Abstract
Purpose
Many patients diagnosed with head-and-neck cancer are current or former smokers. Despite the well-known adverse effects of smoking, continuation of smoking during cancer treatment is associated with reduced efficacy of that treatment and with cancer recurrence. In the present study, we examined smoking characteristics in patients with head-and-neck cancer near the time of cancer treatment.
Methods
A prospective cohort of patients with head-and-neck cancer who attended a dental oncology clinic before receiving cancer treatment at a regional cancer centre were invited to participate in a study that involved completing an interviewer-administered questionnaire to assess smoking characteristics, intention to quit, motivation to quit, and strategies perceived to potentially aid in successful cessation.
Results
The study enrolled 493 ever-smokers, with a response rate of 96.1% and a self-reported current smoker rate of 37.1% (n = 183). Most of the current smokers reported high nicotine dependence, with 84.7% (n = 155) indicating a time to first cigarette of 30 minutes or less. Most had previously attempted to quit smoking (77.0%), and many had prior unsuccessful quit attempts before resuming smoking again. Most were interested in quitting smoking (85.8%), and many (70.5%) were seriously considering quitting smoking within the subsequent 30 days.
Conclusions
Patients with head-and-neck cancer reported high nicotine dependence and high interest in cessation opportunities near the time of treatment for cancer. Those results might provide support for provision of smoking cessation opportunities.
Keywords: Smoking cessation, head-and-neck cancer, nicotine dependence, cigarette smoking, tobacco use
INTRODUCTION
Many head-and-neck cancers are strongly associated with tobacco use1, and continuation of smoking during cancer treatment has been associated with adverse outcomes such as reduced efficacy of cancer treatment2,3, increased symptom burden, oral mucositis2, treatment-related complications3, depression4,5, occurrence of second primary cancers3, and poor survival2,3. Despite the established causal relationship between tobacco smoking and cancer, many patients with cancer continue to smoke after diagnosis. Supporting patients with smoking cessation opportunities is an important component of cancer treatment and an important component in the treatment trajectory forpatients with cancer.
At diagnosis, the proportion of current smokers among patients with head-and-neck cancer appears high (Beynon et al.6, 24%; Sterba et al.7, 41%; Burris et al.8, 56%; Sharp et al.9, 56%), often falling within the 45%–60% range reported in a recent review of patients with cancer3. However, there are potential benefits to smoking cessation near the time of treatment10 and beyond11 that could result in improved overall and disease-specific survival10. Many patients with cancer who smoke perceive the practice to be harmful12; however, high rates of continued smoking after diagnosis3 (47%–60%) and of smoking relapse in survivors (50%–83%)3,13 are concerning and underscore the importance of targeting this population for a clinical smoking cessation intervention. As a result, the American Association for Cancer Research and Ontario Health (Cancer Care Ontario) issued policy statements recommending cessation assistance for all patients with cancer11,14.
Northeastern Ontario is a large geographic area in the province of Ontario (400,000 km2), with approximately 565,000 residents representing 4% of the Ontario population15. About 30% of the population resides in a rural area15, a rate much higher than that for Ontario overall (14%). Residents report the highest current-smoker prevalence rate in the province (26%)15, which is substantially higher than the Ontario provincial average of 17.3%15. The estimated 2018 age-standardized incidence for lung cancer (the most common smoking-associated cancer) in the region was 95.6 per 100,000, the highest expected in Ontario. Those high values for smoking prevalence and incidence of smoking-associated cancers in the region have persisted for decades16,17.
The Northeast Cancer Centre is a regional cancer centre that serves residents within Northeastern Ontario who are seeking cancer treatment; it is also one of the few cancer centres in Canada that houses a Dental Oncology program. One facet of the Dental Oncology program is to serve as an adjunct to the head-and-neck cancer treatment program by assessing and monitoring patients with head-and-neck cancer from diagnosis to post-treatment, and the program has implemented a clinical tobacco intervention program that targets the population with head-and-neck cancers. The present prospective observational study was designed to assess intention to quit, motivation level, smoking characteristics, interest in smoking cessation, and cessation rates after an offer of a standardized smoking cessation intervention to a cohort of patients with head-and-neck cancer who attended the Northeast Cancer Centre Dental Oncology clinic for oral assessment before the start of cancer treatment. Here, we focus on the baseline characteristics of the cohort at the time of study recruitment and delivery of the intervention.
METHODS
Study Cohort
Before cancer treatment, newly diagnosed patients with head-and-neck cancer attended the Department of Dental Oncology clinic at the Northeast Cancer Centre (Sudbury, Ontario) for baseline oral assessment. Patients who attended from 21 December 2010 through 10 April 2018 were screened for a history of smoking using the question “Have you ever smoked at least 100 cigarettes in your entire life?” Ever-smokers were invited into the study which involved completing an interviewer-administered questionnaire that assessed intention to quit, motivation level, and smoking characteristics; providing a buccal, saliva, or blood sample for genetic analyses; granting researchers access to medical records; and consenting to future contact for determination of cessation and other health-related outcomes. Other study eligibility criteria required participants be 18 years of age or older; to be capable of providing informed consent; and to be able to speak, read, and write in English (a necessity for completing the questionnaire portion of the study and subsequent follow-ups). Given the study’s genetic collection protocol, patients who had pre-existing medical conditions (hiv or hepatitis C) were excluded.
