. J Huazhong Univ Sci Technolog Med Sci 2016; 36 (6): 916–22. [DOI] [PubMed] [Google Scholar]
- 23. Xie D, Marks R, Zhang M et al Nomograms predict overall survival for patients with small‐cell lung cancer incorporating pretreatment peripheral blood markers. J Thorac Oncol 2015; 10 (8): 1213–20. [DOI] [PubMed] [Google Scholar]
- 24. Sun JM, Choi YL, Ji JH et al Small‐cell lung cancer detection in never‐smokers: Clinical characteristics and multigene mutation profiling using targeted next‐generation sequencing. Ann Oncol 2015; 26 (1): 161–6. [DOI] [PubMed] [Google Scholar]
- 25. Liu S, Guo H, Kong L et al The prognostic factors in the elderly patients with small cell lung cancer: A retrospective analysis from a single cancer institute. Int J Clin Exp Pathol 2015; 8 (9): 11033–41. [PMC free article] [PubMed] [Google Scholar]
- 26. Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune‐inflammation index, based on platelet counts and neutrophil‐lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med 2015; 236 (4): 297–304. [DOI] [PubMed] [Google Scholar]
- 27. Hong S, Zhou T, Fang W et al The prognostic nutritional index (PNI) predicts overall survival of small‐cell lung cancer patients. Tumour Biol 2015; 36 (5): 3389–97. [DOI] [PubMed] [Google Scholar]
- 28. Liu S, Zhang G, Li C et al Prognostic factors and survival of patients with small cell lung cancer in a northeastern Chinese population. Thorac Cancer 2013; 4 (2): 143–52. [DOI] [PubMed] [Google Scholar]
- 29. Wu C, Li F, Jiao SC. Prognostic factors for survival of patients with extensive stage small cell lung cancer‐a retrospective single institution analysis. Asian Pac J Cancer Prev 2012; 13 (10): 4959–62. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, Jiang R, Garces YI et al Prognostic factors for limited‐stage small cell lung cancer: A study of 284 patients. Lung Cancer 2010; 67 (2): 221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arinc S, Gonlugur U, Devran O et al Prognostic factors in patients with small cell lung carcinoma. Med Oncol 2010; 27 (2): 237–41. [DOI] [PubMed] [Google Scholar]
- 32. Torres‐Duran M, Ruano‐Ravina A, Kelsey KT et al Small cell lung cancer in never‐smokers. Eur Respir J 2016; 47 (3): 947–53. [DOI] [PubMed] [Google Scholar]
- 33. Cardona AF, Rojas L, Zatarain‐Barrón ZL et al Multigene mutation profiling and clinical characteristics of small‐cell lung cancer in never‐smokers vs. heavy smokers (Geno1.3‐CLICaP). Front Oncol 2019; 9: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varghese A, Zakowski M, Yu H et al Small‐cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol 2014; 9 (6): 892–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


