Abstract
Background
Follicular unit extraction (FUE) is the most popular method of hair transplantation in today's world. Hair transplantation in androgenetic alopecia (AGA) in males can restore the frontal hairline and provide hair density in alopecic areas to the satisfaction of most patients.
Methods
Consecutive male patients of AGA who underwent hair transplantation by FUE method in two centers between the period of January 2016 and June 2017 have been included in this study based on inclusion and exclusion criteria. Photographic images, trichoscopy and Likert's scale were used to assess patient's improvement in hair density after the transplantation procedure. Statistical methods using SPSS software was used to analyze the results. Institutional ethical clearance and patients' written consent for procedure and images was obtained. The study was an observational retrospective study using data and images from records for which consent and ethical clearance was obtained from patients and the institution.
Results
Average number of follicular units transplanted in patients was 1290 (improvement in hair density: of 30.61 follicular units/sq cm). There was a statistically significant difference in improvement in hair density in patients younger than 33 years and in patients with Norwood classification below stage 4a. Forty-nine patients were satisfied with the results after assessment by the Likert scale.
Conclusion
Hair transplantation by follicular extraction method provides good hair cover in AGA in males. This modern dermatosurgical technique with its many innovations is a very helpful technique to improve quality of life in male pattern baldness.
Keywords: Hair transplantation, Follicular unit extraction, Androgenetic alopecia, Hair density, Follicles, Implantation
Introduction
Hair transplantation for androgenetic alopecia (AGA) has come a long way from the age where large punches were used to extract hair and also to implant them back. It has evolved mainly with the aim of making the results look more and more natural. It is in the present day, one of the most exciting and innovative fields in dermatological surgery and in general esthetic practise. Hair transplantation is mainly performed by two methods presently: follicular unit transplantation (FUT) and follicular unit extraction (FUE). In FUT, donor hair harvesting is carried out using single-strip method with elliptical excision of donor hair form occipital scalp, followed by suturing. The disadvantage of this technique is the resulting linear donor scar. In addition, an entire team was required to be present for microdissecting the donor strip into individual follicles. With better understanding of the anatomy of the hair follicle units, FUE has nowadays become the procedure of choice in hair transplantation. We herein present an observational study of the procedure and results of hair transplantation by FUE procedure.
Materials and methods
This retrospective observational study included 52 patients of AGA who underwent hair transplantation by FUE method in two hospitals between January 2016 and October 2017. The inclusion criteria were, patients between age range of 24–50 years and having Norwood grading 2-5 staging. Young patients in whom the alopecia was still evolving, rapidly progressing AGA, patients with Norwood grade 6 or 7 with poor density and grade 1 where hair loss was very minimal, unrealistic expectations and significant systemic health problems were not included. Patients having any medical contraindications like hypertension, diabetes, coronary artery disease, and other systemic illnesses were not included. Patients who were willing to take medications and follow the postoperative and preoperative advise strictly were alone included. Trichoscopy and photographic images were used to assess the improvement of hair growth. The patients were divided into two groups based on the median age and stage of the disease.1 The comparisons were made between the two groups based on age and stage of disease. The assessment was carried out at baseline, i. e., before transplantation and 9 months after the transplantation procedure. The Likert scale (1 = no growth, 2 = very less growth, 3 = satisfactory growth, 4 = good growth, 5 = excellent growth) was used to assess the satisfaction expressed by the patients. Statistical calculations were performed using the SPSS software and included estimation of median, standard error of means, t test, chi-square test, and Mann-Whitney U test for the various variables. This being a novel study, all participants during the study period fulfilling the criteria were included in the study. Post hoc power calculation for the two primary outcome measures, i.e., age and stage as well as their association with mean hair density after treatment was 91.82% and 94.82%, respectively. The study was cleared by the Institutional Ethics Committee of both the centers. A written informed consent was taken from all patients undergoing the procedure.
Procedure
Preprocedure steps
The patients were chosen meticulously for the procedure. Preprocedure advise included stoppage of minoxidil 2 weeks before the procedure as it may increase bleeding, to avoid smoking, and intake of aspirin and non steroidal anti-inflammatory drugs (NSAIDS) 7 days before surgery. Preoperative photographs were taken and trichoscopic assessment was performed. Laboratory investigations including hematocrit, clotting parameters, blood chemistry profile including sugar, renal and liver functions, urine routine, electrocardiogram, chest radiograph, antibodies for hepatitis B surface antigen and hepatitis C, human immunodeficiency tests and venereal disease research laboratory test (VDRL) were carried out.
