Abstract
After sternal tumor resection, reconstruction of chest wall defects is still a challenging part of thoracic surgery. Three‐dimensional (3D)‐printed titanium alloy prosthesis implants provide an effective solution. The bionic bone trabecular micropore structure, which is beneficial to the human body, increases stability and robustness of the prosthesis. Here, we report a successful case of a customized prosthesis using a 3D‐printed titanium alloy to repair and reconstruct bone defects in a patient with sternal osteosarcoma who underwent radical resection of the whole sternum.
Keywords: Chest wall reconstruction, chest wall resection, sternal tumor, titanium alloy prosthesis

We report a successful case of a customized prosthesis using a 3D‐printed titanium alloy to repair and reconstruct bone defects in a patient with sternal osteosarcoma who underwent radical resection of the whole sternum.
Introduction
Previous studies have shown that the extent of thoracic rib tumor resection determines patients' survival rate 1 , 2 If the tumor has invaded the muscles and skin, the soft tissue cutting edge should be more than 4 cm away from the tumor and removed together with the affected skin and muscle 3 Because radical resection can improve the five‐year survival rate in patients, and the chest wall defect caused by palliative resection is larger, traditional materials are difficult to use in repairing such huge defects. These include titanium, which not only has poor tissue connection and stability, but also carries a high risk of postoperative infection, and methylmethacrylate sandwich or e‐PTFE mesh materials, which have a rough surface and edges, leading to a higher risk of soft tissue hematoma and infection after surgery, all of which make them unsuitable for use in sternal reconstruction 4 A three‐dimensional (3D)‐printed titanium alloy prosthesis solves the thorny problem of bone reconstruction, achieving personalized and accurate chest wall reconstruction in clinical applications.
Case report
A 66‐year‐old woman was found to have a 10 × 5 cm chondrosarcoma of the sternum. Computed tomography (CT) showed that one‐third of the sternum was involved and the tumor had invaded the ribs (Fig 1). The patient subsequently underwent radiation therapy (Fig 1b). The tumor size was 5 × 13 cm, and pathological biopsy before surgery confirmed that it was osteosarcoma. No distant metastasis was found, and the tumor has involved the sternum body and the sternum stem, and the upper edge of the fifth rib of the sternum stem has been invaded. That is why the tumor was expanded and resected. Preoperative multidisciplinary ethics were discussed, as the tumor involved the body of the sternum and the sternal stem, and the upper edge of the fifth rib of the sternal stem had been invaded. According to tumor resection principles, the resection margin should be more than 3‐4cm to tumor to ensure that there is no residual tumor. Total sternum resection was therefore deemed necessary and the committee approved a 3D‐printed titanium thoracic reconstruction procedure.
Figure 1.
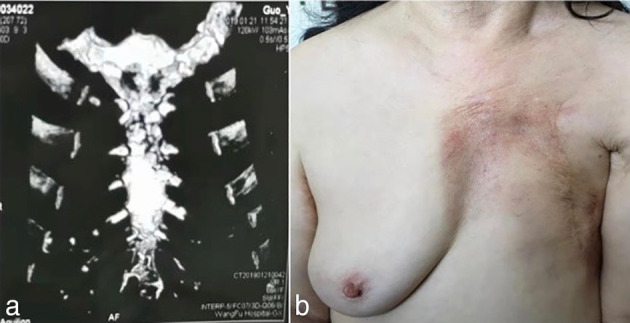
(a) Sternal sarcoma invasion of the rib. (b) Left breast after cancer surgery.
The prosthesis was designed and printed after a preoperative high‐precision 3D CT scan. The reconstruction of the sternosacral joint and the sternal muscle group were taken into account when designing the sternal stem (Fig 2). The middle of the sternum was a metal mesh and rib porous design to reduce the weight of the titanium alloy prosthesis and facilitate embedding the device in tissue, which helps to stabilize the implant. The 3D‐printed chest rib model was produced using polylactic acid (PLC) material, with physical proofreading and improvement (Fig 2b). It is able to completely reconstruct the anatomical structure, meanwhile can be customized and more lighter. Surgery was performed via mid‐thoracic incision and enlarged thoraco‐ribs (3 to 4 cm from the edge of the tumor). The scope of resection is expanded to reduce the recurrence rate. The resection included the bilateral internal mammary artery and surrounding lymph nodes and soft tissues (Fig 2c,d).
