Abstract
Repurposing small molecule drugs and drug candidates is considered as a promising approach to revolutionise the treatment of snakebite envenoming. In this study, we investigated the inhibiting effects of the small molecules varespladib (nonspecific phospholipase A2 inhibitor), marimastat (broad spectrum matrix metalloprotease inhibitor) and dimercaprol (metal ion chelator) against coagulopathic toxins found in Crotalinae (pit vipers) snake venoms. Venoms from Bothrops asper, Bothrops jararaca, Calloselasma rhodostoma and Deinagkistrodon acutus were separated by liquid chromatography, followed by nanofractionation and mass spectrometry identification undertaken in parallel. Nanofractions of the venom toxins were then subjected to a high-throughput coagulation assay in the presence of different concentrations of the small molecules under study. Anticoagulant venom toxins were mostly identified as phospholipases A2, while procoagulant venom activities were mainly associated with snake venom metalloproteinases and snake venom serine proteases. Varespladib was found to effectively inhibit most anticoagulant venom effects, and also showed some inhibition against procoagulant toxins. Contrastingly, marimastat and dimercaprol were both effective inhibitors of procoagulant venom activities but showed little inhibitory capability against anticoagulant toxins. The information obtained from this study aids our understanding of the mechanisms of action of toxin inhibitor drug candidates, and highlights their potential as future snakebite treatments.
KEY WORDS: Snakebite, Antivenom, Varespladib, Marimastat, Dimercaprol, Chelators, Nanofractionation
Abbreviations: ACN, acetonitrile; CTL, C-type lectins; DMSO, dimethyl sulfoxide; FA, formic acid; HTS, high-throughput screening; LC, liquid chromatography; MS, mass spectrometry; NOI, no observed inhibition; PBS, phosphate buffered saline; PLA2, phospholipase A2; PN, partly neutralised at 20 μmol/L inhibitor concentrations; SVMP, snake venom metalloproteinase; SVSP, snake venom serine protease; TIC, total ion current; WHO, World Health Organization; XIC, extracted ion current
Graphical abstract
This study investigated the small molecule inhibitors varespladib, marimastat and dimercaprol in neutralising coagulopathic Crotalinae (pit vipers) snake venom toxins by using nanofractionation analytics which combines bioassays, liquid chromatography and mass spectrometry in parallel. Results obtained highlight their potential as future snakebite treatments.
1. Introduction
Snake envenoming is a highly relevant public health issue that causes substantial morbidity and mortality in tropical regions (more than 138,000 deaths every year)1, but remains largely neglected by funding bodies, public health authorities, the pharmaceutical industry and health advocacy groups2. The true burden of snakebite remains unknown, although it has been estimated to be as high as 1,841,000 envenomings annually3. However, since many tropical snakebite victims first seek traditional treatment rather than presenting at a healthcare facility, this number may be an underestimate, as it is known that many snakebite victims die at home and those deaths remain unrecorded2,4. Specific antivenom therapies, which consist of immunoglobulins purified from serum or plasma of animals following hyperimmunization by selected venom(s), represent life-threatening treatments of snake envenoming5. They are given intravenously in a clinical environment and can neutralise the coagulopathic, haemorrhagic and hypotensive actions of snake venoms, among other signs of systemic envenoming6. However, antivenoms are highly specific in neutralising only one or several venoms from species of a certain geographical location7,8, due to extensive variation in venom composition among different snake species9. In addition, antivenom-associated adverse reactions are common10, with both acute reactions (anaphylactic shock or immediate hypersensitivity) and delayed reactions (treatment-induced serum sickness) reported11. Finally, antivenoms are often limited in terms of their availability and/or poorly distributed, and are typically unaffordable to those who live in impoverished rural regions of the tropics—i.e., those who are at the greatest risk of snakebite12.
A promising approach for solving the critical therapeutic gap between a snakebite occurring and delayed presentation to a hospital setting, is the use of small molecule toxin inhibitors that can be administered orally and are able to (at least partially) neutralise enzymatic snake venom toxicities13, 14, 15, 16, 17. The venoms of many snakes, particularly those of vipers (Viperinae and Crotalinae; both family Viperidae) cause coagulopathic and haemorrhagic toxicities by the combined actions of different toxin isoforms of enzymatic families, such as phospholipase A2s (PLA2s), snake venom serine proteases (SVSPs), and snake venom metalloproteinases (SVMPs)6,7. Snake venom hydrolytic enzymes are associated with a number of systemic clinical syndromes, of which coagulopathy is one of the most important in terms of contribution towards mortality5,6. PLA2s can hydrolyse glycerophospholipids and hence degrade membranes, or damage sarcolemma by hydrophobic interactions18. SVSPs can proteolytically degrade fibrinogen and regulate factor V and plasminogen to perturb haemostasis19,20. SVMPs can activate prothrombin and/or factor X, hydrolyse and degrade endothelial cell basement membranes, and preclude muscle fibre regeneration, all of which may contribute to perturbations of blood coagulation and induce severe haemorrhaging21. These enzymes can potentially be inhibited by small molecule toxin inhibitors, such as the PLA2 inhibitor varespladib22, 23, 24 and the metalloproteinase inhibitor marimastat17,25,26. Varespladib, which is a nonspecific PLA2 inhibitor and was a drug candidate for treating atherosclerotic lesions27, coronary heart disease28,29, and acute chest syndrome induced by sickle cell disease23, has been the focus of considerable recent research as a potential therapeutic candidate for snakebite treatment13,14,22,24. Varespladib has showed efficient inhibition of venom-induced PLA2 activity13,22 and has been demonstrated to reduce haemorrhage, myotoxicity and neurotoxicity in murine models after snake envenomation13,24, as well as protect against venom-induced lethality caused by certain snake species and, thus, is now considered as a potential pre-referral drug candidate for treating snakebite22.
Similarly, other small molecule inhibitor-based antidote candidates have been studied for their potential to inhibit SVMP and SVSP toxin families, and therefore serve as alternative (or adjunct), non-antibody based, future treatments for snakebite7,30. One such molecule is marimastat, which is a water-soluble broad spectrum matrix metalloprotease inhibitor31,32, and which binds to the active site of matrix metalloproteinases to form non-covalent complexes17,33. Hence it is considered as a potential inhibitor of SVMPs which share regions of structural similarity to matrix metalloproteinases26,34,35. Marimastat has been evaluated for inhibition of angiogenesis36 and the spread and growth of cancers25,32. Because SVMP toxins are major constituents of many snake venoms, marimastat was repurposed as a candidate to treat snake envenomation and has since been shown to effectively inhibit the metalloprotease activity of snake venoms in vitro and neutralise systemic toxicity and lethality in vivo in mice envenomed with viper venoms17,37.
SVMPs are Zn2+-dependent proteinases, which become inactive after Zn2+ removal from their active site38. Many metal chelator treatments have been proven to be safe in humans and are used as marketed drugs for chelating heavy metals after heavy metal poisoning39,40. A small number of these drugs have been shown to be effective in neutralising the venom-induced proteolytic, myotoxic, haemorrhagic and coagulation activities in murine snakebite models26,41. Dimercaprol, which was developed during World War II by British biochemists42 and is listed by the World Health Organization (WHO) as an essential licensed medicine43, is a widely used antidote in treating heavy metal poisoning44,45, and is recommended for treating Wilson's disease46. A recent study from Albulescu et al.16 showed that dimercaprol could effectively inhibit SVMP activity, counteract coagulopathic effects and neutralise lethal effects of envenoming caused by certain snake species (Viperinae: Echis) in murine models.
In this study we used a combination of nanofractionation analytics and high-throughput coagulation assaying to investigate the potential of varespladib, marimastat and dimercaprol as drug repurposing candidates for new, non-antibody-based, snakebite treatments against coagulopathic pit viper (Crotalinae) snake venoms. The coagulation activities of separated toxins from the venoms of the medically important pit vipers Bothrops asper, Bothrops jararaca, Calloselasma rhodostoma and Deinagkistrodon acutus were evaluated in the presence of the various drug repurposing candidates by using a high-throughput screening (HTS) coagulation assay after nanofractionation by liquid chromatography (LC) with parallel mass spectrometry (MS). We then identified the coagulopathic toxins, including those that were neutralised by the various small molecule toxin inhibitors, by correlating the resulting bioactivity chromatograms to the parallel obtained MS and proteomics data. Our results show that varespladib, marimastat and/or dimercaprol exhibit different specificities and potencies against coagulopathic venom toxins, but that all show promise as novel therapeutics for treating coagulopathic snakebites.
