Abstract
Purpose
The optimal approach to identify SARS-CoV-2 infection among college students returning to campus is unknown. Recommendations vary from no testing to two tests per student. This research determined the strategy that optimizes the number of true positives and negatives detected and reverse transcription polymerase chain reaction (RT-PCR) tests needed.
Methods
A decision tree analysis evaluated five strategies: (1) classifying students with symptoms as having COVID-19, (2) RT-PCR testing for symptomatic students, (3) RT-PCR testing for all students, (4) RT-PCR testing for all students and retesting symptomatic students with a negative first test, and (5) RT-PCR testing for all students and retesting all students with a negative first test. The number of true positives, true negatives, RT-PCR tests, and RT-PCR tests per true positive (TTP) was calculated.
Results
Strategy 5 detected the most true positives but also required the most tests. The percentage of correctly identified infections was 40.6%, 29.0%, 53.7%, 72.5%, and 86.9% for Strategies 1–5, respectively. All RT-PCR strategies detected more true negatives than the symptom-only strategy. Analysis of TTP demonstrated that the repeat RT-PCR strategies weakly dominated the single RT-PCR strategy and that the thresholds for more intensive RT-PCR testing decreased as the prevalence of infection increased.
Conclusion
Based on TTP, the single RT-PCR strategy is never preferred. If the cost of RT-PCR testing is of concern, a staged approach involving initial testing of all returning students followed by a repeat testing decision based on the measured prevalence of infection might be considered.
Keywords: COVID-19, College students, Testing, Decision analysis
Implications and Contribution.
Testing all college students on arrival to campus and again 7 days later will detect the greatest number of COVID-19 cases. A single test could miss almost half of all infected students. At higher prevalence of infection, repeat testing will detect more true positives per test.
See Related Editorial on p.1
The COVID-19 pandemic disrupted higher education in the U.S. [1]. Colleges and universities transitioned learning to virtual instruction and required students to vacate campuses [[2], [3], [4], [5]]. The pandemic affected the education of approximately 26 million students and the operations of more than 4,200 higher education institutions nationwide [6]. As COVID-19 transmission appeared to be slowing and testing capacity was increasing, leadership of many colleges and universities began developing plans to resume in-person instruction for the fall semester. A critical component of these plans should include the evaluation of potential outcomes of repopulating a large congregate setting.
At present, there is no consensus on the most appropriate approach for testing students for COVID-19 on repopulating college campuses. Most institutions plan to implement campaigns for basic preventive measures (e.g., face coverings, physical distancing, and handwashing), but proposed policies for initial testing for COVID-19 vary. The Centers for Disease Control and Prevention (CDC) does not recommend universal testing of all individuals on entry to campus [7]. Some institutions simply encourage, but do not require, students to undergo initial testing on arrival to campus [8]. Others plan to distribute a screening questionnaire to all students and require testing for at-risk and symptomatic students [9]. The Higher Education Subcommittee of the Reopen Connecticut Task Force recommends that colleges and universities test students on arrival to campus and again 7–14 days later to account for false negative results on the first test [10].
To best inform the procedures for reopening college campuses, data about disease and testing characteristics should guide decision-making [11,12]. Greater rates of asymptomatic disease in younger people [[13], [14], [15]] and low testing sensitivity early in infection may limit testing strategies for coronavirus infection. To the best of our knowledge, no decision analysis has been conducted to systematically quantify the results of different testing strategies. Thus, this research aimed to evaluate five COVID-19 testing strategies to determine the optimal approach for detecting infections as students repopulate college campuses.
Methods
Target population
This research was based on a target population of college students. A reference of 20,000 students, based on the estimated number of students returning to campus at the authors' institution, was used to aid in the interpretation of the analyses.
Testing strategies
Five strategies for screening students for COVID-19 on arrival to campus were developed, with each strategy building on the previous approach. Each strategy represents an arm of the decision tree used for the analyses.
