Abstract
Background
The prognostic nutritional index (pni) is a simple metric calculated using serum albumin and the peripheral lymphocyte count. It was reported that a low pni score is significantly associated with major postoperative complications and poor prognosis. The purpose of the present study was to investigate the effects of perioperative oral management (pom) on the perioperative pni profiles of patients with digestive system or urinary cancers.
Study Design
The medical records of 181 patients with cancer who underwent surgery and for whom a pni could be calculated were retrospectively reviewed.
Results
The intervention rate with pom was 34.8%. The median preoperative pni score was 48.25 in all patients with a pom intervention [25% to 75% interquartile range (iqr): 44.38–54.13] and 47.25 in those without an intervention (iqr: 42.0–53.5). Compared with patients not receiving pom, those who received pom had significantly higher pni scores from the early postoperative period (p < 0.05). Notably, of patients who could resume oral intake within 3 days after surgery, those who received pom intervention, compared with those who did not, had significantly higher pni scores from the early postoperative period (p < 0.05).
Conclusions
Perioperative oral management interventions might have positive effects on the postoperative pni scores of patients with cancer.
Keywords: Perioperative oral management, prognostic nutritional index, digestive cancer, urinary cancer, serum albumin, C-reactive protein, crp
INTRODUCTION
During cancer treatment, patients can experience various systemic and local complications, including oral complications. Some studies suggest that a patient’s dental and oral condition is associated with systemic and surgical complications. In Japan, perioperative oral management (pom) became available under the national health insurance system in 2012. Patients with cancer, cardiovascular diseases, and organ transplantation can receive dental and oral pom during the perioperative period, and studies indicate that pom has positive effects in preventing complications such as postoperative pneumonia, surgical site infection, and prolonged hospital stay, among others1–14.
The prognostic nutritional index (pni), first reported by Onodera et al.15, is a simple metric calculated using serum albumin and the peripheral lymphocyte count. Low pni scores were reported to be significantly associated with major postoperative complications and poor prognosis in patients after gastrointestinal surgery15. Other reports have examined the clinical significance and usefulness of the pni as a prognostic factor in some cancers16–20.
Unfavourable dental and oral conditions, including increased oral bacteria, dental infections, oral mucositis, decreased saliva, missing teeth, masticatory disturbance, and neurosensory changes cause a decline of oral function, negatively affecting the nutrition condition in patients with cancer. However, no report has yet investigated the association between decline of oral function and the pni. Perioperative oral management during cancer surgery could have a positive effect by maintaining or improving a patient’s nutrition and immune condition, which might consequently reduce perioperative complications.
The purpose of the present study was to investigate the effects of pom on the perioperative pni profile in patients with digestive system and urinary cancers. The oral cavity is a part of the digestive tract, which is essential to nutrition, and so the direct effects of pom were investigated in patients undergoing digestive system surgery for cancer. Given that urologic diseases are not directly related to functions of the oral cavity, the indirect effects of pom were also investigated in patients undergoing surgery for urologic cancer.
METHODS
The study protocol was approved by the Committee on Medical Research of Shinshu University (no. 3788). We published the research plan on the hospital Web site and guaranteed the opportunity for opt-out.
Medical records of patients who underwent cancer surgery at the departments of digestive surgery and urology of Shinshu University in 2016 were retrospectively reviewed. The primary outcome was an increase or decrease of the perioperative pni score. Predictor variables were defined as patient factors (age, sex), surgical treatment factors (surgical site, operation time, blood loss, and postoperative time to the start of oral intake), and the presence or absence of a pom intervention. Pre- and postoperative blood parameters, including peripheral lymphocyte count and serum albumin, were obtained before the surgery (<1 week) and regularly afterward (until 6 weeks postoperatively). The pni was calculated using the formula of Onodera et al.15:
In our hospital, all patients with cancer are recommended and encouraged to receive pom before initiation of cancer treatments. The criteria for a pom intervention accorded with the report published by Yamagata et al.21. In general, pom was initiated at the time of the decision for hospitalization (before initiation of cancer therapy) and included oral health instruction, removal of dental calculus (scaling), professional mechanical tooth cleaning, removal of the tongue coating with a toothbrush, cleaning and adjustment of dentures, and extraction of teeth in cases of severe periodontitis showing pain, discharge of pus, mobility problems, or marked alveolar bone loss by radiographic examination. The pom interventions continued regularly after surgery.
