Abstract
Background
Circulating genetically abnormal cells (CACs) with specific chromosome variations have been confirmed to be present in non‐small cell lung cancer (NSCLC). However, the diagnostic performance of CAC detection remains unclear. This study aimed to evaluate the potential clinical application of the CAC test for the early diagnosis of NSCLC.
Methods
In this prospective study, a total of 339 participants (261 lung cancer patients and 78 healthy volunteers) were enrolled. An antigen‐independent fluorescence in situ hybridization was used to enumerate the number of CACs in peripheral blood.
Results
Patients with early‐stage NSCLC were found to have a significantly higher number of CACs than those of healthy participants (1.34 vs. 0.19; P < 0.001). The CAC test displayed an area under the receiver operating characteristic (ROC) curve of 0.76139 for discriminating stage I NSCLC from healthy participants with 67.2% sensitivity and 80.8% specificity, respectively. Compared with serum tumor markers, the sensitivity of CAC assays for distinguishing early‐stage NSCLC was higher (67.2% vs. 48.7%, P < 0.001), especially in NSCLC patients with small nodules (65.4% vs. 36.5%, P = 0.003) and ground‐glass nodules (pure GGNs: 66.7% vs. 40.9%, P = 0.003; mixed GGNs: 73.0% vs. 43.2%, P < 0.001).
Conclusions
CAC detection in early stage NSCLC was feasible. Our study showed that CACs could be used as a promising noninvasive biomarker for the early diagnosis of NSCLC.
Key points
What this study adds: This study aimed to evaluate the potential clinical application of the CAC test for the early diagnosis of NSCLC.
Significant findings of the study: CAC detection in early stage NSCLC was feasible. Our study showed that CACs could be used as a promising noninvasive biomarker for the early diagnosis of NSCLC.
Keywords: Biomarker, circulating genetically abnormal cells, early detection, non‐small‐cell lung cancer, small nodules
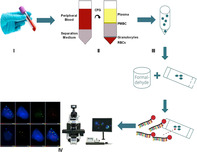
Workflow of circulating genetically abnormal cells enumeration.
Circulating genetically abnormal cell detection in early stage non‐small‐cell lung cancer was feasible.
Circulating genetically abnormal cell test has good stability and adaptability for different patient populations.
Circulating genetically abnormal cells could be used as a promising noninvasive biomarker for the early diagnosis of NSCLC.
Introduction
NSCLC is the major pathological subtype of lung cancer and the leading cause of cancer death. 1 , 2 At present, the five‐year survival rate of lung cancer is only 16.6%, and more than 70% of patients are diagnosed at advanced stages due to the lack of early clinical symptoms. 2 , 3 Early‐stage diagnosis of lung cancer contributes to effective cancer therapy, which could significantly increase the five‐year survival rate after lung cancer resection. 4 Thus, early detection of lung cancer is crucial to reducing mortality.
Various diagnostic methods for early‐stage lung cancer are available in clinics, such as low‐dose computed tomography (LDCT) screening, percutaneous biopsy, positron emission tomography (PET) and liquid biopsy. 5 , 6 , 7 , 8 , 9 However, LDCT has a high false positive rate. 10 For invasive methods (such as percutaneous biopsy) or imaging examination, there are also disadvantages such as insufficient discrimination of malignant and benign nodules in small nodules and ground‐glass nodules (GGNs). 11 , 12 , 13 Additionally, noninvasive methods including testing for traditional serum tumor biomarkers, circulating genetically abnormal cells (CTCs), and circulating nucleic acids have insufficient sensitivity in early diagnosis, which limits their utility in large screening programs. 14 , 15 , 16 Thus, an effective, noninvasive method that is sufficiently sensitive and specific for use in the early diagnosis of lung cancer is urgently needed.
It is well known that CTCs are of great interest in translational oncology. CTCs generally refer to cancer cells in the peripheral blood that have disseminated from the primary tumor or metastatic sites. 17 However, because the detection of CTCs relies upon expression of epithelial markers, the diagnostic sensitivity of CTC detection may be drastically reduced if the epithelial markers are absent. 18 , 19 Interestingly, in recent years, CACs with specific chromosome variations have been detected in lung cancer patients. 18 , 20 It is reported that CACs contain similar genetic abnormalities to the primary tumor in patients, and have a strong correlation with early tumorigenesis of NSCLC, suggesting that CACs may be an effective and specific biomarker for cancer diagnosis. 18 In particular, CACs assays may be used as adjuncts to the diagnosis of indeterminate lung nodules.
To date, an assay for CAC enrichment and detection has been established but only validated with a small patient set (n = 59). The value of CAC detection for the early diagnosis of NSCLC remains unknown. Here, we conducted a prospective study to evaluate the diagnostic value of CACs at early‐stage NSCLC using blood samples from lung cancer patients and healthy controls. We also compared the sensitivity of CACs and traditional serum tumor biomarkers, including carcinoembryonic antigen (CEA), neuron‐specific enolase (NSE), total prostate specific antigen (TPSA), squamous cell carcinoma antigen (SCC‐Ag), progastrin‐releasing peptide (ProGRP), carbohydrate antigen 19‐9 (CA19‐9), and cytokeratin 19 fragment 21‐1 (CYFRA21‐1), to further validate its performance.
