Abstract
Background
Depression appears to be a common complication in patients during and post–COVID-19 infection. Understanding the mechanism of action of cytokines such as interleukin-6, interleukin-10 and others in depression and in cytokine storm syndrome, the core component of COVID- 19, could shine a new light on future treatment options for both disorders.
Objective
This review demonstrates the role of interleukins in COVID-19 pathogenesis and their role in depression.
Results
We described cases we have treated as an example for the dual role interleukins have in COVID-19 infection and depression and reviewed approximately 70 articles focusing on the role of interleukins in cytokine storm syndrome and depression.
Conclusion
This review highlights the key features of cytokines in both diseases. As the scientific community has more time to recover and process the effect of the current pandemic, we believe that additional research will pave the way to diverse pathways to treat depression in these patient and others.
Keywords: Inflammatory cytokines, Interleukin-6, Interleukin-10, Depression, SARS-CoV-2
Highlights
-
•
This review highlights the key features of cytokines in both diseases.
-
•
We believe that additional research will pave the way to diverse pathways to treat depression in COVID-19 patients and others.
1. Introduction
The worldwide impact of the COVID-19 pandemic is significant and growing with the escalation of global cases, leading to increased mortality and morbidity of patients who survive (Guo et al., 2020). As of October 25, 2020, there have been 42,522,402 total cases worldwide with 1,148,490 deaths (Covid-19 dashboard by the, 2020). The impact of the pandemic is not only limited to case numbers and mortality rates, but also deeply extends into the mental health of the global population. These cases are further compounded by the psychological pressure stemming from the ever-growing economic concerns (Guo et al., 2020). Studies of patients who were diagnosed with acute respiratory syndrome (SARS) have shown that patients who survived and were discharged from the hospital displayed varying degrees of mental health issues (Guo et al., 2020). When we examined survivors of previous infections, it became apparent that they were prone to depression (Kuhlman et al., 2018), anxiety (Wheaton et al., 2012), adjustment disorder (van Hoek et al., 2011) and post-traumatic stress disorder (Flu, 2013). This literature review will analyze the psychiatric impact of COVID-19 infection while referencing the naïve patient population experiencing new onset depressive symptoms, the population with active COVID-19 infection, and the population recovering from COVID-19 infection. Contemporary literature suggests that new onset depression may be caused by inflammation initiated during the active phase of the COVID-19 infection which results in a cytokine surge (Liu et al., 2020a). Over the past 2 decades, an increasing amount of literature supports the hypothesis that inflammation contributes to new onset depression (Lee et al., 2018). In addition, this hypothesis suggests that the immune system and its response plays a significant role in the pathophysiology of mood disorders.
1.1. Case I
42-year-old male with history of asthma, type II diabetes mellitus, ischemic cardiomyopathy requiring coronary angioplasty with stent placement, hypertension, obesity and no previous psychiatric history, presented to the Emergency Department (ED) with 1-week history of worsening cough, wheezing, chest pain and fever (103 F). The patient reported a history of travel to Disney World, Florida 1 month prior to presentation. Upon his return, he developed wheezing and cough. He was seen by his primary care physician and was prescribed prednisone taper and albuterol inhaler for asthma exacerbation. The patient reported worsening shortness of breath despite treatment. On presentation, he was tachypneic with respiratory rate 40–52 breaths per minute, tachycardic with pulse of 98 beats per minute and blood pressure 108/58 mmHg, pulse oximetry showed oxygen saturation of 80%. Physical exam was positive for wheezing, shortness of breath and tenderness of left lower chest. Chest radiograph showed bilateral infiltrates. Laboratory results showed the following: white cell count: 4.9 k/ul, hemoglobin 12.1 g/dL, hematocrit 37.7% and platelets 166 k/uL, absolute lymphocytes 0.1 K/μL, segmented neutrophils 92.2%, band 4.3%, D-Dimer 665 ng/dL, ferritin 2,037 ng/ml, C-reactive protein (CRP) 0.28 mg/dl, interleukin -6 was 25 pg/mL and procalcitonin 0.11 ng/mL. Comprehensive chemistry was within normal limits except glucose 163 mg/dL, calcium 8.2 mg/dL, aspartate aminotransferase 147 units/L and alanine aminotransferase 70 unit/L. Renal function was normal. QTc was 429 and he tested positive for COVID-19.
The patient received azithromycin, steroids, ceftriaxone, magnesium, and terbutaline. The patient began to deteriorate after admission and met criteria for intensive care unit (ICU) admission and intubation due to hypoxic respiratory failure and hypotension. While intubated in the intensive care unit, the patient was treated with fentanyl, midazolam and empiric antibiotic (piperacillin, tazobactam vancomycin). The patient was also treated with hydroxychloroquine 200 mg every 12 h, azithromycin, tocilizumab, zinc sulfate 220 twice daily to target COVID-19 infection.
The patient’s hospital course was complicated by sepsis, seizure like activity with brain dysfunction on EEG consistent with metabolic encephalopathy (delirium) in the context of a negative head CT. The patient began to improve and was extubated on admission day 18. Psychiatry was consulted on admission day 33 to evaluate the patient for depression following suicidal statements. The patient admitted to depression with frequent crying, getting easily frustrated, and demands to leave the hospital against medical advice. He endorsed hopelessness, helplessness, and isolation with a preoccupation on dying. His sleep was poor with difficulty falling asleep and maintaining his sleep. He endorsed poor appetite, anxiety, and panic attacks. Furthermore, the patient’s wife reported that the patient was confused and unable to retain new information. Hospital Anxiety and Depression Scale (HADS) was administered to the patient and he scored 19 on depression (severe) and 16 (severe) on anxiety. For reference, an asymptomatic value on the scale is less than 7 and severe range is 15–21.