All current smokers, regardless of study enrolment status, were offered an intensive tobacco intervention. The opt-out approach18 was used in conjunction with motivational interviewing and nicotine replacement therapy or pharmacotherapy. Patients can opt out of the program at any point during the study or can refuse the intervention. The study was approved by the Health Sciences North Research Ethics Board, and all participants provided written informed consent.
Baseline Questionnaire
The interviewer-administered questionnaire included standardized questions that assessed smoking history (age started, duration, cigarettes per day, quit attempts, characteristics of previous cessation attempts, current smoking status) and nicotine dependence level for all ever-smokers. Current smokers were asked questions pertaining to interest in smoking cessation, motivation, and strategies that they believed would achieve cessation (Table I). Many interviewer questions were adapted from the Canadian Tobacco Use Monitoring Survey19 or the National Enhanced Cancer Surveillance System20. Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (ftnd)21, the Heaviness of Smoking Index22, and the time to first cigarette (ttfc) metric23.
TABLE I.
Questionnaire administered to current smokers to assess interest in smoking cessation, motivations, and cessation strategies
| Are you interested in quitting smoking? | |
| □ Yes | □ No |
|
| |
| Are you seriously considering quitting smoking within the next 30 days? | |
| □ Yes | □ No |
|
| |
| Are you seriously considering quitting smoking within the next 6 months? | |
| □ Yes | □ No |
|
| |
| If you are considering quitting smoking, what are your motivations for doing so? (Check all that apply) | |
| □ Health | □ Pregnancy or baby in the household |
| □ Cost of cigarettes | □ Less stress in life |
| □ Smoking is less socially acceptable nowadays | □ Other |
|
| |
| If you are seriously considering quitting smoking, what cessation strategies and/or products do you believe will help you successfully quit? (Check all that apply) | |
| □ Motivational counselling | |
| □ Motivational support from family or loved ones | |
| □ Nicotine replacement therapy (for example, patch, gum, etc.) | |
| □ Prescription medication (for example, Champixa, Zybanb, etc.) | |
| □ None (“cold turkey”) | □ Other |
Pfizer Canada, Kirkland, QC.
GlaxoSmithKline Canada, Mississauga, ON.
Clinical Intervention
All current smokers were offered individual and personalized counselling for smoking cessation by staff who were trained and certified under the Centre for Addiction and Mental Health’s Training Enhancement in Applied Counselling and Health and who used the 3A’s of tobacco cessation treatment (Ask, Advise, Arrange)24–26. The opt-out approach for tobacco intervention was advocated for all patients18. All current smokers were offered individual intensive clinical tobacco counselling, with pharmacotherapy or nicotine replacement if appropriate, and they received follow-up consultations 1–2 weeks after the intervention. Follow-up for the tobacco intervention took place weekly during active radiation therapy for their head-and-neck cancer, and post-treatment follow-up took place 4 weeks, 8 weeks, 6 months, and 1 year later.
Statistical Analyses
Descriptive statistics and frequencies summarize the study variables. Odds ratios (ors), adjusted ors (age at enrolment, sex), and 95% confidence intervals (95% cis) were used to evaluate the association of smoking status at enrolment with tobacco use behaviours and nicotine dependence. Total years of smoking and smoking pack–years were substantially skewed and were recoded into tertiles based on the overall distribution in the cohort. The ftnd scores were calculated by summing responses to the 6 ftnd questions, using the response scale provided with the ftnd. The Heaviness of Smoking Index was calculated by summing the responses to ftnd item 1 and ftnd item 4, using the ftnd response scale. The ttfc was calculated from ftnd item 1 and recoded into two groups: 31 or more minutes, and 30 minutes or less.
RESULTS
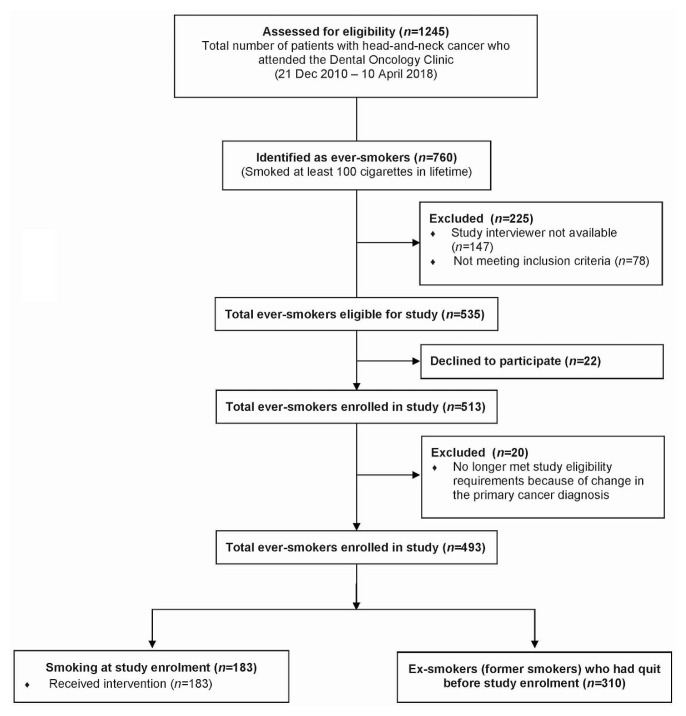
During the study interval, 1245 patients with head-and-neck cancer attended the Dental Oncology clinic, and 760 (61.0%) self-identified as ever-smokers. Of those ever-smokers, 147 were not approached because a study interviewer was not available, and 78 were not eligible for study because they did not meet the study inclusion criteria or had pre-existing medical issues. Of the remaining 535 ever-smokers eligible and approached, 22 (4.1%) did not choose to participate in the study. Of those 22 eligible participants who declined to participate, 77.3% were men, with a median age of 64 years. Of the 513 ever-smokers enrolled in the study (95.9%), 20 (3.9%) were subsequently removed during the analysis (based on an updated primary cancer diagnosis), leaving 493 ever-smokers enrolled in the study (96.1%). At enrolment, 183 ex-smokers (37.1%) self-reported as current smokers (Figure 1).