Correlations between variables and pni profiles were analyzed statistically using the Fisher exact test, the Wilcoxon test, and the univariate repeated-measures analysis least-squares method. Statistical analyses were performed using the JMP software application (version 13: SAS Institute, Cary, NC, U.S.A.), with p values less than 0.05 being considered significant.
RESULTS
Of the 349 patients who underwent cancer surgery at the departments of digestive surgery and urology, 168 were excluded because of insufficient data. The analysis therefore included 181 patients (132 men, 49 women; mean age: 67.8 ± 10.9 years; age range: 33–89 years; Table I). Of those 181 patients, 63 (34.8%) received pom interventions. Of 74 patients who underwent surgery for digestive system cancers, 47 received pom (63.5%); and of the 107 who underwent surgery for urologic cancers, 16 received pom (15.0%). Patients with digestive system cancer received pom more frequently.
TABLE I.
Patient characteristics
| Variable | Overall cohort | Digestive system cancer subgroup | Urologic cancer subgroup | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| POM | No POM | POM | No POM | POM | No POM | |
| Patients [n (%)] | 63 (34.8) | 118 (65.2) | 47 (63.5) | 27 (36.5) | 16 (15.0) | 91 (85.0) |
|
| ||||||
| Mean age (years) | 67.0±10.4 | 68.1±10.5 | 66.3±11.3 | 69.7±11.1 | 69.0±9.7 | 67.6±9.8 |
| NSa (p=0.430) | NSa (p=0.724) | NSa (p=0.341) | ||||
|
| ||||||
| Sex (men:women) | 42:21 | 90:28 | 27:20 | 17:10 | 15:1 | 75:16 |
| NSb (p=0.219) | NSb (p=0.806) | NSb (p=0.459) | ||||
|
| ||||||
| Preoperative PNI score | ||||||
| Median | 48.3 | 47.3 | 49.5 | 49.5 | 44.5 | 46.3 |
| IQR | 44.4–54.1 | 42.0–53.5 | 44.5–55.0 | 40.3–57.3 | 41.0–47.5 | 42.0–51.1 |
| NSa (p=0.171) | NSa (p=0.797) | NSa (p=0.166) | ||||
|
| ||||||
| Operation time (minutes) | ||||||
| Median | 297 | 212 | 336.5 | 100 | 223 | 207 |
| IQR | 233–404 | 165.5–268 | 278.3–412.5 | 215–330 | 166–244.5 | 165–244.5 |
| p<0.001a | p<0.05a | NSa (p=0.722) | ||||
|
| ||||||
| Blood loss (mL) | ||||||
| Median | 100 | 55 | 100 | 30 | 150 | 100 |
| IQR | 40–350 | 10–200 | 20–330 | 10–160 | 77.5–712.5 | 10–200 |
| p<0.05a | p<0.05a | p<0.05a | ||||
|
| ||||||
| Resumption of oral intake after surgery [n (%)] | ||||||
| Within 3 days | 23 (12.7) | 91 (50.3) | 9 (12.2) | 12 (16.2) | 14 (13.1) | 79 (73.8) |
| At 4 days or more | 40 (22.1) | 27 (14.9) | 38 (51.4) | 15 (20.3) | 2 (1.9) | 12 (11.2) |
| p<0.01b | p<0.05b | NSb (p=1.000) | ||||
By Wilcoxon rank-sum test.
By Fisher exact test.
POM = perioperative oral management; PNI = prognostic nutritional index; IQR = 25%–75% interquartile range.