The patient was diagnosed with depression disorder according to the Diagnostic and Statistical Manual of Mental Disorder, 5th Edition (DSM-5). In accordance to psychiatry’s recommendation, the patient began treatment with escitalopram 10 mg daily, later titrated to 20 mg. His insomnia was treated with melatonin with poor response. The patient was temporarily started on mirtazapine 15 mg for anxiety and ongoing insomnia which was discontinued once the patient’s insomnia and anxiety subsided. The patient then remained on escitalopram 20 mg, moving forward. The patient’s inflammatory cytokines (IL-6) level, which were elevated during the first stages of the disease, normalized over time along with the D-dimmer and lymphopenia.
The patient’s depressive symptoms subsided approximately 6 weeks following the initiation of treatment with antidepressants and normalization of the inflammatory cytokines. The medications were discontinued while the patient attended rehabilitation.
1.2. Case II
75-year-old female with past medical history of hypertension, coronary artery disease, type II diabetes mellitus and no psychiatric history presented to the ED with generalized weakness, poor oral intake, dry cough, shortness of breath and exertional dyspnea, in the context of poor glycemic control. On evaluation she had a temperature of 101.5 F, pulse 104 beats per minute, respiratory rate 30 breaths per minute, blood pressure 140/80 mmHg. Pulse oximetry showed oxygen saturation of 87%. Chest X ray showed hazy opacities and peribronchial thickening in the left lower lung field, highly suspicious for pneumonia. Her blood workup showed white cell count 8.1 k/uL, hemoglobin 14 g/dL, hematocrit 42.0%, platelets 250 10 k/uL, neutrophils absolute percent 79.0%, neutrophils absolute 6.4 k/uL, lymphocytes percent 13.5%, lymphocytes absolute 1.1 k/uL. Comprehensive metabolic panel was significant for sodium 132 mmol/L, potassium 5.3 mmol/L, glucoses 367 mg/dL, Urea Nitrogen (BUN) 21 mg/dL, creatinine 1.03 mg/dL, and anion gap 20.3. Beta-hydroxybutyrate was 2.1. D-Dimer 1,272 ng/dL, ferritine 1200.6 ng/m, C-reactive protein 20.45 mg/dl, procalcitonin 1.30 ng/mL and interleukin-6 was 26.7 pg/mL. Viral panel was negative for influenza A and B. Her viral PCR for SARS-CoV-2 returned positive. She was placed on non-rebreather (NRB) mask and started on Zithromax, hydroxychloroquine and solumedrol.
She began to deteriorate and developed hypoxic respiratory failure requiring Intensive Care Unit (ICU) care. She was placed on 15L optiflow and an NRB mask for ongoing desaturation.
Psychiatry was consulted on admission day 30 to assist with assessment of the patient’s mood. The hospital nursing staff observed an increase in anxiety, low mood, withdrawn appearance, and refusing to accept calls from her family. She reportedly made passive suicidal statements such as “I don’t want to live anymore.” In addition, the patient reported sadness, fatigue, and poor energy and appetite. Patient’s granddaughter reported to the nutritionist that patient had made comments regarding decreased appetite, which she believed was due to her being depressed and isolated from family. Hospital Anxiety and Depression Scale (HADS) was administered to the patient and she scored 14 on depression (abnormal/moderate) and 13 (abnormal/mild) on anxiety. As stated previously, asymptomatic value on the scale is less than 7 and severe range is 15–21.
The patient was diagnosed with depression disorder and anxiety disorder according to the Diagnostic and Statistical Manual of Mental Disorder, 5th Edition (DSM-5). The patient did not exhibit symptoms of delirium including disturbance of attention and awareness, sleep-wake cycle disturbance, hallucinations or disturbance of cognition. Delirium was ruled out by Confusion Assessment Method for the ICU (CAM-ICU). The Montreal cognitive assessment (MOCA) was administered and showed mild cognitive impairment (MCI) with score of 22.
Under psychiatry’s recommendation, the patient began treatment with mirtazapine 7.5 mg nightly and sertraline 25 mg daily to address her symptoms. The patient’s inflammatory cytokines (IL-6) level, which were elevated during the first stages of the disease normalized over time in addition to the D-dimmer and lymphopenia. The patient’s depression improved as the cytokines level normalized. Over the following 10 days, the patient’s sleep had improved along with cognitive engagement. She showed an interest in watching TV and communicating with her family. She endorsed feeling less depressed as the sertraline dose was increased to 50 mg daily.
Discussions regarding discharge home halted when the patient’s conditioned worsened. Her oxygen requirement began to deteriorate, and she became septic due to a pressure wound resulting in mechanical ventilation. She was placed on IV steroids and a feeding tube was inserted. She developed an upper gastrointestinal bleeding which was subsequently stabilized. She received covalence plasma and continued with antidepressant treatment by gastric tube. During the next few days, the patient became hemodynamically unstable and she was found pulseless.
1.3. Literature review
To further the investigation of the underlying mechanism contributing to the outcomes of the respective cases described above, the review begins with an exploration of the Coronaviradae family which is widely distributed in humans and other mammals (Huang et al., 2020).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also called coronavirus 2 disease (COVID) is caused by a novel beta–coronavirus: an enveloped, non-segmented, positive sense RNA virus characterized by club-like spikes that project from the surface. The majority of the human coronavirus infections are mild and cause benign upper respiratory infections. However, the severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), two beta coronaviruses, caused pandemics with 37% mortality rate (Huang et al., 2020; Cheng et al., 2004).
