Abstract
Background
HS‐1‐associated protein‐1 (HAX1) has been reported to be overexpressed in non‐small cell lung cancer (NSCLC) tissues. However, the underlying mechanism of HAX1 in NSCLC has not previously been demonstrated. The present study investigated the role and underlying mechanism of HAX1 in NSCLC.
Methods
The HAX1 expression were confirmed in NSCLC tissues through TCGA database and qRT‐PCR. Moreover, we performed qRT‐PCR, Western blotting, Transwell assays, TUNEL assays and so on to evaluate the role of HAX1 in A549 and H1299 cell lines.
Results
mRNA expression of HAX1 was overexpressed in NSCLC tissues compared to adjacent normal tissues according to The Cancer Genome Atlas (TCGA) database. QRT‐PCR assays showed that HAX1 mRNA expression was upregulated in NSCLC tissues. The high HAX1 mRNA levels were found to be positively associated with tumor size, TNM stage and lymphatic metastasis. Silencing of HAX1 promoted apoptosis and reduced invasion of A549 and H1299 cells by inhibiting the AKT/mTOR and MDM2/P53 signal pathway. AKT agonist SC79 could inhibit apoptosis and promote proliferation, migration and invasion of A549 and H1299 cells transfected with si‐HAX1.
Conclusions
The present study provided a better understanding of HAX1 mechanism in NSCLC and potential therapeutic target for NSCLC.
Keywords: AKT, HS‐1‐associated protein‐1, non‐small cell lung cancer
HAX1 enhances the survival, invasion and epithelial–mesenchymal transition of NSCLC cells through the AKT/mTOR signaling pathway. HAX1 play an important role in NSCLC and should be taken into consideration.
Introduction
Lung cancer is one of the most lethal cancers in the world, which causes a heavy socioeconomic burden. 1 , 2 , 3 There are two major histological types including small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC) and NSCLC accounts for more than 80% of all lung cancer cases.4 NSCLC includes adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma, and large cell carcinoma. Despite huge efforts in the fight against the disease, the prognosis for NSCLC is still poor and a five‐year survival rate is no more than 15%. 5 , 6 , 7 The development of lung cancer is a multistep process, including changes in oncogenes and tumor suppressor genes, leading to gradual dysregulation of pathways related to cell proliferation and survival. 8 Therefore, the discovery that new molecular targets play a regulatory role in the uncontrolled proliferation of tumor cells is of great significance for the diagnosis and treatment of NSCLC. 9
HS‐1 related protein X‐1 (HAX1), a 35 kDa protein, is an important regulator of apoptosis and was first discovered to interact with HS‐1, a substrate of serine/tyrosine kinases. 10 Its gene is located on human chromosome lq21.3 and encodes 279 amino acid residues. HAX1 plays an antiapoptotic function in many tissues due to a homology domain of the BCL‐2 family and a weak homology with the apoptosis‐related protein NIP3. 11 Recent studies have shown that HAX1 can bind to a variety of intracellular apoptosis‐related proteins (such as caspase 9, OMI/HtrA2, VPR, K15, AKT) and exert antiapoptotic effects. HAX1 may also participate in the cell migration process through molecules such as Galphal3, integrin aVl36, IL‐1a, GRB7, NF‐κB. 12 , 13 , 14 , 15 In addition, some studies have confirmed that HAX1 also binds 3 ′ translation region mRNAs and acts as a new transactivating factor in the post‐transcriptional regulation of Pol β expression. 16 HAX1 is upregulated in lung cancer, hepatoma, lymphoma, melanoma, leukemia and myeloma according to Oncomine, a cancer microarray database. 17 In lung cancer tissues, upregulation has been observed in the most advanced stages of the disease (stages III–IV, 0.049 for lung cancer, respectively). 18 However, the role of HAX1 in NSCLC metastasis and recurrence as well as the underlying mechanism have not been previously reported.
Here, we explored the relationship between HAX1 expression and the apoptosis and metastasis of NSCLC. Our study confirmed HAX1 expression in NSCLC samples, and revealed that HAX1 expression is significantly elevated in NSCLC tissues, suggesting that HAX1 might play an important role in EMT in NSCLC cells and in the promotion of NSCLC metastasis.
Methods
Clinical specimen collection
A total of 35 paired tumor tissues and their matched adjacent normal tissues were obtained from March 2015 to June 2019 in Xinhua Hospital (Shanghai, China). Each fresh resected specimen was snap‐frozen in liquid nitrogen and stored at −80° prior to RNA isolation. This study was approved by the Medical Ethics Committee of the Xinhua Hospital and all experiments were performed in accordance with the Declaration of Helsinki.
Cell culture
NSCLC cell lines H1299 and A549 were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in DMEM high‐glucose medium (Gibco, USA) with 10% fetal bovine serum (Gibco, USA) at 37°C in a humidified environment with 5% CO2.
RNAi and transfection
HAX1‐siRNAs (si‐HAX1) were designed and purchased from Sangon Biotech, China. The target sequences for HAX1 were as follows: Sense: GGAUACGUUUCCACGAUAAdTdT; Antisense: UUAUCGUGGAAACGUAUCCdTdT. Cells were seeded on six‐well plates for 70% confluency and transfected with si‐HAX1 using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer's instructions. Cells were obtained for the following experiments after 48 hours.
Quantitative real‐time polymerase chain reaction (qRT‐PCR)
Total RNA was obtained from tissue samples or cells by using Trizol reagent (Beyotime, China) according to the manufacturer's instruction. Subsequently, cDNA synthesis and qRT‐PCR were performed by one‐step RT qPCR SYBRGreen Kit (DBI, Germany) and the ABI 7500 Fast Real‐Time PCR System (Life Tech, Carlsbad, California, USA). 2−ΔΔCt method was used to calculate the relative expression of genes, which were normalized to β‐actin.
Western blotting
Cells were lysed using RIPA buffer containing 10% PMSF (Beyotime, China) and total protein obtained. Then, 30 μg protein lysate were separated by 8% to 12% SDS‐PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and electro‐transferred to PVDF (polyvinylidene difluoride). PVDF were blocked in 5% skimmed milk for 2 hours. The membranes were then incubated with primary antibodies at 4 °C overnight. Primary antibodies are listed as follows: β‐actin (Beyotime, China, 1:1000), Cytochrome C (Abcam, UK, 1:1000), Caspase 9 (Proteintech, USA, 1:1000), Bcl‐2 (Proteintech, USA, 1:1000), Bax (Proteintech, USA, 1:1000), Caspase 3 (Abcam, UK, 1:1000), AKT (CST, USA, 1:1000), p‐AKT (CST, USA, 1:1000), mTOR (CST, USA, 1:1000), p‐mTOR (CST, USA, 1:1000), MDM2 (CST, USA, 1:1000), p‐MDM2 (CST, USA, 1000), p53 (CST, USA, 1:1000), and HAX1 (Proteintech, USA, 1:1000). Membranes were washed with three times and subsequently incubated with horseradish peroxidase (HRP)‐conjugated IgG secondary antibodies (Beyotime, China, 1:2000) at room temperature for 1 hour. The target protein was visualized using an ECL kit (Millipore, Billerica, MA, USA). GAPDH was regarded as an endogenous reference.