Procedural steps
-
1.
Hair was trimmed to 1.5–2 mm size by using hair clippers or patient was asked to take a zero haircut 36 h before procedure.
-
2.
Scalp was washed with betadine, spirit, and then saline.
-
3.
Local anesthesia solution: 15 ml of lignocaine, 2% with adrenaline, and 15 ml saline with 5 ml of 0.5% bupivacaine. Tumescent anesthesia solution: 90 ml saline with 1 ampule 1:1 vial triamcinolone acetonide 40 mg/ml and 5 ml 0.5% bupivacaine.
-
4.Donor area preparation
-
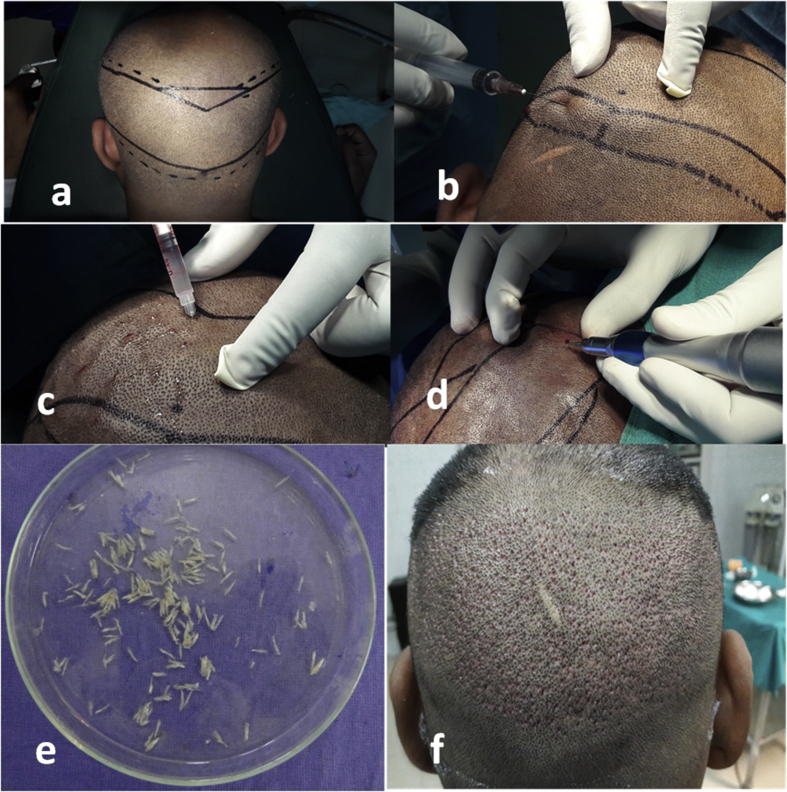
a.Marking of safe donor area: Using paper scale and permanent marker as shown in Fig. 1a.
- b.
-
c.Extraction of follicles was carried out by first creating punches in the scalp with sharp 0.8–0.9 mm punches held in an electrical micromotor (Fig. 1d) and then extracting them with single forceps or double forceps method. Magnification headgears were used (2.5×) to aid better viewing.
-
d.Extracted follicles were collected in Petri dishes filled with cold ringer lactate solution (Fig. 1e). Single hair follicular units were separated out for hairline use at this stage itself.
-
e.Each Petri dish was kept on iceboxes to maintain cold temperature and transferred to the refrigerator handle once 200 grafts were collected in them.
-
f.Donor area (Fig. 1f) was then dressed.
-
a.