The COVID-19 strain of the virus emerged in Wuhan, China in December 2019 and is the third known lethal human coronavirus discovered, joining SARS and MERS (Liu et al., 2020a). The endemic infection spread rapidly without sparing a continent and became a global pandemic (Bi et al., 2020). The mortality rate of critically sick patients is high, approximately 49–61.5% or higher, 79% (Liu et al., 2020a; Horowitz et al., 2020).
The SARS–CoV and SARS- CoV-2 use the angiotensin-converting enzyme-related carboxypepetide (ACE2) receptor to gain entry into cells. This receptor was found to be expressed in cardiopulmonary tissues, monocytes, and macrophages (Moore and June 2020). Lymphopenia, a low lymphocyte count in the peripheral blood, is an important feature in COVID-19 infection and is correlated closely with disease severity (Moore and June 2020). It is recognized that SARS-CoV infects primarily human monocytes and dendritic cells leading to dendritic cell dysfunction resulting in T-cell apoptosis and exhaustion which contribute to the immunopathology (Moore and June 2020). The infection of monocytes, macrophages, and dendrites result in a cascade leading to secretion of IL-6 and other cytokines.
COVID-19-infected patients often present with fever, cough, fatigue, shortness of breath, pneumonia diagnosed by imaging, and other respiratory tract symptoms (Bi et al., 2020). However, as time progressed, it became apparent that other systems are involved including the CNS, kidneys, gastrointestinal tract, heart, and immune system. It also became evident that while some patients developed mild disease, others developed progressive, fulminate disease ending in death (Liu et al., 2020b). Patients with mild cases show symptoms of fever, dry cough, fatigue, abnormal chest CT findings but with good prognosis. Patients with severe disease are likely to develop acute respiratory distress syndrome (ARDS) and multiple organ failure with death rates ranging from 4.3% to 15% (Liu et al., 2020b). ARDS and multiorgan failure are often the leading cause of death in severely ill patients (Liu et al., 2020a). Clinical information received from various hospitals indicate that 17.7%–32% of patients with COVID-19 required ICU care and that the average length of stay was 9.5–12 days. The most common causes for ICU admission were ARDS (67%), acute kidney injury (29%), acute cardiac injury (23%) and liver dysfunction (29%) (Liu et al., 2020a).
Significant clinical data is emerging regarding the manifestation of neuropsychiatric symptoms in COVID-19. Reports originating from China and the U.S show that close to 50% of patients hospitalized for severe disease exhibited cerebrovascular manifestations such as strokes, encephalitis, and muscle injuries (Troyer et al., 2020).
In a retrospective study by Ling Mao et al. reported 78 patients of 214 (36.4%) had neurological manifestations. The neurological symptoms were divided into 3 categories: 1) central nervous system (CNS) manifestations such as headaches, altered level of consciousness, acute strokes (CVA), ataxia and seizure activity. 2) peripheral nervous system symptoms (PNS) including anosmia (dysfunction of olfaction/smell), ageusia (dysfunction of taste), vision impairment, and nerve pain. 3). Skeletal muscle injury (Mao et al., 2020). Furthermore, those who presented with CNS symptoms such as headaches, dizziness, and ataxia showed low blood lymphocyte count, and patients with myalgia also exhibited an increase in C-reactive protein (CRP) (Troyer et al., 2020).
Common and early CNS-symptoms described by patients with COVID-19 were anosmia and ageusia (Troyer et al., 2020). Prior information from infection with human alpha-coronavirus (HC0V-229E) demonstrated disruption of the ciliary nasal epithelium and it is speculated that this may be the mechanism behind SARS- CoV-2. It has also become apparent that olfactory epithelial cells express the angiotensin-converting enzyme 2 (ACE2).
Encephalopathy or delirium (alteration in consciousness) is another CNS manifestation of COVID-19 described in 20% of patients (Troyer et al., 2020). In those patients “cytokine storm syndrome” (CSS) or “cytokine release syndrome” (CRS) was evident with elevated blood plasma levels of pro-inflammatory cytokines such as Interleukin-6 (IL-6), tumor necrosis factor-a (TNF-a), Interleukin-8 (IL-8), Interleukin-10 (IL-10) and Interleukin-2R (IL-2R) (Liu et al., 2020b). These patients often present to the hospital with altered mental status as their only symptom and were later found to be COVID-19 positive. The inflammatory cytokines including IL-6, IL-10, IL-1B, IL-2 and monocyte chemoattractant protein 1 (MCP-1) were significantly elevated in patients with COVID-19 (Liu et al., 2020a).
Studies have shown that 63%–70.3% of patients with severe COVID-19 exhibit lymphopenia, and it is believed that the virus affects T lymphocytes, particularly CD4 and CD8 T cells (Liu et al., 2020b). Researchers also found that lymphopenia observed in patients with severe disease course was related to a decrease in absolute T-cell count, especially CD8 T cells, and elevated levels of proinflammatory cytokines including TNF-a, IL-6, IL-8 and IFN–γ are seen in patients with significant lung disease and result in poor outcome (Liu et al., 2020b). Research suggests that patients who presented with severe COVID-19 had high concentrations of IL-6, IL-10 and IL-2 and IFN- γ pointing to cytokines associated with the disease (Liu et al., 2020b). In addition, previous studies that investigated SARS and MERS have also shown an increase in neutrophil count which suggests the possibility that the intensity of the inflammatory response in COVID-19 is similar and related to disease severity (Liu et al., 2020b).