Symptom-based screening only (Figure A1) relies on monitoring symptoms suggestive of COVID-19 (e.g., fever, cough, and shortness of breath [[16], [17], [18]]), but no reverse transcription polymerase chain reaction (RT-PCR) testing occurs. The tree begins with a pair of branches representing the presence and absence of COVID-19. Individuals without disease can either not have or have COVID-19-like symptoms. Those with disease can either have asymptomatic disease or disease that will eventually become symptomatic. Individuals in the latter group are in either the incubation period (without symptoms) or the post-incubation period (with symptoms). Students who do not present with symptoms suggestive of COVID-19 are considered uninfected, whereas those who present with symptoms are considered to have COVID-19.
For symptom-based RT-PCR testing (Figure A2), all students are screened for COVID-19 symptoms, and those who are symptomatic undergo RT-PCR testing. This tree begins with the branches in Strategy 1, but students with symptoms receive RT-PCR testing. In this and screening strategies 3–5, negative RT-PCR tests can be either true or false negatives. Positive RT-PCR tests can be either true or false positives.
In universal, single RT-PCR testing (Figure A3), all students receive a single RT-PCR test. This tree begins with the branches in Strategy 2, but students without symptoms also receive RT-PCR testing.
In repeat RT-PCR testing based on symptoms (Figure A4), all individuals undergo RT-PCR testing, and students with symptoms suggestive of COVID-19 (anytime from testing to a week post-testing) with an initial negative result are retested with RT-PCR 7 days later. This tree begins with the branches for Strategy 3, but individuals who had COVID-19 symptoms and a negative first RT-PCR test receive a second RT-PCR test.
Finally, in universal, repeat RT-PCR testing (Figure A5), all individuals are tested, and those with a negative test are retested via RT-PCR 7 days later. This tree begins with the branches in Strategy 4, but individuals without symptoms and a negative first RT-PCR are also retested.
Baseline parameters
The seven parameters used in the primary analysis were calculated based on the best available data from published literature and national guidelines (e.g., CDC; Table 1 ). The baseline prevalence of COVID-19 in the U.S. was calculated as .0045 based on the total number of active cases and population estimates at the time of analysis (1,473,944 cases/329,517,756 people) [19,20]. The proportion of symptomatic cases was estimated as .5679 based on 159 symptomatics/280 cases in three representative cohort samples (Iceland, Indiana, and Vo’) included in Oran et al. [21]. To account for individuals presenting with symptoms that mimic COVID-19 disease that would prompt testing or positive diagnosis in the absence of testing, an estimate of .0957 was used based on the prevalence of college students who develop influenza-like illness in November (the earliest month with data available for the first semester) [22].
Table 1.
Parameters for decision tree analysis
| Variable | Estimate (range) | Source | Distributiona |
|---|---|---|---|
| COVID-19 prevalence | .0045 (0–.1000) | [19,20] | Beta |
| Proportion of symptomatic cases | .5679 (.2000–.8000) | [21,24] | Beta |
| Proportion of symptomatic without COVID-19 | .0957 (.0500–.2000) | [22] | Beta |
| Proportion of symptomatic cases in pos-tincubation periodb | .7143 | ||
| RT-PCR sensitivity | |||
| Incubation period (Days 1–4) | .0850 (±30%) | [23] | Beta |
| Post-incubation period (Days 5–14) | .7173 (±30%) | [23] | Beta |
| RT-PCR specificity | 1.0000 (.9897–1.0000) | [23] | Beta |
RT-PCR = reverse transcription polymerase chain reaction.
Distribution used in second-order Monte Carlo analysis.
Based on a median 5-day incubation period and a post-incubation period of 10 days.
To account for the potential difference in the sensitivity of the RT-PCR testing at different points of viral shedding and disease progression, estimates were dichotomized based on the median incubation period: .0850 for the incubation period and .7173 for the post-incubation period. These estimates were made by averaging the daily sensitivity values derivable from a study by Kucirka et al. for Days 1–4 (incubation period) and for Days 5–14 (post-incubation period) [23]. When weighted by the proportion of cases in the incubation period (4/14 days) and post-incubation period (10/14 days), the combined sensitivity of a single RT-PCR test performed any time during illness was estimated as 53.7% (28.57% of days with a sensitivity of 8.5% and 71.43% of days with a sensitivity of 71.73%). The sensitivity of symptom-based screening was estimated as 40.57% (56.79% of students ever symptomatic times 71.73% in the post-incubation period). The specificity of the RT-PCR testing was estimated as 1.00 based on that reported by Kucirka [23].
