Highlights
-
•
The prevalence of acute/subacute infarcts was the most common in COVID-19 patients with neurologic symptoms.
-
•
Cerebral microhemorrhages (6.9 %) and spontaneous ICH (5.4 %) were similarly prevalent.
-
•
The prevalence of encephalitis/encephalopathy (3.3 %) was less frequent.
Abbreviations: ADEM, acute disseminated encephalomyelitis; CI, confidence interval; COVID-19, coronavirus disease 2019; ICH, intracranial hemorrhage; PRES, posterior reversible encephalopathy syndrome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; I2, Higgin’s inconsistency index
Keywords: COVID-19, Severe acute respiratory syndrome coronavirus 2, Brain diseases, Neuroimaging, Prevalence
Abstract
Purpose
To comprehensively evaluate the incidences of abnormal neuroimaging findings in patients with COVID-19 via a systematic review and meta-analysis.
Method
PubMed-MEDLINE and EMBASE were searched for original articles reporting imaging findings of the brain in adult patients with COVID-19 between January 1, 2020 and October 9, 2020. Abnormal neuroimaging findings were categorized as (1) cerebral microhemorrhages, (2) acute spontaneous intracranial hemorrhage (ICH), (3) acute to subacute infarcts, and (4) encephalitis or encephalopathy. Pooled incidences of neuroimaging findings were assessed using random-effects modeling. Between-study heterogeneity was explored by using the χ2 statistic for pooled incidences and the inconsistency index I2. The quality of the studies was evaluated using the Risk of Bias Assessment Tool for Nonrandomized Studies. Subgroup meta-regression analysis was performed to identify potential sources of heterogeneity.
Results
Twenty-one eligible papers, including 2125 patients, were identified. The pooled incidences of cerebral microhemorrhages, acute spontaneous ICH, acute/subacute infarcts, and encephalitis/encephalopathy were 6.9 % (95 % confidence interval [CI], 4.9 %–8.9 %), 5.4 % (95 % CI, 3.1 %–7.6 %), 24.0 % (95 % CI, 16.1 %–31.8 %), and 3.3 % (95 % CI, 1.9 %–4.7 %), respectively. Substantial heterogeneities were noted for all neuroimaging findings (I2 = 87 %–97 %). Significant publication biases were present in the pooled incidences. In the subgroup meta-regression analysis, patients with mean or median ages over 65 years showed a significantly lower incidence of encephalitis/encephalopathy (P < 0.001). Furthermore, studies reported that patients in ICU had significantly higher incidences of cerebral microhemorrhages (P < 0.001) and encephalitis/encephalopathy (P < 0.001).
Conclusions
Considerable incidences of abnormal neuroimaging findings have been reported in patients with COVID-19. Acute to subacute cerebral infarction was the most prevalent neuroimaging finding.
1. Introduction
In December 2019, the epidemic of coronavirus disease 2019 (COVID-19)—caused by the severe acute respiratory syndrome virus (SARS-CoV-2)—was first reported in Wuhan, China. The rapid spread of COVID-19 has made it a public health emergency of international concern [1]. As of October 21, over 40 million people were confirmed to be infected with SARS-CoV-2 globally, with over 700,000 confirmed deaths worldwide [2]. While SARS-CoV-2 is mostly known for causing severe respiratory distress, a growing number of neurologic manifestations have been reported [3,4]. In one study from Wuhan, China, 28.2 % of the patients with COVID-19 showed an altered mental status or acute cerebrovascular disease [3]. Also, in patients who recovered from COVID-19, neurologic symptoms were reported to be present in 55 % of the patients [5].
Since the initial case reports describing COVID-19-associated acute hemorrhagic necrotizing encephalopathy [6], other neuroimaging findings in patients with COVID-19 have been reported, including encephalitis/meningitis [[7], [8], [9]], hemorrhagic posterior reversible encephalopathy syndrome (PRES) [10,11], acute disseminated encephalomyelitis (ADEM) [12,13], cerebral venous thrombosis [[14], [15], [16]], and acute ischemic stroke [8,16,17]. Over the past several months, the number of publications reporting COVID-19-related neuroimaging findings since the outbreak of SARS-CoV-2 has been unprecedented and evolving. However, most publications are case reports/series, which have low evidence levels to establish the prevalence of specific neuroimaging findings in COVID-19.
Considering the dire nature of the pandemic, the current meta-analysis aims to collate currently available literature reporting various neuroimaging findings of COVID-19 to provide more research evidence, thereby improving radiologists’ diagnostic confidence upon encountering COVID-19-related neuroimaging findings.
2. Materials and methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. The institutional review board of our institution approved this study.
2.1. Literature search
PubMed-MEDLINE and EMBASE databases were searched for studies published between January 1, 2020 and July 30, 2020. The search terms were as follows: (Coronavirus disease OR Novel coronavirus OR 2019-nCoV OR SARS-CoV-2 OR Covid-19 OR Severe Acute Respiratory Syndrome Coronavirus 2) AND (Brain) AND (CT OR computed tomography OR MRI OR magnetic resonance imaging). The search was updated on October 9, 2020. The references of the selected articles were further screened to search for potentially relevant articles. Two reviewers (Y.C. and M.K.L.) independently performed the literature search, and any disagreement regarding study inclusion was resolved by consensus. The authors were not blinded to the authors, institutions, or journals while selecting studies or extracting data. EndNote version X9 (Thomas Reuters, New York City, NY, USA) was used for literature management.
2.2. Eligibility criteria
Studies investigating imaging findings (CT or MRI) of the brain in patients with COVID-19 were eligible for inclusion. The included studies met the following criteria: (1) Population: Patients diagnosed with COVID-19 with a sample size greater than five patients. (2) Study design: Both retrospective and prospective observational studies were included. (3) Outcomes: Studies reporting sufficient details on imaging findings of the brain from either CT or MRI (i.e., cerebral microhemorrhages, acute/subacute infarction, acute spontaneous intracranial hemorrhage (ICH), encephalitis/encephalopathy).
The exclusion criteria were as follows: (1) reviews, editorials, and letters; (2) case reports or case series < 5 patients; (3) partially overlapping patient cohorts; (4) articles not written in English, and (5) non-human studies. Two reviewers independently reviewed the literature in consensus.