Methods
Study design and patients
This was a prospective clinical trial (NCT03790735) conducted at Tianjin Medical University Cancer Institute and Hospital between May 2018 and December 2018. Patients of either gender, no less than 18 years of age, with a pulmonary nodule diameter less than 30 mm (as measured via computed tomography [CT] scan) and with suspected lung cancer who underwent resection were eligible to enter the study. Patients who received any prior treatments or had a history of malignancy were excluded. All blood samples were obtained just prior to surgery. In total, 261 patients were enrolled. In addition to diseased patients, 78 healthy controls were also recruited for this study. Healthy participants were eligible for enrollment if there were no nodules or masses on the lungs (as determined via CT scan) and they had no other pulmonary diseases.
The blood samples of enrolled patients were processed to enumerate CACs before identification of tumor stage by paraffin pathology. All patients were diagnosed by histopathological examination after surgery and NSCLC staging was performed according to the eighth edition of the tumor, node, metastasis (TNM) classification. 21 This clinical trial was conducted in accordance with the Declaration of Helsinki, and the ethical Good Clinical Practice Guidelines. The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. Written informed consent was obtained from all patients.
Measurement of CACs
A total of 10 mL peripheral blood was collected using K2EDTA vacuum tubes (BD, USA). All specimens were immediately stabilized after collection with a blood collection kit following the manufacturer's guidelines (Sanmed Biotech Ltd., China). The stabilized sample could be stored or transferred at room temperature for up to 96 hours prior to cell processing. The peripheral blood mononuclear cells (PBMCs) were enriched by Ficoll (GE Healthcare, US) density gradient centrifugation using 5 mL of stabilized sample (equal to 2.5 mL full blood). The collected PBMCs were then applied onto glass slides by Cytospin (Thermo Fisher, US). Subsequent to PBMC isolation and slide making, Multicolor fluorescence in situ hybridization (FISH) was performed using a mononuclear cell chromosomal abnormality detection kit (Sanmed Biotech Ltd., China) to visualize chromosomal changes in the locus of 3p22.1, CEP3, 10q22.3 and CEP10. Samples were washed with sodium citrate acid (SCC) buffer to reduce nonspecific probe binding followed by nuclei labeling using 4′‐6‐diamidino‐2‐phenylindole (DAPI). Imaging of cell fluorescence signals were conducted with the Duet microscope system (BioView, Israel). The overall sample processing procedure is shown in Figure 1.
Figure 1.
The workflow of CAC enumeration. (I) Collection of 10 mL peripheral blood. (II) PBMCs enrichment. (III) Hybridization of FISH probes. (IV) Fluorescence image acquisition and analysis.
Tumor biomarker measurement
A 3 mL anticoagulant peripheral blood sample was collected from each patient to measure the tumor biomarkers (CEA, NSE, TPSA, SCC‐Ag, ProGRP, CA19‐9, and CYFRA21‐1). The serum levels of tumor biomarkers were detected with an electrochemiluminescence immunoassay (ECLI) using the Roche Elecsys E170 analyzer (Roche Diagnostics, Switzerland) on the day of sample collection.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL). Quantitative data were described by means and range. Qualitative data were described by number or rate and ratio. The normality of the data was tested with the Kolmogorov–Smirnov test. For the continuous variable, if the data distribution was normal, Student's t‐test was used for comparison between the two groups. If the data distribution was asymmetrical, the Mann‐Whitney U test was used. For the discontinuous variable, the χ2 test was used for analysis. Diagnostic performance of the CAC test was assessed by constructing a receiver operating characteristic (ROC) curve and evaluated by calculating the area under the curve (AUC). 22 All statistical tests were two‐sided, with the significance set at P < 0.05 along with 95% confidence intervals (CI).
Results
Patient characteristics
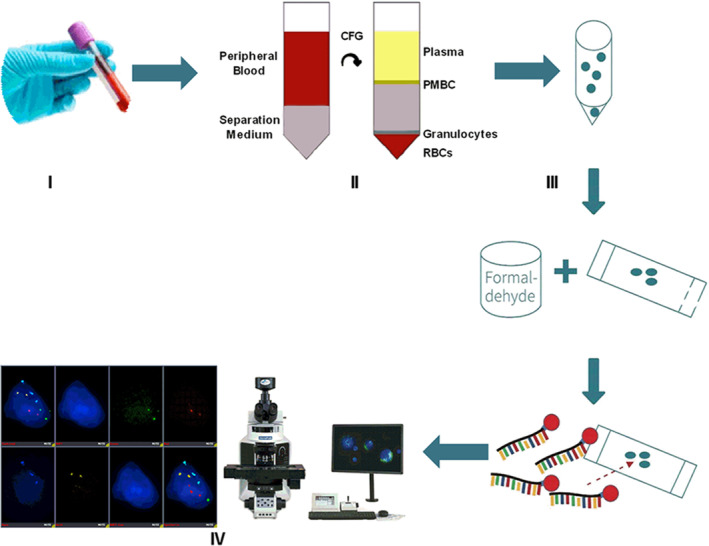
A total of 339 participants (261 patients and 78 healthy volunteers) were enrolled into this single‐center, prospective clinical trial (Fig 2). Among the 261 patients, 248 had NSCLC (232 stage I, 10 stage II, and six stage IIIa), three had small cell carcinoma, and 10 patients were diagnosed with benign lung disease. Stage I NSCLC patients were analyzed in the study. Most stage I NSCLC patients were women (61.2%), with a mean age of 56.0 years. A total of 44.9% of the healthy participants were women, and the mean age of all healthy participants was 45.0 years; and 74 (31.9%) NSCLC patients and 21 (26.9%) healthy participants were either current smokers or had a history of smoking. The demographics and characteristics of stage I NSCLC patients and healthy participants are shown in Table 1.