The study used only published literature and was thus not submitted for review by an institutional review board. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Outcome measures
Outcomes included true positives (i.e., correctly identified infections) and true negatives (i.e., correctly identified as uninfected) detected, RT-PCR tests needed, and the number of RT-PCR tests per true positive (TTP) case detected.
Little information about willingness to trade off RT-PCR tests versus true positives and true negatives is available. In addition, given it is unlikely that true positives and true negatives are valued equally, it is unclear how one should construct a composite of these two outcomes. Both of these issues pose problems for making recommendations for trading off tests and disease classifications. There are, however, two sets of relationships among the five strategies that allow the use of TTP to rank order most of the strategies in terms of preference.
First, Strategies 3–5 have the same specificity, and thus, the TTP ranges where each strategy is preferred would be unaffected by the inclusion of true negatives. In addition, these strategies have higher sensitivities and specificity (at least 53.7% and 100%) compared with Strategy 1 (40.47% and 90.43%). They will therefore yield more true positives and true negatives but will require more RT-PCR tests than Strategy 1 (i.e., none). Thus, the TTP threshold between preference for Strategy 1 and the lowest ranked RT-PCR strategy would represent an upper bound for preferring symptom-based screening only. That is, because Strategy 1 always has more false positives than Strategies 3–5, the threshold for TTP would overestimate Strategy 1's value.
Second, Strategies 2–5 will result in identical numbers of true negatives. Thus, in comparing Strategy 2 with Strategies 3–5, the same logic that was used to justify the lack of effect of true negatives on the ranges where Strategies 3–5 are preferred extends to the ranges where Strategies 2–5 are preferred.
Statistical analysis
A decision tree model was used to quantify the study outcomes. All analyses were based on estimates from students' initial arrival on campus (Day 0) and the assumption that all repeat testing occur within 7 days of the first test.
To rank the strategies and derive ranges of TTPs where the different strategies are preferred, the analysis followed the standard algorithm used in cost-effectiveness analysis (Supplementary Data) [24]. The ranges of TTP were defined for four probabilities of COVID-19 (.4473% [baseline assumption], 1%, 5%, and 10%) and for two sets of sensitivity for RT-PCR (one with the baseline weighted average of 53.7% and one with a weighted average of 70%). Recommendations from the comparison of Strategies 1, 3, 4, and 5 and of Strategies 2–5 were combined to define ranges of TTPs that can be compared with one's own “willingness” (i.e., testing capability, including personnel needs, testing kits and reagent availability, and cost) to test to detect a true positive.
To account for variability in the estimates, we performed five deterministic sensitivity analyses in which we varied the (1) prevalence of infection from 0% to 10%, (2) proportion of symptomatic cases from 20% to 80% (obtained from CDC estimates and widened to adjust for the higher prevalence of asymptomatic infection in the younger target population) [25], (3) sensitivities of RT-PCR during the incubation and post-incubation periods by ±30%, (4) specificity of RT-PCR from .9897 to 1.0 (obtained from a study by Kucirka et al. [23] and Wilson confidence limits for an estimated specificity of 1.0), and (5) proportion of individuals with symptoms not due to COVID-19 from 5% to 20%. To account for sampling uncertainty related to the estimates, we performed second-order Monte Carlo simulation. The probability of COVID-19, the RT-PCR sensitivities and specificity, the probability of eventually symptomatic COVID-19, and the probability of COVID-like symptoms were all represented by beta distributions with real-numbered parameters.
TreeAge Pro Version 2019, R1.1 (TreeAge Software, Williamstown, MA) was used to construct the decision trees and to complete all statistical analyses.
Results
True positives and negatives by strategy
Table 2 provides the results for each of the five testing strategies under the baseline assumption of .4473% infection. It also reports sampling uncertainty derived from the second-order Monte Carlo simulation (incremental analysis reported in Tables A1–3). Universal, repeat RT-PCR testing identified the greatest number and proportion of true positives (mean: .0039, 95% confidence interval [CI]: .0037%–.0040%, 86.90% of cases detected), followed by repeat RT-PCR testing based on symptoms (mean: .0032, 95% CI: .0034%–.0037%, 72.54% detected). Symptom-based RT-PCR testing (mean: .0013, 95% CI: .0012%–.0014%, 29.09% detected) identified the lowest proportion of true positives. Based on an estimate of 89 (20,000 × .004473) students with COVID-19, the total numbers of students identified by Strategies 1–5 were 36, 26, 48, 65, and 78, respectively. In other words, the total number of students with COVID-19 missed (i.e., false negatives) by the strategies were 53 (89–36), 62, 21, 24, and 11, respectively.