2. Materials and methods
2.1. Chemical and biological reagents
Deionized water was purified by a Milli-Q Plus system (Millipore, Amsterdam, The Netherlands). Acetonitrile (ACN) and formic acid (FA) (Biosolve, Valkenswaard, The Netherlands) were used for the HPLC analyses. Calcium chloride (CaCl2 dihydrate; Sigma–Aldrich, Zwijndrecht, The Netherlands) was used to de-citrate plasma to initiate coagulation in the coagulation assay. Phosphate buffered saline (PBS) buffer was prepared by dissolving PBS tablets (Sigma–Aldrich) in Milli-Q according to the manufacturer's instructions, before storage at 4 °C until use, but for no longer than one week prior to use. Bovine plasma was purchased from Biowest (Nuaillé, France; sodium citrated, sterile filtered, 500 mL per bottle), and prior to use was defrosted in a warm water bath and then quickly transferred to 15 mL CentriStar™ tubes (Corning Science, Reynosa, Mexico) once fully defrosted. The 15 mL tubes were then re-frozen immediately and stored at −80 °C until use. Varespladib, marimastat and dimercaprol (Sigma–Aldrich) were dissolved in DMSO (≥99.9%, Sigma–Aldrich) to a concentration of 10 mmol/L and stored at −20 °C. Prior to use, they were diluted in PBS buffer to the concentrations used for testing. Lyophilized venoms from B. asper (Costa Rica “Atlantic”), B. jararaca (Brazil), C. rhodostoma (captive bred, Thailand ancestry) and D. acutus (captive bred, Chinese ancestry) were provided by the Centre for Snakebite Research and Interventions, Liverpool School of Tropical Medicine (UK). This facility and its protocols for the expert husbandry of snakes are approved and inspected by the UK Home Office and the Liverpool School of Tropical Medicine and University of Liverpool Animal Welfare and Ethical Review Boards. The lyophilized venoms were dissolved in water at 5.0 ± 0.1 mg/mL concentrations, and stored at −80 °C until use.
2.2. LC‒MS with parallel nanofractionation
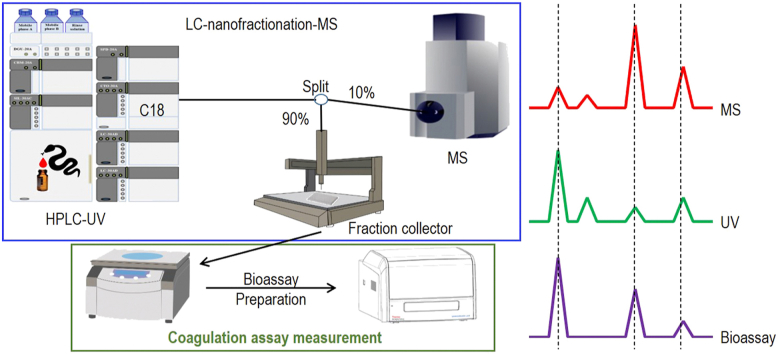
A UPLC system (‘s Hertogenbosch, The Netherlands), which was controlled by a Shimadzu Lab Solutions software by the help of a Shimadzu CBM-20A System Controller, was used for venom separation. For each analysis, 50 μL venom solution (1.0 mg/mL) was injected by a Shimadzu SIL-30AC autosampler after diluting the stock venom solutions (5.0 ± 0.1 mg/mL) in Milli-Q. The gradient separation was performed on a Waters XBridge reversed-phase C18 column (250 mm × 4.6 mm column with 3.5 μm pore-size). The temperature of the column was controlled at 30 °C by a Shimadzu CTO-30A column oven. The total solvent flow rate was 0.5 mL/min and was controlled by two Shimadzu LC-30AD pumps. The mobile phases consisted of eluent A (98% H2O, 2% ACN, and 0.1% FA) and eluent B (98% ACN, 2% H2O, and 0.1% FA). The mobile phase gradients were run as follows: a linear increase of eluent B from 0 to 50% in 20 min followed by a linear increase to 90% B in 4 min, then isocratic elution at 90% for 5 min, subsequently the eluent B was decreased from 90% to 0% in 1 min after which an equilibration of 10 min occurred. A flow split of 9:1 was applied to the column effluent of which the smaller fraction was sent to a Shimadzu SPD-M20A prominence diode array detector and then to the mass spectrometer. The larger fraction was directed to a FractioMate™ nanofractionator (SPARK-Holland & VU, Emmen & Amsterdam, The Netherlands) controlled by FractioMator software (Spark-Holland, Emmen, The Netherlands) or to a modified Gilson 235P robot allowing for nanofractionation onto transparent 384-well plates (F-bottom, rounded square well, polystyrene, without lid, clear, non-sterile; Greiner Bio One, Alphen aan den Rijn, The Netherlands). The nanofractionator was set to collect fractions continuously at a resolution of 6 s/well. After fraction collection, the well plates were dried overnight in a Christ Rotational Vacuum Concentrator (RVC 2-33 CD plus, Zalm en Kipp, Breukelen, The Netherlands), to remove any solvent remaining in the wells. The Vacuum Concentrator was equipped with a cooling trap maintained at −80 °C during operation. The dried plates were then stored at −20 °C before bioassaying.
2.3. Plasma coagulation assay
The HTS coagulation assay was performed as described by Still et al.47, following assay preparation and inhibitor pre-incubation on the vacuum-centrifuge dried nanofractionated well plates. For assay preparation, frozen plasma in 15 mL CentriStar™ tubes was defrosted to room temperature in a warm water bath and then centrifuged at 2000 rpm (805×g) for 4 min in a 5810 R centrifuge (Eppendorf, Germany) to remove possible particulate matter. CaCl2 was dissolved in Milli-Q at room temperature to obtain a 20 mmol/L solution. For the pre-incubation step, the individual inhibitor stock solutions were first diluted in PBS to the required concentrations. Then, from each diluted inhibitor solution prepared, 10 μL was pipetted to all wells of plate wells containing freeze-dried nanofractionated venom fractions by a VWR Multichannel Electronic Pipet, followed by centrifuging the plate for 1 min at 2000 rpm (805×g) in the same 5810 R centrifuge (Eppendorf). Next, a pre-incubation step for 30 min at room temperature was performed. Final concentrations of inhibitor solutions used for the coagulation bioassay were 20, 4 and 0.8 μmol/L (with corresponding DMSO final concentrations of 0.02%, 0.004% and 0.0008%, respectively). Venom-only analyses were performed as control experiments, for which 10 μL PBS instead of inhibitor solution was added to all wells of the vacuum-centrifuge-dried nanofractionated well plates. For initiating the coagulation assay, 20 μL of CaCl2 solution, followed by 20 μL plasma, was pipetted onto all wells of the 384-well plates by a Multidrop™ 384 Reagent Dispenser (Thermo Fisher Scientific, Ermelo, The Netherlands) with rinsing of the Multidrop with deionized water in between dispensing. Immediately after robotically pipetting the CaCl2 and plasma solutions to a plate, a Varioskan™ Flash Multimode Reader (Thermo Fisher Scientific) was used to measure the absorbance of each well kinetically for 100 min at 595 nm at 25 °C. Each nanofractionation analysis was performed in at least duplicate. The slope value of each well was normalized by dividing these slope values with the median value from all wells in that single measurement, as described by Slagboom et al.48 and Xie et al.49. The coagulation chromatograms were then plotted by using the normalized slope values (y-axis) against the time of nanofraction collection (x-axis). For visualizing coagulation activity, the average rate of the kinetic coagulation curve from 0 to 5 min was plotted for very fast coagulation activity, from 0 to 20 min was plotted for slightly/medium increased coagulation activity, and the single reading of the coagulation curve at 100 min was plotted for anticoagulation activity. Procoagulation was plotted in two different ways since all significantly increased procoagulation curves reached their maximum absorbance before 20 min and thus plotting the short 5 min kinetic time window allows discrimination of the most potent acting procoagulation activities.