-
5Recipient area
-
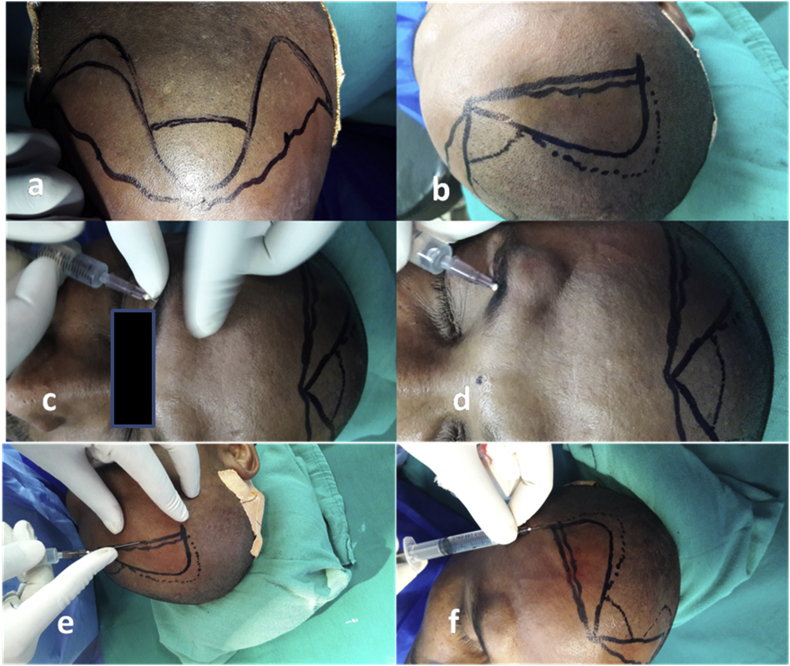
a.The recipient area was marked. The frontal hairline was given a wavy look. Areas which required the most hair were highlighted (Fig. 2a and b).
-
b.Anesthesia: Supratrochlear and supraorbital nerve blocks were carried out on both sides (Fig. 2 c and d). Only the lateral most areas which were not covered by these blocks were injected locally (Fig. 2e and f). Tumescent anesthesia was performed as for donor area. This helped by widening the area for more slits to be placed and also reduced bleeding.
-
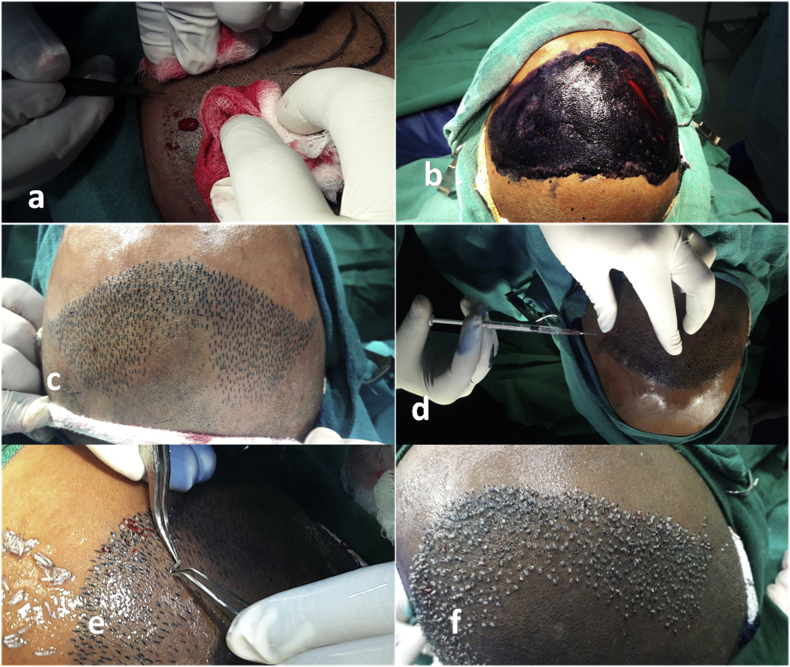
c.Slits were then made in the recipient area using either Kolkata slits or blades fitted into forceps (blades, 1.2mm/1.4 mm wide and 4–5 mm in length) (Fig. 3a). Slits were placed in the direction of already existing hair in the area so that a natural look could be created after transplant. In case there was no hair present in the recipient area, the general direction indicated by hair from surrounding regions or based on standard protocols were used.
- d.
-
e.The harvested follicular units were placed into the slits using two fine-angled forceps. The units were handled with minimal trauma especially to the roots. The slits were slightly enlarged by a pair of forceps held in the left hand, and the grafts were held with a pair of second forceps not touching the bulb and inserted into the slit (Fig. 3e). A small portion of top of the graft was left outside the slit. Two persons implanted on either side to fasten the process.
-
f.The insertion of follicles was carried out from front to back to avoid popping and accidental touching of implanted grafts of back rows while handling front rows (Fig. 3f). The recipient site was cleaned frequently with saline filled in a sprinkler to avoid clogging of blood.