Table 2.
Number of true positives, true negatives, and number of RT-PCR per student for each strategy and estimates for a student population of 20,000
| Strategy | Mean | 95% CI | Percentage detected | Total studentsa (out of 89) |
|---|---|---|---|---|
| True positives | (Out of .004473) | |||
| Symptom-based screening only | .0018 | .0016–.0020 | 40.56 | 36 |
| Symptom-based RT-PCR testing | .0013 | .0012–.0014 | 29.09 | 26 |
| Universal, Single RT-PCR testing | .0024 | .0023–.0025 | 53.66 | 48 |
| Repeat RT-PCR testing based on symptoms | .0032 | .0031–.0034 | 72.54 | 65 |
| Universal, Repeat RT-PCR testing |
.0039 |
.0037–.0040 |
86.90 |
78 |
| True negatives |
(Out of .995527) |
Percentage mistaken diagnosis of well students |
(out of 19,911) |
|
| Symptom-based screening only | .9002 | .8899–.9101 | 9.56 | 18,005 |
| Symptom-based RT-PCR testing | .9955 | .9945–.9955 | 0 | 19,911 |
| Universal, Single RT-PCR testing | .9955 | .9850–.9935 | 0 | 19,911 |
| Repeat RT-PCR testing based on symptoms | .9955 | .9842–.9955 | 0 | 19,911 |
| Universal, repeat RT-PCR testing |
.9955 |
.9747–.9955 |
0 |
19,911 |
| Number of RT-PCR tests/student |
Total testsb |
|||
| Symptom-based screening only | .0000 | .0000–.0000 | 0 | -- |
| Symptom-based RT-PCR testing | .0971 | .0872–.1074 | 1,942 | -- |
| Universal, Single RT-PCR testing | 1.0000 | 1.0000–1.0000 | 20,000 | -- |
| Repeat RT-PCR testing based on symptoms | 1.0965 | 1.0864–1.1068 | 21,930 | -- |
| Universal, repeat RT-PCR testing | 1.9976 | 1.9871–1.9977 | 39,952 | -- |
CI = confidence interval; RT-PCR = reverse transcription polymerase chain reaction.
Rounded to nearest integer.
Number per 20,000 students.
Strategies 2–5 detected a greater proportion of true negatives (mean: .9955 out of .9955, or 100%, with varying 95% CIs; Table 2) than symptom-based screening only (mean: .9002 out of .9955, or 90.43%, 95% CI: .889–.9101; Table 2). Based on an estimate of 19,911 students without COVID-19, Strategy 1 mistakenly identified 1,906 uninfected students as infected (i.e., false positives), whereas the other strategies yielded no false positives.
Number of RT-PCR tests
Strategy 1 relies on symptom-based screening only, so no RT-PCR tests were needed. For a population of 20,000 students, 1,942; 20,000; 21,930; and 39,952 tests were required for Strategies 2–5, respectively (Table 2).