2.3. Data extraction
From the selected articles, the following data were extracted into standardized formats: (a) study characteristics: authors, affiliations, country of origin, study duration, study design, and sample size; (b) patients’ demographic and clinical characteristics: age, sex, imaging modality and its specifications, number of neuroradiologist reviewers and experience, proportion of patients in ICU, reported neurologic symptoms, and indications for imaging; (c) outcomes: incidences of four types of neuroimaging findings (acute spontaneous ICH, acute/subacute infarct, cerebral microhemorrhages, and encephalitis/encephalopathy). One reviewer (blinded) extracted the data, and the second reviewer (blinded) confirmed the data’s validity. The acute spontaneous ICH was defined as acute brain hemorrhage identified on brain CT without a history of trauma; acute/subacute infarct, cerebral microhemorrhages, and encephalitis/encephalopathy were diagnosed based on diffusion-weighted, susceptibility-weighted, and T2-weighted/fluid-attenuated inversion recovery imaging with or without contrast-enhanced T1-weighted imaging, respectively. For encephalitis/encephalopathy, variable neuroimaging findings were included, such as abnormal signal intensities or enhancement in white matter, basal ganglia or cortex, PRES or diffuse leukoencephalopathy.
2.4. Quality assessment
Quality assessments of the included studies were independently performed in consensus by two reviewers using the structured criteria of the Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) [7].
2.5. Statistical analyses
The primary outcomes of this meta-analysis were the pooled incidences of abnormal neuroimaging findings, including cerebral microhemorrhages, acute/subacute infarcts, acute spontaneous ICH, and encephalitis/encephalopathy. Meta-analytic pooling was performed using the inverse variance method to calculate weights. Pooled incidences with 95 % confidence intervals (CIs) were obtained using Der Simonian-Laird random-effects modeling [19]. Subgroup meta-regression analysis was performed based on patients’ mean/median age (>65 or ≤65 years), imaging modality (MRI or CT/MRI), origin of publication (Europe, USA, or Asia), single/multi-center design, indication for imaging (neurologic symptoms or others), and patients in ICU (reported or not reported). Likelihood ratio tests were used to compare the random-effects models in subgroup analysis.
Between-study heterogeneity was calculated by using the χ2 statistics for pooled estimates (P < 0.05, indicating significant heterogeneity) and the Higgin’s inconsistency index (I 2), where I 2 values of 0%–40 %, 30 %–60 %, 50 %–90 %, and 75 %–100 % indicated insignificant, moderate, substantial, and considerable heterogeneity, respectively [8]. Publication bias was evaluated using funnel plots and Egger’s test, with a P-value < 0.1 indicating significant bias [20].
Publication bias-adjusted pooled incidences were also calculated via the trim-and-fill method [21], where agreement between the unadjusted and adjusted pooled incidences and estimates may indicate little publication bias. All statistical meta-analyses were performed using R (v.3.6.1, R Foundation for Statistical Computing, Vienna, Austria). A P-value of <0.05 was considered statistically significant.
3. Results
3.1. Literature search
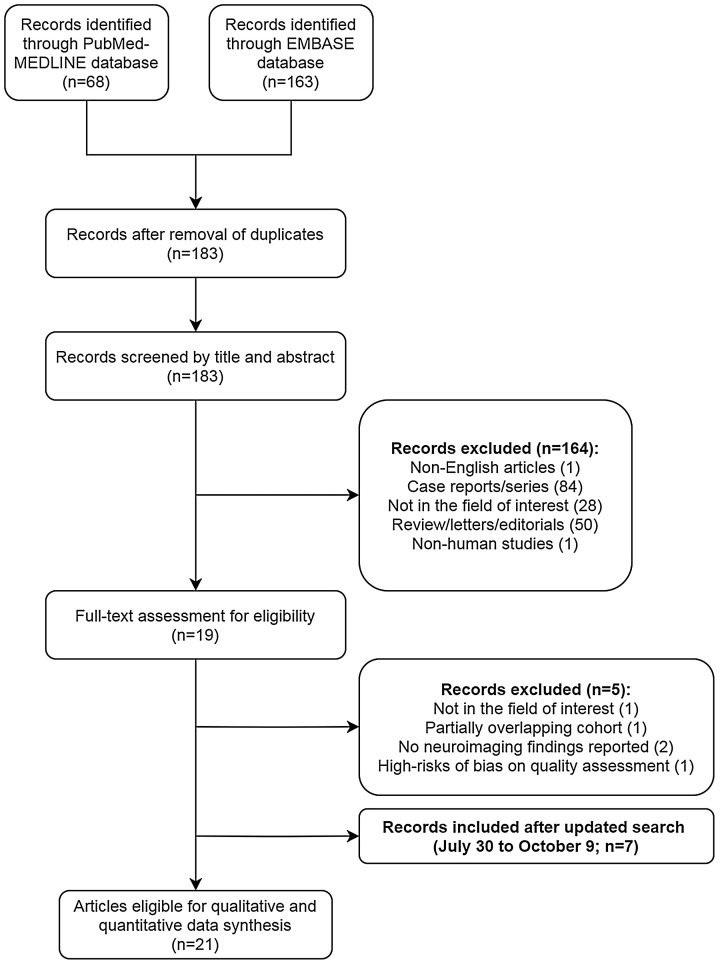
An overview of the selection process for studies from the literature search is depicted in Fig. 1 . The initial literature search identified 231 articles. After the removal of duplicates, 183 articles were screened for eligibility. Among these, 164 were excluded after reviewing their titles and abstracts (84 case reports/series, 50 review/letters/editorials, 28 articles with irrelevant content, 1 non-English article, and 1 non-human study). The full texts of the remaining 19 articles were thoroughly reviewed; five articles were further excluded due to lack of neuroimaging findings [22,23], partially overlapping patient cohorts [24], high risks of bias based on the quality assessment [25], and not in the field of interest [26]. Between the two studies with overlapping cohorts, the study with a more recent study period was chosen. An updated literature search for studies published between July 30, 2020 and October 9, 2020 resulted in 346 studies, of which seven studies met the same eligibility criteria [[27], [28], [29], [30], [31], [32], [33]]. Overall, 21 articles with a total of 2125 patients were included [4,[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]]. No additional eligible study was found after a search of references within the included studies.
Fig. 1.
Flow diagram depicting the study eligibility criteria.