Figure 2.
Flowchart of patients enrolled in the study. NSCLC, non‐small cell lung cancer.
Table 1.
Demographics and characteristics of stage I NSCLC patients and healthy participants
| Characteristics | NSCLC patients (n = 232) | Healthy participants (n = 78) |
|---|---|---|
| Age, years (mean, range) | 56.0 years (28–79) | 45.0 years (21–72) |
| Sex, n (%) | ||
| Male | 90 (38.8%) | 43 (55.1%) |
| Female | 142 (61.2%) | 35 (44.9%) |
| Smoking history, n (%) | ||
| No | 158 (68.1%) | 72 (73.1%) |
| Yes | 74 (31.9%) | 21 (26.9%) |
| Pathological types | ||
| AIS and MIA | 73 (31.5%) | — |
| IAC | 149 (64.2%) | — |
| Others | 10 (4.3%) | — |
| Nodule size on CT, n (%) | ||
| ≤10 mm | 52 (22.4%) | — |
| 10–20 mm | 122 (52.6%) | — |
| >20 mm | 58 (25.0%) | — |
| Nodule type classification on CT | ||
| Solid nodule | 92 (39.7%) | — |
| pGGNs | 66 (28.4%) | — |
| mGGNs | 74 (31.9%) | — |
AIS, adenocarcinoma in situ; CT, computed tomography; IAC, invasive adenocarcinoma; mGGNs, mixed ground‐glass nodules; MIA, minimally invasive adenocarcinoma; NSCLC, non‐small cell lung cancer; pGGNs, pure ground‐glass nodules.
CAC counts in patients
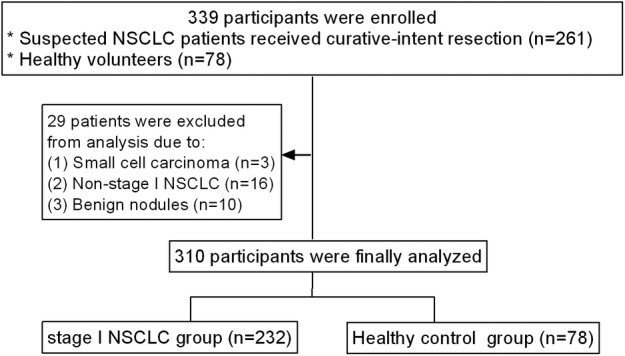
The CACs isolated from NSCLC patients were identified using a four color FISH assay. CACs were defined as cells with gains of at least two probes targeting chromosome loci 3p22.1, CEP3, 10q22.3 and CEP10 (Fig 3a), while normal PBMCs were diploid cells without gains (Fig 3b).
Figure 3.
Diagnostic value of CAC test in patients with stage I NSCLC. (a) Exemplary image of an isolated CAC from a NSCLC patient. 3p22.1: red, CEP3: green, 10q22.3: gold, CEP10: aqua, DAPI: blue. In contrast, (b) normal leukocytes with no significant increase in diploid cells. CACs, circulating genetically abnormal cells; PBMCs, peripheral blood mononuclear cells; FISH, fluorescence in situ hybridization; NSCLC, non‐small cell lung cancer. (c) Distribution of CAC counts in stage I NSCLC patients and healthy participants. (d) ROC curves for CAC counts to discriminate stage I NSCLC patients from healthy participants. CACs, circulating genetically abnormal cells; ROC, receiver operating characteristic; AUC, area under curve; NSCLC, non‐small cell lung cancer. ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %.
) identity %.
Distributions of CAC counts in stage I NSCLC patients and healthy participants are shown in Figure 3c. The CAC counts were significantly higher in stage I NSCLC patients (1.34, range 0–24) than in healthy participants (0.19, range 0–1) (P < 0.001). CACs were detected in 15 of 78 (19.2%) healthy participants, with a CAC count of 1. CACs were detected in 156 of 232 (67.2%) NSCLC patients, with CAC counts of 1–24. The AUC for CAC counts in discrimination between stage I NSCLC patients and healthy participants was 0.769 (95% CI, 0.716–0.822; P < 0.001; Fig 3d). According to the ROC analysis, the cutoff point of CAC counts was estimated to be 1 with sensitivity and specificity of 67.2% and 80.8%, respectively (NSCLC patients, CAC counts ≥1; healthy participants, CAC counts = 0). Overall, these results indicated that CACs would be clinically useful in the diagnosis of early‐stage NSCLC.