FIGURE 1.
Flow chart for enrolment of the study participants.
Median age at enrolment was 66 years, and the cohort consisted mostly of men (76.9%, Table II). Many participants reported a substantial history of smoking, which had begun at a young age (median: 16 years) and continued for a long duration. For example, more than half the current smokers in the study population had smoked for at least 44 years (51.4%, n = 94; Table II). When compared with the ex-smokers, the current smokers had longer smoking durations (adjusted or: 21.42 years; 95% ci: 11.12 years to 41.25 years; Table II), a higher number of cigarettes per day (adjusted or: 3.63; 95% ci: 1.87 to 7.08), and greater smoking pack–years (adjusted or: 6.46; 95% ci: 3.66 to 11.40).
TABLE II.
Characteristics of 493 patients with head-and-neck cancer, overall and by smoking status at study enrolment
| Variable | Smoking status | Crude analysis | Adjusted analysisa | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Overall | Current smoker | Ex-smoker (already quit) | OR | 95% CI | OR | 95% CI | |
| Patients (n) | 493 | 183 | 310 | ||||
|
| |||||||
| Sex [n (%)] | |||||||
| Men | 379 (76.9) | 139 (76.0) | 240 (77.4) | ||||
| women | 114 (23.1) | 44 (24.0) | 70 (22.6) | ||||
|
| |||||||
| Age (years) | |||||||
| Median | 66 | 63 | 69 | ||||
| Range | 37–96 | 38–90 | 37–96 | ||||
|
| |||||||
| Age started to smoke (years) | |||||||
| Median | 16 | 15 | 16 | ||||
| Range | 4–60 | 4–59 | 4–60 | ||||
|
| |||||||
| ICD codes for Dx [n (%)] | |||||||
| Oral cavity (C01–C06) | 180 (36.5) | 80 (43.7) | 100 (32.3) | ||||
| Oropharynx (C09–C10) | 57 (11.6) | 20 (10.9) | 37 (11.9) | ||||
| Larynx (C32) | 89 (18.1) | 34 (18.6) | 55 (17.7) | ||||
| ICD codes not included above | 141 (28.6) | 40 (21.9) | 101 (32.6) | ||||
| D00–D44 | 17 (3.4) | 5 (2.7) | 12 (3.9) | ||||
| Missing | 9 (1.8) | 4 (2.2) | 5 (1.6) | ||||
|
| |||||||
| Duration smoked [n (%)] | |||||||
| 1–30 Years | 189 (38.3) | 28 (15.3) | 161 (51.9) | 1.0 (reference) | 1.0 (reference) | ||
| 31–43 Years | 146 (29.6) | 60 (32.8) | 86 (27.7) | 4.01 | 2.39 to 6.74 | 4.81 | 2.71 to 8.53 |
| 44–75 Years | 157 (31.8) | 94 (51.4) | 63 (20.3) | 8.58 | 5.14 to 14.33 | 21.42 | 11.12 to 41.25 |
| Missing | 1 | 1 | |||||
|
| |||||||
| Smoking per day [n (%)] | |||||||
| ≤10 Cigarettes | 82 (16.6) | 15 (8.2) | 67 (21.6) | 1.0 (reference) | 1.0 (reference) | ||
| 11–20 Cigarettes | 191 (38.7) | 81 (43.8) | 110 (35.5) | 3.29 | 1.75 to 6.17 | 3.24 | 1.70 to 6.18 |
| 21–30 Cigarettes | 148 (30.0) | 65 (35.5) | 83 (26.8) | 3.50 | 1.83 to 6.68 | 3.63 | 1.87 to 7.08 |
| ≥31 Cigarettes | 69 (14.0) | 22 (12.0) | 47 (15.2) | 2.09 | 0.98 to 4.48 | 2.34 | 1.07 to 5.10 |
| Missing | 3 (0.6) | 3 (1.0) | |||||
|
| |||||||
| Pack–years [n (%)] | |||||||
| ≤25 | 177 (35.9) | 35 (19.1) | 142 (45.8) | 1.0 (reference) | 1.0 (reference) | ||
| 26–50 | 178 (36.1) | 82 (44.8) | 96 (31.0) | 3.47 | 2.16 to 5.56 | 4.36 | 2.62 to 7.27 |
| ≥51 | 135 (27.4) | 66 (36.1) | 69 (22.3) | 3.88 | 2.35 to 6.40 | 6.46 | 3.66 to 11.40 |
| Missing | 3 (0.6) | 3 (1.0) | |||||
|
| |||||||