3.2. Characteristics of the included studies
The characteristics of the 21 included studies are summarized in Table 1 . Of the 21 studies, all study designs were retrospective, except for two prospective studies [28,39]. Nine studies were multi-center studies [4,29,31,33,36,38,40,42,43] whereas the other 12 were performed at single centers [27,28,30,32,34,35,37,39,41,[44], [45], [46]]. The cohort sizes ranged from 9 to 454 patients. The countries of origin of the selected studies were heterogeneous, including China [40], Turkey [42], Europe [4,30,[35], [36], [37],39,41,43,45,46], and the USA [27,28,[31], [32], [33], [34],38,44]. One study was a multinational study involving all three continents [29]. Brain MRI was the only neuroimaging modality used in ten of the studies [4,28,32,33,37,39,[41], [42], [43], [44]] whereas ten studies used brain CT and/or MRI [27,[29], [30], [31],[34], [35], [36],38,45,46]; one study used only brain CT [40].
Table 1.
The clinical characteristics of the included studies.
| First author | Study period, all in 2020 | Affiliation | modality (n) | image analysis (experience) | Number of patients with neuroimaging | sex, male (%) | Age, mean ± SD/median (range) | study design | Multicenter study | microbleed | Acute/subacute infarct | non-traumatic ICH | Encephalitis/encephalopathy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fitsiori | NR | University Hospitals of Geneva and Faculty of Medicine of Geneva, Geneva | MRI | 2 neuroradiologists (NR) | 9 | 7 (77.8) | 67.7 ± 9 | retrospective | no | 9 | 2 | 0 | 0 |
| Chougar | March 23 - May 7 | Pitie-Salpetrier Hospital, Paris | MRI | 2 neuroradiologists (NR) | 73 | 48 (65.8) | 58.5 ± 15.6 | retrospective | no | 8 | 17 | 0 | 12 |
| Xiong | January 18 - March 20 | 56 hospitals in Hubei and Sichuan | CT | NR | 28 | NR | NR | retrospective | yes | NR | 10 | 0 | 0 |
| Helms | March 3 - April 3 | Strasbourg, France | MRI | NR | 13 | NR | median, 63 | retrospective | yes | 0 | 3 | 0 | 8 |
| Paterson | April 9 - May 15 | University College London, Queen Square Institute of Neurology, London | MRI (13); CT (3) | NR | 16 | 9 (56.3) | 58.8 ± 12.5 | retrospective | yes | 4 | 8 | 0 | 11 |
| Coolen | 3/31−4/24 | CUB Hôpital Erasme, Brussels | MRI | 3 neuroradiologists (NR) | 19 | 14 (73.7) | mean, 77 (49–94) | prospective | no | 2 | 0 | 1 | 2 |
| Jain | March 1 - April 13 | New York University Langone Health | MRI (48); CT (323 one examination; 131 > 1 examinations) | 4 neuroradiologists (fellowship-trained) | 454 | 275 (60.7) | median, 64 | retrospective | yes | 0 | 26 | 0 | 1 |
| Radmanesh | April 5 - April 25 | NYU Langone Medical Centers | MRI | 2 neuroradiologist2 (6 and 9 years of experience) | 27 | 9 of 11 reported (81.8) | 53 (38–64) (11 patients in ICU) | retrospective | no | 7 | 11 | 4 | 5 |
| Hernández-Fernández | March 1 - April 19 | Hospital Universitario de Albacete, Castilla-La Mancha, Spain | CT (23) / both CT and MRI (6) | 1 neuroradiologist (NR) | 23 | 18 (78.3) | 66.8 | retrospective | no | 4 | 17 | 5 | 1 |
| Kremer | March 23 - April 27 | 16 Hospitals in France | MRI | 3 neuroradiologists (9, 20, 25 years of experience) | 37 | 30 (81.1) | 61 ± 12 | retrospective | yes | 9 | NR | 20 | 27 |
| Kandemirli | March 1 - April 18 | 8 Hospitals in Istanbul, Turkey | MRI | 2 neuroradiologists (both, 29 years of experience) | 27 | 21 (77.8) | 63 (34–87) | retrospective | yes | 0 | 1 | 1 | 12 |
| Radmanesh | March 1 - March 31 | NYU Langone Medical Centers | CT (207) / MRI (11) / both CT and MRI (24) | 1 neuroradiologist (6 years of experience) | 242 | 150 (62) | 68.7 ± 16.5 | retrospective | no | 134 | 13 | 11 | 26 |
| D'Amore | February 21 - May 21 | Hospital of Circolo and Macchi Foundation, Varese, Lombardia, Italy | CT (27) / both CT and MRI (4) | 3 neuroradiologists (6, 7, 10 years of experience) | 27 | 7 (46.7) | mean 68 (21–88) | retrospective | no | 0 | 6 | 4 | 2 |
| Klironomos | March 2 - May 24 | Department of Neuroradiology, Karolinska University Hospital | CT (174) / MRI (43) (both CT and MRI, 32) | 11 neuroradiologists, 1 radiology resident (mean ± SD, 11.5 ± 5.7 years of experience) | 185 | 138 (74.6) | 62 ± 14 | retrospective | no | 29 | 25 | 27 | 31 |
| Yoon | March 3 - May 6 | Department of Radiology, Massachusetts General Hospital | CT (141) / MRI (21) / both CT and MRI (31) | 2 neuroradiologists (NR) | 150 | 98 (65.3) | 63.6 ± 16 | retrospective | no | 7 | 13 | 2 | 7 |
| Sheth | October 30, 2019 – May 20, 2020 | Department of Neurology, Yale University School of Medicine | Portable MRI (20) | 1 neuroradiologist (NR) | 20 | 17 (85) | 60 ± 8 | Prospective | No | 0 | 3 | 1 | 3 |
| Shahjouei | March 27 – May 1 | 99 tertiary centers in 11 countries | CT or MRI | Local radiologists (NR) | 156 | 109 (70) | 66 ± 15 | Retrospective | Yes | 0 | 123 | 27 | 0 |
| Sawlani | March 1 - May 31 | Queen Elizabeth Hospital Birmingham | CT (172) / MRI (36) | 2 neuroradiologists (NR) | 167 | NR | NR | Retrospective | No | 12 | 21 | 3 | 0 |
| Lin | March 4 - May 9 | academic quaternary-care center and affliated community hospital | CT (269) / MRI (51) (both, 42) | 2 neuroradiologists (10 years) | 278 | 165 (59) | 64 (50−75) | Retrospective | No | 3 | 31 | 10 | 0 |
| Freeman | March 1 - June 18 | Perelman School of Medicine at the University of Pennsylvania | MRI | 3 neuroradiologists (NR) | 59 | NR | Nr | Retrospective | No | 2 | 10 | 0 | 0 |
| Agarwal | March 1 - May 10 | 3 tertial care hospitals of an academic medical center | MRI | 2 neuroradiologist (fellow) | 1115 | 82 (71.3) | NR | Retrospective | Yes | 25 | 47 | 0 |
NR = not reported; NA = not applicable; SD = standard deviation; DWI = diffusion-weighted imaging; T1WI = T1-weighted imaging; T2WI = T2-weighted imaging; CE-T1WI = contrast-enhanced T1-weighted imaging; FLAIR = fluid attenuated inversion recovery; SWI = susceptibility-weighted imaging.