Relationship between CAC counts and patient demographics
Next, we analyzed the relationship between CAC counts and patient demographics (Table 2). There was no relationship between CAC status and patient demographics, including age (P = 0.969), gender (P = 0.890) and smoking history (P = 0.942). Adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) lesions may be small and asymptomatic, which greatly hinders their early detection. We evaluated the diagnostic performance of CAC counts in these patients, and they were found in 52 (71.2%) patients with AIS and MIA, compared with invasive adenocarcinoma (IAC), in which 98 (65.8%) patients were CAC positive, and no significant difference was found among different pathological types (P = 0.592, Table 2). With regard to tumor size, CACs were found in 34 (65.4%) patients with a tumor diameter ≤ 10 mm, 83 (68.0%) patients with a tumor diameter of 10–20 mm, and 39 (67.2%) patients with a tumor diameter > 20 mm, without a significant difference (P = 0.944, Table 2). Furthermore, we also compared the CAC counts in different subtypes of malignant pulmonary ground‐glass nodules (GGNs), which were difficult to discriminate using only CT. A total of 44 NSCLC patients with pGGNs were CAC positive (66.7%), 54 with mGGNs were CAC positive (73.0%), and 58 with solid nodules were CAC positive (63.0%). There was no difference in CAC detection between GGN and solid nodules (pP = 0.397, Table 2).
Table 2.
Association between CAC counts and patient characteristics
| Characteristics | CACs positive (n = 156) | CACs negative (n = 76) | P‐value |
|---|---|---|---|
| Age | |||
| <60 years | 94 (67.1%) | 46 (32.9%) | 0.969 |
| ≥60 years | 62 (67.4%) | 30 (32.6%) | |
| Gender | |||
| Male | 61 (67.8%) | 29 (32.2%) | 0.890 |
| Female | 95 (66.9%) | 47 (33.1%) | |
| Smoking history | |||
| No | 106 (67.1%) | 52 (32.9%) | 0.942 |
| Yes | 50 (67.6%) | 24 (32.4%) | |
| Pathological types | |||
| MIA and AIS | 52 (71.2%) | 21 (28.8%) | 0.592 |
| IAC | 98 (65.8%) | 51 (34.2%) | |
| Nonadenocarcinoma | 6 (60.0%) | 4 (40.0%) | |
| Nodule size | |||
| ≤10 mm | 34 (65.4%) | 18 (34.6%) | 0.944 |
| 10–20 mm | 83 (68.0%) | 39 (32.0%) | |
| >20 mm | 39 (67.2%) | 19 (32.8%) | |
| Nodule type | |||
| Solid nodules | 58 (63.0%) | 34 (37.0%) | 0.397 |
| pGGNs | 44 (66.7%) | 22 (33.3%) | |
| mGGNs | 54 (73.0%) | 20 (27.0%) |
AIS, adenocarcinoma in situ; CACs, circulating genetically abnormal cells; IAC, invasive adenocarcinoma; mGGNs, mixed ground‐glass nodules; MIA, minimally invasive adenocarcinoma; pGGNs, pure ground‐glass nodules.
Sensitivity comparison between the CAC test and tumor biomarkers
We next examined the diagnostic performance of selected blood tumor biomarkers, including CEA, NSE, TPSA, SCC, PROGRP, CA19‐9 and CYFRA21‐1, and compared their lung cancer malignancy detection sensitivities with that of CACs (Table 3). CAC counts outperformed individual or combined tumor biomarkers with the highest AUC (0.769, 95 CI: 0.716–0.822) (Table 3, Fig 4). The results indicated that in all NSCLC patients, the CAC test showed significantly higher sensitivity than the combination of tumor biomarker (67.2% vs. 48.7%, P < 0.001, Table 4). Similarly, the CAC test showed significantly higher sensitivity in patients with MIA and AIS (71.2% vs. 35.6%, P < 0.001), IAC (65.8% vs. 53.7%, P = 0.033), small tumor size (≤10 mm: 65.4% vs. 36.5%, P = 0.003; 10–20 mm: 68.0% vs. 50.0%, P = 0.004) and GGNs (pure GGNs: 66.7% vs. 40.9%, P = 0.003; mixed GGNs: 73.0% vs. 43.2%, P < 0.001). There was no significant difference between the sensitivity of the CAC test and the combination of tumor biomarkers (any tumor biomarkers positive) in patients with nodule size of >20 mm (P = 0.251) and solid nodule (P = 0.546).
Table 3.