2.4. Correlation of biological data with MS and proteomics data
To identify the venom toxins that exhibited activity in the plasma coagulation assay, we used the proteomics data and corresponding accurate mass(es) described by Slagboom et al.48. For venoms under study in this project that were not studied by Slagboom et al.48, the same procedure as previously described48 was followed to acquire and process proteomics data on these snake venoms. The UniprotKB database was used to search for information on class and possible known functions for the relevant toxins. To import the proteomics data for the venoms included in this study that were also included in the study of Slagboom et al.48, the fingerprint profile LC‒UV traces (measured at 220, 254 and 280 nm) for the same venom analyses acquired in both studies were used to align chromatograms with each other to accommodate for potential slight differences in chromatographic retention times. Consequently, the coagulopathic bioactivity peaks together with UV data acquired for each nanofractionated venom fraction obtained in this study could be linked to the MS-total ion current (TIC) data, to the extracted ion current (XIC) data, and to the protein IDs from the proteomics data acquired by Slagboom et al.48. In order to construct XICs, an MS spectrum corresponding to each bioactive peak found in the bioassay chromatogram was first extracted from the MS data by averaging the spectra acquired from the 50% and higher peak height range of each bioactive peak. Then, XICs were plotted of the clearly observed m/z-values and were then used to match these peak retention times with the bioactivity peaks in the bioactivity chromatograms. The matched exact masses were assigned to each corresponding bioactive peak observed in the bioassay chromatogram by matching peak shape and retention time. This way, the m/z-values found from the MS data were correlated to each bioactive peak, and the accurate monoisotopic masses were determined by applying the deconvolution option in the MS software.
3. Results
In this study, venoms from a variety of Crotalinae snake species were nanofractionated by LC followed by low volume HTS coagulation assaying to evaluate the inhibiting effects of varespladib, marimastat and dimercaprol on separated venom toxins that induce coagulopathic activities. The bioactive venom toxins identified were assigned by correlating bioactivity chromatograms to parallel obtained MS data and by using proteomics data obtained for proteins present in wells showing coagulopathic activities. Using this approach, the neutralizing specificities of these molecules on a variety of venom toxins were revealed. All analyses were performed at least in duplicate using venom concentrations of 1.0 mg/mL. Note that despite venom toxins being, in general, rather stable during RPLC within the nanofractionation analytics pipeline, some venom toxins might have (partly) denatured and thereby lost their enzymatic activity; consequently, the coagulation traces of venom-only analyses in control experiments may vary slightly from each other.
3.1. The inhibitory effects of varespladib, marimastat and dimercaprol on coagulopathic venom toxins from snake species of the genus Bothrops
Two venoms from the most medically important genus of snakes in Latin America were investigated in this study (B. asper, Costa Rica and B. jararaca, Brazil). Envenomings by Bothrops spp. can result in severe local tissue damage, pain and inflammation, as well as systemic hemotoxicity characterised by haemorrhage and coagulopathy50, 51, 52. The coagulopathic activity of the obtained venom fractions from both species was assayed in the presence of different concentrations of varespladib, marimastat and dimercaprol (Figure 1, Figure 2). Duplicate bioassay chromatograms were measured to assess repeatability and detailed descriptions of each signal peak observed in the chromatograms are given in the Supporting Information Section S1.
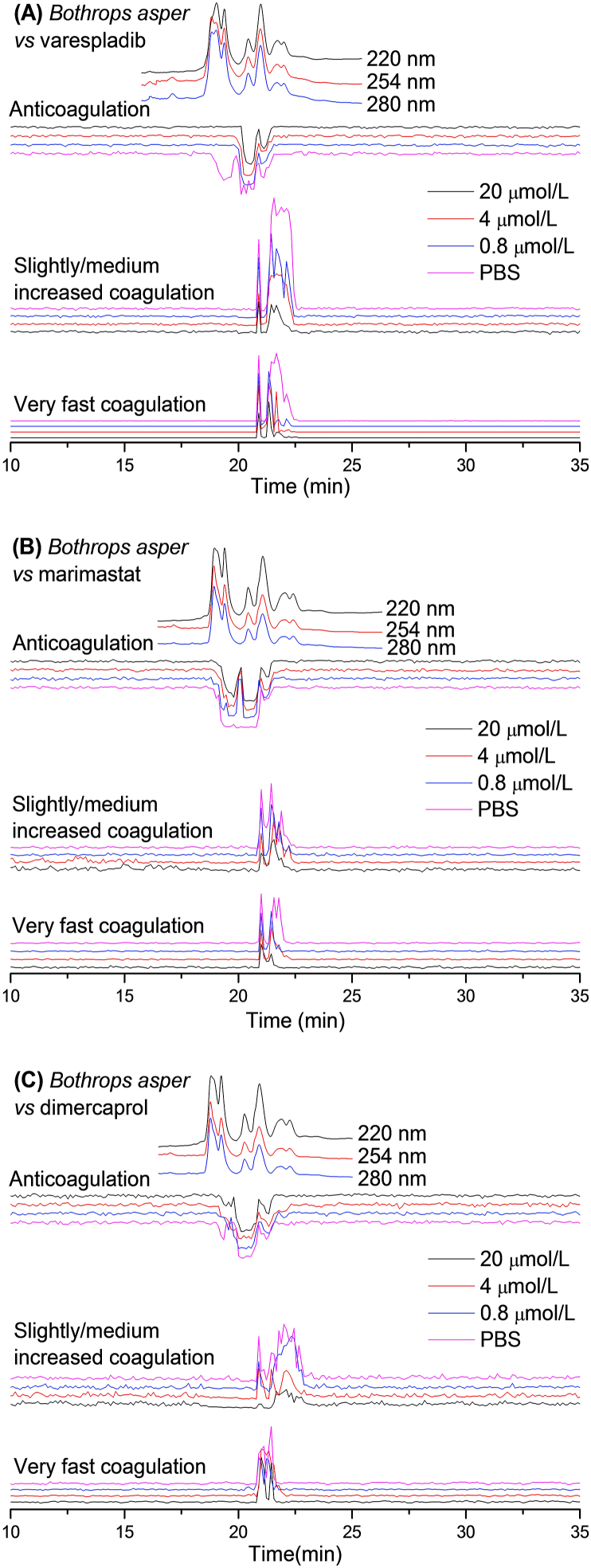
Figure 1.
UV absorbance chromatograms and reconstructed bioassay chromatograms of coagulopathic venom fractions of B. asper in the presence of different concentrations of (A) varespladib, (B) marimastat and (C) dimercaprol. The top superimposed chromatograms are characteristic profiles of the UV trace detected at 220, 254 and 280 nm. PBS indicates venom only samples where PBS was used as a control for the inhibitors.
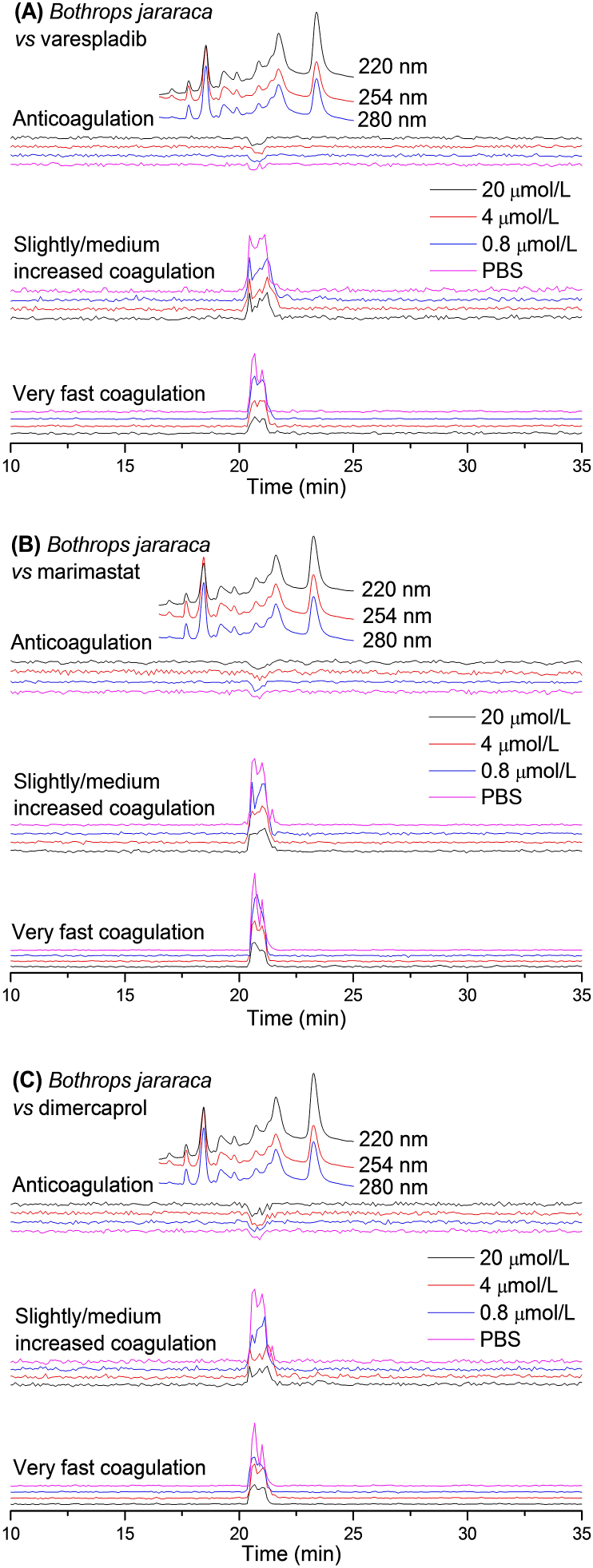
Figure 2.