| Nicotine dependenceb [n (%)] | |||||||
| Very low (0–2) | 96 (19.5) | 14 (7.7) | 82 (26.5) | 1.0 (reference) | 1.0 (reference) | ||
| Low (3–4) | 122 (24.7) | 46 (25.1) | 76 (24.5) | 3.55 | 1.81 to 6.96 | 4.04 | 2.02 to 8.11 |
| Medium (5) | 86 (17.4) | 43 (23.5) | 43 (13.9) | 5.86 | 2.89 to 11.88 | 5.95 | 2.89 to 12.28 |
| High (6–7) | 146 (29.6) | 62 (33.9) | 84 (27.1) | 4.32 | 2.25 to 8.32 | 4.28 | 2.19 to 8.37 |
| Very high (8 to 10) | 38 (7.7) | 18 (9.8) | 20 (6.5) | 5.27 | 2.25 to 12.36 | 4.91 | 2.05 to 11.76 |
| Missing | 5 (1.0) | 5 (1.6) | |||||
|
| |||||||
| Smoking dependencec [n (%)] | |||||||
| Very low | 134 (27.2) | 26 (14.2) | 108 (34.8) | 1.0 (reference) | 1.0 (reference) | ||
| Low–moderate | 112 (22.7) | 55 (30.1) | 57 (18.4) | 4.01 | 2.28 to 7.06 | 4.09 | 2.28 to 7.33 |
| Moderate | 113 (22.9) | 47 (25.7) | 66 (21.3) | 2.96 | 1.68 to 5.22 | 2.90 | 1.62 to 5.20 |
| Very high | 132 (26.8) | 55 (30.1) | 77 (24.8) | 2.97 | 1.71 to 5.15 | 3.06 | 1.74 to 5.39 |
| Missing | 2 (0.4) | 2 (0.6) | |||||
|
| |||||||
| Time to first cigarette [n (%)] | |||||||
| ≥31 Minutes | 140 (28.4) | 28 (15.3) | 112 (36.1) | 1.0 (reference) | 1.0 (reference) | ||
| ≤30 Minutes | 351 (71.2) | 155 (84.7) | 196 (63.2) | 3.16 | 1.99 to 5.03 | 3.23 | 2.00 to 5.21 |
| Missing | 2 (0.4) | 2 (0.6) | |||||
Adjusted for age and sex.
By the Fagerström Test for Nicotine Dependence.
By the Heaviness of Smoking Index.
More than half the cohort (54.8%, n = 270) reported medium or higher nicotine dependence, assessed as a score of 5 or greater on the ftnd. When compared with ex-smokers, current smokers were significantly more likely to report higher levels of nicotine dependence for low and medium dependence levels (adjusted or: 5.95; 95% ci: 2.89 to 12.28) and for high and very high dependence levels (Table II). Smokers scored significantly higher on the Heaviness of Smoking Index (adjusted or: 3.06; 95% ci: 1.74 to 5.39) and also reported shorter ttfc [30 minutes or less (adjusted or: 3.23; 95% ci: 2.00 to 5.21), Table II].
Most of the 183 current smokers had previously attempted to quit (77.0%, n = 141), had made frequent attempts to quit (median: 3 quit attempts; range: 0–50 quit attempts; Table III), and had a prior quit attempt that lasted for a duration of 1 week or 1 month (Table III). When asked about the longest period of cessation during a prior quit attempt, 19.1% of the current smokers (n = 35) reported less than 1 month or up to 1 month (Table III); 14.8% (n = 27) had been able to cessate for 3–6 months or 7–12 months before resuming smoking again (Table III). In prior quit attempts, many current smokers had used no cessation aids, choosing to quit “cold turkey” (48.5%), although 23.8% had also tried nicotine replacement therapy for assistance (Table III). Ex-smokers in our cohort also reported multiple quit attempts, with short periods of success, which appeared similar to reports from the current smokers in the study (Table III). Ex-smokers predominately attributed a “cold turkey” strategy to their successful quit attempt (69.7%, Table III).
TABLE III.