-
g.Dressing: A bandage was given for the donor area for about 5 days to prevent bleeding and unpleasant sensations while lying down. No dressing was given for recipient area. To prevent swelling of periorbital regions, a headband kind of bandage was tied on the forehead just above the eyebrows which was to be removed after 24 h.
-
a.
Fig. 1.
Donor area preparation: (a) Marking of safe donor area, (b) local anesthesia, (c) tumescent anesthesia, (d) punch extraction of follicles, (e) collection of follicles in ringer lactate Petri dishes, and (f) dressing after extraction.
Fig. 2.
Preparation of recipient area: (a & b) Highlighting the areas requiring the most hair, (c & d) supratrochlear and supraorbital nerve blocks, and (e & f) injecting lateral areas not covered by the blocks.
Fig. 3.
(a) Making slits, (b & c) making the slits more prominent using methylene blue application, (d) tumescent anesthesia after slit making, (e) implantation of follicles into the premade slits, and (f) front to back implantation.
Postprocedure steps
The patient was detained in the (Opertion Theatre) OT 30 min after procedure. Postoperative medication advise included oral prednisolone 40 mg daily, tablet ibuprofen 400 mg plus paracetamol 500 mg 1 tablet twice daily, tablet ranitidine 150 mg 1 tablet twice daily, capsule Augmentin 1g twice daily, all for 5 days. Patients were instructed to lie straight supine, to spray saline every 2 h for atleast 2 weeks after the procedure to prevent dessication of grafts and not to wear anything which would cause friction on the transplanted hair (either while dressing or undressing) such as T shirts, helmets or any other head gear for 4 weeks. Minoxidil was to be resumed 2 weeks later. Soft shampooing was started after 2 weeks and regular shampooing after 4 weeks.
Results
Of the 52 patients who underwent hair transplantation, age range was between 25 and 48 years. Norwood stages 2–5 were all represented. The average hair density in the recipient areas before transplantation was 6.21 follicular units/sq cm (range: 0–18). Average number of follicular units transplanted was 1290.48 (range: 976–2400). The average recipient area follicular density after transplantation was 36.82 follicular units/sq cm (range: 28–51). Thus an improvement in hair density of 30.61 follicular units/sq cm on an average was achieved. Transection rate was 4% in our study. Implantation speed was 400 per hour in this study. Patient assessment scales showed 4 patients with satisfaction level 5, 25 patients with satisfaction level 4, 20 patients with satisfaction level 3, and 3 patients with satisfaction level 2.
The other important findings of the study were as follows:
-
1.
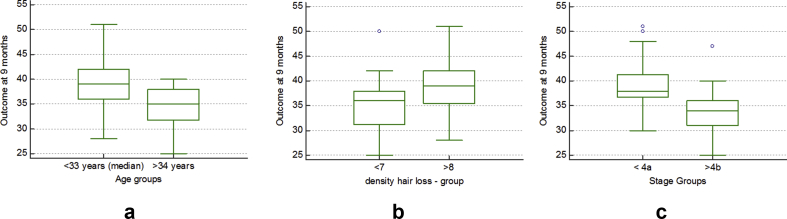
Older the age at transplant, lesser was the improvement in density as expected. With median taken as 33 years, there was a statistically significant difference in improvement in hair density in patients less than 33 years when compared with those above 33 years, as assessed by the t test for difference between means (Table 1).
-
2.
Stage of Norwood classification before transplantation was the other significant variable found to influence the outcome. With hair loss taken as moderate below stage 4a, those with hair loss below stage 4a at initial assessment were found to have statistically significant better outcome than those beyond stage 4a, as assessed by the t test for difference between means (Table 1 and Fig. 4).
-
3.
Initial density was between ranges of 0–18. That did not seem to have a major bearing on the results (Table 1).
-
4.
The satisfaction levels of patients after transplantation had no association with either the age, stage of hair loss, or initial density of hair loss as assessed by the Mann-Whitney U test. The 2-tailed significance was 0.410, 0.551, and 0.951 for the age groups (<33 years and 33 or more years), stage of initial alopecia (<stage 4a, 4b, and beyond) and initial density of hair (<7, 8 and >8 hairs/sq cm), respectively.
-
5.