RT-PCR TTP detected
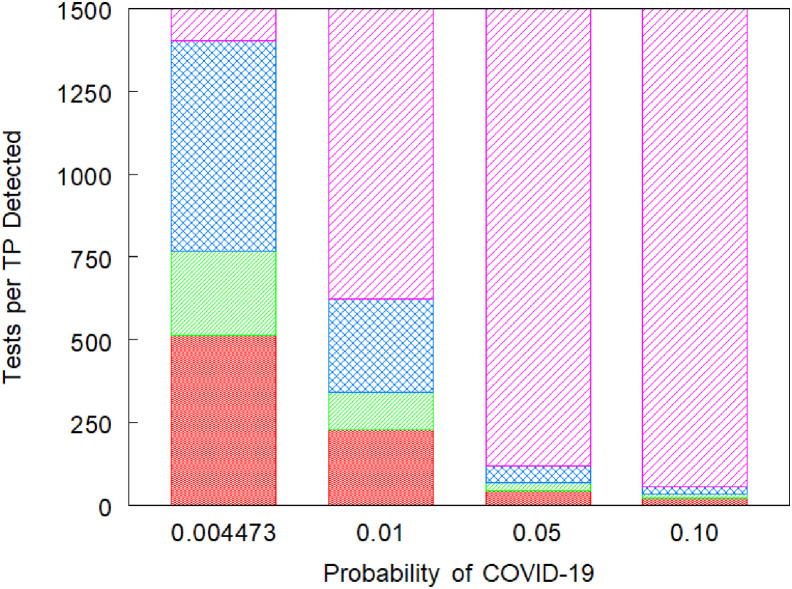
Figure 1 reports the results of the incremental TTP analyses. For a probability of COVID-19 of .4473%, if an institution is unwilling or unable to perform 514 RT-PCRs per true positive detected, and if Strategy 1's additional true positives and fewer tests are more important than its false positives, then it is preferred to Strategy 2. If, instead, Strategy 2's additional true negatives are more important than its additional false negatives and number of tests, then it is preferred. If an institution is willing and able to perform between 514 and 767 TTP, and if Strategy 1's fewer tests are more important than its additional false positives and negatives, then it is preferred to Strategy 4. If, instead, Strategy 4's additional true positives and negatives are more important than its additional number of tests, then it is preferred.
Figure 1.
Ranges of acceptable tests per true positive (TTP) identified from cost-effectiveness for five RT-PCR based strategies. Columns represent values of TTP for which the different screening strategies are preferred for probabilities of COVID-19 of .4473%, 1%, 5%, and 10%. Red narrower cross hatch represents the TTP for which (Strategy 2) symptom-based testing or (Strategy 1) symptom-based screening is preferred; green narrower diagonal represents the TTP for which (Strategy 1) symptom-based screening or (Strategy 4) retesting students with negative tests and symptoms is preferred; blue wider cross hatch represents (Strategy 4) retesting students with negative tests and symptoms is preferred; and magenta wider diagonal represents (Strategy 5) retesting all students with negative tests is preferred. Strategy 3 is never preferred because of weak dominance. RT-PCR, reverse transcription polymerase chain reaction.
If an institution is willing to perform between 766 and 1,403 TTP, then repeat RT-PCR testing based on symptoms is preferred. Finally, if an institution is willing to perform at least 1,403 TTP, then universal, repeat RT-PCR testing was preferred. Universal, single RT-PCR testing is never recommended because it is weakly dominated by both repeat RT-PCR testing strategies (i.e., fewer true positives and a larger TTP than the higher ranked strategies).
Sensitivity analyses
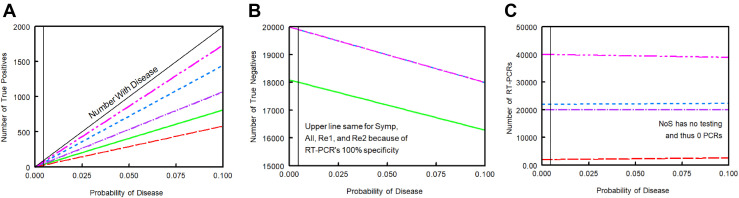
As the probability of COVID-19 increased, the number of true positives for each of the five strategies linearly increased (Figure 2 A), whereas the number of true negatives linearly decreased (Figure 2B). Increasing the probability of disease had minimal effects on the number of RT-PCR tests needed (Figure 2C).
Figure 2.
Sensitivity analysis for primary outcomes with varying probabilities of COVID-19. Results among a population of 20,000 students. (A) Number of true positives; (B) Number of true negatives; (C) Number of RT-PCR tests. For A and C, green line represents Strategy 1: symptom-based screening only (NoS). Red line represents Strategy 2: symptom-based RT-PCR testing (Symp). Purple line represents Strategy 3: universal, single RT-PCR testing (All). Blue line represents Strategy 4: repeat RT-PCR based on symptoms (Re1). Pink line is Strategy 5: universal repeat RT-PCR testing (Re2). For B, green line represents Strategy 1, whereas mixed line represents Strategies 2–5. RT-PCR, reverse transcription polymerase chain reaction.