Transwell invasion assay
Cell invasion assay was carried out using 24‐well transwell plates (Corning, USA) containing Matrigel (BD, USA). Approximately 2 × 105 cells were planted in the upper chamber with medium in triplicate without serum. Medium supplemented with 10% FBS was added to the lower chamber. After incubation for 24 hours, the cells above the Matrigel layer were removed using a cotton swab, and then cells below the membrane were fixed using 4% paraformaldehyde, and stained with crystal violet for 10 minutes. Finally, five randomly chosen fields of each well were counted.
Annexin V‐FITC/PI apoptosis assay
Apoptotic cells were detected with an annexin V‐FACS apoptosis detection kit (BD, USA) according to the manufacturer's instructions. Briefly, after transfection with si‐HAX1 for 48 hours, cells were washed with ice PBS twice times and incubated in 300 μL 1× binding buffer supplemented with 5 μL annexin V‐FITC for 10 minutes and then resuspended with PI (propidium iodide) in the dark for 5 minutes at 25°C. The stained cells were detected using an FC 500 flow cytometer (Beckman Coulter, Brea, CA, USA) within 1 hour according to the manufacturer's protocol.
TUNEL assays
Cells were seeded on the coverslips and then transfected with si‐HAX1 for 48 hours. After being fixed using 4% paraformaldehyde, cells were penetrated with 0.3% Triton X‐100 for 5 minutes. Then, 50 μl TUNEL was added to each coverslip and incubated for 1 hour. Finally, images were photographed by microscopy (Olympus BX51).
Statistical analysis
All data are presented as mean ± standard deviation (SD). Paired sample t‐test was used to calculate the expression differences of HAX1 between NSCLC tissues and adjacent noncancerous tissues. Independent sample Student's t‐test was used to calculate the differences between groups. Pearson's correlation coefficient was used to analyze the expression correlation of HAX1. All experiments were performed in triplicate. The difference was considered to be statistically significant when P‐values were less than 0.05. ***P < 0.001, **P < 0.01, *P < 0.05. Statistical analysis was carried out in SPSS 20.0 (SPSS, USA).
Results
HAX1 upregulated in NSCLC tissues according to TCGA database
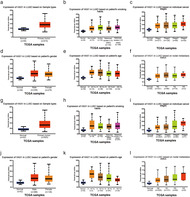
HAX1 has been reported to be overexpressed in lung cancer. 18 To confirm whether HAX1 was overexpressed in NSCLC, we searched the express information of HAX1 in the Cancer Genome Atlas (TCGA) database. We found that expression of HAX1 was upregulated in LUAD based on sample types (Fig 1a). The P‐value of each group is shown in Tables 1 and 2. There was no significant difference in HAX1 expression in LUAD on cancer stage, patients’ smoking habits, gender, age and nodal metastasis status (Fig 1b–f). In addition, expression of HAX1 was also overexpressed in LUSC based on sample types (Fig 1g). HAX1 expression was not significantly different in LUSC on cancer stage, patients’ smoking habits, gender, age and nodal metastasis status (Fig 1h–l). The HAX1 expression was explored in TCGA database and revealed that HAX1 was overexpressed in NSCLC and high levels might be positively related to the clinicopathological characteristics of NSCLC patients.
Figure 1.
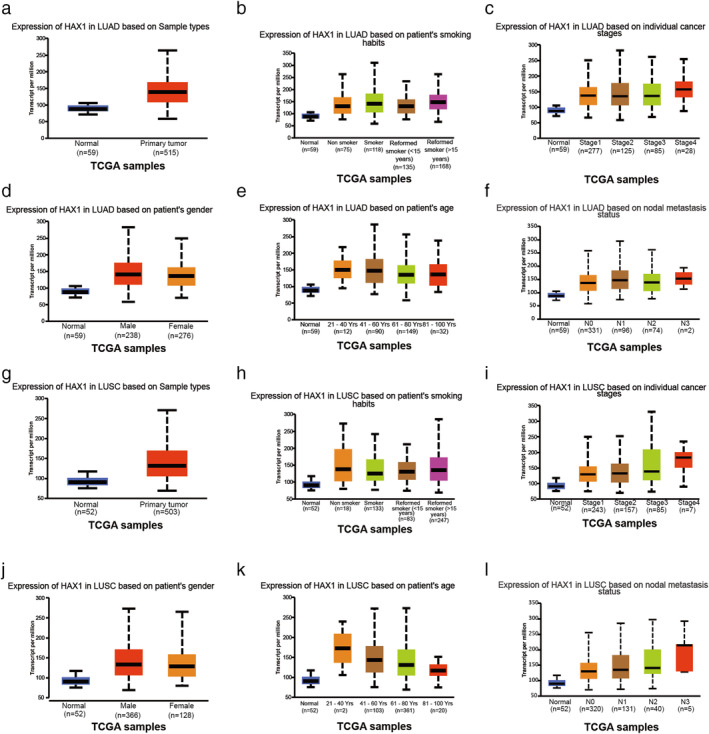
Information of HAX1 expression in TCGA database. (a) Expression of HAX1 in LUAD based on sample type. (b) Expression of HAX1 in LUAD based on patients’ smoking habits. (c) Expression of HAX1 in LUAD based on individual cancer stages. (d) Expression of HAX1 in LUAD based on patients’ gender. (e) Expression of HAX1 in LUAD based on patients’ age. (f) Expression of HAX1 in LUAD based on nodal metastasis status. (g) Expression of HAX1 in LUSC based on sample type. (h) Expression of HAX1 in LUSC based on patients’ smoking habits. (i) Expression of HAX1 in LUSC based on individual cancer stages. (jJ) Expression of HAX1 in LUAD based on patients’ gender. (k) Expression of HAX1 in LUSC based on patients’ age. (l) Expression of HAX1 in LUSC based on nodal metastasis status. LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; N0, no regional lymph node metastasis; N1, metastases in 1–3 axillary lymph nodes; N2, metastases in 4–9 axillary lymph nodes; N3, metastases in 10 or more axillary lymph nodes.
Table 1.