3.3. Pooled incidences of COVID-19-associated abnormal neuroimaging findings
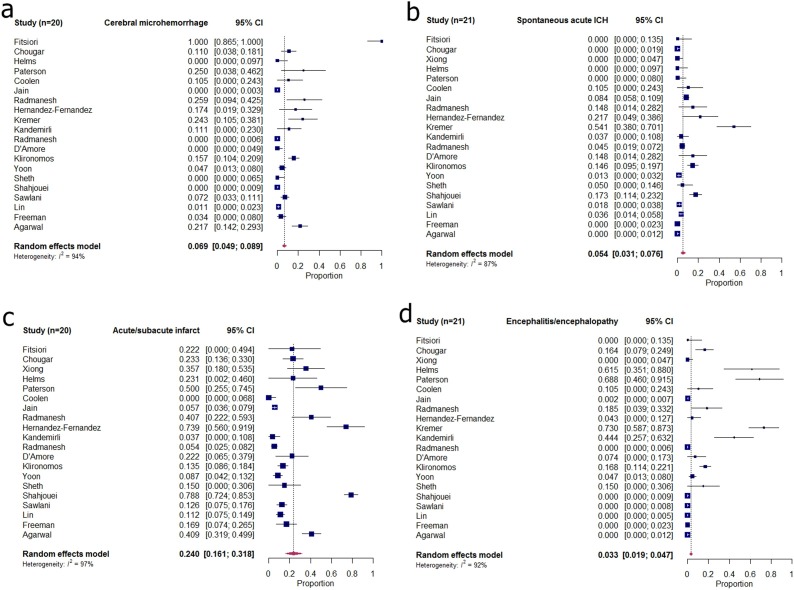
In the included studies, 42.6 % (906/2125) of the patients showed abnormal neuroimaging findings on brain CT or MRI. The pooled incidence of cerebral microhemorrhages in 20 studies (excluding one study that used brain CT alone [40]) was 6.9 % (95 % CI: 4.9 %–13.8 %; I 2 = 94 %) [Table 2 and Figs. 2,3 (a)]. The pooled incidence of acute spontaneous ICH in the included studies (n = 21) was 5.4 % (95 % CI: 3.1 %–7.6 %; I 2 = 87 %) [Table 2 and Fig. 3 (b)]. In 20 studies (excluding one study that excluded patients with infarction) [47], the pooled incidence of acute to subacute infarct was 24.0 % (95 % CI: 16.1 %–31.8 %; I 2 = 97 %) [Table 2 and Fig. 3(c)]. The pooled incidence of encephalitis or encephalopathy was 3.3 % (95 % CI: 1.9 %–4.7 %; I 2 = 92 %) [Table 2 and Fig. 3 (d)].
Table 2.
Summary of the meta-analytically pooled incidences for abnormal neuroimaging findings in patients with COVID-19.
| Summary estimate | Trim-and-fill estimate | |||||
|---|---|---|---|---|---|---|
| Pooled incidences (%) [95 % CI] | P-value for heterogeneitya | I2b (%) | P-value for publication biasc | No. of missing studies | Adjusted pooled proportions (%) [95 % CI] | |
| Cerebral microhemorrhages | 6.9 [4.9–8.9] | <0.001 | 94 | <0.001 | 10 | 1.6 [0–3.7] |
| Spontaneous acute ICH | 5.4 [3.1–7.6] | <0.001 | 87 | <0.001 | 4 | 4.0 [1.7–6.4] |
| Acute/subacute infarct | 24.0 [16.1–31.8] | <0.001 | 97 | 0.014 | 0 | 24.1 [16.3–31.9] |
| Encephalitis/encephalopathy | 3.3 [1.9–4.7] | <0.001 | 92 | <0.001 | 10 | 0.8 [0–2.5] |
ICH = intracranial hemorrhage; CI = confidence interval; I2=Higgins’ inconsistency index.
P-value by the Cochran’s Q method to test the heterogeneity of the pooled data (P < 0.05 indicates significant heterogeneity).
Higgins’ inconsistency index (0–40 % may indicate insignificant heterogeneity; 30–60 %, 50–90 %, and 75–100 % may indicate moderate, substantial, and considerable heterogeneity, respectively).
Egger’s test (P < 0.10 indicates significant publication bias).
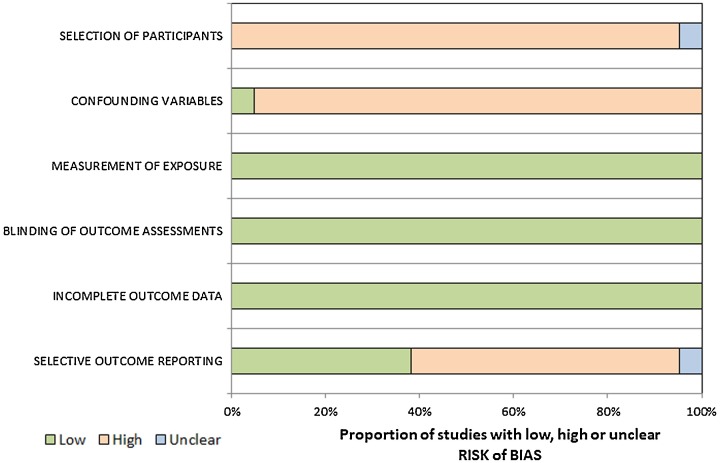
Fig. 2.
The RoBANS criteria of the included studies.
RoBANS = Risk of Bias Assessment Tool for Nonrandomized Studies.
Fig. 3.