Table I summarizes the characteristics of the patients. No significant differences in sex and age were observed between the patients who did and did not receive pom. The median preoperative pni score was 48.3 for patients who received pom [25%–75% interquartile range (iqr): 44.4–54.1], and 47.3 for those who did not (iqr: 42.0–53.5). The median preoperative pni for patients with digestive system cancers was the same for patients who received pom (median: 49.5; iqr: 44.5–55.1) and for those who did not (median: 49.5; iqr: 40.3–57.3; Table I). The median preoperative pni for patients with urologic cancers was slightly lower in the patients who received pom (median: 44.5; iqr: 41.0–47.5) than in those who did not (median: 46.3; iqr: 42.0–51.1; Table I). No significant differences in preoperative pni were evident between the patients who did and did not receive pom (Wilcoxon p = 0.171). Operation times were significantly longer in patients who received pom than in those who did not, except when the operation was for urologic cancer (all patients, p < 0.001; patients with digestive system cancers, p < 0.05; patients with urologic cancers, p = 0.722). Blood loss was significantly greater in patients receiving pom than in those not receiving pom (all patients: Wilcoxon p < 0.01; patients with digestive system and urologic cancers: Wilcoxon p < 0.05). In the cohort overall, resumption of oral intake occurred significantly earlier in patients not receiving pom than in those receiving pom (p < 0.01). The result was the same for patients undergoing surgery for digestive system cancers, (p < 0.05), but not for those undergoing surgery for urologic cancer.
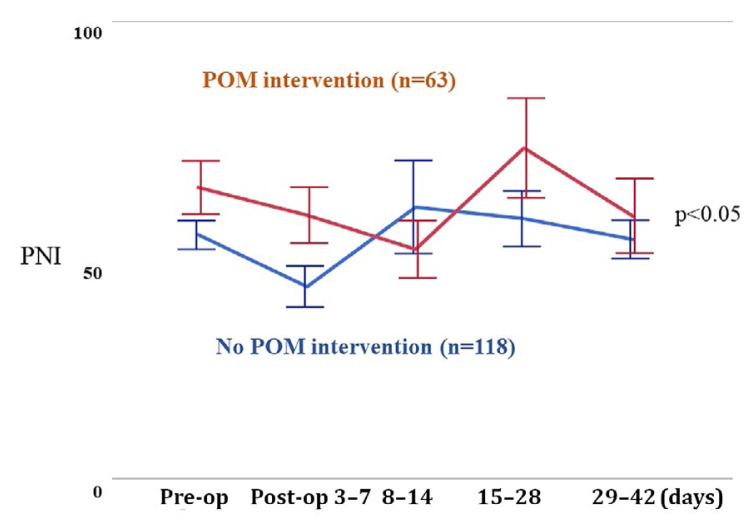
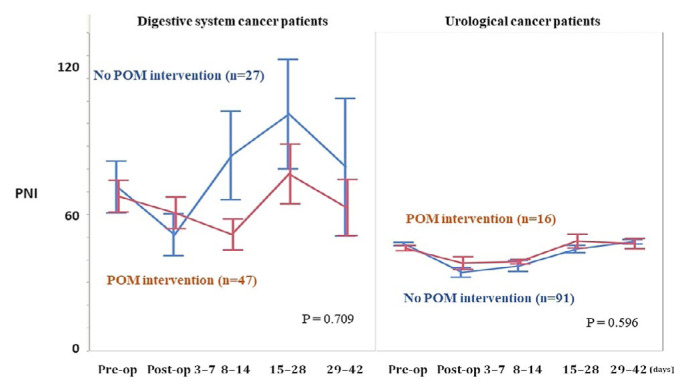
Figure 1 compares the perioperative pni profiles during the perioperative period for all patients. Before surgery and throughout the postoperative period, pni scores were significantly higher in patients receiving pom than in patients not receiving pom (univariate repeated-measures analysis least-squares method, p < 0.05). Figure 2 compares the perioperative pni profiles for patients with digestive system and urologic cancers. In patients with digestive system cancers, the pni score was higher during the early postoperative days, but later became lower, in patients who received pom compared with those who did not receive pom, although the difference was nonsignificant (univariate repeated-measures analysis least-squares method, p = 0.709). In contrast, in patients with urologic cancers, pni scores throughout the perioperative period were higher in patients receiving pom than in those not receiving pom, although that difference also did not reach significance (univariate repeated-measures analysis least-squares method, p = 0.596).
FIGURE 1.
Perioperative change of the prognostic nutritional index (PNI) profile in 181 patients. Compared with those who did not receive perioperative oral management (POM) interventions, those who did had significantly higher PNI scores from the early postoperative period (p < 0.05).
FIGURE 2.