The P‐value of each group in LUAD samples in Fig 1
| Patients’ smoking habit | Cancer stages | ||
|---|---|---|---|
| Comparison | P‐value | Comparison | P‐value |
| Normal vs. non‐smoker | <0.05 | Normal vs. stage 1 | <0.05 |
| Normal vs. smoker | <0.05 | Normal vs. stage 2 | <0.05 |
| Normal vs. reformed smoker 1 | <0.05 | Normal vs. stage 3 | <0.05 |
| Normal vs. reformed smoker 2 | <0.05 | Normal vs. stage 4 | <0.05 |
| Non‐smoker vs. smoker | >0.05 | Stage 1 vs. stage 2 | >0.05 |
| Non‐smoker vs. reformed smoker 1 | >0.05 | Stage 1‐ vs. stage 3 | >0.05 |
| Non‐smoker vs. reformed smoker 2 | >0.05 | Stage 1 vs. stage 4 | >0.05 |
| Smoker vs. reformed smoker 1 | >0.05 | Stage 2 vs. stage 3 | >0.05 |
| Smoker vs. reformed smoker 2 | >0.05 | Stage 2 vs. stage 4 | >0.05 |
| Reformed smoker 1 vs. reformed smoker 2 | >0.05 | Stage 3 vs. stage 4 | >0.05 |
| Patients’ gender | |||
|---|---|---|---|
| Comparison | P‐value | Comparison | P‐value |
| Normal vs. male | <0.05 | ||
| Normal vs. female | <0.05 | ||
| Male vs. female | >0.05 | ||
| Patients’ age | Nodal metastasis status | ||
|---|---|---|---|
| Comparison | P‐value | Comparison | P‐value |
| Normal vs. age (21–40 years) | >0.05 | Normal vs. N0 | <0.05 |
| Normal vs. age (41–60 years) | <0.05 | Normal vs. N1 | <0.05 |
| Normal vs. age (61–80 years) | <0.05 | Normal vs. N2 | <0.05 |
| Normal vs. age (81–100 years) | <0.05 | Normal vs. N3 | >0.05 |
| Age (21–40 years) vs. age (41–60 years) | >0.05 | N0 vs. N1 | >0.05 |
| Age (21–40 years) vs. age (61–80 years) | >0.05 | N0 vs. N2 | >0.05 |
| Age (21–40 years) vs. age (81–100 years) | >0.05 | N0 vs. N3 | >0.05 |
| Age (41–60 years) vs. age (61–80 years) | >0.05 | N1 vs. N2 | <0.05 |
| Age (41–60 years) vs. age (81–100 years) | >0.05 | N1 vs. N3 | >0.05 |
| Age (61–80 years) vs. age (81–100 years) | >0.05 | N2 vs. N3 | >0.05 |
N0, no regional lymph node metastasis; N1, metastases in 1 to 3 axillary lymph nodes; N2, metastases in 4 to 9 axillary lymph nodes; N3, metastases in 10 or more axillary lymph nodes.
Table 2.
The P‐value of each group in LUSC samples in Fig 1
| Patients’ smoking habit | Cancer stages | ||
|---|---|---|---|
| Comparison | P‐value | Comparison | P‐value |
| Normal vs. non‐smoker | <0.05 | Normal vs. stage 1 | <0.05 |
| Normal vs. smoker | <0.05 | Normal vs. stage 2 | <0.05 |
| Normal vs. reformed smoker 1 | <0.05 | Normal vs. stage 3 | <0.05 |
| Normal vs. reformed smoker 2 | <0.05 | Normal vs. stage 4 | <0.05 |
| Non‐smoker vs. smoker | >0.05 | Stage 1 vs. stage 2 | >0.05 |
| Non‐smoker vs. reformed smoker 1 | >0.05 | Stage 1 vs. stage 3 | <0.05 |
| Non‐smoker vs. reformed smoker 2 | >0.05 | Stage 1 vs. stage 4 | >0.05 |
| Smoker‐ vs. reformed smoker 1 | >0.05 | Stage 2 vs. stage 3 | <0.05 |
| Smoker vs. reformed smoker 2 | <0.05 | Stage 2 vs. stage 4 | >0.05 |
| Reformed smoker 1 vs. reformed smoker 2 | >0.05 | Stage 3 vs. stage 4 | >0.05 |
| Patients’ gender | |||
|---|---|---|---|
| Comparison | P‐value | Comparison | P‐value |
| Normal vs. male | <0.05 | ||
| Normal vs. female | <0.05 | ||
| Male vs. ‐female | >0.05 | ||
| Patients’ age | Nodal metastasis status | ||
|---|---|---|---|
| Comparison | P‐value | Comparison | P‐value |
| Normal‐ vs. age (21–40 years) | >0.05 | Normal vs. N0 | <0.05 |
| Normal vs. age (41–60 years) | <0.05 | Normal vs. N1 | <0.05 |
| Normal vs. age (61–80 years) | <0.05 | Normal vs. N2 | <0.05 |
| Normal vs. age (81–100 years) | <0.05 | Normal vs. N3 | <0.05 |
| Age (21–40 years) vs. age (41–60 years) | >0.05 | N0 vs. N1 | >0.05 |
| Age (21–40 years) vs. age (61–80 years) | >0.05 | N0 vs. N2 | >0.05 |
| Age (21–40 years) vs. age (81–100 years) | >0.05 | N0 vs. N3 | >0.05 |
| Age (41–60 years) vs. age (61–80 years) | >0.05 | N1 vs. N2 | <0.05 |
| Age (41–60 years vs. age (81–100 years) | <0.05 | N1 vs. N3 | >0.05 |
| Age (61–80 years vs. age (81–100 years) | <0.05 | N2 vs. N3 | >0.05 |
N0, no regional lymph node metastasis; N1, metastases in 1 to 3 axillary lymph nodes; N2, metastases in 4 to 9 axillary lymph nodes; N3, metastases in 10 or more axillary lymph nodes.
Upregulation of HAX1 observed in NSCLC tissues compared to adjacent normal tissues
We performed qRT‐PCR to measure the mRNA expression of HAX1 in NSCLC and adjacent normal tissues. The results showed that HAX1 mRNA expression was higher in NSCLC tissues than adjacent normal tissues (Fig 2a). Furthermore, we observed that the expression level of HAX1 was positively associated with tumor size (P = 0.027*) (Table 3). HAX1 expression level was not significantly associated with gender, age, tumor status progression or lymphatic metastasis. Together, our data suggested that increased HAX1 expression might be involved in NSCLC progression.
Figure 2.