Research indicates that Cytokine Release Syndrome (CRS) often plays a role in severe cases resulting in death (Liu et al., 2020a). We define CRS as an “uncontrolled and overwhelming release of pro-inflammatory mediators by an overly activated immune system (Liu et al., 2020b).” CRS is common in patients with COVID-19, and elevated serum IL-6 is strongly correlated with ARDS, respiratory failure and poor outcome. C-reactive protein (CRP) level is also elevated in patients with COVID-19 and its expression is stimulated by IL-6 (Moore and June 2020). Postmortem examinations of deceased patients from the disease revealed elevated inflammatory cytokines, tissue necrosis, and infiltration of intestinal macrophages and monocytes in lungs, heart, gastrointestinal mucosa (Liu et al., 2020a). Severe lymphopenia with hyperactivated proinflammatory T–cells (Liu et al., 2020a) and decrease regulatory T-cells (Liu et al., 2020b) were also observed in patients with severe disease process. These changes are an indication of dysregulation of the immune response.
Studies have shown that severe diseases due to intense cytokine release syndrome were identified even in low viral load patients suggesting “exuberant host immune response rather than viral virulence, is possibly responsible for tissue pathogenesis (Liu et al., 2020b).” The levels of fibrinogen, D-dimer, total bilirubin, aspartate transaminase, alanine transaminase, lactate dehydrogenase, creatinine kinase, C-reactive protein (CRP), ferritin, and serum amyloid A protein (SAA) in the peripheral blood of the severely ill patients were higher when compared to the values observed in patients with mild disease (Liu et al., 2020b). Changes in inflammatory cytokine level including IL-2, IL-7, IL-10, Il-6 and TNF-a, were also seen in patients with COVID-19 (Liu et al., 2020b). The changes, however, were minor in patients with mild disease and significant in patients with severe disease (Liu et al., 2020b). Those cytokines reached their peak in the serum at 3–6 days after the disease onset and IL-6 and IL-10 levels showed sustained increase with the reduction in levels by day 16 (Liu et al., 2020b). IL-6 and IL-10 showed sustained increase in the severe group compared to the mild group. Elevated serum level of IFN-γ was also seen in patients with severe disease during the first 4–6 days (Liu et al., 2020b). Patients with mild and severe COVID-19 disease did not show difference in the level of IL-2, IL-4 and TNF-a throughout the disease course (Liu et al., 2020b).
In many immunological based diseases such as graft-versus-host disease (GVHD), macrophage activation syndrome (MAS), sepsis, acute respiratory distress syndrome (ARDS), hemophagocytic lymphohistocytosis (HLH), chimeric antigen receptor (CAR) T-cell chemotherapy for leukemia, CRS is the major factor in pathogenesis (Liu et al., 2020b).
A link between the development of mood disorder and inflammation with activation of inflammatory response was seen in animal models and humans and has been a focus of interest in recent research (Benros et al., 2013). When examining the connection between the immunological based disorders and depression, accumulative evidence is seen that implicates the immune system in the pathogenesis of major depression (Kappelmann et al., 2020). High levels of CRP are found in about 25% of patients with depression disorder (Kappelmann et al., 2020). In addition to high CRP, the proinflammatory IL-6, its upstream stimulator, has been associated with depressive symptoms such as low mood, increase in appetite, sleep irregularity, low energy, and inability to focus and concentrate (Kappelmann et al., 2020). An association was also found between an increased level of inflammatory markers, especially IL-6 and suicidality and it is evident that patients who suffer from chronic inflammatory illness, such as Inflammatory Bowel Disease, tends to exhibit an increased in suicide rate (Kappelmann et al., 2020). This association between IL-6 signaling, depression and suicidality may have clinical importance in managing depression and suicidality. Suicidality can be an additional sign to a high level of CRP seen in inflammatory activity which can be used for immunotherapy for depression and suicide behavior. It can also be helpful for the evaluation of effectiveness of immunotherapy for depression and suicide ideation and behavior (Kappelmann et al., 2020). Raison showed in his study that infliximab, a tumor necrosis factor alpha, was effective in patients with depression and suicidality who had high level of CRP prior to treatment (Kappelmann et al., 2020; Raison et al., 2013).
An immunological illness such as graft versus host disease (GVHD) is a main complication of allogenic haemopoietic–cell transplantation (HCT), a potential curative treatment of different hematological malignancies. During allogeneic bone marrow transplantation, lymphocytes are introduced and are engrafted and proliferated within the immunocompromised host (Henden and Hill, 2015). The donor T-cells recognize the recipient cells as foreign, which results in the destruction of leukemic or other malignant cells via an activation of pathways of “adaptive immune response.” This mechanism is called “Graft versus host leukemia” (GVL). Since the effect is not specific to malignant cells, damage and destruction of healthy recipient cells occur simultaneously giving rise to GVHD (Henden and Hill, 2015). GVHD can affect multiple organs including the skin, liver, gastrointestinal tract, and lungs and is defined as multisystem inflammatory disease characterized by tissue fibrosis and mucosal lichenoid plaques (MacDonald et al., 2017). Total body irradiation or busulphan-based chemotherapy is used in myeloblastive stem cell transplantation to reduce the number of malignant cells and to ablate residual immune response to permit engraftment of the donor cells. The use of those agents also cause damage to the GI mucosa leading to “Cytokine storm” which is characterized by the release of proinflammatory cytokines, specifically TNF, IL-1 and IL-6 (Henden and Hill, 2015).
Studies have shown that in addition to the previous mechanism, microbial products such as lipopolysaccharides (LPS), which leak through the damaged GI tract mucosa or skin, can stimulate secretion of pro-inflammatory cytokines through “Toll –like receptors.” The intestinal mucosa is especially vulnerable to injury from TNF-a and it has an important role in amplification and propagation of the cytokines storm typical of GVHS (Ferrara et al., 2009). In addition, LPS “danger-associated molecular patterns” originated from GI microbiota and have an important role in GVHS (Henden and Hill, 2015).