Forest plots of pooled incidences of (a) cerebral microhemorrhage, (a) acute spontaneous ICH, (c) acute/subacute infarct, and (d) encephalitis/encephalopathy in patients with COVID-19. COVID-19=coronavirus disease 2019; ICH = intracranial hemorrhage.
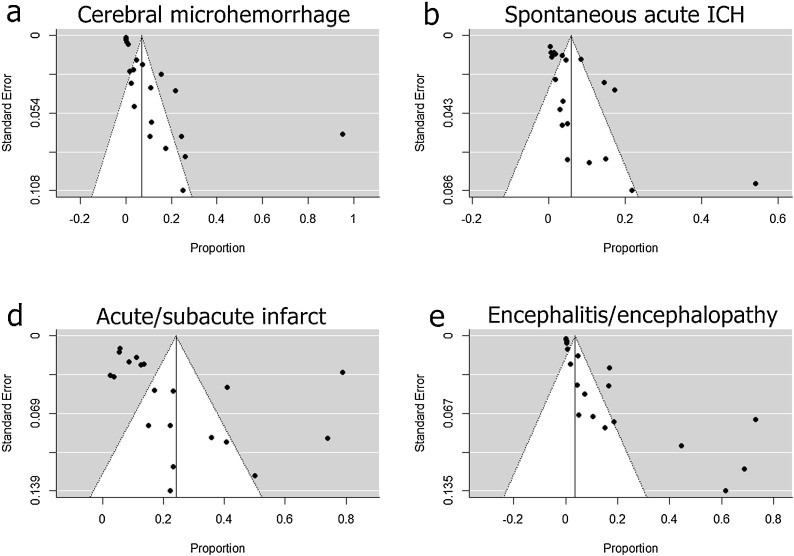
All four types of abnormal neuroimaging findings demonstrated significant publication bias on funnel plots (i.e., asymmetric distribution of studies) (Fig. 4 ) and in Egger’s tests (P < 0.05). Moreover, except for the acute/subacute infarct category, the trim-and-fill estimates were inconsistent with the pooled incidences, indicating publication bias (Table 2). All included studies had significant between-study heterogeneities (P < 0.001).
Fig. 4.
Funnel plots of pooled incidences of (a) cerebral microhemorrhage, (b) acute spontaneous ICH, (c) acute/subacute infarct, and (d) encephalitis/encephalopathy in patients with COVID-19. COVID-19=coronavirus disease 2019; ICH = intracranial hemorrhage.
3.4. Subgroup meta-regression analysis
The results of the subgroup meta-regression analysis are summarized in Table 3 . In the subgroup analysis of cerebral microhemorrhages, studies using only MRI demonstrated significantly higher incidences (13.8 %) than those using either CT or MRI (3.1 %) (P < 0.001). Regionally, studies published in Europe showed higher pooled incidences of cerebral microhemorrhages (14.0 %) than those in the USA (4.1 %) or Asia (3.0 %) (P < 0.001). Moreover, studies that reported patients in ICU showed higher pooled incidences (11.8 %) than those not reporting patients in ICU (3.2 %) (P < 0.001).
Table 3.
Subgroup meta-regression analysis of COVID-19 related neuroimaging findings.
| Covariate | Cerebral microhemorrhages | Acute/subacute infarct | Spontaneous acute ICH | Encephalitis/encephalopathy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Imaging modality | Pooled incidences (%) [95 % CI] | I2 (%) | P | Pooled incidences (%) [95 % CI] | I2 (%) | P | Pooled incidences (%) [95 % CI] | I2 (%) | P | Pooled incidences (%) [95 % CI] | I2 (%) | P |
| MRI | 13.8 [10.5–17.2] | 96 | <0.001 | 20.0 [7.9–32.2] | 89 | 0.413 | 3.9 [0.6–7.3] | 83 | 0.458 | 6.9 [4.2–9.7] | 95 | <0.001 |
| CT or MRI | 3.1 [1.0–5.2] | 86 | 27.1 [16.4–37.7] | 98 | 6.1 [3.3–8.9] | 86 | 2.1 [0.4–3.7] | 88 | ||||
| Regiona | ||||||||||||
| Europe | 14.0 [10.6–17.4] | 96 | <0.001 | 22.5 [14.7–30.3] | 89 | 0.389 | 7.1 [3.4–10.8] | 89 | 0.149 | 12.9 [9.3–16.4] | 96 | <0.001 |
| USA | 4.1 [1.1–7.1] | 88 | 17.0 [9.1–24.8] | 92 | 3.5 [0.1–7.0] | 87 | 1.8 [0–4.7] | 64 | ||||
| Asia | 3.0 [0–8.8] | 63 | 13.9 [1.7–26.0] | 82 | 2.4 [0–7.8] | 0 | 2.6 [0–7.4] | 91 | ||||
| Mean/median ageb | ||||||||||||
| >65 years | 10.1 [5.6–14.7] | 98 | 0.326 | 33.4 [17.2–49.6] | 99 | 0.27 | 10.6 [4.8–16.4] | 76 | 0.25 | 1.7 [0–5.2] | 8 | <0.001 |
| ≤65 years | 6.9 [3.4–10.3] | 88 | 18.0 [5.6–30.4] | 81 | 6.6 [2.9–10.2] | 89 | 10.5 [7.6–13.4] | 96 | ||||
| Multicenter | ||||||||||||
| Yes | 5.7 [1.8–9.6] | 87 | 0.119 | 30.4 [16.8–44.0] | 99 | 0.257 | 6.1 [2.6–9.5] | 92 | 0.525 | 3.3 [1.1–5.5] | 95 | 0.545 |
| No | 9.9 [6.7–13.1] | 96 | 20.0 [9.0–31.1] | 88 | 5.0 [1.8–8.1] | 78 | 4.3 [2.1–6.4] | 85 | ||||
| Indication for imagingc | ||||||||||||
| Neurologic symptoms | 6.1 [3.8–8.3] | 95 | 0.053 | 23.2 [12.4–33.9] | 98 | 0.624 | 7.8 [4.4–11.1] | 88 | 0.574 | 5.0 [2.9–7.1] | 93 | 0.054 |
| Not reported or other clinical indication | 11.8 [5.6–17.9] | 78 | 29.2 [8.6–49.7] | 78 | 4.3 [0–10.2] | 85 | 9.7 [4.7–14.7] | 95 | ||||
| Patients in ICU | ||||||||||||
| Reported | 11.8 [8.8–14.8] | 96 | <0.001 | 17.2 [5.3–29.1] | 79 | 0.118 | 6.2 [2.8–9.5] | 87 | 0.544 | 11.1 [8.2–14.0] | 96 | <0.001 |
| Not reported | 3.2 [0.6–5.7] | 84 | 30.9 [19.1–42.7] | 98 | 4.8 [1.6–7.9] | 88 | 0.7 [0–2.7] | 63 |
CI = confidence interval; ICH = intracranial hemorrhage; I2=Higgin’s inconsistency index.