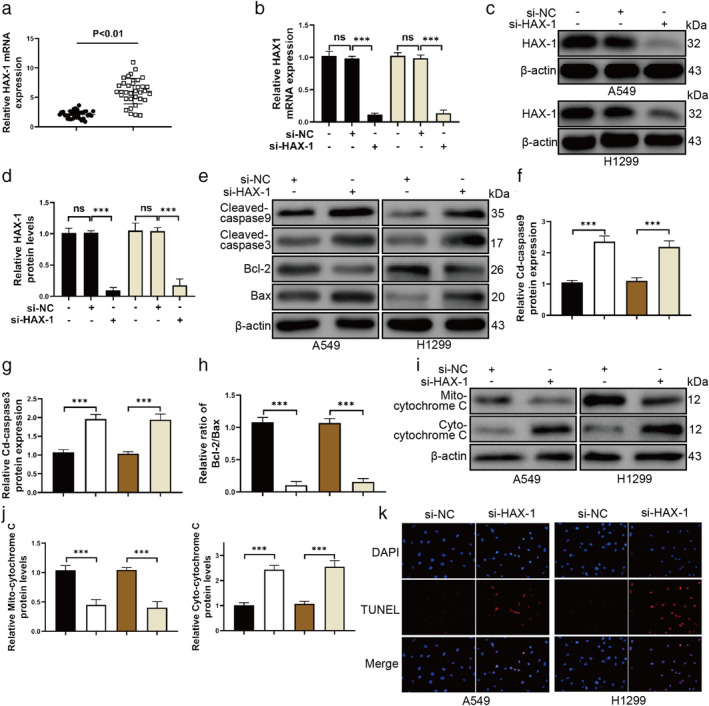
HAX1 knockdown increases apoptosis of A549 and H1299 cells. (a) HAX1 mRNA expression was increased in NSCLC tissue samples. ( ) Adjacent tissues and (
) Adjacent tissues and ( ) cancer tissues. (b) HAX1 mRNA expression was reduced remarkably by si‐HAX1 in A549 and H1299 cells. (
) cancer tissues. (b) HAX1 mRNA expression was reduced remarkably by si‐HAX1 in A549 and H1299 cells. ( ) A549 and (
) A549 and ( ) H1299. (c) HAX1 protein expression was decreased remarkably by si‐HAX1 in A549 and H1299 cells. (d) Quantitative analysis of protein HAX1 expression in A549 and H1299. (
) H1299. (c) HAX1 protein expression was decreased remarkably by si‐HAX1 in A549 and H1299 cells. (d) Quantitative analysis of protein HAX1 expression in A549 and H1299. ( ) A549 and (
) A549 and ( ) H1299. (e) Western blotting for cleaved‐caspase 9, cleaved‐caspase 3, Bcl‐2, Bax and β‐actin in A549 and H1299 cells. (f–h) Quantitative analysis of protein cleaved‐caspase 9, cleaved‐caspase 3, Bcl‐2, Bax expression in A549 and H1299. (f) (
) H1299. (e) Western blotting for cleaved‐caspase 9, cleaved‐caspase 3, Bcl‐2, Bax and β‐actin in A549 and H1299 cells. (f–h) Quantitative analysis of protein cleaved‐caspase 9, cleaved‐caspase 3, Bcl‐2, Bax expression in A549 and H1299. (f) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (g) (
) H1299/si‐HAX1. (g) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (h) (
) H1299/si‐HAX1. (h) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (i) Western blotting for cytochrome C and β‐actin in A549 and H1299 cells. (j) Quantitative analysis of protein cytochrome C expression in A549 and H1299. (
) H1299/si‐HAX1. (i) Western blotting for cytochrome C and β‐actin in A549 and H1299 cells. (j) Quantitative analysis of protein cytochrome C expression in A549 and H1299. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (
) H1299/si‐HAX1. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (k) TUNEL assays for apoptosis of A549 and H1299 cells. ***P < 0.001, **P < 0.01, *P < 0.05.
) H1299/si‐HAX1. (k) TUNEL assays for apoptosis of A549 and H1299 cells. ***P < 0.001, **P < 0.01, *P < 0.05.
Table 3.
The association of HAX1 expression in 35 NSCLC patients with clinicopathological characteristics
| Expression of HAX1 | ||||
|---|---|---|---|---|
| Characteristics | Patients | High‐HAX1 | Low‐HAX1 | P‐value |
| Total | 35 | 18 | 17 | |
| Gender | 0.315 | |||
| Male | 20 | 12 | 8 | |
| Female | 15 | 6 | 9 | |
| Age (years) | 0.601 | |||
| ≤60 | 16 | 9 | 7 | |
| ≥60 | 19 | 9 | 10 | |
| TNM stage | 0.499 | |||
| I–II | 20 | 9 | 11 | |
| III–IV | 15 | 9 | 6 | |
| Tumor size | 0.027* | |||
| <3 cm | 14 | 4 | 10 | |
| >3 cm | 21 | 14 | 7 | |
| Lymphatic metastasis | 0.489 | |||
| No | 12 | 5 | 7 | |
| Yes | 23 | 13 | 10 | |
Difference was considered to be statistically significant when P values were less than 0.05. ***p < 0.001, **p < 0.01, *p < 0.05. Statistical analysis was carried out in SPSS 20.0 (SPSS, USA).
HAX1 knockdown increases apoptosis of NSCLC cell lines
To explore the role and underlying mechanism of HAX1, we constructed the siRNA to silence the HAX1 expression in A549 and H1299 cell lines. First, qRT‐PCR was used to measure the mRNA expression in A549 and H1299 cell lines transfected with si‐HAX1. The results of qRT‐PCR confirmed that si‐HAX1 caused a significant decrease of HAX1 in A549 and H1299 cells (Fig 2b). Western blotting also confirmed si‐HAX1 worked in A549 and H1299 cells (Fig 2c,d). Second, we wanted to investigate whether HAX1 knockdown caused A549 and H1299 cells apoptosis. Thus, western blotting and TUNEL assays were used to investigate the apoptosis of A549 and H1299 cells. HAX1 knockdown increased the protein levels of cleaved‐caspase 9 and cleaved‐caspase 3 and decreased the ratio of Bcl‐2/Bax (Fig 2e–h). HAX1 knockdown also promoted the flux of cytochrome 3 from mitochondria to cytoplasm in A549 and H1299 cells (Fig 2i,j). TUNEL assay results showed that HAX1 knockdown caused the apoptosis of A549 and H1299 cells (Fig 2k). These results showed that HAX1 knockdown could cause NSCLC cell apoptosis.
Silencing of HAX1 reduces invasion and epithelial–mesenchymal transition of NSCLC cells
To investigate whether HAX1 regulates the metastasis of NSCLC cells, si‐HAX1 was transfected into A549 and H1299 cell lines. As shown in Fig 3a–f, HAX1 knockdown reduced the protein expression of Vimentin, Slug, Snail and Twist, whereas E‐cadherin protein expression was increased. Silencing of HAX1 remarkably reduced the epithelial‐mesenchymal transition of NSCLC cells. In addition, transwell assay results showed that HAX1 knockdown caused a significant decrease of invasion in A549 and H1299 cells transfected with si‐HAX1 (Fig 3g,h). Silencing of HAX1 reduced the invasion and epithelial–mesenchymal transition of NSCLC cells.
Figure 3.