UV absorbance chromatograms and reconstructed bioassay chromatograms of coagulopathic venom fractions of B. jararaca in the presence of different concentrations of (A) varespladib, (B) marimastat and (C) dimercaprol. The top superimposed chromatograms are characteristic profiles of the UV trace detected at 220, 254 and 280 nm. PBS indicates venom only samples where PBS was used as a control for the inhibitors.
For B. asper venom, in the venom-only analysis (indicated as “PBS” in the figures) revealed a sharp intense positive peak (20.9 min) followed by a broad intense positive peak (21.2–22.4 min) in both the very fast coagulation activity chromatograms and the slightly/medium increased coagulation activity chromatograms. A broad negative peak (18.9–21.5 min) was also observed in the anticoagulation activity chromatograms, demonstrating that this venom exhibits both pro- and anticoagulant activities. The broad peaks observed are likely the result of co-eluting bioactive venom toxins. When testing the venom fractions in the presence of small molecule toxin inhibitors, we found that varespladib inhibited some of the observed anticoagulation activities and reduced the potency of a number of the procoagulant venom toxins. Contrastingly, marimastat and dimercaprol inhibited procoagulant venom effects to varying extents, but had little inhibitory impact on anticoagulant toxins. For the very fast coagulation activity, the first eluting sharp positive peak (20.9 min) decreased in size with increasing concentrations of both varespladib and marimastat. The intensity of the broad intense positive peak (21.2–22.4 min) was also reduced substantially by varespladib and marimastat, and the tailing part was fully neutralised by 20 μmol/L of both varespladib and marimastat. In contrast, both peaks (20.9 and 21.2–22.4 min) showed limited change in the presence of dimercaprol. However, the slightly/medium increased coagulation activity was almost fully neutralised by 20 μmol/L dimercaprol. The front sharp positive peak (20.9 min) was inhibited by all the three inhibitors in a concentration-response manner, while the majority of the broad intense positive peak (21.2–22.4 min) was inhibited by 20 μmol/L varespladib, but only the tailing part of this peak was inhibited by 20 μmol/L marimastat. For anticoagulant venom activity, the front part of the broad negative peak (21.2–22.4 min) was fully neutralised by a concentration as low as 0.8 μmol/L varespladib, while the remaining part was not inhibited at all by this molecule. Surprisingly, the broad negative peak was split into two individual peaks (18.9–20.0 and 20.1–21.0 min) by 0.8 μmol/L marimastat, but both peaks remained unchanged in the presence of increasing concentrations of marimastat. No inhibition of anticoagulation activity was observed for dimercaprol.
Next, B. jararaca venom was fractionated and the fractions assessed in the presence of varespladib, marimastat and dimercaprol, as outlined above for B. asper. The resulting bioassay chromatograms of B. jararaca analyses are shown in Fig. 2. A positive peak with a co-eluting shoulder peak (20.2–21.7 min) was observed in both the very fast coagulation chromatogram and the slightly/medium increased coagulation chromatogram in the venom-only analysis. This peak decreased in a relatively comparable manner with increasing concentrations of varespladib, marimastat and dimercaprol, although full inhibition was not achieved at the highest concentration of any of the inhibitors tested (20 μmol/L). Contrasting strongly with its congener B. asper, only a very weak negative peak (20.5–21.1 min) was detected, demonstrating limited anticoagulant venom activity; this peak was not inhibited by varespladib, marimastat or dimercaprol at any of the concentrations tested.
3.2. The inhibitory effects of varespladib, marimastat and dimercaprol on coagulopathic venom toxins from C. rhodostoma and D. acutus
Next, we assessed the inhibitory capability of the same small molecule toxin inhibitors on two monotypic medically important Crotalinae snake species, namely C. rhodostoma from Southeast Asia53, and D. acutus from southern China and northern Vietnam54. Venoms from both species are abundant in hemotoxic compounds that can deregulate blood coagulation and cause severe coagulopathy in snakebite victims55,56. In this study, their venoms were fractionated and the coagulopathic activity of the obtained fractions was assessed in the presence of varespladib, marimastat and dimercaprol. The resulting bioassay chromatograms are shown in Figure 3, Figure 4. The duplicate bioassay chromatograms of the C. rhodostoma and D. acutus venom analyses, including detailed descriptions of each bioactivity peak, are provided in the Supporting Information Section S2.
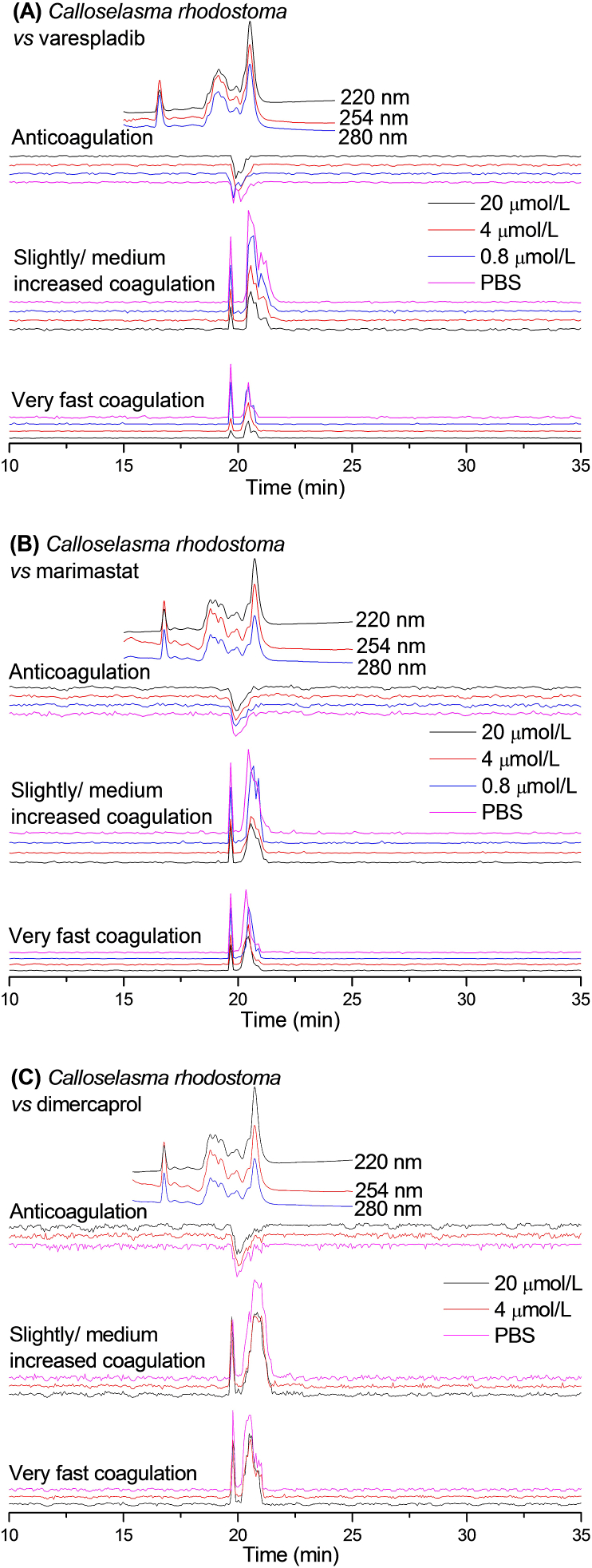
Figure 3.
UV absorbance chromatograms and reconstructed bioassay chromatograms of coagulopathic venom fractions of C. rhodostoma in the presence of different concentrations of (A) varespladib, (B) marimastat and (C) dimercaprol. The top superimposed chromatograms are characteristic profiles of the UV trace detected at 220, 254 and 280 nm. PBS indicates venom only samples where PBS was used as a control for the inhibitors.
Figure 4.