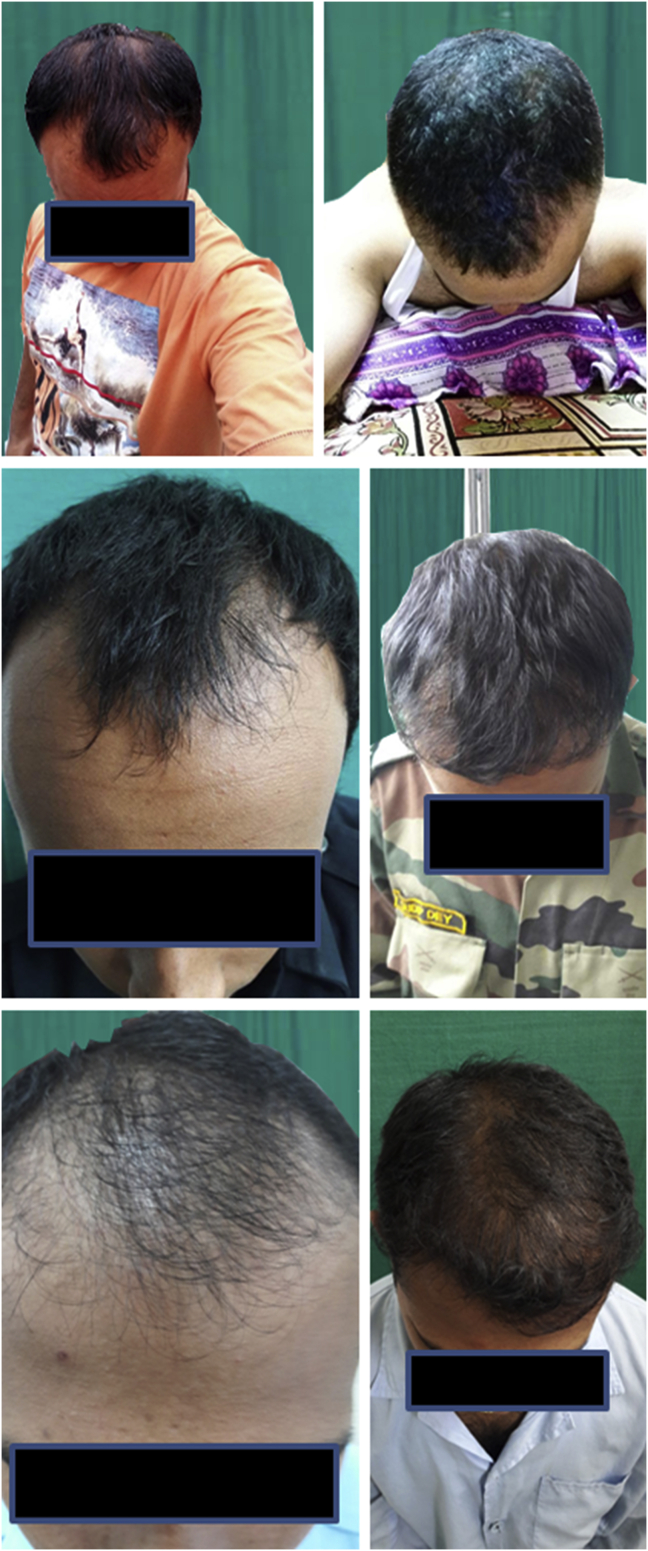
Younger patients with grade 2–3 Norwood had better improvements in density even with lower number of follicular units transplanted after duration of 9 months (Fig. 5).
-
6.
Hairline was satisfactory in all patients.
-
7.
All patients had well healed scars after 8 weeks of surgery in donor area.
-
8.
Good hair growth was obtained after 9 months of surgery.
Table 1.
t test for difference between means for outcome of hair density per sq cm for various variables after procedure.
| Variable | n = 52 | Mean hair density per sq cm | Standard error mean |
t test for equality of means |
|
|---|---|---|---|---|---|
| Mean difference | Significance (2-tailed) | ||||
| Age groups | |||||
| <33 years (median) | 27 | 39.00 | 1.08 | 4.52 | 0.002 |
| >34 years | 25 | 34.48 | 0.81 | ||
| Stage of initial alopecia before procedure | |||||
| <4a | 29 | 38.97 | 0.90 | 4.84 | 0.001 |
| >4b | 23 | 34.13 | 1.01 | ||
| Initial density of hair per sq cm | |||||
| <7 | 31 | 35.42 | 0.89 | −3.49 | 0.694 |
| >8 | 21 | 38.90 | 1.19 | ||
Fig. 4.
Graphs showing the outcome with respect to (a) age, (b) hair density, and (c) stage of hair loss.