A multinational study by Shahjouei et al involving all three continents was not included.
Three studies (Sawlani et al, Freeman et al, and Agarwal et al) not reporting mean/median ages were excluded for cerebral microhemorrhages; four studies (Xiong et al, Sawlani et al, Freeman et al, and Agarwal et al) were excluded in other three categories for the same reason.
Three studies (Lin et al, Freeman et al, and Agarwal et al) not reporting indication for neuroimaging were excluded.
For the incidence of encephalitis/encephalopathy, imaging modality (MRI, 6.9 % vs. CT/MRI, 2.1 %; P < 0.001), region (Europe, 12.9 % vs. USA, 1.8 % and Asia, 2.6 %; P < 0.001), median/mean age (>65 years, 1.7 % vs. ≤65 years, 10.5 %; P < 0.001), and patients in ICU (11.1 % vs. not reported, 0.7 %; P < 0.001) demonstrated significant differences.
While considerable heterogeneities remained in most subgroup analyses, little heterogeneity was observed in encephalitis/encephalopathy for older patients (I 2 = 8%).
3.5. Quality assessment
The quality assessment, according to RoBANS, indicated moderate overall quality, with all studies satisfying at least three of the six criteria (Fig. 2). Notably, for the “selection of participants” domain, none of the studies confirmed neuroimaging findings among the study participants at study initiation.
4. Discussion
In the present meta-analysis evaluating the pooled incidences of abnormal neuroimaging findings in patients with COVID-19, acute to subacute infarcts were the most common [24.0 %, 95 % CI = 16.1 %–31.8 %], followed by cerebral microhemorrhages [6.9 %, 95 % CI = 4.9 %–8.9 %], acute spontaneous intracerebral hemorrhages [5.4 %, 95 % CI = 3.1 %–7.6 %], and encephalitis/encephalopathy [3.3 %, 95 % CI = 1.9 %–4.7 %]. Substantial heterogeneities were observed across studies, and subgroup analysis did not reveal specific sources of potential heterogeneity. Substantial publication biases were present among all types of imaging findings. These results add evidence for the considerable incidence of abnormal neuroimaging findings in patients with COVID-19. Therefore, our results may increase the awareness of COVID-19-related neuroimaging findings among radiologists.
A recent systematic review by Nepal et al. comprehensively reviewed the various COVID-19-related neurologic manifestations, emphasizing clinical symptoms [48]. They found that the most common symptoms were smell and taste disorders and headaches, indicating the involvement of the central nervous system. The difference between their study and the present study was that their study consisted of many case reports/series without neuroimaging findings, and meta-analytic assessment was not available. The current study attempted to focus on specific COVID-19-related neuroimaging findings with meta-analytic outcomes.
As for the possible pathogeneses underlying various neuroimaging findings, the severe coagulopathy often present in COVID-19 patients might be responsible for the relatively common incidence of disseminated cerebral microhemorrhages [49]—as a milder form of acute spontaneous ICH. However, a study by Radmanesh et al. showed that among seven critically ill COVID-19 patients with cerebral microhemorrhages, none showed overt disseminated intravascular coagulation [44]. They postulated that cerebral microhemorrhages could be a late complication of critical-stage COVID-19 related to hypoxemia or a form of small vessel vasculitis. Therefore, the underlying mechanism of the development of cerebral microhemorrhages remains to be elucidated.
Patients with encephalitis and encephalopathy were grouped since their imaging patterns were often non-specific, including leptomeningeal enhancement [43], acute hemorrhagic necrotizing encephalopathy [44], diffuse leukoencephalopathy [44], and increased white matter signal intensities after accounting for age [34], ADEM, and PRES [39,41,45]. These manifestations suggest that SARS-CoV-2 causes acute brain injury via unexplained mechanisms. Although these aspects are not fully elucidated, a recent review by Li et al. suggested various potential routes of invasion of SARS-CoV-2 into the central nervous system [50]. According to Li et al., the potential entry routes include the vascular, peripheral nerve, lymphatic, and cerebrospinal fluid pathways. Thus, the various abnormal neuroimaging findings might have different underlying routes of invasion, as suggested by Li et al. However, concrete associations between routes of invasion and neuroimaging findings cannot be established without sufficient evidence.
Notably, the included studies demonstrated substantial heterogeneity and publication bias, indicating that interpretations of pooled incidences should be performed with caution. Even in subgroup analysis, the potential sources of heterogeneities were only found for encephalitis/encephalopathy in older patients—demonstrating minor heterogeneities. Interestingly, plausible sources of heterogeneity, such as study regions and multi-center settings, did not cause heterogeneities. Thus, the various COVID-19-related neuroimaging findings might involve a broad spectrum of underlying interrelated mechanisms, making it difficult to identify sources of heterogeneities.
Importantly, the pooled incidences of abnormal neuroimaging findings in the current study should not be interpolated to the overall population of patients with COVID-19; the included studies consisted mostly of patients who had severe neurologic manifestations, were admitted to the ICU, or were under mechanical ventilation. Furthermore, the mean and median patients ages in all included studies were over 58 years, indicating that the patients belonged to older age groups that are more vulnerable to severe disease [47]. Therefore, such predispositions would make the patients included in this study more susceptible to developing abnormal neuroimaging findings.
Several limitations need to be addressed. First, the literature search period was limited to several months; however, due to the ever-increasing concerns on the spread of COVID-19 globally, rapid collation of available evidence is needed to understand the various COVID-19-associated neuroimaging findings. Additionally, in most selected studies, confounding variables such as underlying comorbidities were not statistically adjusted for the neuroimaging findings, nor were there control groups. Again, considering the urgency of the COVID-19 pandemic, a careful review of patients’ medical records must have been difficult. Moreover, the causal relationship between COVID-19 infection and abnormalities in imaging findings is not fully established; abnormal neuroimaging findings might be due to systematic confounding factors such as DM, mechanical ventilation, and the multi-drug regimen for respiratory distress with hypoxia. Finally, nearly all the diagnoses of encephalitis and encephalopathy were not definite without serologic confirmation. These limitations necessitate a future prospective study with adjustment for comorbidities and a more detailed analysis to confirm the association between COVID-19 and neuroimaging findings.