Recent observational studies have identified that chronic GVHD is a risk factor for depression following an HCT due to the release of pro-inflammatory cytokines, which is seen in other medical conditions accompanied by inflammation such as cancer, autoimmune disease and post viral infections (Tsamakis et al., 2020). In GVHD, pro-inflammatory cytokines are able to activate central nervous system circuits leading to neurobehavioral and affective responses such as depressive mood, fatigue, sleep disturbance, anorexia and decrease in cognition, seen in other chronic inflammatory disorders. It is believed that anti-depressant medications have a role in suppressing immune mediated disorders in addition to providing treatment for depression (Tsamakis et al., 2020). Furthermore, research also suggests that anti-inflammatory drugs can improve depressive symptoms in patients with chronic inflammatory diseases independent of improvement in the physical illness (Kappelmann et al., 2020).
High prevalence of depression is often seen in individuals with immunological diseases such as autoimmune diseases (rheumatoid arthritis and other connective tissue diseases), inflammatory bowel disease, and infections such as COVID-19. The role of inflammation involving C-reactive protein (CRP) and inflammatory cytokines in depression were shown when the improvement in inflammation was evident following treatment with anti-depressants.
In a prospective cohort study consisting of 78 million individuals, Benros at el, demonstrated that infection and autoimmune diseases increase the risk of developing mood disorders. Infection is the most common risk factor occurring in 32% of patients without a history of depression, while autoimmune disease was represented in only 5% of patients (Benros et al., 2013). In addition, patients with autoimmune disease who also acquire infection, experience an increased risk of developing depression; more than 4 times the general population (Benros et al., 2013). When a patient had both infection and autoimmune disease, the risk of mood disorder was compounded (Benros et al., 2013). It was observed that severe infections are more likely to affect the brain than less severe infections, due to more intense inflammatory response. It was also observed in this study that patients hospitalized for influenza and coronavirus showed mood disorders, especially depression (Benros et al., 2013; Okusaga et al., 2011). “Sickness behavior,” a drastic change in experience and behavior occurring in physically ill patients, which includes a decrease in appetite, apathy, fatigue, impairment in concentration, sleep disturbance and lack of interest in social interaction, can be induced by systemic inflammation. It is a normal response to infection and is characterized by endocrine, autonomic and behavioral changes and is induced by pro-inflammatory cytokines (Dantzer et al., 2008). The clinical presentation of “sickness behavior” is similar to that seen in depression and in vulnerable individuals with prolonged systemic disease (Benros et al., 2013). However, in addition to “sickness behavior,” pro-inflammatory cytokines can induce true depressive illness in the physically ill patients with no previous history of mental illness (Dantzer et al., 2008).
Inflammation occurring in autoimmune disease and infections are likely to alter the brain due to a compromise of the blood-brain barrier, leading to increase in permeability leaving the brain susceptible to infectious agents and immune components such as cytokines (Benros et al., 2013).
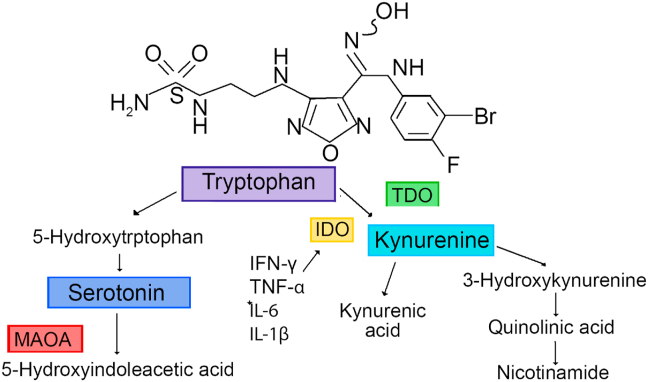
It is apparent that the pro-inflammatory cytokines affect depression through the tryptophan-Kynurenine-pathway which regulates serotonin production and N-methyl-aspartate glutamate (NMDA) receptor activity (Benros et al., 2013; Dantzer et al., 2008). Tryptophan, as an essential ammino acid, is actively transported into the brain for the synthesis of serotonin. The level of plasma tryptophan decreases in patients undergoing immunotherapy by the activation of tryptophan 2,3 dioxygenase (TDO) leading to its oxygenation with the production of kynurenine and Nicotinamide and by indoleamine 2,3 dioxygenase (IDO) leading to the production of pro-inflammatory cytokines. Kynurenine is transported into the brain where it is metabolized in the perivascular macrophages, microglia and astrocytes to produce 3 hydroxykynurenine (3-HK) and quinolinic acid (QA). QA is an NMDA receptor agonist (Fig. 1).
Fig. 1.
This figure shows the metabolism of tryptophan and its relation to pro-inflammatory cytokines.
Increased stimulation of the immune system can enhance the activity of the hypothalamic pituitary adrenal axis leading to depression. The major hormonal output is glucocorticoid such as cortisol, ultimately regulating the immune system (Benros et al., 2013).
Depression was viewed as an “inflammatory state,” as patients with depression were found to have high levels of CRP and inflammatory cytokines. Those can normalize after treatment with anti-depressants (Tsamakis et al., 2020).
Peripheral blood samples of patients with depression showed an activation of the inflammatory pathways, which was manifested by an increase in pro-inflammatory cytokines, acute-phase proteins, expression of chemokine and adhesion molecules (Raison et al., 2006). The levels of IL-6, IL-8, IL- β, tumor necrosis factor (TNF)-a and CRP were observed to be elevated in the peripheral blood and the central nervous system in patients with depression (Raison et al., 2006). Acute–phase proteins such as a-1–acid glycoprotein, a-1–antichymotrypsin and haptoglobin were also present (Raison et al., 2006).