Perioperative change of the prognostic nutritional index (PNI) profile in 74 patients with digestive system cancers and 107 patients with urologic cancers. Perioperative PNI scores were not significantly different in patients who received perioperative oral management (POM) and in those who did not (digestive cancer: p = 0.709; urologic cancer: p = 0.596).
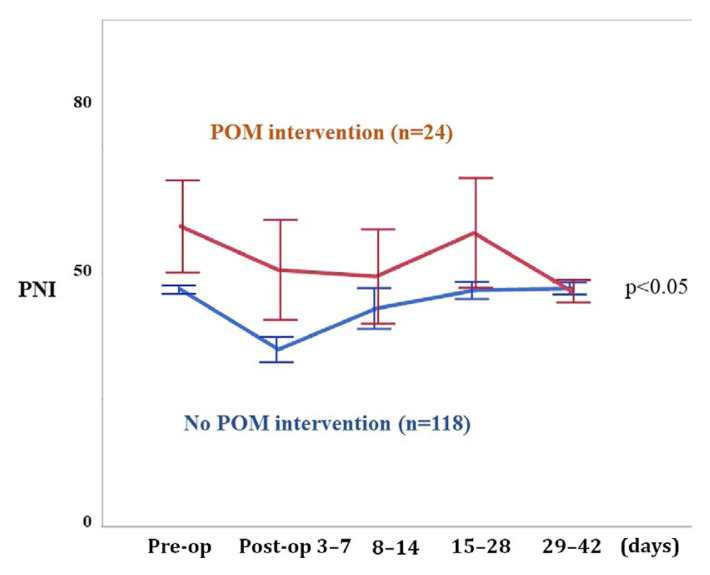
Postoperative pni profiles were influenced by surgical intensity and the postoperative alimentation method. The pni profiles of patients who resumed oral intake within 3 days after surgery were therefore compared separately (Figure 3). The pni scores from the early postoperative period were significantly higher in patients who received pom than in patients who did not receive pom (univariate repeated-measures analysis least-squares method, p < 0.05).
FIGURE 3.
Perioperative change of the prognostic nutritional index (PNI) profile in 142 patients who could resume oral intake by postoperative day 3. Of the patients who could resume oral intake within 3 days postoperatively, PNI scores were significantly higher for those who received perioperative oral management (POM) from the early postoperative period, compared with those who did not (p < 0.05).
DISCUSSION
In Japan, pom has been available under the national health insurance system since 2012 for patients receiving cancer treatment, organ transplantation, cardiovascular surgery, and orthopedic implant surgery. The oral cavity has been reported to possibly be a large reservoir of pathogenic bacteria that can cause infections in multiple organs22–25, and professional oral care has been reported to reduce microbial counts in the oropharynx, aiding in the prevention of aspiration pneumonia26. However, because of a lack of prospective randomized clinical trials investigating pom, evidence of its efficacy has not been fully established. Several recent reports have described positive effects of pom in patients with cancer, with many studies indicating that pom interventions might reduce the prevalence of postoperative pneumonia in patients who undergo cancer surgery1,2,4,5,8,10–12,14. Other reported effects of pom include reduced numbers of bacteria and bacterial species detected by endotracheal bacteriologic examination3, shortened postoperative systemic inflammatory response syndrome3, fewer postoperative hospitalization days6–9,11, a shorter postoperative fasting period6,7,9, lower C-reactive protein11, and a reduced prevalence of surgical site infections13. It is easy to understand that the reduction of oral and dental bacteria and infections could lead to reductions in local and systemic infections. Perioperative oral management includes not only oral care, but also dental and functional restoration of the oral cavity. That functional and dental management might improve a patient’s nutrition status and, consequently, their immunologic condition. However, no reports about the association between pom and change in perioperative nutrition markers have been published. In the present study, we therefore examined the possible effects of pom on nutrition status and immunologic condition.
The pni has been reported to be an easy and useful prognostic factor for survival and postoperative complications in some patients with cancer. A low pni score has been reported to be significantly associated with poor survival15,17–20 and to be useful for predicting nutrition status and mortality in patients undergoing peritoneal dialysis16. Additionally, low pni scores have been associated with postoperative complications in colorectal and lung cancer15,17,20.