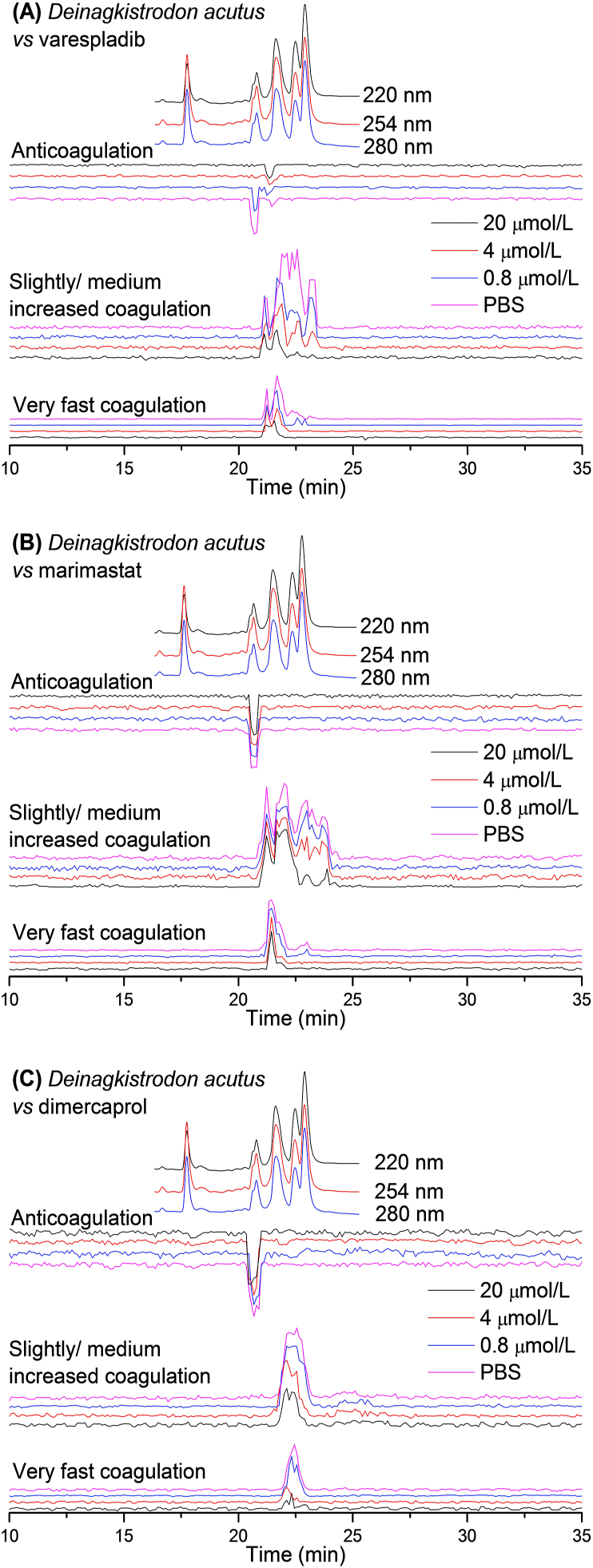
UV absorbance chromatograms and reconstructed bioassay chromatograms of coagulopathic venom fractions of D. acutus venom in the presence of different concentrations of (A) varespladib, (B) marimastat and (C) dimercaprol. The top superimposed chromatograms are characteristic profiles of the UV trace detected at 220, 254 and 280 nm. PBS indicates venom only samples where PBS was used as a control for the inhibitors.
The bioactivity chromatogram of C. rhodostoma venom (Fig. 3) showed two sharp positive peaks (19.7 and 20.4 min) for the very fast coagulation activity as well as for the slightly/medium increased coagulation activity, while a moderate negative peak (19.9 min) was observed for the anticoagulation activity. Both procoagulation activities were gradually reduced by increasing concentrations of varespladib and marimastat. These procoagulation activities, however, were not fully neutralised by the highest varespladib or marimastat concentrations tested (20 μmol/L), suggesting perhaps a non-specific inhibitory effect. In contrast to these findings, we observed very limited inhibition of both procoagulant peaks with dimercaprol. The negative anticoagulation peak (19.9 min) observed was not inhibited by any of the inhibitory molecules tested.
The bioassay chromatograms constructed from the measurement of LC nanofractions of D. acutus venom in the presence of the different concentrations of varespladib, marimastat and dimercaprol are shown in Fig. 4. In the venom-only analysis, a co-eluting intense positive peak (21.0–22.2 min) was followed by a very weak positive peak (22.7–23.2 min) in the very fast coagulation activity chromatogram. The later eluting weak positive peak (22.7–23.2 min) was fully neutralised by both varespladib and marimastat at a 4 μmol/L concentration. The broad intense positive peak (21.0–22.2 min) was substantially reduced by varespladib in a concentration-dependent fashion, but was only slightly inhibited by the highest marimastat concentration tested (20 μmol/L). In the slightly/medium increased coagulation activity chromatogram, several positive peaks eluted close together (21.2–23.4 min) and were observed as non-baseline separated peaks in the venom-only analysis. They were all reduced by increasing varespladib concentrations and the tailing part of the broad peak was fully neutralised at 20 μmol/L varespladib. Marimastat had a limited effect on these peaks, with only the tailing part of the peaks partly neutralised at the highest concentration tested (20 μmol/L). For both the very fast coagulation activity and the slightly/medium increased coagulation activity, dimercaprol showed no inhibitory effect at 0.8 μmol/L, though showed moderate inhibition at 4 and 20 μmol/L. For anticoagulant venom activity, a sharp intense negative peak (20.7 min), followed by a very weak negative peak (21.5 min), was observed in the venom-only analysis. The intense negative peak (20.7 min) was reduced in a dose dependent manner by varespladib, with complete inhibition achieved at the 4 μmol/L concentration, whereas the weak negative peak (21.5 min) was not affected by varespladib. Neither of these anticoagulation activity peaks was affected by marimastat and dimercaprol.
3.3. Identification of coagulopathic venom toxins neutralised by the small molecule inhibitors varespladib, marimastat and dimercaprol
The venom toxins responsible for the coagulation activities observed for each of the four snake venoms were tentatively identified by correlating the acquired data with MS and proteomics data previously obtained by Slagboom et al.48 (Table 1). Bioactivities were linked to accurate molecular masses and tentative protein identities by aligning the characteristic LC‒UV chromatograms obtained for each venom. For those toxins where no exact mass data was acquired by LC‒MS, only the proteomics data obtained from the Mascot searches is provided.
Table 1.
Correlated MS and proteomics data matching coagulopathic venom toxins.
| Species | Peak retention time (min) | Peak activity | Mascot results matching the exact mass | Exact mass from MS data | Exact mass calculated from Mascot data | Toxin class | Dose required for full inhibition |
|---|---|---|---|---|---|---|---|
| B. asper | 18.9–20.0 | Anticoagulation | PA2H2_BOTAS | 13,714.565 | 13,715 | PLA2 | PN |
| 20.1–21.0 | Anticoagulation | PA2HA_BOTAS | 13,912.465 | 13,897 | PLA2 | PN | |
| 20.1–21.0 | Anticoagulation | PA2H3_BOTAS | 13,765.581 | 13,766 | PLA2 | PN | |
| 21.0–21.5 | Anticoagulation | PA2B3_BOTAS | 13,957.533 | 13,957 | PLA2 | PN | |
| 21.0–21.5 | Anticoagulation | PA2A2_BOTAS | – | 14,194 | PLA2 | PN | |
| 21.0–21.5 | Anticoagulation | VM2_BOTAS | – | 53,564 | SVMP | NOI | |
| 20.8–22.4 | Procoagulation | VSPL_BOTAS | – | 28,019 | SVSP | PN | |
| 20.8–22.4 | Procoagulation | VM1B1_BOTAS | – | 45,936 | SVMP | PN | |
| 21.2–22.4 | Procoagulation | SLA_BOTAS | 7084 | CTL | PN | ||
| B. jararaca | 20.5–21.1 | Anticoagulation | – | – | – | – | NOI |
| 20.2–21.7 | Procoagulation | VSPA_BOTJA | – | 25,584 | SVSP | PN | |
| 20.2–21.7 | Procoagulation | VSP1_BOTJA | – | 25,742 | SVSP | PN | |
| 20.2–21.7 | Procoagulation | VSP2_BOTJA | – | 27,894 | SVSP | PN | |
| 20.2–21.7 | Procoagulation | VSP12_BOTJA | – | 27,988 | SVSP | PN | |
| 20.2–21.7 | Procoagulation | VSP14_BOTJA | – | 27,843 | SVSP | PN | |
| 20.2–21.7 | Procoagulation | VSP20_BOTJA | – | 27,815 | SVSP | PN | |
| C. rhodostoma | 19.3–20.5 | Anticoagulation | PA2BD_CALRH | 13,665.085 | 13,665 | PLA2 | NOI |
| 19.3–20.5 | Anticoagulation | PA2AB_CALRH | – | 14,352 | PLA2 | NOI | |
| 19.3–20.5 | Anticoagulation | VSPF1_CALRH | – | 26,570 | SVSP | NOI | |
| 19.3–20.5 | Anticoagulation | SLEA_CALRH | – | 15,962 | CTL | NOI | |
| 19.3–20.5 | Anticoagulation | SLEB_CALRH | – | 15,190 | CTL | NOI | |
| 19.3–21.6 | Procoagulation | VSPF2_CALRH | – | 29,145 | SVSP | PN | |
| 19.9–21.6 | Procoagulation | SLYA_CALRH | – | 15,796 | CTL | PN | |
| 19.9–21.6 | Procoagulation | SLYB_CALRH | – | 16,770 | CTL | PN | |
| D. acutus | 20.4–20.9 | Anticoagulation | PA2A_DEIAC | – | 14,820 | PLA2 | 4 μmol/L varespladib |
| 20.4–20.9 | Anticoagulation | SL_DEIAC | – | 18,332 | CTL | 4 μmol/L varespladib | |
| 21.4–21.7 | Anticoagulation | – | – | – | – | NOI | |
| 21.0–22.7 | Procoagulation | VSP1_DEIAC | – | 29,480 | SVSP | PN | |
| 21.0–22.7 | Procoagulation | VSPA_DEIAC | – | 26,132 | SVSP | PN | |
| 21.4–22.7 | Procoagulation | SLCB_DEIAC | – | 17,133 | CTL | PN | |
| 21.4–22.7 | Procoagulation | VM1AC_DEIAC | – | 47,690 | SVMP | PN | |
| 21.4–22.7 | Procoagulation | VM11_DEIAC | – | 47,845 | SVMP | PN | |
| 21.4–22.7 | Procoagulation | VM1H5_DEIAC | – | 46,518 | SVMP | PN | |
| 21.4–22.7 | Procoagulation | VM3AK_DEIAC | – | 69,752 | SVMP | PN | |
| 22.7–23.4 | Procoagulation | VM3A2_DEIAC | – | 27,151 | SVMP | 20 μmol/L varespladib/4 μmol/L marimastat | |
| 22.7–23.4 | Procoagulation | VM3AH_DEIAC | – | 70,721 | SVMP | 20 μmol/L varespladib/4 μmol/L marimastat |
Peak retention times are adapted from Figure 1, Figure 2, Figure 3, Figure 4; PLA2 = phospholipase A2; SVMP = snake venom metalloproteinase; SVSP = snake venom serine protease; CTL=C-type lectin; PN = partly neutralised at 20 μmol/L inhibitor concentrations; NOI = no observed inhibition; ‒ Not applicable.