Patients with depression disorder show an increase in innate immune cytokines and soluble receptors in both their peripheral blood and cerebrospinal fluid (CSF). Evidence suggests an increase in chemokines and adhesion molecules in the peripheral blood as well (Miller, 2009).
One of the first cytokines that was studied by multiple researchers to understand the connection between cytokines and depression was interferon (IFN)-a. It served as a model for cytokine-induced depression (Raison et al., 2006). Approximately, 30–50% of patients treated with interferon IFN-a met criteria of depression (Miller, 2009). When the cytokine IFN-a was used for the treatment of infectious disease such as hepatitis–C and cancers, patients developed a behavioral syndrome that appeared similar to depression and responded to treatment with antidepressant agents (Raison et al., 2006). Symptoms associated with depression disorder include anhedonia, feelings of guilt, suicidal thoughts, anxiety, as well as cognitive symptoms such as loss of concentration, disturbance of memory, difficulty in word finding, confusion, and indecisiveness (Miller, 2009). Additional depressive neurovegetative symptoms include fatigue, loss of energy, abnormal sleep and appetite, and psychomotor retardation (Miller et al., 2008). Some patients may exhibit somatic complaints (Miller et al., 2008). Those symptoms were seen in medically healthy people when the inflammatory system was activated and in patients with cardiovascular disease, cancer, rheumatologic disease, gastrointestinal disease, and post viral infection (Raison et al., 2006). IFN-a is a potent inducer of pro-inflammatory cytokines, primarily IL-6 but also IL-1β and TNF-a. It also became apparent that IFN-a is associated with alterations in serotonin metabolism and it has an effect on CRH function by leading to an increase in adrenocorticotropic hormone ACTH and cortisol (Raison et al., 2006).
Depression is a common comorbidity in patients with Inflammatory Bowel Diseases (IBD) which significantly affects their quality of life. Depression in these patients is not completely explained by psychosocial stressors alone.
C. D Moulton et al. argued that depressive symptoms are extra-intestinal manifestations of inflammation in patients with IBD and that inflammation is not only the cause of depressive symptoms but also the cause of somatic symptoms associated with depression such as fatigue and sleep disturbance (Moulton et al., 2019). Inflammatory cytokines such as IL-6, and acute base proteins such as CRP, are associated with an increase in depression over time (Moulton et al., 2019). Symptoms consistent with depression are also associated with T cell activation, which is manifested by an increase in soluble IL-2 receptors (sIL-2Rs), sCD8 concentration, as well as an increased activation of effector T–cell response including increase in IL-2, interferon (IFN)–gamma, and IL-17 (Moulton et al., 2019). Patients with IBD often experience somatic symptoms of depression more frequently than depressive symptoms. Fatigue is reported by 44–86% of patients with active disease and 22–41% of patients in remission (Moulton et al., 2019). About 77% of patients with active IBD and 49% of patients with inactive disease reported poor sleep.
In a cross–sectional study of 96 individuals with Crohn’s Disease (CD) and healthy controls, an increase in CRP measured by standard sensitivity and increase in erythrocyte sedimentation rate (ESR) were strongly correlated with poor sleep and fatigue.
The use of anti-tumor necrosis factor (TNF) agents changed the outcome of patients with IBD with significant decrease in the need for surgery and complications.
Anti-TNF agents can neutralize soluble TNF but also exert their effect through binding membrane–bound TNF (Moulton et al., 2019) leading to apoptosis in the proinflammatory T lymphocytes, reducing the levels of T-cell–derived proinflammatory cytokines resulting in a decrease in active inflammatory cells in the intestinal mucosa (Moulton et al., 2019).
In a retrospective study of 69 patients with IBD, a treatment with an anti-TNF showed significant improvement in Patient Health Questionnaire-9 (PHQ-9) depression scores independently of IBD severity symptoms and it was correlated with change in CRP (Moulton et al., 2019). It was also observed that the use of Vedolizumab improved depression and sleep in patients with IBD (Moulton et al., 2019).
Rheumatoid arthritis (RA) is another example where we see a relationship between cytokines and depression. Margarida Figueiredo–Braga et al. examined the relationship between cytokines and depression in RA. A cohort of 209 patients was recruited for this study. During the study, laboratory workup including CBC, CRP level and cytokines including IL-6, Il-10 and TNF-a, were measured using ELISA along with psychological interviews, and clinical evaluations. Hospital Anxiety and Depression Scale (HADS) for depression in physically ill patient, and Pittsburgh Sleep Quality Index (PSQI) for measurement of sleep and fatigue were utilized (Figueiredo-Braga et al., 2018). It became apparent that IL-6 and TNF-a were positively correlated with depressive symptoms. Higher levels of IL-10 were correlated with more depressive symptoms. IL- 10 was associated with depression in patients with RA and SLE, but higher levels were measured in RA versus lower level in SLE. It was also found that treatment with IL-6 inhibitor, such as tocilizumab could decrease depressive symptoms and disease activity in RA patients (Figueiredo-Braga et al., 2018).
Carolina David Wiener et al. studied the relationship between depression disorder and cytokines in a cross-sectional study involving 1034 young adults aged 18–35. In this study the psychiatric evaluation was performed using Mini International Neuropsychiatric Interview (MINI), which is a short-structured interview lasting 15–30 min in duration. The serum cytokines were analyzed with a sandwich- ELISA immunoassay. The study found that serum IL-6 and IL-10 levels were positively correlated to depression disorder but not in healthy control subjects. Further evaluation showed that the increase in IL-6 during depression lead to an induction of IL-10 release (Wiener et al., 2019). It was also hypothesized that an increase in cytokine level may be related to an increase HPA axis activity as manifested by an increase in cortisol, which normally has an anti-inflammatory effect. However, dysregulated HPA axis seen in depression will result in poor anti-inflammatory effects resulting in an increase in pro-inflammatory cytokines.