Fig. 5.
Good results in 3 young patients with preprocedure images on the left and post procedure images on the right.
The complications noted were poor growth in 2 patients, small pustules in the recipient area which subsided with antibiotics in 1 patient, mild paresthesia in the donor region lasting for 21 days in one patient and heaviness of the head in the recipient area lasting for 10 days in one patient.
We also noticed that the following methods were very user-friendly and contributed to the good results and patient comfort:
-
1.
Use of sterilized garden sprinkler to sprinkle saline
-
2.
Needles of 0.5 and 1.5 inch were used for nerve block and injection locally into area along with insulin syringes for tumescence and were found to be the least painful.
-
3.
Adding tumescence injections after the slits are made to the recipient area, was found to reduce bleeding further and also made implantation faster.
-
4.
Dressing with the forehead band led to a zero incidence of periorbital edema.
Discussion
Androgenetic alopecia (AGA) is the commonest form of hair loss in men.1 The loss especially which occurs with increasing age in genetically susceptible individuals follows a stereotypical pattern beginning with bitemporal region and progressing to the vertex. The occipital area is usually spared.2
Hair transplantation can be performed by 2 main methods: in the first, method called, follicular unit transplantation (FUT), a strip of hair is harvested from occipital scalp, whereas in the second method called follicular unit extraction (FUE)., the individual follicular units are harvested from the occipital scalp.3 The advantages of FUE over FUT are that no scar is usually present in donor area and therefore patients can keep hair short after surgery. The FUE method is ideal for patients who have less scalp laxity, those who have undergone the strip method in the past, those who are prone to hypertrophic scarring, where hair from other parts of the body has to be used and where the postoperative care needs to be minimal. FUT is labor intensive, time consuming, needs more assistants, and also needs microscopic dissection of hairs, which requires more skill.4
In the standard FUE procedure, extraction time for all the grafts varies from 1 to 3 h. In our study, it was 2.5–3 h (400/hr X 2 extractors working on both sides). Good tumescence and a waiting period of 10–15 min after giving local anesthesia before any of the procedural steps is important for maintaining a pain-free surgery period and to reduce bleeding. The tumescent anesthesia helps in two ways: to lift up the follicles and to get a better angle for extraction. It also helps to lift up the site of extraction away from the blood vessels and therefore reduces bleeding. It also reduces the volume of local anesthetic used and hence reduces the chances of any side effects. The area becomes turgid after infiltration and gives a good base too for extraction.
Good lighting, quality punches, and good forceps are essential for efficacy of extraction. This minimizes transection of follicles.5 Ideal transection rate is now as low as 6%.6 In our study, the transection rate was satisfactory at 4%.
Proper cooling and hydration of donor grafts are very important for survival of grafts throughout the surgery. The extraction leaves behind tiny wounds which heal well within 2 weeks almost leaving nonvisible scars in the donor area. In our study, all patients had well-healed scars after about 8 weeks of surgery.
Planning of the hairline is the most artistic part as also important part of the transplantation. A saw-toothed irregular hairline is ideal. Slits can also be made with No. 18/20/23 gauge needle in the same pattern. About 250–300 follicular units are required to make a good hairline. This must be remembered while counting the number of slits to be made. Single hair grafts are usually implanted in the frontal hairline to create a wavy natural look. Further behind 2–3 hair units are used to bring up the bulk. Hairline was satisfactory in all patients in our study.