Characteristics of quit attempts made and cessation methods previously used by smokers
| Question | Smoking status [n (%)] | |
|---|---|---|
|
| ||
| Current smokers (n=183) | Ex-smokers (n=310) | |
| Have you ever attempted to quit smoking? | ||
| No | 41 (22.4) | 41 (13.2) |
| Yes | 141 (77.0) | 269 (86.8) |
| Patients with missing data | 1 (0.5) | |
|
| ||
| About how many attempts have you made to quit smoking? | ||
| Median | 3 | 2 |
| Range | 0–50 | 0–100 |
| Patients with missing data | 42 | 44 |
|
| ||
| How many of your attempts to quit smoking have lasted more than a week? | ||
| Median | 1 | 1 |
| Range | 0–40 | 0–25 |
| Patients with missing data | 45 | 49 |
|
| ||
| How many of your attempts to quit smoking have lasted more than a month? | ||
| Median | 1 | 1 |
| Range | 0–30 | 0–10 |
| Patients with missing data | 45 | 47 |
|
| ||
| What is the longest period of time you have gone without smoking since you began? | ||
| <1 Month | 35 (19.1) | 29 (9.4) |
| 1 Month up to 2 months | 18 (9.8) | 5 (1.6) |
| 3–6 months | 27 (14.8) | 29 (9.4) |
| 7 Months up to 1 year | 27 (14.8) | 29 (9.4) |
| 2–5 Years | 11 (6.0) | 41 (13.2) |
| 6–10 Years | 7 (3.8) | 19 (6.1) |
| 11–20 Years | 0 (0.0) | 32 (10.3) |
| >20 Years | 1 (0.5) | 63 (20.3) |
| Patients with missing data | 57 | 63 |
|
| ||
| What cessation method(s) have you used in your attempts to quit smoking? (Check all that apply) | ||
| Nicotine replacement therapy | 48 (23.8) | 52 (16.8) |
| Champixa | 32 (15.8) | 19 (6.1) |
| Zybanb | 12 (5.9) | 9 (2.9) |
| Motivational counselling | 3 (1.5) | 0 (0.0) |
| None (“cold turkey”) | 98 (48.5) | 216 (69.7) |
| Other | 9 (4.5) | 14 (4.5) |
| Patients with missing data | 42 | 43 |
Pfizer Canada, Kirkland, QC.
GlaxoSmithKline Canada, Mississauga, ON.
Of patients who were currently smoking, most were interested in quitting (85.8%), and many were seriously considering quitting smoking within the subsequent 30 days (70.5%) or subsequent 6 months (84.7%, Table IV). The primary motivation for quitting was health concerns (85.2%). Many anticipated that a successful cessation strategy would include nicotine replacement therapy (25.1%) or prescription medications such as bupropion or varenicline [Zyban (GlaxoSmithKline Canada, Mississauga, ON) Champix/Chantix (Pfizer Canada, Kirkland, QC)] (38.8%); others (27.9%) anticipated quitting “cold turkey” (Table IV).
TABLE IV.
Interest in cessation and proposed cessation strategies among 183 current smokers
| Question | Responses [n (%)] |
|---|---|
| Are you interested in quitting smoking? | |
| Yes | 157 (85.8) |
| No | 23 (12.6) |
| Missing | 3 (1.6) |
|
| |
| Are you seriously considering quitting smoking within the next 30 days? | |
| Yes | 129 (70.5) |
| No | 28 (15.3) |
| Missing | 26 (14.2) |
|
| |
| Are you seriously considering quitting smoking within the next 6 months? | |
| Yes | 155 (84.7) |
| No | 1 (0.5) |
| Missing | 27 (14.8) |
|
| |
| If you are considering quitting smoking, what are your motivations for doing so? (Check all that apply) | |
| Health | 150 (85.2) |
| Pregnancy or baby in the household | 1 (0.6) |
| Cost of cigarettes | 10 (5.7) |
| Less stress in life | 5 (2.8) |
| Smoking is less socially acceptable nowadays | 6 (3.4) |
| Other | 4 (2.3) |
| Health plus any other selectiona | 15 |
| Missing | 28 |
|
| |
| If you are seriously considering quitting smoking, what cessation strategies and/or products do you believe will help you successfully quit? (Check all that apply) | |
| Motivational counselling | 5 (2.7) |
| Motivational support from family or loved ones | 1 (0.5) |
| Nicotine replacement therapy (for example, patch, gum, etc.) | 46 (25.1) |
| Prescription medication (for example, Champixa, Zybanb, etc.) | 71 (38.8) |
| None (“cold turkey”) | 51 (27.9) |
| Other | 9 (4.9) |
| Missing | 32 |
Pfizer Canada, Kirkland, QC.
GlaxoSmithKline Canada, Mississauga, ON.
DISCUSSION
The main finding in the present study is that most of the current smokers (85.8%) who were preparing to receive treatment for head-and-neck cancer were interested in smoking cessation opportunities, and many (70.5%) were seriously considering quitting within the subsequent 30 days. Those proportions appear substantially higher than the approximately 50% that has been reported in some earlier quantitative research studies27,28 and more similar to recent reports of 73% and 81.8% in populations similar to our study cohort29,30. Motivation level appeared higher than the 56% interested in quitting within the month in another study29. The motivation for quitting smoking in our cohort was primarily health, which is consistent with a “teachable moment” related to a cancer diagnosis13,31. High interest and enhanced motivation might also have been related to our opt-out and intensive clinical tobacco intervention approach.
The foregoing findings are important because they support a patient’s willingness to receive a clinical tobacco intervention from oncology providers and other support staff at or near the time of diagnosis and treatment. Some authors have argued that tobacco control is at least partly the role of an oncologist32, including advising patients to stop smoking33, and that strong initial advice from a physician might help to avoid smoking relapse31. However, some oncology treatment providers have reported a perceived lack of patient motivation to be a patient-related barrier to providing smoking cessation services34. Other barriers include not being aware of cessation resources for referral35,36 and time constraints36.
The prevalence of current smokers in our sample appears lower than the prevalence at diagnosis of 45%–60% reported in a large review study3, but it is within the range of other reports in head-and-neck cancer populations. For example, previously published data showed that, at diagnosis, current smokers constituted 26.4%37 of a head-and-neck cancer population and 37%38 of a head-and-neck and non-small-cell lung cancer sample. However, issues of self-reporting bias and non-standardized operational definitions across studies have resulted in a needed call for uniform tobacco assessment8.