By comparing Table 1 with Figure 1, Figure 2, Figure 3, Figure 4, the inhibiting effects of varespladib, marimastat and dimercaprol on individual Crotalinae venom toxins were assessed. Most anticoagulant toxins identified were PLA2s. The PLA2s in B. asper venom were partly neutralised by 20 μmol/L varespladib, and in D. acutus venom were fully neutralised at a concentration of 4 μmol/L varespladib. However, no anticoagulant toxin was identified from the Mascot results for B. jararaca venom, and no inhibition was observed on the anticoagulation peaks from C. rhodostoma venom by varespladib, marimastat and dimercaprol. The tentatively identified procoagulant toxins were mainly SVMPs, SVSPs and C-type lectins (CTL). Only two SVMPs in D. acutus venom identified as procoagulant toxins were fully inhibited by 4 μmol/L marimastat, and by 20 μmol/L varespladib. The potential inhibition of SVMPs by varespladib can indicate that varespladib may not exert its effect solely by inhibition of the phospholipid hydrolysing activity of PLA2s, though further research is required to robustly test this hypothesis. Critically, as several venom toxins were found to co-elute in regions where bioactives were observed, the unambiguous identification of the individual contribution of each toxin to each coagulation bioactivity peak, and their relative contributions to coagulotoxicity, are difficult to interpret. Additionally, some non-polar (late eluting) venom toxins might (partially) denature during LC separation and become biologically inactive and as such may not be detected by the coagulopathic bioassay. A detailed description of the results discussed here is provided in the Supporting Information Section S3.
4. Discussion
Snake envenoming by pit vipers is usually characterized by coagulopathy, haemorrhage and local effects, such as swelling and tissue damage around the site of the bite1. Our study investigated the neutralizing capabilities of promising small molecule-based drug candidates on the coagulopathic activities of pit viper venoms by using nanofractionation analytics. All venoms showed both procoagulation activities and anticoagulation activities, albeit only trace anticoagulant activity was observed with B. jararaca venom. Our findings reveal that the PLA2 inhibitor varespladib inhibited many of the observed anticoagulation activities, and surprisingly also exhibited varying inhibitory effects on several of the observed procoagulation activities. However, future work is required to unravel the mechanisms of this inhibition of procoagulant activity, given that such toxins are unlikely to be PLA2s. We cannot rule out that non-specific effects at high inhibitor concentrations are responsible for these observations. Snake venom PLA2s hydrolyse phospholipids to release fatty acids and lysophospholipids, resulting in damage to cell membranes, or prolong/delay prothrombin time to prevent blood clotting. These toxins, often acting synergistic with other venom toxin families such as SVMPs and SVSPs57,58, contribute to various toxicities observed following snake envenoming, including hemotoxic, myotoxic and neurotoxic effects, and thus are one of the main players in resulting morbidity and mortality48,59,60. By using varespladib, the enzymatic toxicities mediated by PLA2s can be alleviated, delayed and/or abrogated14,22,24,61. However, additional work is required to fully understand the inhibitory potential of varespladib for snakebite. For example, Wang et al.13 previously demonstrated that varespladib was capable of fully inhibiting the haemorrhagic toxicity induced by D. acutus venom when it was administered subcutaneously or intramuscularly to mice, whilst also having some inhibitory effect of the myotoxic activities caused by this venom. Despite these findings, our results show that not all coagulopathic toxins found in D. acutus venom were inhibited by varespladib, and indeed similar observations were observed with the other venoms under study. Thus, while varespladib undoubtedly remains a highly promising snakebite therapeutic, more work is required to fully understand its toxin-neutralising specificity.
The SVMP inhibitors marimastat and dimercaprol only partly inhibited the procoagulation activities revealed in this study, and showed little inhibitory activity against anticoagulant toxins (mostly identified as PLA2s). These findings are in line with the anticipated specificity of their mechanism of action, which interact via different mechanisms on the active sites of SVMPs16,17,37. Despite previous studies showing that marimastat and dimercaprol inhibited SVMP activity from the venoms of Crotalus atrox and Echis spp.16,17,37, here we found that marimastat and dimercaprol showed only a degree of inhibition against certain procoagulant toxins present in the venoms of the pit vipers under study. However, these findings can be rationalised, given that many of the procoagulant toxins tentatively identified here were not SVMPs (Table 1). Interestingly, neither marimastat nor dimercaprol had a noticeable inhibitory effect on the coagulotoxicity of C. rhodostoma venom, and indeed no SVMP toxins were identified among the bioactives detected in this study. Thus, specific inhibitors against C-type lectins and/or SVSP toxins may be required to prevent procoagulant venom effects caused by this species. It is worth noting that Albulescu et al.37 recently demonstrated that the serine protease inhibitor nafamostat was capable of broadly neutralised the SVSP activity of a variety of viper venoms (including B. asper) in a dose-dependent manner, and thus use of this molecules seems likely to be a logical starting point for future inhibition studies using C. rhodostoma venom.
It is worth highlighting that these inhibitor-based therapeutic candidates have different specificities and act on different toxin classes found in snake venoms. Therefore, in the long term, utilising combinations of such molecules (e.g., the PLA2 inhibitor varespladib, SVMP inhibitor marimastat and/or chelator dimercaprol, together with serine protease inhibitors and other venom toxin inhibitors) seems likely to be a potentially viable strategy for the development of adjunct and/or stand-alone treatments of snakebite, that may be of great value for use as early interventions in remote rural areas where treatment delays contribute to poor patient outcomes. In the recent study of Albulescu et al.37, we demonstrated that a therapeutic combination of varespladib and marimastat protected experimental animals from the lethal effects of a variety of viper venoms, including that of B. asper studied here. A combination of various different inhibitors (or indeed modalities, e.g., small molecules and monoclonal antibodies or nanobodies) may prove to be the only viable approach to obtain affordable broad-spectrum therapeutics for treating snakebite globally9,22,26. However, considerable further work is required to robustly explore this view, ideally via the application of both in vitro and in vivo studies, using both a diversity of chemical entities as potential inhibitory agents and a variety of snake venoms that represent full (global) toxin diversity. In the longer term, such in vivo studies should also explore appropriate dosing schedules and administration routes of identified lead candidates to robustly predict their translational potential for use in human clinical trials. Our findings here reinforce the notion that small molecule based toxin inhibitors are viable entities as next-generation snakebite therapeutics, and though hurdles associated with overcoming variable venom compositions and distinct toxin inhibiting specificities remain, there is a strong rationale for future research programmes to continue to evaluate these molecules as valuable repurposing candidates for snakebite.