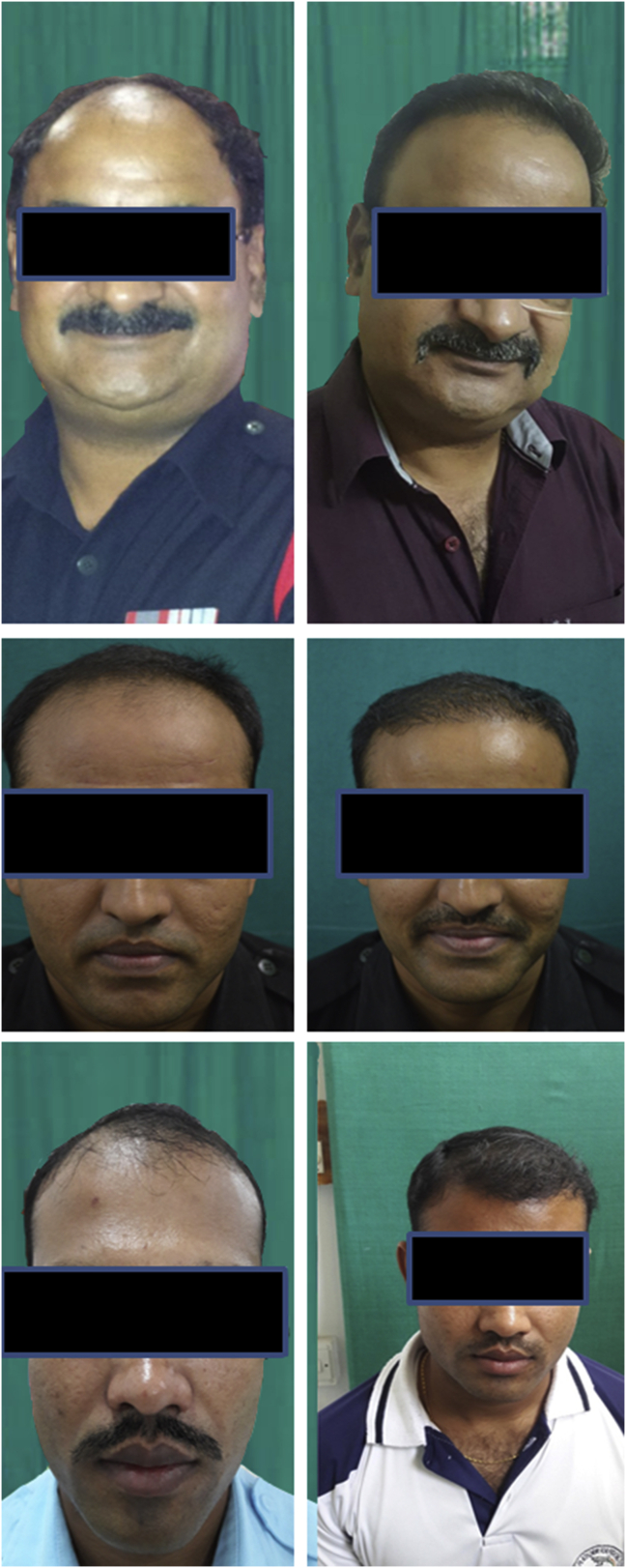
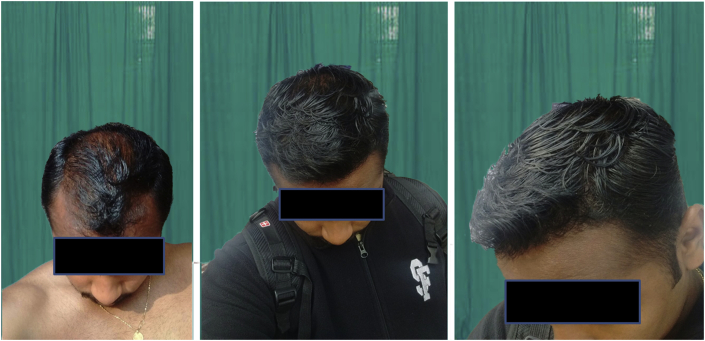
After transplantation, the implanted hair loose their fat and most of the dermis in about 2–4 weeks and may either continue to grow or fall off leaving roots intact. The new hair start growing in the roots at about 12 weeks after the transplantation. Appreciable growth can be seen usually in about 6–9 months and was seen in our study too (Fig. 6, Fig. 7).
Fig. 6.
Good growth of hair after 9 months.
Fig. 7.
Good growth with proper frontal hairline after 9 months.
It is generally agreed that minimum density required for good cosmetic results is about 30–40/sq cm.7 Most of our patients achieved this density after transplantation. In a study on direct hair transplantation, hair growth became visible after 2–3 months and the acceptable “good” results were obtained after a follow-up from 8 to 18 months in 27 patients of 29.8 In our study, only 2 patients had less than 30/sq cm density after transplantation, thereby indicating that results and hair growth were good, both objectively as assessed by photography and trichoscopy and subjectively as assessed by the Likert scale.
Complications are rare. In one study, complications were reported in 4.7% of patients. The complications included hypertrophic scar, folliculitis, donor area necrosis, bleeding, and infection.9 Other complications which can occur are hypoaesthesia or paresthesia in the donor area which is usually only temporary. In our study, one patient had paresthesia in the donor area which subsided after 21 days.