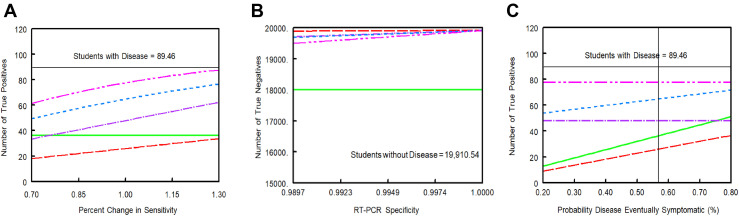
Increasing the sensitivity of the RT-PCR test resulted in greater numbers of true positives identified for Strategies 2–5 (Figure 3 A). Reducing the specificity of the RT-PCR test yielded fewer numbers of true negatives (more false positives) detected by Strategies 2–5 (Figure 3B). Increasing the probability of eventually symptomatic COVID-19 resulted in greater numbers of true positives for the symptom-based strategies (Strategies 1, 2, and 4) but did not affect Strategy 3 or Strategy 5 (Figure 3C).
Figure 3.
Sensitivity analysis for primary outcomes with varying RT-PCR sensitivity and specificity and for proportion of (eventually) symptomatic COVID-19 cases. (A) Number of true positives with varying sensitivity; (B) Number of true negatives with varying specificity; (C) Number of true positives with varying proportions of eventually symptomatic disease. Green line represents Strategy 1: symptom-based screening only. Red line represents Strategy 2: symptom-based RT-PCR testing. Purple line represents Strategy 3: universal, single RT-PCR testing. Blue line represents Strategy 4: repeat RT-PCR based on symptoms. Pink line represents Strategy 5: universal repeat RT-PCR testing. RT-PCR, reverse transcription polymerase chain reaction.
Increasing the probability of COVID-like symptoms in those without infection reduced the number of true negatives for Strategy 1 and increased the number of RT-PCR tests needed for Strategies 2 and 4 (Figure A6).
With regard to TTP thresholds, as the prevalence of infection increased, the TTP thresholds for moving from one strategy to the next, more test intensive strategy decreased (Figure 1). For example, at probabilities of disease of .05 and .10, it is harder to justify substituting one of the less effective strategies for the universal, repeat RT-PCR testing strategy. When the composite test sensitivity was assumed to be 70%, the range for which Strategy 4 was preferred grew in magnitude, with a lower threshold for moving from Strategy 1 or 2 to Strategy 4 and a higher threshold for moving from Strategy 4 to 5 (Figure A7). At this higher sensitivity of RT-PCR, the single RT-PCR strategy was still never preferred.
Discussion
To the best of our knowledge, this is the first study that used a formal decision tree analysis to evaluate the true positives and negatives and the number of RT-PCR tests needed for a comprehensive range of COVID-19 testing strategies for repopulating a university campus. No strategy detected all infected students. The strategy that relied solely on symptoms without RT-PCR testing identified more cases (40.56%) and used fewer tests (0) than the strategy that relied on using symptoms to identify students for RT-PCR testing (29.09%). However, it also resulted in 9.53% false positive students identified. Strategy 5, which retested all individuals with a negative first RT-PCR test, identified the most true positives (and was equal to the other RT-PCR strategies with regard to 100% true negatives), but it required the greatest number of tests and still failed to detect 13.1% of cases. CDC guidelines, which align with Strategy 2 included in this analysis, would lead to an estimated 71% of infections missed. Varying the RT-PCR sensitivity and specificity and the proportion of symptomatic cases did not substantially impact the comparisons among strategies.
Based on the analysis of TTP, there is no value of willingness to trade off RT-PCR tests for true positive students detected for which Strategy 3 (universal, single RT-PCR testing) was preferred. There is at least one (lowest) TTP range where either Strategy 1or 2 was preferred, and there may be ranges where each strategy is preferred. Determining which of these two strategies is preferred requires consideration of trade-offs between all three outcomes, true positives, true negatives, and RT-PCR tests needed. Strategies 4 and 5 each have TTP ranges in which they are preferred.