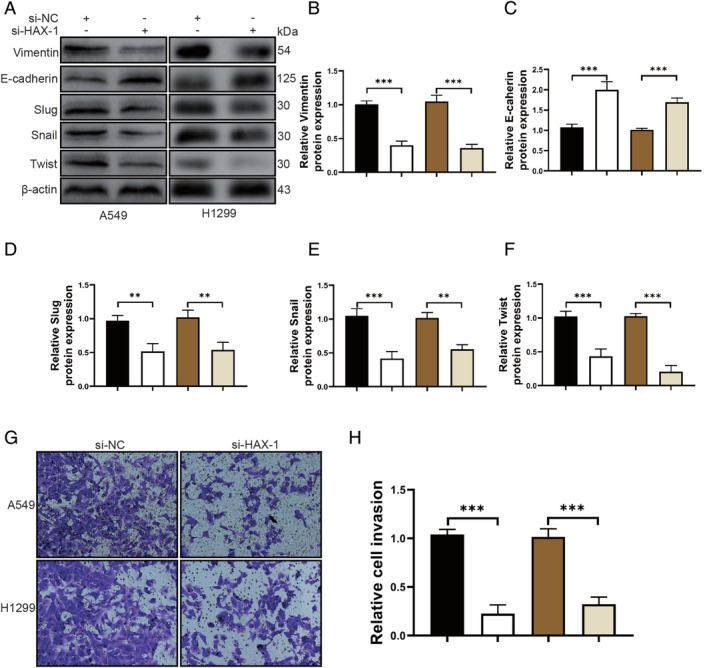
Silencing of HAX1 reduces the invasion and epithelial–mesenchymal transition of NSCLC cells. (a) Western blotting for Vimentin, E‐cadherin, Slug, Snail, Twist and β‐actin in A549 and H1299 cells. (b–f) Quantitative analysis of protein Vimentin, E‐cadherin, Slug, Snail and Twist expression in A549 and H1299 cells. (b) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (c) (
) H1299/si‐HAX1. (c) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (d) (
) H1299/si‐HAX1. (d) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (e) (
) H1299/si‐HAX1. (e) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (f) (
) H1299/si‐HAX1. (f) ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1.(g) Transwell assays were used to determine the invasion of A549 and H1299 cells. (h) Quantitative analysis of the invasion of A549 and H1299 cells. (
) H1299/si‐HAX1.(g) Transwell assays were used to determine the invasion of A549 and H1299 cells. (h) Quantitative analysis of the invasion of A549 and H1299 cells. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. ***P < 0.001, **P < 0.01, *P < 0.05.
) H1299/si‐HAX1. ***P < 0.001, **P < 0.01, *P < 0.05.
HAX1 suppresses NSCLC cell apoptosis through inhibiting AKT/mTOR signal pathway
A recent study reported that HAX1 prevents glioblastoma cells from apoptosis through the Akt1 pathway, 15 and it has also been reported that the AKT/mTOR signal pathway plays an important role in NSCLC. AKT/mTOR signal pathway induces gefitinib‐resistant of NSCLC. 19 FAM83D has been reported to improve epithelial‐mesenchymal transition, invasion and cisplatin resistance through activating the AKT/mTOR pathway in NSCLC. 20 The oncogene IARS2 promotes the occurrence of NSCLC by activating the AKT/MTOR pathway. 21 Therefore, we wanted to explore whether HAX1 protected the apoptotic NSCLC cells through inhibiting AKT/mTOR signal pathway. We found that HAX1 knockdown could decrease the levels of p‐AKT, p‐mTOR and p‐70S6K in A549 and H1299 cells (Fig 4a,b). Furthermore, we performed AKT agonist SC79 (5 μg/mL for 1 hour) to treat A549 and H1299 cells after silencing of HAX1. As shown in Fig 4c,d, SC79 was not able to change the protein expression of HAX1, which suggested the AKT/mTOR signal pathway was not able to regulate HAX1. However, SC79 was capable of promoting the levels of p‐AKT and p‐mTOR in A549 and H1299 cells after silencing of HAX1. To investigate whether SC79 could attenuate A549 and H1299 cell apoptosis, we performed a TUNEL assay to detect the apoptosis of A549 and H1299 cells. As shown in Fig 4f, we determined that SC79 could promote the survival of A549 and H1299 cells after HAX1 knockdown. These results showed that HAX1 increases the apoptotic NSCLC cells through inhibiting the AKT/mTOR signal pathway.
Figure 4.
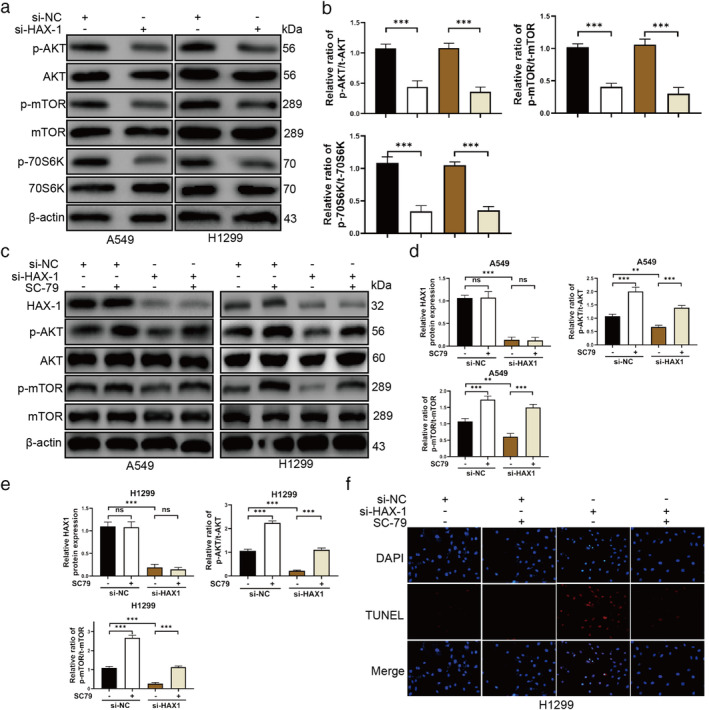
HAX1 promotes the survival of NSCLC cells via AKT/mTOR signaling pathway. (a) Phosphorylation of AKT, mTOR and 70S6K was inhibited in A549 and H1299 cells transfected with si‐HAX1. (b) Quantitative analysis of protein p‐AKT, p‐mTOR and p‐70S6K expression in A549 and H1299 cells. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (
) H1299/si‐HAX1. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (
) H1299/si‐HAX1. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1.(c) SC79 promoted the phosphorylation of AKT and mTOR in A549 and H1299 cells transfected with si‐HAX1. (d, e) Quantitative analysis of protein HAX1, p‐AKT, and p‐mTOR expression in A549 and H1299 cells. (f) TUNEL assays for apoptosis of A549 and H1299 cells transfected with si‐HAX1 after treatment of SC79. ***P < 0.001, **P < 0.01, *P < 0.05.
) H1299/si‐HAX1.(c) SC79 promoted the phosphorylation of AKT and mTOR in A549 and H1299 cells transfected with si‐HAX1. (d, e) Quantitative analysis of protein HAX1, p‐AKT, and p‐mTOR expression in A549 and H1299 cells. (f) TUNEL assays for apoptosis of A549 and H1299 cells transfected with si‐HAX1 after treatment of SC79. ***P < 0.001, **P < 0.01, *P < 0.05.