AUC comparison of CAC counts with established tumor biomarkers
| NSCLC versus healthy participants | AUC (95% CI) |
|---|---|
| CAC counts | 0.769 (0.716–0.822) |
| CEA | 0.528 (0.456–0.600) |
| NSE | 0.511 (0.438–0.585) |
| TPSA | 0.520 (0.447–0.593) |
| SCC | 0.528 (0.456–0.600) |
| ProGRP | 0.502 (0.428–0.576) |
| CA19‐9 | 0.520 (0.447–0.592) |
| CYFRA21‐1 | 0.516 (0.442–0.589) |
| All tumor biomarkers † | 0.558 (0.485–0.631) |
All tumor biomarkers: CEA + NSE + TPSA + SCC + ProGRP + CA19‐9 + CYFRA21‐1.
AUC, area under curve; CA19‐9, carbohydrate antigen 19‐9; CACs, circulating genetically abnormal cells; CEA, carcinoembryonic antigen; CYFRA21‐1, cytokeratin 19 fragment 21‐1; NSCLC, non‐small cell lung cancer; NSE, neuron‐specific enolase; ProGRP, progastrin‐releasing peptide; SCC‐Ag, squamous cell carcinoma antigen; TPSA, total prostate specific antigen.
Figure 4.
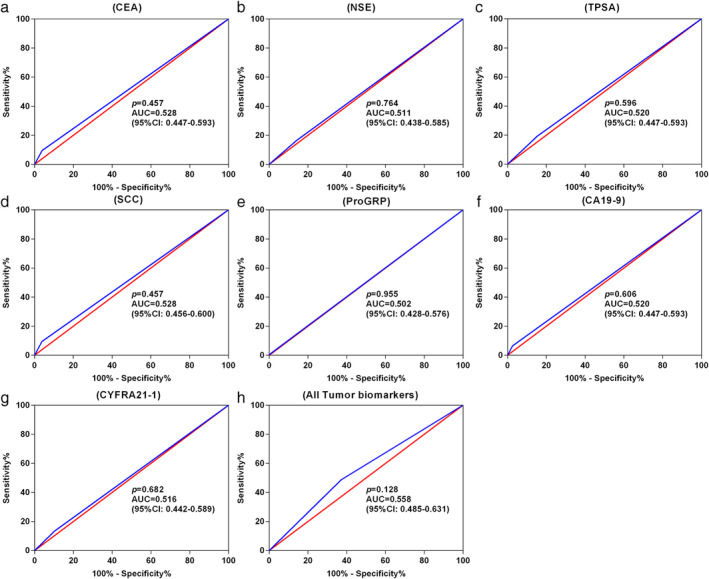
ROC curves for various tumor biomarkers to discriminate stage I NSCLC patients from healthy participants. (a) CEA; (b) NSE; (c) TPSA; (d) SCC; (e) ProGRP; (f) CA19‐9; (g) CYFRA21‐1; and (h) all tumor biomarkers (including CEA, NSE, TPSA, SCC, ProGRP, CA19‐9 and CYFRA21‐1). ROC, receiver operating characteristic; AUC, area under curve; CEA, carcinoembryonic antigen; NSE, neuron‐specific enolase; TPSA, total prostate specific antigen; SCC‐Ag, squamous cell carcinoma antigen; ProGRP, progastrin‐releasing peptide; CA19‐9, carbohydrate antigen 19‐9; CYFRA21‐1, cytokeratin 19 fragment 21‐1. (a) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %. (b) (
) identity %. (b) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %. (c) (
) identity %. (c) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %. (d) (
) identity %. (d) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %. (e) (
) identity %. (e) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %. (f) (
) identity %. (f) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %. (g) (
) identity %. (g) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %. (h) (
) identity %. (h) ( ) Sensitivity % and (
) Sensitivity % and ( ) identity %.
) identity %.
Table 4.
Sensitivity comparison between CACs and tumor biomarker in patients
| Positive (sensitivity) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | CACs | Tumor biomarkers † | TPSA | SCC | PRO | NSE | CA19 | CEA | CYPFRA21 |
| Total | 156 (67.2%) | 113 (48.7%)** | 45 (19.4%)** | 22 (9.5%)** | 1 (0.4%)** | 38 (16.4%)** | 15 (6.5%)** | 22 (9.5%)** | 31 (13.4%)** |
| Adenocarcinoma types | |||||||||
| MIA and AIS | 52 (71.2%) | 26(35.6%)** | 11(15.1%)** | 7(9.6%)** | 0 (0) ** | 8 (10.96%)** | 3 (4.1%)** | 1(1.4%)** | 6(8.2%)** |
| IAC | 98 (65.8%) | 80 (53.7%)* | 33(22.1%)** | 13(8.7%)** | 1 (0.7%)** | 28 (18.79%)** | 10 (6.7%)** | 18 (12.1%)** | 21 (14.1%)** |
| Nodule size | |||||||||
| ≤10 mm | 34 (65.4%) | 19(36.5%)* | 10(19.2%)** | 4(7.7%)** | 0 (0)** | 5 (9.62%)** | 2 (3.8%)** | 1(1.9%)** | 6 (11.5%)** |
| 10–20 mm | 83 (68.0%) | 61(50.0%)* | 23(18.9%)** | 12(9.8%)** | 1 (0.8%)** | 21 (17.21%)** | 8 (6.6%)** | 13 (10.7%)** | 15 (12.3%)** |
| >20 mm | 39 (67.2%) | 33 (56.9%) | 12 (20.7%)** | 6(10.3%)** | 0 (0)** | 12 (20.69%)** | 5 (8.6%)** | 8 (13.8%)** | 10 (17.2%)** |
| Nodule type | |||||||||
| Solid nodules | 58 (63.0%) | 54 (58.7%) | 17 (18.5%)** | 12 (13.0%)** | 1(1.1%)** | 13 (14.13%)** | 9 (9.8%)** | 18 (19.6%)** | 13 (14.1%)** |
| pGGNs | 44 (66.7%) | 27 (40.9%)* | 12 (18.2%)** | 8 (12.1%)** | 0 (0)** | 10 (15.15%)** | 1 (1.5%)** | 2 (3.0%)** | 8 (12.1%)** |
| mGGNs | 54 (73.0%) | 32(43.2%)** | 16 (21.6%)** | 2 (2.7%)** | 0(0)** | 15 (20.27%)** | 5 (6.8%)** | 2 (2.7%)** | 10 (13.5%)** |
P < 0.05 compared with CACs;
P < 0.001 compared with CACs.