It has been known for some time that cytokines, a heterogeneous group of polypeptides, play a key role in the immune activation during states of depression and stress (Raison et al., 2006). The cytokines produced and released in the periphery are carried by transport systems to the brain. In the brain, glial cells can produce cytokines, which in turn can affect neurotransmitters, especially monoamines that play important role in depression. Research has shown that the plasma concentration of IL-6 increases 1 h after restraint stress and following the initial peak, a gradual and attenuated decrease was seen. Other studies show that the elevation in IL-6 level during stress was independent of the intestinal microflora (Nukina et al., 2001).
Research has investigated the relationship between various types of depression and IL-6. It became evident that patients with melancholic depression characterized by psychomotor retardation, anxiety, loss of appetite and change in sleep pattern, show higher activity of IL-6 compared to healthy control individuals. Patients with melancholic depression also demonstrate higher IL-6 levels that did not normalize despite clinical response to electroconvulsive therapy (ECT). Patients with atypical depression with hypersomnia, increase in appetite and weight gain also demonstrated elevated levels of IL-6. These findings are also found in postpartum depression and dysthymic disorders (Rush et al., 2016).
Emily Yi-Chith et al. investigated the relationship between elevated IL-6 levels in patients with depression and their brain morphology. It was apparent that there is an inverse correlation between the serum IL-6 level and prefrontal cortex thickness. Sleep disturbance and impairment in sustained attention was positively related to an increase in IL-6 level (Ting et al., 2020). Patients with cancers such as lung, ovarian, colorectal, pancreatic, and others show higher levels of IL-6 compared to healthy controls. It was found that improvement in depressive symptoms in these patients was correlated with normalization of IL-6 levels. Symptoms such as anorexia, insomnia, and fatigue were significantly associated with elevated IL-6 levels (Ting et al., 2020).
The pathogenesis of these phenomena is complicated and multiple mechanisms are involved. It is believed that when encountering a foreign antigen, whether it is a virus, bacteria, or cancer cell, monocyte immune cells secrete cytokines including IL-6. Following the antigen presentation, dendritic cells mature producing cytokines, which stimulate lymphocytes. Due to circulating inflammatory factors, there is an increase in the blood brain barrier permeability allowing access of the pro-inflammatory cytokines produced by macrophages and monocytes. The leaky blood brain barrier can lead to access of other microorganisms (Wiener et al., 2019). Studies have shown that depression responds to treatment with decreasing IL-6 levels. This could be achieved by using IL-6R antibodies (e.g., tocilizumab) or IL-6 antibodies (e.g., sirulumab) (Nukina et al., 2001).
2. Conclusions
In the two cases we observed in the early phase of the pandemic we found association between depression and cytokines such as IL-6, which are the core components in the cytokine storm syndrome in COVID-19. This was also found by the previous research we reviewed. This association was also seen in chronic medical conditions with inflammatory components such as graft-versus-host disease, autoimmune diseases, inflammatory bowel disease, rheumatoid arthritis, cancer and infections. We observed patients who often developed depression during or post-infection. This mechanism is separate from other mechanisms of depression during this pandemic such as social isolation, psycho-socioeconomic stressors. This suggests that using medications that lower cytokine activity can ameliorate the symptoms of depression, since the normalization of pro-inflammatory cytokines decreased depression with or without anti-depressant treatment.
As the scientific community has more time to recover and process the effects of the current pandemic, we believe that there will be ongoing research that will shine a greater light on the diverse and extensive pathways that can contribute to depression. Since we observed an association between depression and cytokines such as IL-6, there is a need for additional research to investigate the effects caused by the other interleukins involved in the cytokine storm reaction, to gain further understanding of the process. The cytokine storm is a direct part of the infectious process and its correlation with serotonin and dopamine is well understood.
As the world comes to terms with this current reality, it is important to maintain a multidisciplinary approach in treating the psychological effects of this pandemic. Treatment regimens that can focus on diminishing the effect of the virus from a cytokine perspective can be effective during the acute phase of the disease. However, they do not serve to replace the SSRIs and other standards of treatment that would be effective once the acute inflammatory process subsides and the patients return back to a non-infective state.
When we examined the cases presented, it became apparent that those patients experienced cytokine storm described above. The role of the immune system is to fight various infections, however, an excessive and dysregulated immune response seen in patients affected by COVID-19 contribute to ARDS and multiorgan failure. We observed that those patients presented with high levels of various pro-inflammatory cytokines such as lnterleukin-6, Interleukin-10 and others and chemokines (CCL2, CCL-5). These pro-inflammatory cytokines are elevated in patients with depression and we observe the connection between the two in the patients discussed. Specifically, we observed a resolution of the depression with the decline of pro-inflammatory cytokines with anti-depressant treatment.
Treatment with tocilizumab and SSRIs anti-depressants in both cases lead to a decrease in the level of cytokines and improvement in depressive symptoms.
Since delirium could have complicated the diagnosis of depression, this was ruled out in our patients through clinical evaluation of the patient and the Mini Mental Status Exam.
Declaration of competing interest
This is to document that there are no conflicts of interest regarding the article submitted for publication.
Contributor Information
Orna Alpert, Email: oalpert1@gmail.com.
Leonid Begun, Email: Leonidx.begun@hackensackmeridian.org.
Patrik Garren, Email: garrenp@sas.upenn.edu.