As a downstream of AKT, MDM2/p53 signal pathway has been reported to play an important role in glioblastoma cells and endothelial progenitor cells. 15 We further investigated whether the MDM2/p53 signal pathway participates in NSCLC cells transfected with si‐HAX1. We observed the protein level of p‐MDM2 was reduced and p53 was increased in A549 and H1299 cells after si‐HAX1 transfection (Fig 5a,b). MDM2, as a negative regulator of p53, can bind to p53 to bind to p53 to limit its function, and ubiquitinate p53 to promote degradation. 22 , 23 , 24 To investigate whether HAX1 inhibits NSCLC cells apoptosis through the AKT/MDM2/p53 signal pathway, we detected the protein levels of p‐MDM2 and p53 after AKT agonist SC79 treatments in NSCLC cells. SC79 increased the protein levels of p‐MDM2 in A549 and H1299 cells (Fig 5c,d). Although HAX1 was silenced, SC79 caused an increased expression of p‐MDM2 in A549 and H1299 cells (Fig 5c,d). SC79 did not change the expression of p53 in A549 and H1299 cell (Fig 5c,d). However, SC79 could reduce the p53 protein expression in A549 and H1299 cells transfected with si‐HAX1 (Fig 5c,d). These results confirmed that HAX1 also prevented NSCLC cells apoptosis through promoting AKT/MDM2/p53 signal pathway.
Figure 5.
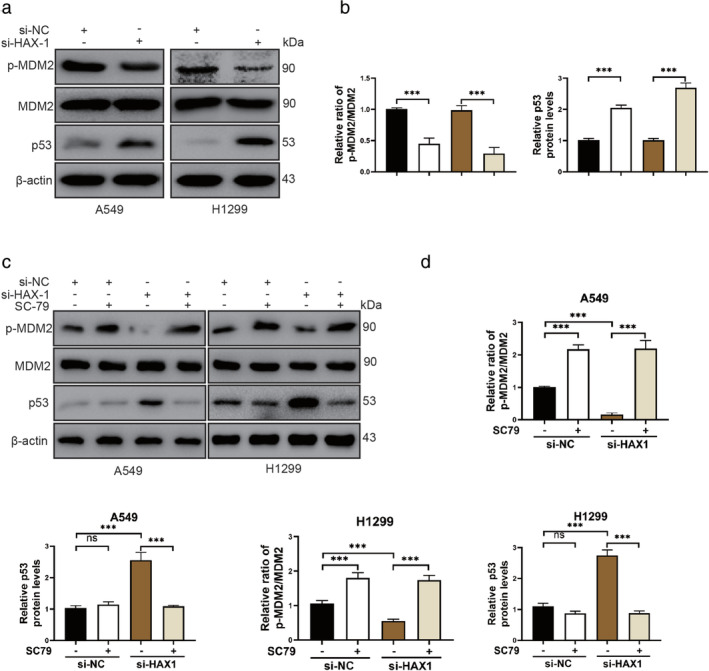
AKT/MDM2/p53 signaling pathway participates in the protective effect of HAX1 in lung cancer. (a) Phosphorylation of MDM2 was reduced and p53 levels in A549 and H1299 cells transfected with si‐HAX1. (b) Quantitative analysis of protein p‐MDM2 and p53 expression in A549 and H1299 cells. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1. (
) H1299/si‐HAX1. ( ) A549/si‐NC, (
) A549/si‐NC, ( ) H1299/si‐NC, (
) H1299/si‐NC, ( ) A549/si‐HAX1 and (
) A549/si‐HAX1 and ( ) H1299/si‐HAX1.(c) SC79 promoted the phosphorylation of MDM2 and reduced p53 level in A549 and H1299 cells transfected with si‐HAX1. (d) Quantitative analysis of protein p‐MDM2, and p53 expression in A549 and H1299 cells. ***P < 0.001, **P < 0.01, *P < 0.05.
) H1299/si‐HAX1.(c) SC79 promoted the phosphorylation of MDM2 and reduced p53 level in A549 and H1299 cells transfected with si‐HAX1. (d) Quantitative analysis of protein p‐MDM2, and p53 expression in A549 and H1299 cells. ***P < 0.001, **P < 0.01, *P < 0.05.
Discussion
NSCLC is one of the most prevalent cancers in the world with a high risk of patients developing subsequent metastasis. Patients with local or distant metastasis have a poor prognosis. 25 , 26 Therefore, early diagnosis and treatment are important for NSCLC patients.
HAX1 serves as a multifunctional protein and exerts an important role in many metabolisms through various cellular proteins and viral proteins. 11 , 14 , 27 , 28 , 29 , 30 , 31 Many studies have reported that HAX1 participated in multiple cellular processes and signaling pathways, such as cancer cell apoptosis, invasion, proliferation, and migration. 31 , 32 Upregulation of HAX1 is found in many tumors. In breast cancer, HAX1 mRNA expression has been reported to be higher in tumor tissues than paired adjacent noncancerous tissues. 18 Upregulation of HAX1 has also been observed in hepatocellular carcinoma and was associated positively with grade of differentiation, tumor thrombus and tumor satellite. 14 A recent study showed that HAX1 is upregulated in lung cancer. 18 However, the role and underlying mechanism of HAX1 are still unclear. In this study, we searched the expression information of HAX1 in TCGA database and found that HAX1 was overexpressed in NSCLC tissues. We then analyzed HAX1 expression in NSCLC tissues and paired adjacent normal tissues by using qRT‐PCR and western blotting. The results showed that HAX1 mRNA expression was higher in NSCLC tissues than paired adjacent normal tissues. We also found that high HAX1 levels were related positively with tumor status progression, tumor size and lymphatic metastasis.
HAX1 acts as antiapoptotic protein and maintains various cell survival. 12 , 33 , 34 Many studies have previously reported that HAX1 protects various cells from apoptosis including cardiomyocytes, neurons as well as many cancer cells. 34 , 35 , 36 , 37 Therefore, we investigated the role of HAX1 in NSCLC apoptosis. Si‐HAX1 was designed to silence the expression of HAX1 in A549 and H1299 cells. Silencing of HAX1 caused an increased expression of cleaved‐caspase 9, cleaved‐caspase 3 and decreased ratio of Bcl‐2/Bax. Importantly, HAX1 knockdown increased the flux of cytochrome C from mitochondria to cytoplasm. TUNEL assays confirmed that apoptotic cells were observed in A549 and H1299 cells. Furthermore, our results demonstrated that HAX1 could reduce the invasion and epithelial‐mesenchymal transition of NSCLC cells. These results suggested that HAX1 plays an important role in maintaining the survival and metastasis of NSCLC cells.