The pni is calculated using serum albumin and the peripheral lymphocyte count15. Lymphocytes act as activators in the adaptive immune system to clear tumour from the body and to halt its development and dispersion26. Serum albumin has been reported to reflect an individual’s nutrition and inflammatory status27. Low levels of serum albumin and lymphocytes have been reported to promote inflammatory tumour development and the spread and metastasis of cancer28. In patients with colorectal cancer, hypoalbuminemia was found to reflect malnutrition and immunosuppression, increasing disease severity, progression of the tumour, and poor prognosis29. Additionally, early postoperative reduction in serum albumin was found to be an independent risk factor for severe postoperative complications and poor prognosis30,31.
Our results suggested that pom interventions might have the positive effects of preserving and improving the perioperative pni score in patients with cancer. The number of decayed teeth was reported to correlate with lower serum albumin32. A significant association between mean clinical attachment loss and lower serum albumin was reported in elderly patients33, and a close relationship between periodontitis and lower serum albumin has been demonstrated33–36. Tooth loss was reported to possibly be a predictor of low energy and protein intake, and low serum albumin37, and prosthodontic treatments such as partial dentures were reported to significantly increase serum albumin38. Those results suggest that pom—including oral care, abatement of chronic dental infections, and prosthodontic treatments—has a positive effect on maintaining and increasing perioperative nutrition status, including serum albumin and lymphocyte counts, resulting in higher perioperative pni scores.
However, in digestive cancer surgery, the pni level more than a week after surgery was higher in patients who did not receive pom than in those who received pom. In the present retrospective study, our investigations showed that pom had been applied in patients who underwent long and intensive surgery (with more blood loss). It is logical to speculate that the patients who did not receive pom underwent less- intensive surgery, and thus their pni scores recovered better than did those for patients who received pom. Additionally, oral intake was resumed significantly later in patients who received pom than in those who did not receive pom.
Postoperative parenteral nutrition might have a significant influence on the postoperative pni score. The patients who resumed oral intake within 3 days after surgery were therefore analyzed separately, with pni profiles being compared for those who did and did not receive pom (Figure 3). A better pni profile was observed in the patients who received pom than in those who did not. In addition, in patients with digestive system cancers, pni scores were lower in the patients who received pom than in those who did not, especially in the early postoperative period when the postoperative alimentation method was not different between the groups. Those results also suggest that pom has a positive effect on the perioperative pni profile.
Our report is the first to examine the effects of pom interventions on the perioperative pni score in patients who undergo cancer surgery. However, the study has some limitations. One limitation is the retrospective nature of the study, which was based on a relatively small case series at a single institute. Because of insufficient data, especially lack of laboratory data before surgery (<1 week) and postoperatively (regularly until 6 weeks postoperatively), more than half of the 349 eligible patients had to be excluded from the study. Those exclusions might have affected the study results. Although our study detected positive effects of pom interventions on the perioperative pni score, other factors, such as the primary site and surgical method, might have affected the pni score. Although the patients who received pom underwent more intensive surgery, their pni profiles were better than those of the patients who did not receive pom. Further prospective investigations, with larger numbers of cases and multicentre analyses, will be needed to clarify the significant independent risk factors for perioperative pni score in patients with cancer.