The current smokers in our population displayed high nicotine dependence and had an associated increase in prior attempts at cessation, with few sustained durations of cessation before resuming smoking. The significantly higher nicotine dependence levels of current smokers in our study suggest that those who cease might require enhanced follow-up and support to deal with a high probability of smoking relapse39. Further, support should also be extended to ex-smokers, because many of them also display moderate-to-high nicotine dependence, and they might need the enhanced support system to maintain their cessated status.
Most study participants indicated that they would likely use prescription medication (38.8%) to assist with a successful cessation; “cold turkey” (27.9%) and nicotine replacement therapies (25.1%) were the next most likely approaches. In prior unsuccessful quit attempts, participants indicated that they had tried the “cold turkey” (48.5%) or nicotine replacement therapy (23.8%) approaches to assist in their attempts. Choosing to quit “cold turkey” in prior cessation attempts was also the most frequent approach used in a sample of smokers diagnosed with head-and-neck cancer40. Providing access to tobacco cessation supports might increase the probability of success3.
Our study is strengthened by its prospective design using standardized tobacco assessments, a high response rate (96.1%), and a large sample size (n = 493), with a low study refusal rate (3.9%) similar to that reported for enrolment in a large smoking cessation clinical trial in patients with cancer41. The high level of interest in our study and in cessation by our patient population might be related to the individualized and intensified in-person method of study recruitment by a dentist or dental staff member trained in clinical tobacco intervention and education, the use of the opt-out approach18, and the opportunity to participate and receive a cessation intervention and pharmacotherapy, if appropriate—all at the time of study enrolment. A more modest interest in smoking cessation was reported in a southern regional cancer centre in Ontario that used less-intensive screening, referral, and follow-up opportunities26. Our study’s sex distribution (76.9% men) reflects the predominance of men in incident head-and-neck cancer cases in the general population, and it is similar to the sex distribution in other published findings38. We think that our study findings are generalizable to the population of patients with head-and-neck cancer in Northeastern Ontario and that our study does not suffer from extensive selection bias.
We collected nicotine dependence data using the ftnd, because that instrument has been reported extensively—although some have cautioned that the ftnd might have suboptimal psychometric properties42,43. However, the ttfc and the cigarettes per day components of the ftnd have been reported to be reliable over time and to be predictors of quitting behaviour44,45. The ttfc is considered reliable and valid44,46, to be predictive of cotinine level and nicotine uptake46, apparently to be associated with other biomarkers of exposure such as nicotine equivalents and serum and blood carboxyhemoglobin47, to be associated with risk for some oral cancers48, and to be predictive of short-term quitting outcomes39,44. The most appropriate method to assess nicotine dependence and the importance of other measures of smoking behaviour (for example, cognitive, affective, and environmental) in predicting smoking cessation remain important topics for future research.
A major limitation of our study was that we used self-report, with no biochemical verification of smoking status, and we are unclear about the level of misclassification of smokers. Other authors have shown true tobacco use to be misrepresented in similar populations49,50. At study enrolment, 37.1% of our cohort self-reported current smoking, similar to the self-reported 37% smokers found by Cooley et al.38 in a cohort of patients with lung and head-and-neck cancers. Their estimate of smokers increased to 45% when defined biochemically. However, Karam-Hage et al.2 observed 90% agreement in a small percentage (10%) of their cessated patient population between measured CO in expired breath samples and self-reported smoking status during follow-up appointments (reported unpublished findings), which supports the use of self-reported assessments. We also did not collect other sociodemographic data such as marital status or education level, or clinicopathologic data such as tumour stage at diagnosis, and we were unable to adjust our estimates for those variables. It is possible that, given the higher rates of smoking in our region15 and the higher rates of smoking-related diseases in our population, a cancer diagnosis with a higher stage or grade might potentially influence the behaviours and responses to the smoking cessation intervention. Our study was also conducted at a single institution. However, the Dental Oncology clinic is the only provider of pretreatment services for residents of this geographic area who seek cancer treatment at the Northeast Cancer Centre, and we would not expect any associated bias to be substantial.
CONCLUSIONS
Our study suggests that there is a high willingness and motivation to receive an individualized smoking cessation intervention near or at the time of cancer diagnosis and treatment in patients with head-and-neck cancers; however, the high levels of nicotine dependence in current smokers suggest that relapse could be likely. Enhanced follow-up and support should therefore be considered. Future research that assesses the effect of the intervention or that incorporates an economic analysis of the costs and benefits of intensive clinical tobacco intervention in the oncology setting is important.