Emerging evidence has revealed that HAX1 protects glioblastoma cells from apoptosis through the AKT pathway. 15 The AKT signal pathway exerts an important role in protecting cells from apoptosis. In osteoblasts, PMSC6 has been reported to increase osteoblast apoptosis via inhibiting AKT signaling pathway activation in an OVX mouse model. 38 MiR‐208 has also been reported to protect neonatal rat cardiomyocyte apoptosis in simulated ischemia‐reperfusion injury via AKT signaling pathway. 39 However, inhibition of the AKT signaling pathway can promote the apoptosis of gastric cancer cells, breast cancer, lung cancer and so on. 40 , 41 , 42 Importantly, we found that HAX1 knockdown suppressed the phosphorylation of AKT, mTOR and S6K. Subsequently, we performed AKT agonist SC79 to treat A549 and H1299 cells transfected with si‐HAX1. The results of western blotting showed that SC79 could promote the phosphorylation of AKT and mTOR, whereas there was no change in HAX1 expression. SC70 also reduced the apoptosis of A549 and H1299 cells caused by HAX1 knockdown. These results indicated that HAX1 protected NSCLC cells from apoptosis by regulating the AKT signaling pathway.
MDM2, as a negative regulator of p53, can bind to p53 to limit its function, and ubiquitinate p53 to promote degradation. 22 , 23 , 24 MDM2 maintains the tumor characteristics of lung cancer via suppressing the function and expression levels of p53. 43 , 44 , 45 , 46 For example, Cai et al. reported miR‐305 restricts the progression of NSCLC through inhibiting MDM2. 43 Ning et al. also reported that ZCCHC10 inhibits lung cancer progression and cisplatin resistance by attenuating MDM2‐mediated p53 ubiquitination and degradation. 46 It has also been reported that P53, a famous tumor suppressor gene, inhibits NSCLC cell proliferation, invasion and epithelial‐mesenchymal transition. 47 , 48 , 49 AKT also regulates the tumor characteristics of tumor by MDM2/p53 signal pathway. 50 , 51 , 52 Our results indicated that AKT/MDM2/p53 pathway participates in the protective effect of HAX1 in lung cancer.
In conclusion, our findings indicate that HAX1 may be an essential factor in the development of NSCLC. Therefore, the present study provided a potential target for NSCLC therapy and a better understanding for the mechanism of NSCLC.
Disclosure
The authors confirm that there are no conflicts of interest.
Acknowledgments
We thank all members of our laboratories for useful discussions.
This work was supported by grants from the National Natural Science Foundation of China (No. 81772459), Ningbo Natural Science Foundation (No. 2016A610167) and Zhejiang Provincial Medicine and Health Technology Project (No. 2017KY580).
Contributor Information
Xinjian Li, Email: dxjs1961@sina.com.
Mingsong Wang, Email: wangmingsong@xinhuamed.com.cn.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 4. Kim IM, Ackerson T, Ramakrishna S et al The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res 2006; 66: 2153–61. [DOI] [PubMed] [Google Scholar]
- 5. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non‐small cell lung cancer. Nature 2018; 553: 446–54. [DOI] [PubMed] [Google Scholar]
- 6. Kunda NK. Antimicrobial peptides as novel therapeutics for non‐small cell lung cancer. Drug Discov Today 2020; 25: 238–47. [DOI] [PubMed] [Google Scholar]
- 7. Zhang T, Zhang DM, Zhao D et al The prognostic value of osteopontin expression in non‐small cell lung cancer: A meta‐analysis. J Mol Histol 2014; 45: 533–40. [DOI] [PubMed] [Google Scholar]
- 8. Osada H, Takahashi T. Genetic alterations of multiple tumor suppressors and oncogenes in the carcinogenesis and progression of lung cancer. Oncogene 2002; 21: 7421–34. [DOI] [PubMed] [Google Scholar]
- 9. Ji L, Ni T, Shen Y et al Transformer 2beta (Tra2beta/SFRS10) positively regulates the progression of NSCLC via promoting cell proliferation. J Mol Histol 2014; 45: 573–82. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki Y, Demoliere C, Kitamura D, Takeshita H, Deuschle U, Watanabe T. HAX‐1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol 1997; 158: 2736–44. [PubMed] [Google Scholar]
- 11. Yap SV, Koontz JM, Kontrogianni‐Konstantopoulos A. HAX‐1: A family of apoptotic regulators in health and disease. J Cell Physiol 2011; 226: 2752–61. [DOI] [PubMed] [Google Scholar]
- 12. Radhika V, Onesime D, Ha JH, Dhanasekaran N. Galpha13 stimulates cell migration through cortactin‐interacting protein Hax‐1. J Biol Chem 2004; 279: 49406–13. [DOI] [PubMed] [Google Scholar]
- 13. Ramsay AG, Keppler MD, Jazayeri M et al HS1‐associated protein X‐1 regulates carcinoma cell migration and invasion via clathrin‐mediated endocytosis of integrin alphavbeta6. Cancer Res 2007; 67: 5275–84. [DOI] [PubMed] [Google Scholar]
- 14. Hu YL, Feng Y, Ma P et al HAX‐1 promotes the migration and invasion of hepatocellular carcinoma cells through the induction of epithelial‐mesenchymal transition via the NF‐kappaB pathway. Exp Cell Res 2019; 381: 66–76. [DOI] [PubMed] [Google Scholar]
- 15. Deng X, Song L, Zhao W, Wei Y, Guo XB. HAX‐1 protects glioblastoma cells from apoptosis through the Akt1 pathway. Front Cell Neurosci 2017; 11: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarnowska E, Grzybowska EA, Sobczak K et al Hairpin structure within the 3'UTR of DNA polymerase beta mRNA acts as a post‐transcriptional regulatory element and interacts with Hax‐1. Nucleic Acids Res 2007; 35: 5499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhodes DR, Yu J, Shanker K et al ONCOMINE: A cancer microarray database and integrated data‐mining platform. Neoplasia 2004; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trebinska A, Rembiszewska A, Ciosek K et al HAX‐1 overexpression, splicing and cellular localization in tumors. BMC Cancer 2010; 10: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang D, Han LL, Du F et al FGFR1 induces acquired resistance against gefitinib by activating AKT/mTOR pathway in NSCLC. Onco Targets Ther 2019; 12: 9809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yin C, Lin X, Wang Y et al FAM83D promotes epithelial‐mesenchymal transition, invasion and cisplatin resistance through regulating the AKT/mTOR pathway in non‐small‐cell lung cancer. Cell Oncol (Dordr) 2020; 43: 395–407. [DOI] [PubMed] [Google Scholar]
- 21. Di X, Jin X, Ma H et al The oncogene IARS2 promotes non‐small cell lung cancer tumorigenesis by activating the AKT/MTOR pathway. Front Oncol 2019; 9: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang J, Zhang ZQ, Li FQ et al Triptolide interrupts rRNA synthesis and induces the RPL23‐MDM2‐p53 pathway to repress lung cancer cells. Oncol Rep 2020; 43: 1863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamer I, Daniel‐Meshulam I, Zadok O et al Stromal‐MDM2 promotes lung cancer cell invasion through tumor‐host feedback signaling. Mol Cancer Res 2020; 18: 926–37. [DOI] [PubMed] [Google Scholar]