CONCLUSIONS
Positive effects of pom interventions in patients undergoing cancer surgery were demonstrated. In the early postoperative period, pni scores were significantly higher in patients receiving pom than in patients not receiving pom. Of patients who could resume oral intake within 3 days after surgery especially, pni scores from the early postoperative period were significantly higher for those who received pom than for those who did not receive pom. Those results suggest that pom interventions have positive effects on the postoperative improvement of the pni score in patients with cancer. We intend to conduct a nationwide retrospective study to investigate the efficacy of pom for perioperative serum albumin using data from the Japanese Stomatological Society.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Akutsu Y, Matsubara H, Okazumi S, et al. Impact of preoperative dental plaque culture for predicting postoperative pneumonia in esophageal cancer patients. Dig Surg. 2008;25:93–7. doi: 10.1159/000121903. [DOI] [PubMed] [Google Scholar]
- 2.Ktaoka T, Umeda M, Minamikawa T, et al. Prophylactic effect of oral health care of postoperative pneumonia after oral cancer surgery [Japanese] Jpn J Oral Diag. 2008;21:1–6. [Google Scholar]
- 3.Uejima S, Sakai K, Naganawa Y, et al. Efficacy of professional oral care for the patients who underwent esophagectomy [Japanese] J Jpn Soc Surg Infect. 2009;6:183–8. [Google Scholar]
- 4.Hiramatsu T, Sugiyama M, Kuwabara S, Tachimori S, Nishioka M. Effectiveness of an outpatient preoperative care bundle in preventing postoperative pneumonia among esophageal cancer patients. Am J Infect Control. 2014;42:385–8. doi: 10.1016/j.ajic.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Soutome S, Yanamoto S, Funahara M, et al. Effect of perioperative oral care on prevention of postoperative pneumonia associated with esophageal cancer surgery: a multicenter case–control study with propensity score matching analysis. Medicine (Baltimore) 2017;96:e7436. doi: 10.1097/MD.0000000000007436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tozawa S, Nishimaki F, Kamijou R, et al. A retrospective clinical study on the effect of perioperative oral management for patients who underwent gastrointestinal cancer surgery [Japanese] JJMCP. 2015;24:214–23. [Google Scholar]
- 7.Urauno H, Nakashima D, Kasamatsu A, et al. Evaluation of the effectiveness of perioperative oral care in patients with oral cancer [Japanese] Oral Sci Japan. 2015;2015:93–6. [Google Scholar]
- 8.Yamamura Y, Takizawa H, Matsumoto F, et al. Effects of perioperative oral management in thoracoscopic lung lobectomy: retrospective study [Japanese] Jpn J Oral Care. 2016;10:106–10. [Google Scholar]
- 9.Aizawa H, Shimane S, Sinobu U, Kamada T, Koyama Y, Kurita H. Clinical study on the effect of the perioperative oral management in surgical patients of liver cancer [Japanese] Jpn J Oral Care. 2016;11:43–7. [Google Scholar]
- 10.Soutome S, Funahara M, Oho T, et al. The effect of perioperative oral management on prevention of postoperative pneumonia in patients undergoing esophageal resection. [Japanese] J Jpn Stomatol Soc. 2016;65:324–9. [Google Scholar]
- 11.Nishino T, Takizwa H, Sawada T, et al. Perioperative oral management can prevent postoperative pneumonia after lung cancer surgery [Japanese] Jpn J Chest Surg. 2017;31:432–8. doi: 10.2995/jacsurg.31.432. [DOI] [Google Scholar]
- 12.Kajihara R, Yamada K, Nishimaki F, et al. A retrospective study of the efficacy of perioperative oral management on prevention of postoperative pneumonia associated with lung cancer surgery [Japanese] Shinshu Med J. 2018;66:249–56. [Google Scholar]
- 13.Nobuhara H, Yanamoto S, Funahara M, et al. Effect of perioperative oral management on the prevention of surgical site infection after colorectal cancer surgery: a multicenter retrospective analysis of 698 patients via analysis of covariance using propensity score. Medicine (Baltimore) 2018;97:e12545. doi: 10.1097/MD.0000000000012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwata E, Hasegawa T, Yamada S, et al. Effects of perioperative oral care on prevention of postoperative pneumonia after lung resection: multicenter retrospective study with propensity score matching analysis. Surgery. 2019;165:1003–7. doi: 10.1016/j.surg.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients [Japanese] Nihon Geka Gakkai Zasshi. 1984;85:1001–5. [PubMed] [Google Scholar]