Figure 2.
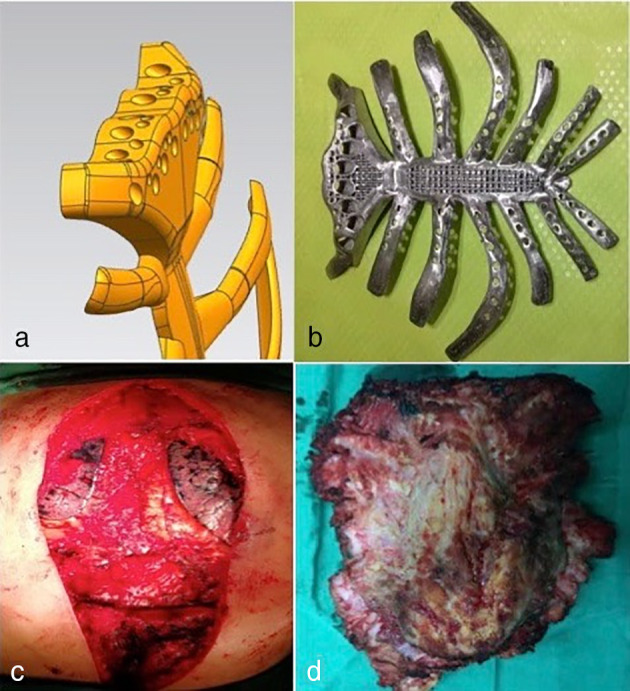
(a) Design of the sternal stem. (b) 3D‐printed titanium alloy prosthesis. Slice thickness was 1.25 mm; the length of each rib exceeded the expected cutting edge by 3 to 4 cm, providing sufficient length for the rib link. (c) Removal of the entire sternum and bilateral ribs 1–7. (d) Thoracic rib resection (15 × 14 cm).
With Bard Composix E/X mesh pleura, implanted prosthetic ribs and rib resection stumps were fixed with titanium rib claws. The sternocleidomastoid sternal end tendon and sternum thymus muscle were freely dissected, and then suture fixation of the sternohyoid muscle and titanium alloy prosthesis in the upper sternum was performed. With a ligament advanced reinforcement system (LARS), an artificial ligament link fixed the titanium alloy prosthesis and clavicle to reconstruct the sternal‐lock joint. A free left latissimus dorsi muscle flap was used to cover the titanium alloy prosthesis to repair a soft‐tissue defect of the chest wall. The patient did not receive postoperative respiratory physiokinesytherapy. We followed the enhanced recovery after surgery (ERAS) principles for patients to carry out routine breathing exercises after surgery. Preoperative lung function (seven days before surgery) was compared at 60 and 90 days postsurgery (Table 1), including measuring forced vital capacity (FVC), 1‐second forced expiratory volume (FEV1), maximal voluntary ventilation (MVV), oxygen partial pressure (POP), and oxygen saturation (OS). The implanted prosthesis weighed 121 g (Fig 3), and the implanted thoracic ribs prosthesis was 17 × 22 cm (374 cm2). The amount of blood loss was 300 mL. After 5–10 days, the drainage tube was removed. One week after surgery, the chest CT titanium alloy prosthesis was well fixed, and the patient recovered after two weeks (Fig 3b).
Table 1.