To the extent that Strategies 1, 2, or both are preferred, it is for low values of TTP. For Strategy 5 to be preferred, the acceptable TTP must fall into the highest range. At low probabilities of COVID-19, this range begins at approximately 1,400 TTP, whereas at a 5% probability, it begins at approximately 121 TTP. Changing the weighted sensitivity of RT-PCR increased the threshold for which Strategy 5 is favored over Strategy 4 but did not have a major impact on the TTP ranges.
The number of RT-PCR tests needed per identified case is a useful metric to rank order strategies and to aid institutions in their decision-making on testing strategies for returning students. Choosing between screening strategies depends on a willingness to “pay,” which relates to ability and resources for testing to detect true positive students. For example, if RT-PCR testing costs $100 per test on average (personal communication, Michael Feldman, MD) and the baseline prevalence of infection is 1%, an institution with 20,000 students that adopts the universal, repeat RT-PCR testing strategy with its TTP of 624.7 (Supplementary Data, last page) would have to be willing to pay at least $62,470 ($100 × 624.7) for each case identified. Its total cost would be $3,989,200 ($100 × 39,892 tests—data not shown), and the strategy would miss about 26 of the 200 cases of infection (200 infected × .131 missed).
In contrast, if the institution adopts repeat RT-PCR testing based on symptoms, with its potential TTP of 343.2 (Supplementary Data, last page), it would have to be willing to pay at least $34,320 for each case identified at a total cost of $2,194,800 ($100 × 21,947.9 tests—data not shown). This is $1,794,400 less than the universal repeat testing strategy and would miss approximately 55 (200 × .275) infected students. If the prevalence is higher than 1%, then institutions should favor more repeat RT-PCR testing.
This decision analysis had several limitations. Data on the prevalence of disease in the target population age range were not available, so estimates were based on the general population in the U.S. In addition, the dichotomization of the incubation period (4 days) and post-incubation period (10 days) was calculated from estimates for the median incubation period and average duration of disease, which could alter the sensitivity of RT-PCR testing. We addressed these potential limitations in two ways. First, the parameter estimates were calculated based on the best data available at the time. Second, we performed extensive sensitivity analyses to estimate how varying the parameters affected our estimates. Although some estimates for RT-PCR sensitivity are higher than our baseline estimate, they are typically from studies of symptomatic/post-incubation cases, thus overestimating the sensitivity of the test when it is applied to the general population [23]. Nonetheless, using estimates of test sensitivity substantially higher than our baseline had minimal effects on the results, particularly the TTP analyses. Third, we focused on maximizing the identification of infected students on arrival to college campuses. Analyses of the effects of testing strategies throughout the semester are beyond the scope of this study. However, the number of initially undetected cases can influence the subsequent dynamics of transmission.
The underlying probability of disease among returning students may alter administrators' use of these findings. If the probability is low, they may want to adopt Strategy 1 or 2. If it is high, they may want to implement Strategy 4 or 5. Of course, knowledge of the actual prevalence of disease in returning students to any particular institution is unlikely to be known with accuracy. One solution may involve the adoption of a phased approach. First, determine if sufficient resources are available to perform RT-PCR testing on all students on return to campus. If they are not available, then, depending on one's weighting of true positives and negatives as well as testing “costs,” adopt either symptom-based only screening or symptom-based testing. Even for a symptom-based only screening, students with symptoms may end up receiving diagnostic RT-PCR tests. However, institutions should be aware that while reducing false positives, these extra tests will lead to more false negatives and therefore reduce the number of actual infections identified. Second, if RT-PCR testing is to be performed on all students, test them and calculate a more accurate prevalence of disease. Third, based on the revised estimate and available resources, decide whether to withhold repeat testing, retest those with symptoms and a negative first test, or retest all with a negative first test. While withholding repeat testing is weakly dominated by strategies that perform repeat testing, at a very low disease prevalence, rejecting a more intensive testing strategy may be necessary because of unavailable resources for retesting.
In summary, strategies that include RT-PCR testing will identify more COVID-19 cases than symptom-based screening, but all approaches will fail to detect a proportion of infected students. A two-test strategy is superior in identifying infections but will cost more. If RT-PCR testing is to be performed, a staged approach involving initial testing of all returning students followed by a retesting decision based on the revised prevalence of infection might be considered in the setting of limited testing capacity.