- 24. Xie J, Zhang W, Liang X. RPL32 promotes lung cancer progression by facilitating p53 degradation. Mol Ther Nucleic Acids 2020; 21: 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma J, Jemal A, Fedewa SA et al The American Cancer Society 2035 challenge goal on cancer mortality reduction. CA Cancer J Clin 2019; 69: 351–62. [DOI] [PubMed] [Google Scholar]
- 26. Chen W, Zheng R, Baade PD. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 27. Simmen T. Hax‐1: A regulator of calcium signaling and apoptosis progression with multiple roles in human disease. Expert Opin Ther Targets 2011; 15: 741–51. [DOI] [PubMed] [Google Scholar]
- 28. Balcerak A, Trebinska‐Stryjewska A, Wakula M et al HAX1 impact on collective cell migration, cell adhesion, and cell shape is linked to the regulation of actomyosin contractility. Mol Biol Cell 2019; 30: 3024–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trebinska‐Stryjewska A, Szafron L, Rembiszewska A et al Cytoplasmic HAX1 is an independent risk factor for breast cancer metastasis. J Oncol 2019; 6375025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu G, Zhou W, Pan X et al miR‐100 reverses cisplatin resistance in breast cancer by suppressing HAX‐1. Cell Physiol Biochem 2018; 47: 2077–87. [DOI] [PubMed] [Google Scholar]
- 31. Mazel‐Sanchez B, Boal‐Carvalho I, Silva F, Dijkman R, Schmolke M. H5N1 Influenza A virus PB1‐F2 relieves HAX‐1‐mediated restriction of avian virus polymerase PA in human lung cells. J Virol 2018; 92: e00425–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu H, Chen J, Wang Q. Abnormal expression of HAX1 is associated with cellular proliferation and migration in human hypopharyngeal squamous cell carcinoma. Mol Med Rep 2017; 16: 4664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu J, Mu C, Hao J. Cerebral ischemia reduces expression of Hs1‐associated protein X‐1 (Hax‐1) in mouse brain. Neurosci Lett 2013; 534: 338–43. [DOI] [PubMed] [Google Scholar]
- 34. Lam CK, Zhao W, Cai W et al Novel role of HAX‐1 in ischemic injury protection involvement of heat shock protein 90. Circ Res 2013; 112: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klein C, Grudzien M, Appaswamy G et al HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet 2007; 39: 86–92. [DOI] [PubMed] [Google Scholar]
- 36. Jitkaew S, Trebinska A, Grzybowska E et al N(alpha)‐tosyl‐L‐phenylalanine chloromethyl ketone induces caspase‐dependent apoptosis in transformed human B cell lines with transcriptional down‐regulation of anti‐apoptotic HS1‐associated protein X‐1. J Biol Chem 2009; 284: 27827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trebinska A, Hogstrand K, Grandien A, Grzybowska EA, Fadeel B. Exploring the anti‐apoptotic role of HAX‐1 versus BCL‐XL in cytokine‐dependent bone marrow‐derived cells from mice. FEBS Lett 2014; 588: 2921–7. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y, Cao X, Li P et al PSMC6 promotes osteoblast apoptosis through inhibiting PI3K/AKT signaling pathway activation in ovariectomy‐induced osteoporosis mouse model. J Cell Physiol 2020 Jul; 235 (7‐8): 5511–5524. [DOI] [PubMed] [Google Scholar]
- 39. Shi HH, Wang ZQ, Zhang S. MiR‐208a participates with sevoflurane post‐conditioning in protecting neonatal rat cardiomyocytes with simulated ischemia‐reperfusion injury via PI3K/AKT signaling pathway. Eur Rev Med Pharmacol Sci 2020; 24: 943–55. [DOI] [PubMed] [Google Scholar]
- 40. Lu Y, Li L, Wu G, Zhuo H, Liu G, Cai J. Effect of PI3K/Akt signaling pathway on PRAS40Thr246 phosphorylation in gastric cancer cells. Iran J Public Health 2019; 48: 2196–204. [PMC free article] [PubMed] [Google Scholar]
- 41. Pentimalli F, Forte IM, Esposito L et al RBL2/p130 is a direct AKT target and is required to induce apoptosis upon AKT inhibition in lung cancer and mesothelioma cell lines. Oncogene 2018; 37: 3657–71. [DOI] [PubMed] [Google Scholar]
- 42. Jaglanian A, Tsiani E. Rosemary extract inhibits proliferation, survival, Akt, and mTOR signaling in triple‐negative breast cancer cells. Int J Mol Sci 2020 Jan 27; 21(3): 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cai Y, Hao Y, Ren H et al miR‐1305 inhibits the progression of non‐small cell lung cancer by regulating MDM2. Cancer Manag Res 2019; 11: 9529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shen A, Chen Y, Liu L et al EBF1‐mediated upregulation of ribosome assembly factor PNO1 contributes to cancer progression by negatively regulating the p53 signaling pathway. Cancer Res 2019; 79: 2257–70. [DOI] [PubMed] [Google Scholar]
- 45. Wang T, Li K, Song H. p53 suppression is essential for oncogenic SPAG5 upregulation in lung adenocarcinoma. Biochem Biophys Res Commun 2019; 513: 319–25. [DOI] [PubMed] [Google Scholar]
- 46. Ning Y, Hui N, Qing B. ZCCHC10 suppresses lung cancer progression and cisplatin resistance by attenuating MDM2‐mediated p53 ubiquitination and degradation. Cell Death Dis 2019; 10: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou Q, Hou Z, Zuo S et al LUCAT1 promotes colorectal cancer tumorigenesis by targeting the ribosomal protein L40‐MDM2‐p53 pathway through binding with UBA52. Cancer Sci 2019; 110: 1194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fei Z, Gao W, Xie R, Feng G, Chen X, Jiang Y. Synaptotagmin‐7, a binding protein of P53, inhibits the senescence and promotes the tumorigenicity of lung cancer cells. Biosci Rep 2019 Feb 8; 39(2): BSR20181298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xing Y, Liu Y, Liu T et al TNFAIP8 promotes the proliferation and cisplatin chemoresistance of non‐small cell lung cancer through MDM2/p53 pathway. Cell Commun Signal 2018; 16: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu Y, Dai B, Zhang H, Shi G, Shen Y, Ye D. Long non‐coding RNA LOC572558 inhibits bladder cancer cell proliferation and tumor growth by regulating the AKT‐MDM2‐p53 signaling axis. Cancer Lett 2016; 380: 369–74. [DOI] [PubMed] [Google Scholar]
- 51. Tu Y, Kim E, Gao Y, Rankin GO, Li B, Chen YC. Theaflavin‐3, 3′‐digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin‐resistant ovarian cancer A2780/CP70 cells. Int J Oncol 2016; 48: 2657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gao J, Xia R, Chen J. Inhibition of esophageal‐carcinoma cell proliferation by genistein via suppression of JAK1/2‐STAT3 and AKT/MDM2/p53 signaling pathways. Aging 2020; 12: 6240–59. [DOI] [PMC free article] [PubMed] [Google Scholar]