In conclusion, the current systematic review and meta-analysis demonstrated a considerable incidence of abnormal neuroimaging findings related to COVID-19. The findings of this study may help increase awareness of the wide range of COVID-19-related neuroimaging findings among radiologists worldwide.
Funding
This study was supported by Research Fund of Seoul St. Mary’s Hospital, The Catholic University of Korea.
CRediT authorship contribution statement
Yangsean Choi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Min Kyoung Lee: Data curation, Investigation, Resources, Supervision, Validation.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
The authors declare no acknowledgement.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ejrad.2020.109393.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Map: Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. (Accessed October 21,2020.
- 3.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., Anheim M., Meziani F. Neurologic features in severe SARS-COV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y., Li X., Geng D., Mei N., Wu P.-Y., Huang C.-C., Jia T., Zhao Y., Wang D., Xiao A. Cerebral micro-structural changes in COVID-19 patients–An MRI-based 3-month follow-up study. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: C.T. And MRI features. Radiology. 2020 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B., She J., Wang Y., Ma X. Venous thrombosis and arteriosclerosis obliterans of lower extremities in a very severe patient with 2019 novel coronavirus disease: a case report. J. Thromb. Thrombolysis. 2020;50(1):229–232. doi: 10.1007/s11239-020-02084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-olama M., Rashid A., Garozzo D. COVID-19-associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir. 2020;162(7):1495–1499. doi: 10.1007/s00701-020-04402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschi A.M., Ahmed O., Giliberto L., Castillo M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR Am. J. Neuroradiol. 2020;41(7):1173–1176. doi: 10.3174/ajnr.A6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogg J., Baker A., Tung G. Posterior reversible encephalopathy syndrome (PRES): another imaging manifestation of COVID-19. Interdiscip. Neurosurg. Adv. Techniques Case Manage. 2020;22 doi: 10.1016/j.inat.2020.100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020:1–4. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemasian H., Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev. Neurol. 2020;176(6):521–523. doi: 10.1016/j.neurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy-Gash F., Marine D.M., Jean-Michel D., Herve V., Raphael B., Nicolas E. COVID-19-associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features. Crit. Care. 2020;24(1) doi: 10.1186/s13054-020-03131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou B., She J., Wang Y., Ma X. A case of coronavirus disease 2019 with concomitant acute cerebral infarction and Deep vein thrombosis. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg M.F., Goldberg M.F., Cerejo R., Tayal A.H. Cerebrovascular disease in COVID-19. AJNR Am. J. Neuroradiol. 2020;41(7):1170–1172. doi: 10.3174/ajnr.A6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duval S., Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J., Shen B., Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Alexia B., Sandri M.T., Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kremer S., Lersy F., Anheim M., Merdji H., Schenck M., Oesterlé H., Bolognini F., Messie J., Henri-Feugeas M.C., Khalil A., Gaudemer A., Carré S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Jager L., Nesser P., Mba Y.T., Hmeydia G., Benzakoun J., Oppenheim C., Ferré J.C., Maamar A., Carsin-Nicol B., Comby P.O., Ricolfi F., Thouant P., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Desal H., Boulouis G., Berge J., Kazémi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Constans J.M., Zorn P.E., Mathieu M., Baloglu S., Ardellier F.D., Willaume T., Brisset J.C., Caillard S., Collange O., Mertes M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., Meyer N., Helms J., Cotton F. Neurologic and neuroimaging findings in COVID-19 patients: a retrospective multicenter study. Neurology. 2020 doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 25.Scullen T., Keen J., Mathkour M., Dumont A.S., Kahn L. Coronavirus 2019 (COVID-19)–associated encephalopathies and cerebrovascular disease: The New Orleans Experience. World Neurosurg. 2020 doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aznab M. Evaluation of COVID 19 infection in 279 cancer patients treated during a 90-day period in 2020 pandemic. Int. J. Clin. Oncol. 2020 doi: 10.1007/s10147-020-01734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon B.C., Buch K., Lang M., Applewhite B.P., Li M.D., Mehan W.A., Jr., Leslie-Mazwi T.M., Rincon S.P. Clinical and neuroimaging correlation in patients with COVID-19. AJNR Am. J. Neuroradiol. 2020;41(10):1791–1796. doi: 10.3174/ajnr.A6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheth K.N., Mazurek M.H., Yuen M.M., Cahn B.A., Shah J.T., Ward A., Kim J.A., Gilmore E.J., Falcone G.J., Petersen N., Gobeske K.T., Kaddouh F., Hwang D.Y., Schindler J., Sansing L., Matouk C., Rothberg J., Sze G., Siner J., Rosen M.S., Spudich S., Kimberly W.T. Assessment of brain injury using portable, low-field magnetic resonance imaging at the bedside of critically ill patients. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahjouei S., Naderi S., Li J., Khan A., Chaudhary D., Farahmand G., Male S., Griessenauer C., Sabra M., Mondello S., Cernigliaro A., Khodadadi F., Dev A., Goyal N., Ranji-Burachaloo S., Olulana O., Avula V., Ebrahimzadeh S.A., Alizada O., Hancı M.M., Ghorbani A., Vaghefi far A., Ranta A., Punter M., Ramezani M., Ostadrahimi N., Tsivgoulis G., Fragkou P.C., Nowrouzi-Sohrabi P., Karofylakis E., Tsiodras S., Neshin Aghayari Sheikh S., Saberi A., Niemelä M., Rezai Jahromi B., Mowla A., Mashayekhi M., Bavarsad Shahripour R., Sajedi S.A., Ghorbani M., Kia A., Rahimian N., Abedi V., Zand R. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawlani V., Scotton S., Jacob S., Nader K., Jen J.P., Patel M., Gokani K., Denno P., Thaller M., Englezou C., Janjua U., Bowen M., Hoskote C., Veenith T., Hassan-Smith G. COVID-19-related intracranial imaging findings: a large single-centre experience. Clin. Radiol. 2020 doi: 10.1016/j.crad.