Acknowledgments
The authors thank Dr. P.J. Brennan, Dr. Michael Levy, Dr. Jennifer Pinto-Martin, and Dr. Michael Weisberg for their insights on repopulating a college campus.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jadohealth.2020.09.038.
Funding Sources
None.
Supplementary Data
References
- 1.DePietro A. Here’s a look at the impact of coronavirus (COVID-19) on colleges and universities in the U.S. https://www.forbes.com/sites/andrewdepietro/2020/04/30/impact-coronavirus-covid-19-colleges-universities/#120839661a68 Available at:
- 2.Baker M., Hartocollis A., Weise K. First U.S. colleges close classrooms as virus spreads. More could follow. https://www.nytimes.com/2020/03/06/us/coronavirus-college-campus-closings.html Available at:
- 3.COVID-19 – moving classes online, other updates. https://www.harvard.edu/covid-19-moving-classes-online-other-updates News release. Harvard University; March 10, 2020. Available at:
- 4.Update from Penn President Amy Gutmann and provost Wendell Pritchett on campus planning regarding the COVID-19 pandemic. https://coronavirus.upenn.edu/node/89 News release. University of Pennsylvania; March 12, 2020. Available at:
- 5.Svrluga S., Anderson N. Amherst College switches to online learning, as universities nationally scramble to respond to covid-19 outbreak. https://www.washingtonpost.com/education/2020/03/09/princeton-requires-lectures-seminars-go-online-only-temporary-move-amid-covid-19-outbreak/ Available at:
- 6.COVID-19: Higher education resource center. Entangled Solutions. https://www.entangled.solutions/coronavirus-he/ Available at:
- 7.Centers for Disease Control and Prevention Interim considerations for institutions of higher education administrators for SARS-CoV-2 testing. https://www.cdc.gov/coronavirus/2019-ncov/community/colleges-universities/ihe-testing.html Centers for Disease Control and Prevention; 2020. Available at:
- 8.Fall 2020 re-opening: Operational plan. http://www.famu.edu/coronavirus/Reopening%20Plan%20Version%205.27%20final(1)%20(1).pdf News release. Florida Agricultural and Mechanical University; May 28, 2020. Available at:
- 9.UF reopening plan. https://coronavirus.ufl.edu/media/coronavirusufledu/Reopening-Plan.pdf News release. University of Florida; June 12, 2020. Available at:
- 10.Subcommittee on Higher Education Report of the higher education subcommittee reopen Connecticut. https://portal.ct.gov/-/media/Office-of-the-Governor/News/20200506-Recommendations-to-Governor-Lamont-for-a-phased-reopening-of-colleges-and-universities.pdf The office of governor Ned Lamont; 2020. Available at:
- 11.Marcotte L.M., Liao J.M. Incorporating test characteristics into SARS-CoV-2 testing policy-sense and sensitivity. JAMA Health Forum. 2020;1:e200448. doi: 10.1001/jamahealthforum.2020.0448. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi M., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the Achilles’ heel of current strategies to control COVID-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z., Song C., Xu C. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw Open. 2020;3:e2010182. doi: 10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies N.G., Klepac P., Liu Y. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 16.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu L., Wang B., Yuan T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/#countries Worldometer. Available at:
- 20.United States Census Bureau U.S. and world population clock. https://www.census.gov/popclock/ Available at:
- 21.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichol K.L., D’Heilly S., Ehlinger E. Colds and influenza-like illnesses in university students: Impact on health, academic and work performance, and health care use. Clin Infect Dis. 2005;40:1263–1270. doi: 10.1086/429237. [DOI] [PubMed] [Google Scholar]
- 23.Kucirka L.M., Lauer S.A., Laeyendecker O. Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020;173:262–267. doi: 10.7326/M20-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glick H.A., Doshi J.A., Sonnad S.S. 2rd ed. Oxford University Press; Oxford: 2014. Economic evaluation in clinical trials. [Google Scholar]
- 25.Centers for Disease Control and Prevention COVID-19 pandemic planning scenarios. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios-h.pdf Centers for Disease Control and Prevention; 2020. Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.