Ramon Solhkhah, Email: ramon.solhkhah@hackensackmeridian.org.
References
- Benros M.E., Waltoft B.L., Nordentoft M., Ostergaard S.D., Eaton W.W., Krogh J. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. 2013;70(8):812–820. doi: 10.1001/jamapsychiatry.2013.1111. [DOI] [PubMed] [Google Scholar]
- Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect. Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.K., Tsang J.S., Ku K.H., Wong C.W., Ng Y.K. Psychiatric complications in patients with severe acute respiratory syndrome (SARS) during the acute treatment phase: a series of 10 cases. Br. J. Psychiatry. 2004;184:359–360. doi: 10.1192/bjp.184.4.359. [DOI] [PubMed] [Google Scholar]
- Covid-19 Dashboard by the Center for Systems Science and engineering(CSSE) at. Johns Hopkins University; October 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara J.L., Levine J.E., Reddy P., Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo-Braga M., Cornaby C., Cortez A., Bernardes M., Terroso G., Figueiredo M. Influence of biological therapeutics, cytokines, and disease activity on depression in rheumatoid arthritis. J Immunol Res. 2018;2018:5954897. doi: 10.1155/2018/5954897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flu Noone P. Q-fever-related absence and PTSD in reservists. Occup. Med. 2013;63(4):311. [Google Scholar]
- Guo Q., Zheng Y., Shi J., Wang J., Li G., Li C. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: a mixed-method study. Brain Behav. Immun. 2020;88:17–27. doi: 10.1016/j.bbi.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henden A.S., Hill G.R. Cytokines in graft-versus-host disease. J. Immunol. 2015;194(10):4604–4612. doi: 10.4049/jimmunol.1500117. [DOI] [PubMed] [Google Scholar]
- Horowitz R.I., Freeman P.R., Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir Med Case Rep. 2020;30:101063. doi: 10.1016/j.rmcr.2020.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelmann N., Arloth J., Georgakis M.K. Dissecting the association between inflammation, metabolic dysregulation, and specific depressive symptoms: a genetic correlation and 2-sample mendelian randomization study. JAMA Psychiatry. 2020 doi: 10.1001/jamapsychiatry.2020.3436. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman K.R., Robles T.F., Dooley L.N., Boyle C.C., Haydon M.D., Bower J.E. Within-subject associations between inflammation and features of depression: using the flu vaccine as a mild inflammatory stimulus. Brain Behav. Immun. 2018;69:540–547. doi: 10.1016/j.bbi.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Subramaniapillai M., Brietzke E., Mansur R.B., Ho R.C., Yim S.J. Anti-cytokine agents for anhedonia: targeting inflammation and the immune system to treat dimensional disturbances in depression. Ther Adv Psychopharmacol. 2018;8(12):337–348. doi: 10.1177/2045125318791944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K.P., Blazar B.R., Hill G.R. Cytokine mediators of chronic graft-versus-host disease. J. Clin. Invest. 2017;127(7):2452–2463. doi: 10.1172/JCI90593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009;23(2):149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Ancoli-Israel S., Bower J.E., Capuron L., Irwin M.R. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J. Clin. Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Moulton C.D., Pavlidis P., Norton C., Norton S., Pariante C., Hayee B. Depressive symptoms in inflammatory bowel disease: an extraintestinal manifestation of inflammation? Clin. Exp. Immunol. 2019;197(3):308–318. doi: 10.1111/cei.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina H., Sudo N., Aiba Y., Oyama N., Koga Y., Kubo C. Restraint stress elevates the plasma interleukin-6 levels in germ-free mice. J. Neuroimmunol. 2001;115(1-2):46–52. doi: 10.1016/s0165-5728(01)00260-0. [DOI] [PubMed] [Google Scholar]
- Okusaga O., Yolken R.H., Langenberg P. Association of seropositivity for influenza and coronoviruses with history of mood disorders and suicide attampts. J. Affect. Disord. 2011;130(1-2):220–225. doi: 10.1016/j.jad.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison C.L., Rutherford R.E., Woolwine B.J. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):13–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush G., O’Donovan A., Nagle L., Conway C., McCrohan A., O’Farrelly C. Alteration of immune markers in a group of melancholic depressed patients and their response to electroconvulsive therapy. J. Affect. Disord. 2016;205:60–68. doi: 10.1016/j.jad.2016.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting E.Y., Yang A.C., Tsai S.J. Role of interleukin-6 in depressive disorder. Int. J. Mol. Sci. 2020;21(6) doi: 10.3390/ijms21062194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamakis K., Mueller C., Tsirigotis P., Tsiptsios D., Tsamakis C., Charakopoulos E. Depression following graft-versus-host disease in a patient with acute lymphoblastic leukaemia: a case report. Mol Clin Oncol. 2020;12(3):208–211. doi: 10.3892/mco.2019.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoek A.J., Underwood A., Jit M., Miller E., Edmunds W.J. The impact of pandemic influenza H1N1 on health-related quality of life: a prospective population-based study. PloS One. 2011;6(3) doi: 10.1371/journal.pone.0017030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton M.G., Abramowitz J.S., Berman N.C., Fabricant L.E., Olatunji B.O. Psychological predictors of anxiety in response to the H1N1 (swine flu) pandemic. Cognit. Ther. Res. 2012;36(3):210–218. [Google Scholar]
- Wiener C.D., Moreira F.P., Portela L.V., Strogulski N.R., Lara D.R., da Silva R.A. Interleukin-6 and Interleukin-10 in mood disorders: a population-based study. Psychiatr. Res. 2019;273:685–689. doi: 10.1016/j.psychres.2019.01.100. [DOI] [PubMed] [Google Scholar]