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin E., Lantos J.E., Strauss S.B., Phillips C.D., Campion T.R., Jr., Navi B.B., Parikh N.S., Merkler A.E., Mir S., Zhang C., Kamel H., Cusick M., Goyal P., Gupta A. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman C.W., Masur J., Hassankhani A., Wolf R.L., Levine J.M., Mohan S. COVID-19-Related disseminated leukoencephalopathy (CRDL): a retrospective study of findings on brain MRI. AJR Am. J. Roentgenol. 2020 doi: 10.2214/AJR.20.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agarwal S., Jain R., Dogra S., Krieger P., Lewis A., Nguyen V., Melmed K., Galetta S. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. 2020;51(9):2649–2655. doi: 10.1161/STROKEAHA.120.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radmanesh A., Raz E., Zan E., Derman A., Kaminetzky M. Brain imaging use and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. AJNR Am. J. Neuroradiol. 2020;41(7):1179–1183. doi: 10.3174/ajnr.A6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernández-Fernández F., Valencia H.S., Barbella-Aponte R.A., Collado-Jiménez R., Ayo-Martín Ó., Barrena C., Molina-Nuevo J.D., García-García J., Lozano-Setién E., Alcahut-Rodriguez C., Martínez-Martín Á., Sánchez-López A., Segura T. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain : J. neurology. 2020 doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain : a J. neurol. 2020 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitsiori A., Pugin D., Thieffry C., Lalive P., Vargas M.I. Unusual microbleeds in brain MRI of Covid-19 patients. J. Neuroimaging. 2020 doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain R., Young M., Dogra S., Kennedy H., Nguyen V., Jones S., Bilaloglu S., Hochman K., Raz E., Galetta S., Horwtiz L. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J. Neurol. Sci. 2020;414 doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coolen T., Lolli V., Sadeghi N., Rovaï A., Trotta N., Taccone F.S., Creteur J., Henrard S., Goffard J.C., De Witte O., Naeije G., Goldman S., De Tiège X. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020 doi: 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
- 40.Xiong W., Mu J., Guo J., Lu L., Liu D., Luo J., Li N., Liu J., Yang D., Gao H., Zhang Y., Lin M., Shen S., Zhang H., Chen L., Wang G., Luo F., Li W., Chen S., He L., Sander J.W., Zhou D. New onset neurologic events in people with COVID-19 infection in three regions in China. Neurology. 2020 doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]
- 41.Chougar L., Shor N., Weiss N., Galanaud D., Leclercq D., Mathon B., Belkacem S., Stroër S., Burrel S., Boutolleau D., Demoule A., Rosso C., Delorme C., Seilhean D., Dormont D., Morawiec E., Raux M., Demeret S., Gerber S., Trunet S., Similowski T., Degos V., Rufat P., Corvol J.C., Lehéricy S., Pyatigorskaya N. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. 2020 doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., Kaya Y., Yildirim D., Tuzuner F., Yildirim M.S., Ozluk E., Gucyetmez B., Karaarslan E., Koyluoglu I., Demirel Kaya H.S., Mammadov O., Kisa Ozdemir I., Afsar N., Citci Yalcinkaya B., Rasimoglu S., Guduk D.E., Kedir Jima A., Ilksoz A., Ersoz V., Yonca Eren M., Celtik N., Arslan S., Korkmazer B., Dincer S.S., Gulek E., Dikmen I., Yazici M., Unsal S., Ljama T., Demirel I., Ayyıldız A., Kesimci I., Bolsoy Deveci S., Tutuncu M., Kizilkilic O., Telci L., Zengin R., Dincer A., Akinci I.O., Kocer N. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020 doi: 10.1148/radiol.2020201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kremer S., Lersy F., de Sèze J., Ferré J.C., Maamar A., Carsin-Nicol B., Collange O., Bonneville F., Adam G., Martin-Blondel G., Rafiq M., Geeraerts T., Delamarre L., Grand S., Krainik A., Caillard S., Marc Constans J., Metanbou S., Heintz A., Helms J., Schenck M., Lefèbvre N., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Lacalm A., Oesterlé H., Bolognini F., Messie J., Hmeydia G., Benzakoun J., Oppenheim C., Bapst B., Megdiche I., Henri-Feugeas M.C., Khalil A., Gaudemer A., Jager L., Nesser P., Talla Mba Y., Hemmert C., Feuerstein P., Sebag N., Carré S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Comby P.O., Ricolfi F., Thouant P., Desal H., Boulouis G., Berge J., Kazémi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Zorn P.E., Mathieu M., Baloglu S., Ardellier F.D., Willaume T., Brisset J.C., Boulay C., Mutschler V., Hansmann Y., Mertes P.M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., David J.S., Meyer N., Anheim M., Cotton P.F. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radmanesh A., Derman A., Lui Y.W., Raz E., Loh J.P., Hagiwara M., Borja M.J., Zan E., Fatterpekar G.M. COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020 doi: 10.1148/radiol.2020202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Amore F., Vinacci G., Agosti E., Cariddi L.P., Terrana A.V., Vizzari F.A., Mauri M., Giorgianni A. Pressing issues in COVID-19: probable cause to seize SARS-CoV-2 for its preferential involvement of posterior circulation manifesting as severe posterior reversible encephalopathy syndrome and posterior strokes. Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klironomos S., Tzortzakakis A., Kits A., Öhberg C., Kollia E., Ahoromazdae A., Almqvist H., Aspelin Å., Martin H., Ouellette R., Al-Saadi J., Hasselberg M., Haghgou M., Pedersen M., Petersson S., Finnsson J., Lundberg J., Falk Delgado A., Granberg T. Nervous system involvement in COVID-19: results from a retrospective consecutive neuroimaging cohort. Radiology. 2020 doi: 10.1148/radiol.2020202791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yavarpour-Bali H., Ghasemi-Kasman M. Update on neurological manifestations of COVID-19. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nepal G., Rehrig J.H., Shrestha G.S., Shing Y.K., Yadav J.K., Ojha R., Pokhrel G., Tu Z.L., Huang D.Y. Neurological manifestations of COVID-19: a systematic review. Crit. Care. 2020;24(1) doi: 10.1186/s13054-020-03121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maas M.B. Critical medical illness and the nervous system. Contin. Lifelong Learn. Neurol. 2020;26(3):675–694. doi: 10.1212/CON.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 50.Li Z., Liu T., Yang N., Han D., Mi X., Li Y., Liu K., Vuylsteke A., Xiang H., Guo X. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020:1–9. doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.