ACKNOWLEDGMENTS
The authors acknowledge Justin Allemano for his early work on this study. This research was supported through a principal investigator grant to MSCC from the Northern Cancer Foundation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Jethwa AR, Khariwala SS. Tobacco-related carcinogenesis in head and neck cancer. Cancer Metastasis Rev. 2017;36:411–23. doi: 10.1007/s10555-017-9689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karam-Hage M, Oughli HA, Rabius V, et al. Tobacco cessation treatment pathways for patients with cancer: 10 years in the making. J Natl Compr Canc Netw. 2016;14:1469–77. doi: 10.6004/jnccn.2016.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang EHE, Braith A, Hitsman B, Schnoll RA. Treating nicotine dependence and preventing smoking relapse in cancer patients. Expert Rev Qual Life Cancer Care. 2017;2:23–39. doi: 10.1080/23809000.2017.1271981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moubayed SP, Sampalis JS, Ayad T, et al. Predicting depression and quality of life among long-term head and neck cancer survivors. Otolaryngol Head Neck Surg. 2015;152:91–7. doi: 10.1177/0194599814557772. [DOI] [PubMed] [Google Scholar]
- 5.Bloom EL, Oliver JA, Sutton SK, Brandon TH, Jacobsen PB, Simmons VN. Post-operative smoking status in lung and head and neck cancer patients: association with depressive symptomatology, pain, and fatigue. Psychooncology. 2015;24:1012–19. doi: 10.1002/pon.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beynon RA, Lang S, Schimansky S, et al. Tobacco smoking and alcohol drinking at diagnosis of head and neck cancer and all-cause mortality: results from Head and Neck 5000, a prospective observational cohort of people with head and neck cancer. Int J Cancer. 2018;143:1114–27. doi: 10.1002/ijc.31416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterba KR, Garrett-Mayer E, Carpenter MJ, et al. Smoking status and symptom burden in surgical head and neck cancer patients. Laryngoscope. 2017;127:127–33. doi: 10.1002/lary.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burris JL, Studts JL, DeRosa AP, Ostroff JS. Systematic review of tobacco use after lung or head/neck cancer diagnosis: results and recommendations for future research. Cancer Epidemiol Biomarkers Prev. 2015;24:1450–61. doi: 10.1158/1055-9965.EPI-15-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp L, McDevitt J, Carsin AE, Brown C, Comber H. Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: findings from a large, population-based study. Cancer Epidemiol Biomarkers Prev. 2014;23:2579–90. doi: 10.1158/1055-9965.EPI-14-0311. [DOI] [PubMed] [Google Scholar]
- 10.Jerjes W, Upile T, Radhi H, et al. The effect of tobacco and alcohol and their reduction/cessation on mortality in oral cancer patients: short communication. Head Neck Oncol. 2012;4:6. doi: 10.1186/1758-3284-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS on behalf of the aacr Subcommittee on Tobacco and Cancer. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19:1941–8. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alton D, Eng L, Lu L, et al. Perceptions of continued smoking and smoking cessation among patients with cancer. J Oncol Pract. 2018;14:e269–79. doi: 10.1200/JOP.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karam-Hage M, Cinciripini PM, Gritz ER. Tobacco use and cessation for cancer survivors: an overview for clinicians. CA Cancer J Clin. 2014;64:272–90. doi: 10.3322/caac.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ontario Health (Cancer Care Ontario) [oh(cco)] Smoking Cessation Information for Healthcare Providers [Web page] Toronto, ON: OH(CCO); 2019. [Available at: https://www.cancercareontario.ca/en/guidelines-advice/cancer-continuum/prevention/smoking-cessation; cited 25 October 2019] [Google Scholar]
- 15.Health Quality Ontario. Health in the North: A Report on Geography and the Health of People in Ontario’s Two Northern Regions. Toronto, ON: Queen’s Printer for Ontario; 2017. [Google Scholar]
- 16.Conlon MSC, Lightfoot NE, Bissett RJ, Fehringer GM. Cancer incidence and mortality in Northeastern Ontario, 1991–1998. Can J Public Health. 2002;93:380–5. doi: 10.1007/BF03404574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lightfoot NE, Fehringer GM, Bissett RJ, McChesney DC, White JJ. Cancer incidence and mortality trends in Northeastern Ontario. Can J Public Health. 1996;87:17–24. [PubMed] [Google Scholar]
- 18.Nahhas GJ, Wilson D, Talbot V, et al. Feasibility of implementing a hospital-based “opt-out” tobacco-cessation service. Nicotine Tob Res. 2017;19:937–43. doi: 10.1093/ntr/ntw312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistics Canada. Canadian Tobacco Use Monitoring Survey, Cycle 2, 2007 (CTUMS) [archived questionnaire] Ottawa, ON: Stats Can; 2012. [Downloadable from: http://www23.statcan.gc.ca/imdb/p3Instr.pl?Function=getInstrumentList&Item_Id=52109&UL=1V&; cited 1 November 2019] [Google Scholar]
- 20.Johnson KC, Hu J, Mao Y on behalf of the Canadian Cancer Registries Epidemiology Research Group. Passive and active smoking and breast cancer risk in Canada, 1994–97. Cancer Causes Control. 2000;11:211–21. doi: 10.1023/A:1008906105790. [DOI] [PubMed] [Google Scholar]