5. Conclusions
The coagulation activities of toxins isolated from the venoms of the Crotalinae snakes B. asper, B. jararaca, C. rhodostoma and D. acutus were investigated by the application of a recently developed HTS coagulation assay after venom nanofractionation by LC. The inhibitory potential of the small molecule toxin inhibitors varespladib, marimastat and dimercaprol against the bioactivities of nanofractionated pit viper venom toxins was then evaluated. All inhibitors under study are either registered drugs or at least phase II approved candidates known to inhibit PLA2 or protease activity. This implies that these compounds are clinically safe and as such will this significantly increase the chances of these compounds to be developed into eventual snakebite treatments as compared to studying non-clinically tested compounds. Coagulopathic venom toxins were tentatively identified by correlating bioassay activity chromatograms to MS and proteomics data obtained in parallel. Our results showed that the three repurposed drug candidates exhibited varying inhibitory effects against distinct pro- and anticoagulant venom toxins identified in each venom. We conclude that combinations of small molecule drugs capable of inhibiting distinct toxin families may be required to ensure broad neutralization of a diversity of snake venoms.
Acknowledgments
Chunfang Xie was funded by a China Scholarship Council (CSC) fellowship (201706250035). Nicholas R. Casewell acknowledged support from a UK Medical Research Council (MRC) Research Grant (MR/S00016X/1) and Confidence in Concept Award (CiC19017, UK), and a Sir Henry Dale Fellowship (200517/Z/16/Z, UK) jointly funded by the Wellcome Trust and Royal Society.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.09.005.
Author contributions
Chunfang Xie: data curation, formal analysis, funding acquisition, methodology, validation, visualization, writing—original draft; Julien Slagboom: methodology, writing—review & editing; Laura-Oana Albulescu: writing—review & editing; Govert W. Somsen: writing—review & editing; Freek J. Vonk: conceptualization, methodology; Nicholas R. Casewell: conceptualization, funding acquisition, investigation, resources, writing—review & editing; Jeroen Kool: conceptualization, funding acquisition, investigation, project administration, resources, supervision, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gutiérrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite envenoming. Nat Rev Dis Primers. 2017;3:1–21. doi: 10.1038/nrdp.2017.79. [DOI] [PubMed] [Google Scholar]
- 2.Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D. Strategy for a globally coordinated response to a priority neglected tropical disease: snakebite envenoming. PLoS Neglected Trop Dis. 2019;13:7059–7080. doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chippaux J.P. Snakebite envenomation turns again into a neglected tropical disease! J Venom Anim Toxins incl Trop Dis. 2017;23:38. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maduwage K., Isbister G.K. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Neglected Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slagboom J., Kool J., Harrison R.A., Casewell N.R. Haemotoxic snake venoms: their functional activity, impact on snakebite victims and pharmaceutical promise. Br J Haematol. 2017;177:947–959. doi: 10.1111/bjh.14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ainsworth S., Slagboom J., Alomran N., Pla D., Alhamdi Y., King S.I. The paraspecific neutralisation of snake venom induced coagulopathy by antivenoms. Commun Biol. 2018;1:34. doi: 10.1038/s42003-018-0039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso F.C., Ferraz C.R., Arrahman A., Xie C., Casewell N.R., Lewis R.J. Multifunctional toxins in snake venoms and therapeutic implications: from pain to hemorrhage and necrosis. Front Ecol Evol. 2019;7:218–236. [Google Scholar]
- 9.Casewell N.R., Jackson T.N., Laustsen A.H., Sunagar K. Causes and consequences of snake venom variation. Trends Pharmacol Sci. 2020;41:570–581. doi: 10.1016/j.tips.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Silva H.A., Ryan N.M., de Silva H.J. Adverse reactions to snake antivenom, and their prevention and treatment. Br J Clin Pharmacol. 2016;81:446–452. doi: 10.1111/bcp.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morais V., Massaldi H. Snake antivenoms: adverse reactions and production technology. J Venom Anim Toxins Incl Trop Dis. 2009;15:2–18. [Google Scholar]
- 12.Habib A.G., Warrell D.A. Antivenom therapy of carpet viper (Echis ocellatus) envenoming: effectiveness and strategies for delivery in West Africa. Toxicon. 2013;69:82–89. doi: 10.1016/j.toxicon.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Zhang J., Zhang D., Xiao H., Xiong S., Huang C. Exploration of the inhibitory potential of varespladib for snakebite envenomation. Molecules. 2018;23:391–403. doi: 10.3390/molecules23020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bittenbinder M.A., Zdenek C.N., Op den Brouw B., Youngman N.J., Dobson J.S., Naude A. Coagulotoxic cobras: clinical implications of strong anticoagulant actions of African spitting Naja venoms that are not neutralised by antivenom but are by LY315920 (varespladib) Toxins. 2018;10:516–526. doi: 10.3390/toxins10120516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulfone T.C., Samuel S.P., Bickler P.E., Lewin M.R. Developing small molecule therapeutics for the initial and adjunctive treatment of snakebite. J Trop Med. 2018;2018:4320175. doi: 10.1155/2018/4320175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albulescu L.O., Hale M.S., Ainsworth S., Alsolaiss J., Crittenden E., Calvete J.J. Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.aay8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Layfield H.J., Williams H.F., Ravishankar D., Mehmi A., Sonavane M., Salim A. Repurposing cancer drugs batimastat and marimastat to inhibit the activity of a group I metalloprotease from the venom of the Western diamondback rattlesnake, Crotalus atrox. Toxins. 2020;12:309. doi: 10.3390/toxins12050309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JMa Gutiérrez, Ownby C.L. Skeletal muscle degeneration induced by venom phospholipases A2: insights into the mechanisms of local and systemic myotoxicity. Toxicon. 2003;42:915–931. doi: 10.1016/j.toxicon.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Serrano S.M., Maroun R.C. Snake venom serine proteinases: sequence homology vs. substrate specificity, a paradox to be solved. Toxicon. 2005;45:1115–1132. doi: 10.1016/j.toxicon.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Kisiel W. Effect of snake venoms on factor V. In: Tu Anthony., editor. Handbook of natural toxin. Marcel Dekker Inc.; New York: 2018. pp. 253–264. [Google Scholar]
- 21.Williams H.F., Mellows B.A., Mitchell R., Sfyri P., Layfield H.J., Salamah M. Mechanisms underpinning the permanent muscle damage induced by snake venom metalloprotease. PLoS Neglected Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewin M., Samuel S., Merkel J., Bickler P. Varespladib (LY315920) appears to be a potent, broad-spectrum, inhibitor of snake venom phospholipase A2 and a possible pre-referral treatment for envenomation. Toxins. 2016;8:248. doi: 10.3390/toxins8090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abraham E., Naum C., Bandi V., Gervich D., Lowry S.F., Wunderink R. Efficacy and safety of LY315920Na/S-5920, a selective inhibitor of 14-kDa group IIA secretory phospholipase A2, in patients with suspected sepsis and organ failure. Crit Care Med. 2003;31:718–728. doi: 10.1097/01.CCM.0000053648.42884.89. [DOI] [PubMed] [Google Scholar]
- 24.Bryan-Quirós W., Fernández J., Gutiérrez J.M., Lewin M.R., Lomonte B. Neutralizing properties of LY315920 toward snake venom group I and II myotoxic phospholipases A2. Toxicon. 2019;157:1–7. doi: 10.1016/j.toxicon.2018.11.292. [DOI] [PubMed] [Google Scholar]
- 25.King J., Zhao J., Clingan P., Morris D. Randomised double blind placebo control study of adjuvant treatment with the metalloproteinase inhibitor, marimastat in patients with inoperable colorectal hepatic metastases: significant survival advantage in patients with musculoskeletal side-effects. Anticancer Res. 2003;23:639–645. [PubMed] [Google Scholar]