- 16.Kang SH, Cho KH, Park JW, Yoon KW, Do JY. Onodera’s prognostic nutritional index as a risk factor for mortality in peritoneal dialysis patients. J Korean Med Sci. 2012;27:1354–8. doi: 10.3346/jkms.2012.27.11.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–92. doi: 10.1007/s00268-013-2156-9. [DOI] [PubMed] [Google Scholar]
- 18.Broggi MS, Patil D, Baum Y, et al. Onodera’s prognostic nutritional index as an independent prognostic factor in clear cell renal cell carcinoma. Urology. 2016;96:99–105. doi: 10.1016/j.urology.2016.05.064. [DOI] [PubMed] [Google Scholar]
- 19.Sheng J, Yang YP, Ma YX, et al. Low prognostic nutritional index correlates with worse survival in patients with advanced nsclc following egfr-tkis. PLoS One. 2016;11:e0147226. doi: 10.1371/journal.pone.0147226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okada S, Shimada J, Kato D, Tsunezuka H, Teramukai S, Inoue M. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg. 2017;104:296–302. doi: 10.1016/j.athoracsur.2017.01.085. [DOI] [PubMed] [Google Scholar]
- 21.Yamagata K, Onizawa K, Yanagawa T, et al. A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;38:237–42. doi: 10.1038/sj.bmt.1705429. [DOI] [PubMed] [Google Scholar]
- 22.Mojon P. Oral health and respiratory infection. J Can Dent Assoc. 2002;68:340–5. [PubMed] [Google Scholar]
- 23.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–43. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Han YW, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485–91. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishikawa A, Yoneyama T, Hirota K, Miyake Y, Miyatake K. Professional oral health care reduces the number of oropharyngeal bacteria. J Dent Res. 2008;87:594–8. doi: 10.1177/154405910808700602. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Fuhrman MP, Charney P, Mueller CM. Hepatic proteins and nutrition assessment. J Am Diet Assoc. 2004;104:1258–64. doi: 10.1016/j.jada.2004.05.213. [DOI] [PubMed] [Google Scholar]
- 28.Saroha S, Uzzo RG, Plimack ER, Ruth K, Al-Saleem T. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol. 2013;189:454–61. doi: 10.1016/j.juro.2012.09.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nazha B, Moussaly E, Zaarour M, Weerasinghe C, Azab B. Hypoalbuminemia in colorectal cancer prognosis: nutritional marker or inflammatory surrogate? World J Gastrointest Surg. 2015;7:370–7. doi: 10.4240/wjgs.v7.i12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Wang H, Jiang J, Cao X, Liu Q. Early decrease in postoperative serum albumin predicts severe complications in patients with colorectal cancer after curative laparoscopic surgery. World J Surg Oncol. 2018;16:192. doi: 10.1186/s12957-018-1493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Y, Liu Z, Liang J, et al. Early post-operative serum albumin level predicts survival after curative nephrectomy for kidney cancer: a retrospective study. BMC Urol. 2018;18:111. doi: 10.1186/s12894-018-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshihara A, Hanada N, Miyazaki H. Association between serum albumin and root caries in community-dwelling older adults. J Dent Res. 2003;82:218–22. doi: 10.1177/154405910308200313. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa H, Yoshihara A, Amarasena N, Hirotomi T, Miyazaki H. Association between serum albumin and periodontal disease in community-dwelling elderly. J Clin Periodontol. 2006;33:312–16. doi: 10.1111/j.1600-051X.2005.00901.x. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama T, Yamanaka R, Yokoi A, et al. Relationship between serum albumin concentration and periodontal condition in patients with head and neck cancer. J Periodontol. 2012;83:1110–15. doi: 10.1902/jop.2011.110536. [DOI] [PubMed] [Google Scholar]
- 35.Kaur N, Kaur N, Sarangal V. A study to evaluate the correlation of serum albumin levels with chronic periodontitis. Indian J Dent Res. 2015;26:11–14. doi: 10.4103/0970-9290.156788. [DOI] [PubMed] [Google Scholar]
- 36.Terashima T, Chubachi S, Matsuzaki T, et al. The association between dental health and nutritional status in chronic obstructive pulmonary disease. Chron Respir Dis. 2017;14:334–41. doi: 10.1177/1479972316643076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ioannidou E, Swede H, Fares G, Himmelfarb J. Tooth loss strongly associates with malnutrition in chronic kidney disease. J Periodontol. 2014;85:899–907. doi: 10.1902/jop.2013.130347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanehisa Y, Yoshida M, Taji T, Akagawa Y, Nakamura H. Body weight and serum albumin change after prosthodontic treatment among institutionalized elderly in a long-term care geriatric hospital. Community Dent Oral Epidemiol. 2009;37:534–8. doi: 10.1111/j.1600-0528.2009.00496.x. [DOI] [PubMed] [Google Scholar]