Lung function before and after surgery
| FVC (L) | FEV1 (L) | MVV (L/minute) | POP (mmHg) | OS (%) | |
|---|---|---|---|---|---|
| Preoperative data (7 days) | 2.10 | 1.90 | 64.67 | 90.0 | 96.0 |
| Postoperative data (60 days) | 1.59 (−24.3%) | 1.40 (−26.4%) | 50.38 (−22.1%) | 71.0 (−21.2%) | 94.4 (−2.1%) |
| Postoperative data (90 days) | 1.94 (−7.7%) | 1.57 (−17.4%) | / | 77.5 (−12.5%) | 95.7 (−0.3%) |
FVC, forced vital capacity; FEV1; 1‐second forced expiratory volume; MVV, maximal voluntary ventilation; POP, oxygen partial pressure; OS, oxygen saturation.
Figure 3.
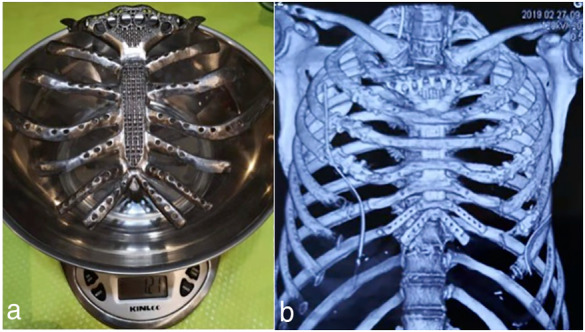
(a) Weight of implanted prosthesis. (b) Chest computed tomography (CT) of titanium alloy prosthesis.
Discussion
In this report, we chose a titanium alloy prosthesis and titanium alloy rib claws to facilitate bone fusion with bone tissue in terms of biocompatibility, which has been shown to produce a stronger histological connection. 5 , 6 3D printed titanium alloy is able to completely reconstruct the anatomical structure, but can also be customized. However, it also has limitations; for example, the implants may fracture, or there may be displacement and infection. In particular, if the implant is large, it affects thoracic movement and impairs respiratory movement and lung function. Functional activity was good at three months follow‐up, which highlights the advantages of personalized treatment using 3D printing. For the rib stump, considering the difference between the titanium alloy prosthesis and rib modulus and the biomechanical properties between the implant and the cortical bone, we used an entangled titanium alloy rib claw to prevent the joint from shifting and breaking. The intraoperative and postoperative chest CT confirmed that the titanium alloy prosthesis and rib fixation were firm and stable (Figure 4). Pleural reconstruction can effectively maintain the stability of the pleural cavity and make the visceral pleura of the lung surface adhere to the artificial pleura, reducing the occurrence of effusion and gas accumulation. 7 , 8 The patient had been treated for left breast cancer 20 years previously, during which radical treatment plus radiotherapy left serious chest wall soft‐tissue defects. Here, we chose the Bard Composix E/X mesh pleura as reconstruction material. The scope of the surgical resection was extensive, increasing the difficulty of the repair. We used the left latissimus dorsi musculocutaneous flap to repair the soft‐tissue defects of the chest wall and a skin graft to repair the defect caused by radiotherapy. According to previous studies, the lung function of patients after chest wall resection was found to be significantly decreased in both the prosthetic and nonprosthetic groups. 9 , 10 Patients with 3D‐printed PEEK implants have been reported to have a decrease in FVC and FEV1 of about 15%. 11 In this case, the FVC and FEV1 at 60 days postsurgery decreased by 24.3% and 26.4%, respectively. The FVC and FEV1 90 days postsurgery decreased by 7.7% and 17.4%, respectively. The patient's lung function was greatly improved after three months; however, she had more significantly impaired lung function than the cases reported in previous studies. A large chest wall defect affected the function of the respiratory muscles in our patient, which caused restrictive dysfunction. We therefore began to guide her in abdominal breathing exercises two months postsurgery to strengthen the respiratory muscles, diaphragm, and abdominal wall muscles in order to eliminate the effects of restrictive ventilatory disorders.
Figure 4.