- 26.Howes J.M., Theakston R.D.G., Laing G. Neutralization of the haemorrhagic activities of viperine snake venoms and venom metalloproteinases using synthetic peptide inhibitors and chelators. Toxicon. 2007;49:734–739. doi: 10.1016/j.toxicon.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Shaposhnik Z., Wang X., Trias J., Fraser H., Lusis A.J. The synergistic inhibition of atherogenesis in apoE−/− mice between pravastatin and the sPLA2 inhibitor varespladib (A-002) J Lipid Res. 2009;50:623–629. doi: 10.1194/jlr.M800361-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenson R.S., Hislop C., McConnell D., Elliott M., Stasiv Y., Wang N. Effects of 1-H-indole-3-glyoxamide (A-002) on concentration of secretory phospholipase A2 (PLASMA study): a phase II double-blind, randomised, placebo-controlled trial. Lancet. 2009;373:649–658. doi: 10.1016/S0140-6736(09)60403-7. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls S.J., Kastelein J.J., Schwartz G.G., Bash D., Rosenson R.S., Cavender M.A. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez J.M., Lomonte B., León G., Rucavado A., Chaves F., Angulo Y. Trends in snakebite envenomation therapy: scientific, technological and public health considerations. Curr Pharm Des. 2007;13:2935–2950. doi: 10.2174/138161207782023784. [DOI] [PubMed] [Google Scholar]
- 31.Curran S., Murray G.I. Matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 1999;189:300–308. doi: 10.1002/(SICI)1096-9896(199911)189:3<300::AID-PATH456>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen H.S., McCann P.P. Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol Ther. 1997;75:69–75. doi: 10.1016/s0163-7258(97)00023-5. [DOI] [PubMed] [Google Scholar]
- 33.Underwood C., Min D., Lyons J., Hambley T. The interaction of metal ions and marimastat with matrix metalloproteinase 9. J Inorg Biochem. 2003;95:165–170. doi: 10.1016/s0162-0134(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D., Botos I., Gomis-Rüth F.X., Doll R., Blood C., Njoroge F.G. Structural interaction of natural and synthetic inhibitors with the venom metalloproteinase, atrolysin C (form d) Proc Natl Acad Sci. 1994;91:8447–8451. doi: 10.1073/pnas.91.18.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagase H., Woessner J.F. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 36.Steward W.P., Thomas A.L. Marimastat: the clinical development of a matrix metalloproteinase inhibitor. Expet Opin Invest Drugs. 2000;9:2913–2922. doi: 10.1517/13543784.9.12.2913. [DOI] [PubMed] [Google Scholar]
- 37.Albulescu L.O., Xie C., Ainsworth S., Alsolaiss J., Crittenden E., Dawson C.A. A combination of two small molecule toxin inhibitors provides pancontinental preclinical efficacy against viper snakebite. bioRxiv. 2020 doi: 10.1038/s41467-020-19981-6. https://www.biorxiv.org/content/10.1101/2020.05.13.094599v1 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda S., Takeya H., Iwanaga S. Snake venom metalloproteinases: structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim Biophys Acta Protein Proteonomics. 2012;1824:164–176. doi: 10.1016/j.bbapap.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Wax P.M. Current use of chelation in American health care. J Med Toxicol. 2013;9:303–307. doi: 10.1007/s13181-013-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawson M.K., Valko M., Cronin M.T.D., Jomová K. Chelators in iron and copper toxicity. Curr Pharmacol Rep. 2016;2:271–280. [Google Scholar]
- 41.Urs A.N.N., Yariswamy M., Ramakrishnan C., Joshi V., Suvilesh K.N., Savitha M.N. Inhibitory potential of three zinc chelating agents against the proteolytic, hemorrhagic, and myotoxic activities of Echis carinatus venom. Toxicon. 2015;93:68–78. doi: 10.1016/j.toxicon.2014.11.224. [DOI] [PubMed] [Google Scholar]
- 42.Peters R.A., Stocken L.A., Thompson R. British anti-lewisite (BAL) Nature. 1945;156:616. doi: 10.1038/156616a0. [DOI] [PubMed] [Google Scholar]
- 43.WHO model list of essential medicines, 20th list (March 2017, amended August 2017). Available from: https://www.who.int/medicines/publications/essentialmedicines/en/.
- 44.Verma S., Kumar R., Khadwal A., Singhi S. Accidental inorganic mercury chloride poisoning in a 2-year old child. Indian J Pediatr. 2010;77:1153–1155. doi: 10.1007/s12098-010-0143-9. [DOI] [PubMed] [Google Scholar]
- 45.Kathirgamanathan K., Angaran P., Lazo-Langner A., Gula L.J. Cardiac conduction block at multiple levels caused by arsenic trioxide therapy. Can J Cardiol. 2013;29 doi: 10.1016/j.cjca.2012.04.004. 130.e5-6. [DOI] [PubMed] [Google Scholar]
- 46.Ioannou P.V., Purchase R. Interaction of British anti-lewisite (BAL) with copper (I) and copper (II) compounds in conjunction with Wilson's disease. Main Group Chem. 2018;17:1–16. [Google Scholar]
- 47.Still K., Nandlal R.S., Slagboom J., Somsen G.W., Casewell N.R., Kool J. Multipurpose HTS coagulation analysis: assay development and assessment of coagulopathic snake venoms. Toxins. 2017;9:382. doi: 10.3390/toxins9120382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slagboom J., Mladić M., Xie C., Kazandjian T.D., Vonk F., Somsen G.W. High throughput screening and identification of coagulopathic snake venom proteins and peptides using nanofractionation and proteomics approaches. PLoS Neglected Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0007802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie C., Slagboom J., Albulescu L.O., Bruyneel B., Still K., Vonk F.J. Antivenom neutralization of coagulopathic snake venom toxins assessed by bioactivity profiling using nanofractionation analytics. Toxins. 2020;12:53. doi: 10.3390/toxins12010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Otero-Patiño R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009;54:998–1011. doi: 10.1016/j.toxicon.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Mora Rodríguez J.F., Mora Rodríguez R., Lomonte B., Gutiérrez J.M. Effects of Bothrops asper snake venom on lymphatic vessels: insights into a hidden aspect of envenomation. PLoS Neglected Trop Dis. 2008;2:e318. doi: 10.1371/journal.pntd.0000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamashita K.M., Alves A.F., Barbaro K.C., Santoro M.L. Bothrops jararaca venom metalloproteinases are essential for coagulopathy and increase plasma tissue factor levels during envenomation. PLoS Neglected Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang E.L.H., Tan C.H., Fung S.Y., Tan N.H. Venomics of Calloselasma rhodostoma, the Malayan pit viper: a complex toxin arsenal unraveled. J Proteomics. 2016;148:44–56. doi: 10.1016/j.jprot.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Chen P.C., Huang M.N., Chang J.F., Liu C.C., Chen C.K., Hsieh C.H. Snake venom proteome and immuno-profiling of the hundred-pace viper, Deinagkistrodon acutus, in Taiwan. Acta Trop. 2019;189:137–144. doi: 10.1016/j.actatropica.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Kraisawat K., Promwang N. Duration after Malayan pit viper bite to detect coagulopathy in Songklanagarind hospital. J Health Sci Med Res. 2020;38:93–101. [Google Scholar]
- 56.Su H.Y., Huang S.W., Mao Y.C., Liu M.W., Lee K.H., Lai P.F. Clinical and laboratory features distinguishing between Deinagkistrodon acutus and Daboia siamensis envenomation. J Venom Anim Toxins Incl Trop Dis. 2018;24:43. doi: 10.1186/s40409-018-0179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bustillo S., García-Denegri M.E., Gay C., Van de Velde A.C., Acosta O., Angulo Y. Phospholipase A2 enhances the endothelial cell detachment effect of a snake venom metalloproteinase in the absence of catalysis. Chem Biol Interact. 2015;240:30–36. doi: 10.1016/j.cbi.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Bustillo S., Gay C.C., Denegri M.E.G., Ponce-Soto L.A., de Kier Joffé E.B., Acosta O. Synergism between baltergin metalloproteinase and Ba SPII RP4 PLA2 from Bothrops alternatus venom on skeletal muscle (C2C12) cells. Toxicon. 2012;59:338–343. doi: 10.1016/j.toxicon.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Montecucco C., Gutiérrez J.M., Lomonte B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: common aspects of their mechanisms of action. Cell Mol Life Sci. 2008;65:2897–2912. doi: 10.1007/s00018-008-8113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lomonte B., Gutiérrez J.M. Phospholipases A2 from viperidae snake venoms: how do they induce skeletal muscle damage?. Acta Chim Slov. 2011;58:647–659. [PubMed] [Google Scholar]
- 61.Lewin M.R., Gilliam L.L., Gilliam J., Samuel S.P., Bulfone T.C., Bickler P.E. Delayed LY333013 (oral) and LY315920 (intravenous) reverse severe neurotoxicity and rescue juvenile pigs from lethal doses of Micrurus fulvius (Eastern coral snake) venom. Toxins. 2018;10:479. doi: 10.3390/toxins10110479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.