Any tumor biomarkers positive: CEA + NSE + TPSA + SCC + ProGRP + CA19‐9 + CYFRA21‐1.
AIS, adenocarcinoma in situ; CA19‐9, carbohydrate antigen 19‐9; CACs, circulating genetically abnormal cells; CEA, carcinoembryonic antigen; CYFRA21‐1, cytokeratin 19 fragment 21‐1; IAC, invasive adenocarcinoma; mGGNs, mixed ground‐glass nodules; MIA, minimally invasive adenocarcinoma; NSE, neuron‐specific enolase; pGGNs, pure ground‐glass nodules; ProGRP, progastrin‐releasing peptide; SCC‐Ag, squamous cell carcinoma antigen; TPSA, total prostate specific antigen.
Discussion
Recently, CACs have been indicated to be related to cancers, and they contain genetic abnormalities similar to the primary tumor. 18 , 23 However, it is only in recent years that reliable and effective methods in detecting CACs have been developed. An antigen‐independent FISH‐based assay has been used to detect CACs in all stages of lung cancer and indicate correlation between the numbers of CACs and cancer, meaning that CAC detection for early diagnosis has become increasingly feasible. 18 However, the clinical significance and diagnostic performance of CAC detection remains unclear.
Here, this prospective clinical trial was performed to evaluate the diagnostic performance of the CAC test in Chinese patients with NSCLC. We confirmed that the CAC test had a significant diagnostic performance in discrimination between NSCLC patients and healthy participants, with an AUC of 0.769, sensitivity of 67.2%, and specificity of 80.8%. The discriminatory capability of this test is acceptable for clinical research. Furthermore, ROC curve analysis showed that the optimal cutoff point for diagnosing stage I NSCLC was 1, which indicated that patients with one or more CACs are diagnosed as CAC positive. Additionally, we found that the CAC positive rate had no relationship with patients' demographics (age, gender and smoking history) or pathological type. These findings indicated that the CAC test has good stability and adaptability for different patient populations.
We evaluated the performance of the CAC test for patients with early‐stage NSCLC. As the diagnostic performance of most biomarkers gradually decreases as the tumor stage decreases, diagnosis of early‐stage cancer is particularly difficult. 24 Katz et al. evaluated the levels of CACs in stage I to IV NSCLC patients without reporting the detection rate of the CACs. 18 Among the 16 stage II and stage III patients who were excluded from our study, 13 patients (81.3%) were found to be CAC‐positive (Table S1). The detection rate of CACs was higher in patients with stage II–III NSCLC than in those with stage I disease, which might support the hypothesis that CAC enumeration is correlated to clinical stage. In the present study, we only enrolled patients with stage I disease and achieved a satisfactory diagnostic performance with an AUC of 0.769. Recently, several other cancer biomarkers have been used in liquid biopsy. However, most biomarkers only showed favorable diagnostic performance in patients with advanced lung cancer. To date, the diagnostic performance in early‐stage NSCLC has been unsatisfactory. For example, Chen et al. found that the sensitivity and specificity of circulating tumor DNA (ctDNA) were only 53.8% and 47.3%, respectively, in early‐stage NSCLC patients. 25 Furthermore, Tanaka et al. observed that CTCs were only detected in 17 of 88 stage I patients with a sensitivity of 19.3%. 26 In contrast, our study showed a sensitivity of 67.2% and specificity of 80.8% for CACs in diagnosing patients with stage I disease. These results suggest that CACs may be a potential marker for the early diagnosis of NSCLC, even in early stage disease.