Postoperative edema was reported as the commonest complication (42.47%) in a study on 73 patients, outlining complications of both FUE and FUT. The swelling of forehead or eyelids occurred 2–6 days after the operation in this study.10 In our study, the use of a forehead bandage, oral prednisolone, and triamcinolone in the tumescent solution probably resulted in absence of this complication.
True infections occur very infrequently. Folliculitis in the donor/recipient area has been observed in up to 20% patients in few studies.11,12 Sterile folliculitis of recipient area is also a frequent complication occurring weeks to months after hair transplantation because of hair growing inwards, foreign body reactions, and piggybacking of grafts.13 Epidermal cysts can be seen at the recipient sites due to graft burial. Drainage may be required in some cases. In our study, 1 patient had pustules in the recipient area, which healed after antibiotics were given.
Delayed hair growth and poor growth can be other major drawbacks. Poor hair growth usually results from inferior technique, wrong patient selection, drying up of grafts or handling them roughly, faulty preparation of the graft, and unpredictable patient factors. Sometimes hair take about 1 year to grow and telogen effluvium can also cause appearance of poor hair growth. In our study, two patients had growth less than anticipated after 9 months.
Conclusion
FUE method of hair transplantation is an important modality of hair restoration in male AGA. Patients less than 33 years and with Hamilton stage 4a and less were found to have better results after the procedure in our study. With good surgical technique and proper patient selection, FUE can offer satisfactory results for cases of AGA in males.
Consent
Patient consent for all images has been obtained.
Conflicts of interest
The authors have none to declare.
References
- 1.Iacobuccia D., Posavac S.S., Kardes F.R., Schneider M.J., Popovich D.L. The median slit: robust, refined, and revived. J Constr Psychol. 2015;25:690–704. [Google Scholar]
- 2.Rebora A. Pathogenesis of androgenetic alopecia. Am Acad Dermatol. 2004;50:777–779. doi: 10.1016/j.jaad.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein R.M., Rassman W.R. Follicular unit transplantation. In: Haber R.S., Stough D.B., editors. Hair Transplantation. Elsevier Saunders; Philadelphia: 2006. pp. 91–98. [Google Scholar]
- 4.Rashid R.M., Morgan Bicknell L.T. Follicular unit extraction hair transplant automation: options in overcoming challenges of the latest technology in hair restoration with the goal of avoiding the line scar. Dermatol Online J. 2012;18:12. [PubMed] [Google Scholar]
- 5.Headington J.T. Transverse microscopic anatomy of the human scalp. Arch Dermatol. 1984;120:449–456. [PubMed] [Google Scholar]
- 6.Harris J.A. New methodology and instrumentation for follicular unit extraction: lower follicle transection rates and expanded patient candidacy. Dermatol Surg. 2006;32:56–61. doi: 10.1111/1524-4725.2006.32006. [DOI] [PubMed] [Google Scholar]
- 7.Limmer B. The density issue in hair transplantation. J CutanAesthetSurg. 1997;23:747–750. doi: 10.1111/j.1524-4725.1997.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 8.Pradeep S., Bansal A. Direct hair transplantation: a modified follicular unit extraction technique. J Cutan Aesthet Surg. 2013;6:100–105. doi: 10.4103/0974-2077.112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salanitri S., Gonçalves A.J., Helene A., Jr., Lopes F.H. Surgical complications in hair transplantation: a series of 533 procedures. Aesthet Surg J. 2009;29:72–76. doi: 10.1016/j.asj.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Eswari L., Sacchidanand S., Divya G., Deepak H.S., Maheshwari N.S., Revathi T.N. Complications of hair restoration surgery: a retrospective analysis. Int J Trichol. 2014;6:168–172. doi: 10.4103/0974-7753.142861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandro S., Gonçalves A.J., Américo H.J., Flavia H.J. Surgical complications in hair transplantation a series of 533 procedures. Aesthet Surg J. 2009;29:72–76. doi: 10.1016/j.asj.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Unger W.P. 3rd ed. Dekker; New York: 1995. Hair Transplantation; pp. 363–374. Complications of hair transplantation. [Google Scholar]
- 13.Vogel J.E., Jimenez F., Cole J. Hair restoration surgery: the state of the art. Aesthet Surg J. 2013;33:128–151. doi: 10.1177/1090820X12468314. [DOI] [PubMed] [Google Scholar]