- 21.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 22.Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84:791–9. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 23.Baker TB, Piper ME, McCarthy DE, et al. on behalf of the Transdisciplinary Tobacco Use Research Center Tobacco Dependence. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(suppl 4):S555–70. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35:158–76. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn VP, Hollis JF, Smith KS, et al. Effectiveness of the 5-As tobacco cessation treatments in nine hmos. J Gen Intern Med. 2009;24:149–54. doi: 10.1007/s11606-008-0865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson SM, Boldt RG, Louie AV. How can we better help cancer patients quit smoking? The London Regional Cancer Program experience with smoking cessation. Curr Oncol. 2018;25:226–30. doi: 10.3747/co.25.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooley ME, Emmons KM, Haddad R, et al. Patient-reported receipt of and interest in smoking-cessation interventions after a diagnosis of cancer. Cancer. 2011;117:2961–9. doi: 10.1002/cncr.25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnoll RA, Rothman RL, Lerman C, et al. Comparing cancer patients who enroll in a smoking cessation program at a comprehensive cancer center with those who decline enrollment. Head Neck. 2004;26:278–86. doi: 10.1002/hed.10368. [DOI] [PubMed] [Google Scholar]
- 29.Sampson L, Papadakos J, Milne V, et al. Preferences for the provision of smoking cessation education among cancer patients. J Cancer Educ. 2018;33:7–11. doi: 10.1007/s13187-016-1035-0. [DOI] [PubMed] [Google Scholar]
- 30.Nightingale CL, Sterba KR, Tooze JA, King JL, Weaver KE. Cessation attitudes and preferences in head and neck cancer patients and implications for cessation program design: a brief report. Glob Adv Health Med. 2019;8:2164956119847117. doi: 10.1177/2164956119847117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gritz ER, Carr CR, Rapkin D, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1993;2:261–70. [PubMed] [Google Scholar]
- 32.Clancy L. Reducing lung cancer and other tobacco-related cancers in Europe: smoking cessation is the key. Oncologist. 2014;19:16–20. doi: 10.1634/theoncologist.2013-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pentz RD, Berg CJ. Smoking and ethics: what are the duties of oncologists? Oncologist. 2010;15:987–93. doi: 10.1634/theoncologist.2010-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver KE, Danhauer SC, Tooze JA, et al. Smoking cessation counseling beliefs and behaviors of outpatient oncology providers. Oncologist. 2012;17:455–62. doi: 10.1634/theoncologist.2011-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren GW, Dibaj S, Hutson A, Cummings KM, Dresler C, Marshall JR. Identifying targeted strategies to improve smoking cessation support for cancer patients. J Thorac Oncol. 2015;10:1532–7. doi: 10.1097/JTO.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Donohue C, DeNofrio T, Vitale Pedulla L, Haddad RI, Rabinowits G. Optimizing tobacco cessation resource awareness among patients and providers. J Oncol Pract. 2016;12:e77–82. doi: 10.1200/JOP.2015.005124. [DOI] [PubMed] [Google Scholar]
- 37.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27:1969–75. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooley ME, Wang Q, Johnson BE, et al. Factors associated with smoking abstinence among smokers and recent-quitters with lung and head and neck cancer. Lung Cancer. 2012;76:144–9. doi: 10.1016/j.lungcan.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yong HH, Borland R, Balmford J, et al. Heaviness of smoking predicts smoking relapse only in the first weeks of a quit attempt: findings from the International Tobacco Control Four-Country Survey. Nicotine Tob Res. 2014;16:423–9. doi: 10.1093/ntr/ntt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khariwala SS, Rubin N, Stepanov I, et al. “Cold turkey” or pharmacotherapy: examination of tobacco cessation methods tried among smokers prior to developing head and neck cancer”. Head Neck. 2019;41:2332–9. doi: 10.1002/hed.25708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren GW, Marshall JR, Cummings KM, et al. Automated tobacco assessment and cessation support for cancer patients. Cancer. 2014;120:562–9. doi: 10.1002/cncr.28440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korte KJ, Capron DW, Zvolensky M, Schmidt NB. The Fagerström Test for Nicotine Dependence: do revisions in the item scoring enhance the psychometric properties? Addict Behav. 2013;38:1757–63. doi: 10.1016/j.addbeh.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etter JF, Duc TV, Perneger TV. Validity of the Fagerström Test for Nicotine Dependence and of the Heaviness of Smoking Index among relatively light smokers. Addiction. 1999;94:269–81. doi: 10.1046/j.1360-0443.1999.94226910.x. [DOI] [PubMed] [Google Scholar]
- 44.Borland R, Yong HH, O’Connor RJ, Hyland A, Thompson ME. The reliability and predictive validity of the Heaviness of Smoking Index and its two components: findings from the International Tobacco Control Four Country study. Nicotine Tob Res. 2010;12(suppl 1):S45–50. doi: 10.1093/ntr/ntq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagerström K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerström Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14:1467–73. doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- 46.Muscat JE, Stellman SD, Caraballo RS, Richie JP., Jr Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev. 2009;18:3415–20. doi: 10.1158/1055-9965.EPI-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muhammad-Kah RS, Hayden AD, Liang Q, Frost-Pineda K, Sarkar M. The relationship between nicotine dependence scores and biomarkers of exposure in adult cigarette smokers. Regul Toxicol Pharmacol. 2011;60:79–83. doi: 10.1016/j.yrtph.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Muscat JE, Liu HP, Livelsberger C, Richie JP, Jr, Stellman SD. The nicotine dependence phenotype, time to first cigarette, and larynx cancer risk. Cancer Causes Control. 2012;23:497–503. doi: 10.1007/s10552-012-9909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103:45–8. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morales NA, Romano MA, Cummings KM, et al. Accuracy of self-reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control. 2013;24:1223–30. doi: 10.1007/s10552-013-0202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]