In addition, in this study we compared the discriminatory capability between CACs and traditional tumor biomarkers. Tumor biomarkers such as CEA, TPSA, NSE, CA19‐9 and CYFRA21‐1 are usually used for diagnosis, progression and prognosis assessment of lung cancer. 27 However, due to the insufficient sensitivity and specificity of these tumor biomarkers as well as a lack of reproducibility, routine use of tumor biomarkers is not recommended in clinical practice, particularly for the diagnosis of early‐stage cancer. 28 , 29 Notably, we found that the CAC test had the higher AUC when compared with the traditional tumor biomarkers, indicating that CACs may be a more effective method for clinical early stage NSCLC diagnosis. Furthermore, sensitivity of the CAC test and tumor biomarkers in different patient populations was compared to further verify the diagnostic strength of the CAC test. In all patients, the sensitivity of the CAC test was significantly higher than that of traditional tumor biomarkers. Additionally, nine patients with stage I NSCLC who underwent surgery were recruited to investigate the change in CACs before and after treatment for the dynamic surveillance study (Fig S1). Our data from the dynamic surveillance study found a significant decrease in the number of postoperative CACs, demonstrating the clinical significance of the CAC test for surveillance of treatment efficiency in patients with NSCLC treated with surgery. These results indicate that CACs may be useful in the treatment response assessment. Overall, these findings suggest that CACs may be a more effective method and provide further evidence for the good discriminatory capability of CACs.
Traditional early diagnostic methods have limited ability to identify small tumors and malignant GGNs, while the evaluation and management of those patients is becoming of increasing importance in clinical practice. 30 Interestingly, according to the stratification analysis results, the detection rate of CACs has not been found to be correlated to tumor size or nodule type. However, CACs showed significantly higher sensitivity than tumor biomarkers in NSCLC patients with small nodules (≤20 mm) and GGNs. The CAC test presented a better diagnostic performance of NSCLC patients with small nodules and GGNs. Thus, it may provide a new perspective in the evaluation and management of small nodules and GGNs.
Although this study reported important findings, the small sample size and the adenocarcinoma‐biased population are open to potential criticism. This study predominantly included adenocarcinoma patients, which may not reflect the general population of early stage NSCLC. Thus, a larger‐scale study including a general population with NSCLC should be performed to verify the findings reported here.
In conclusion, the findings of this study indicated that the CAC test exhibited high sensitivity and specificity and thus may be useful in early stage lung cancer clinical diagnosis. Further investigations on the clinical and prognostic value of the CAC test are warranted.
Disclosure
The authors confirm that there are no potential conflicts of interest.
Supporting information
Figure S1 Dynamic changes of CAC counts in patients receiving curative resection. CACs, circulating genetically abnormal cells.
Table S1 Comparison of CACs test between stage I NSCLC and advanced NSCLC
Acknowledgments
Thanks to Meng Huang and Yan‐Ci Chen (Sanmed Biotech Ltd.) for providing sample processing and analysis assistance. An interim analysis of this paper has been presented as a poster presentation at the IASLC 2019 World Conference on Lung Cancer in Barcelona on September 7–10, 2019.
This work was supported by National Key Research and Development Program of China (grant numbers 2016YFC0905501, 2016YFC0905500); National Natural Science Foundation of China (grant numbers 81772484, 81772488, 81672304); Tianjin Cancer Hospital Clinical Trial Project (grant number C1705); and Program for Guangdong Introducing Innovative and Entrepreneurial Teams (grant number 2019ZT08Y297).
[Correction added on 7 October, after first online publication: affiliation 3 has been added.]
Contributor Information
Wei‐Ran Liu, Email: wrliu@tmu.edu.cn.
Chang‐Li Wang, Email: wangchangli@tjmuch.com.
References
- 1. Heigener DF, Reck M. Lung cancer in 2017: Giant steps and stumbling blocks. Nat Rev Clin Oncol 2018; 15 (2): 71–2. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch FR, Scagliotti GV, Mulshine JL et al Lung cancer: Current therapies and new targeted treatments. Lancet 2017; 389 (10066): 299–311. [DOI] [PubMed] [Google Scholar]
- 3. Conway EM, Pikor LA, Kung SH et al Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med 2016; 193 (2): 116–30. [DOI] [PubMed] [Google Scholar]
- 4. Gesthalter YB, Vick J, Steiling K, Spira A. Translating the transcriptome into tools for the early detection and prevention of lung cancer. Thorax 2015; 70 (5): 476–81. [DOI] [PubMed] [Google Scholar]
- 5. Wille MM, Dirksen A, Ashraf H et al Results of the randomized Danish lung cancer screening trial with focus on high‐risk profiling. Am J Respir Crit Care Med 2016; 193 (5): 542–51. [DOI] [PubMed] [Google Scholar]
- 6. Lee C, Guichet PL, Abtin F. Percutaneous lung biopsy in the molecular profiling era: A survey of current practices. J Thorac Imaging 2017; 32 (1): 63–7. [DOI] [PubMed] [Google Scholar]