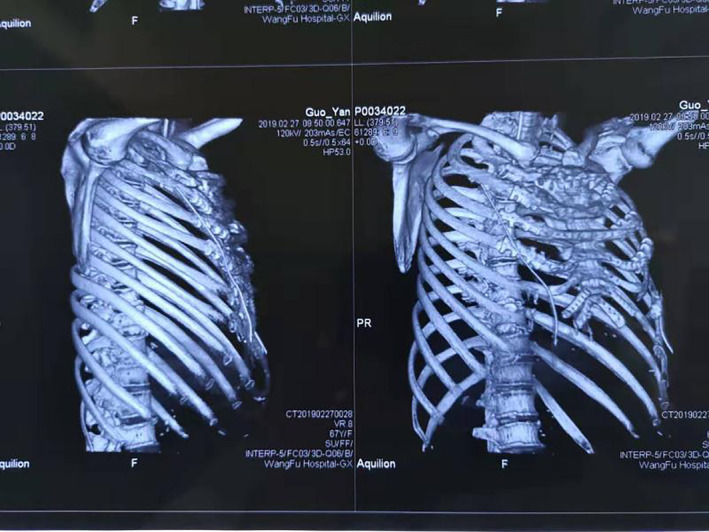
A postoperative chest CT‐scan image.
Disclosure
The authors declare that there are no conflicts of interest.
Contributor Information
Wenzhang Wang, Email: wwz9993@163.com.
Yi Han, Email: hanyeecn@163.com.
References
- 1. Heck RK, Carnesale PG. General principles of tumors In: Canale ST. (ed.). Campbell's Operative Orthopaedics, 10th edn Mosby, Philadelphia, PA: 2003; 733–92. [Google Scholar]
- 2. Gitelis S, Malawer M, MacDonald D, Derman G. Principles of limb salvage surgery In: Chapman MW. (ed.). Chapman's Orthopaedic Surgery, 3rd edn Lippincott Williams and Wikins, Philadelphia, PA: 2001; 3309–81. [Google Scholar]
- 3. Shi Y. Consensus on the diagnosis and treatment of soft tissue sarcoma in China (2015 edition). Chin J Oncol 2016; 38 (4): 310–3. [DOI] [PubMed] [Google Scholar]
- 4. Zarogoulidis P, Grosomanidis V, Tossios P et al Massive chest wall resection and reconstruction for malignant disease. Oncotargets Therap 2016; 9 (1): 2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGilvray KC, Easley J, Seim HB et al Bony ingrowth potential of 3D‐printed porous titanium alloy: A direct comparison of interbody cage materials in an in vivo ovine lumbar fusion model. Spine J 2018; 18 (7): 1250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oron A, Agar G, Oron U, Stein A. Correlation between rate of bony ingrowth to stainless steel, pure titanium, and titanium alloy implants in vivo and formation of hydroxyapatite on their surfacesin vitro. J Biomed Mater Res A 2009; 91 (4): 1006–9. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Li JZ, Hao YJ et al Sternal tumor resection and reconstruction with titanium mesh: A preliminary study. Orthop Surg 2015; 7 (2): 155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiegmann B, Zardo P, Dickgreber N et al Biological materials in chest wall reconstruction: Initial experience with the Peri‐Guard Repair Patch. Eur J Cardiothorac Surg 2010; 37 (3): 602–5. [DOI] [PubMed] [Google Scholar]
- 9. Daigeler A, Druecke D, Hakimi M et al Reconstruction of the thoracic wall‐long‐term follow‐up including pulmonary function tests. Langenbecks Arch Surg 2009; 394: 705–15. [DOI] [PubMed] [Google Scholar]
- 10. Nishida Y, Tsukushi S, Urakawa H et al Post‐operative pulmonary and shoulder function after sternal reconstruction for patients with chest wall sarcomas. Int J Clin Oncol 2015; 20: 1218–25. [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Huang L, Li X et al Three dimensional printing PEEK implant:A novel choice for the reconstruction of chest wall defect. Ann Thorac Surg 2019; 107 (3): 921–8. [DOI] [PubMed] [Google Scholar]