- 7. Volpi S, Ali JM, Tasker A, Peryt A, Aresu G, Coonar AS. The role of positron emission tomography in the diagnosis, staging and response assessment of non‐small cell lung cancer. Ann Transl Med 2018; 6 (5): 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liang W, Zhao Y, Huang W, Liang H, Zeng H, He J. Liquid biopsy for early stage lung cancer. J Thorac Dis 2018; 10 (Suppl 7): S876–s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Humphrey LL, Deffebach M, Pappas M et al Screening for lung cancer with low‐dose computed tomography: A systematic review to update the US Preventive services task force recommendation. Ann Intern Med 2013; 159 (6): 411–20. [DOI] [PubMed] [Google Scholar]
- 10. Aberle DR, Adams AM, Berg CD et al Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med 2011; 365 (5): 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wisnivesky JP, Henschke CI, Yankelevitz DF. Diagnostic percutaneous transthoracic needle biopsy does not affect survival in stage I lung cancer. Am J Respir Crit Care Med 2006; 174 (6): 684–8. [DOI] [PubMed] [Google Scholar]
- 12. Yamagami T, Kato T, Iida S, Hirota T, Nishimura T. Percutaneous needle biopsy for small lung nodules beneath the rib under CT scan fluoroscopic guidance with gantry tilt. Chest 2004; 126 (3): 744–7. [DOI] [PubMed] [Google Scholar]
- 13. Yamauchi Y, Izumi Y, Nakatsuka S et al Diagnostic performance of percutaneous core‐needle lung biopsy under CT scan fluoroscopic guidance for pulmonary lesions measuring </=10 mm. Chest 2011; 140 (6): 1669–70. [DOI] [PubMed] [Google Scholar]
- 14. Plaks V, Koopman CD, Werb Z. Circulating tumor cells. Science 2013; 341 (6151): 1186–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sorber L, Zwaenepoel K, Deschoolmeester V et al Circulating cell‐free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients. Lung Cancer 2017; 107: 100–7. [DOI] [PubMed] [Google Scholar]
- 16. O'Flaherty L, Wikman H, Pantel K. Biology and clinical significance of circulating tumor cell subpopulations in lung cancer. Transl Lung Cancer Res 2017; 6 (4): 431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schochter F, Friedl TWP, deGregorio A et al Are circulating tumor cells (CTCs) ready for clinical use in breast cancer? An overview of completed and ongoing trials using CTCs for clinical treatment decisions. Cell 2019; 8 (11): 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katz RL, He W, Khanna A et al Genetically abnormal circulating cells in lung cancer patients: An antigen‐independent fluorescence in situ hybridization‐based case‐control study. Clin Cancer Res 2010; 16 (15): 3976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mostert B, Kraan J, Sieuwerts AM et al CD49f‐based selection of circulating tumor cells (CTCs) improves detection across breast cancer subtypes. Cancer Lett 2012; 319 (1): 49–55. [DOI] [PubMed] [Google Scholar]
- 20. Feng M, Lin M, Zhou H et al Clinical utility of circulating genetically abnormal cells within low‐dose computed tomography lung cancer screening: A correlative MCPND trial study. J Clin Oncol 2020; 38 (15_Suppl): e15536. [Google Scholar]
- 21. Goldstraw P, Chansky K, Crowley J et al The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11 (1): 39–51. [DOI] [PubMed] [Google Scholar]
- 22. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143 (1): 29–36. [DOI] [PubMed] [Google Scholar]
- 23. Pailler E, Auger N, Lindsay CR et al High level of chromosomal instability in circulating tumor cells of ROS1‐rearranged non‐small‐cell lung cancer. Ann Oncol 2015; 26 (7): 1408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou J, Yu L, Gao X et al Plasma microRNA panel to diagnose hepatitis B virus‐related hepatocellular carcinoma. J Clin Oncol 2011; 29 (36): 4781–8. [DOI] [PubMed] [Google Scholar]
- 25. Chen KZ, Lou F, Yang F et al Circulating tumor DNA detection in early‐stage non‐small cell lung cancer patients by targeted sequencing. Sci Rep 2016; 6: 31985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanaka F, Yoneda K, Kondo N et al Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009; 15 (22): 6980–6. [DOI] [PubMed] [Google Scholar]
- 27. Mishra A, Verma M. Cancer biomarkers: Are we ready for the prime time? Cancers (Basel) 2010; 2 (1): 190–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vargas AJ, Harris CC. Biomarker development in the precision medicine era: Lung cancer as a case study. Nat Rev Cancer 2016; 16 (8): 525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neal JW, Gainor JF, Shaw AT. Developing biomarker‐specific end points in lung cancer clinical trials. Nat Rev Clin Oncol 2015; 12 (3): 135–46. [DOI] [PubMed] [Google Scholar]
- 30. Tao G, Jingying Y, Tan G et al A novel CT‐guided technique using medical adhesive for localization of small pulmonary ground‐glass nodules and mixed ground‐glass nodules (≤20 mm) before video‐assisted thoracoscopic surgery. Diagn Interv Radiol 2018; 24 (4): 209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Dynamic changes of CAC counts in patients receiving curative resection. CACs, circulating genetically abnormal cells.
Table S1 Comparison of CACs test between stage I NSCLC and advanced NSCLC


