Abstract
Purpose
Previous studies have noted increased utilization of perioperative chemotherapy over time. The goal of this study was to determine trends in perioperative chemotherapy use within a contemporary population.
Materials and Methods
The National Cancer Database was queried for patients diagnosed with cT2-4N0M0 urothelial muscle invasive bladder cancer from 2011 to 2015 and underwent subsequent radical cystectomy. We retrospectively analyzed factors associated with perioperative chemotherapy and evaluated overall treatment trends in the use of neoadjuvant and adjuvant chemotherapy. Linear regression, logistic regression, Cox regression, and Kaplan–Meier analysis were performed.
Results
In total, 7,101 patients met inclusion criteria for analysis. The use of perioperative chemotherapy increased from 46.4% in 2011 to 57.2% in 2015 (p=0.003). Neoadjuvant chemotherapy use increased from 22.9% to 32.3% (p=0.007) over the time period analyzed, while adjuvant chemotherapy use experienced no significant change (23.5% to 24.9%, p=0.182). Logistic regression demonstrated that increased age and Charlson Comorbidity Index were predictors of not receiving chemotherapy (p<0.05), while those with increasing T stage, income above $48,000, and insurance other than Medicaid or Medicare were more likely to receive perioperative chemotherapy (p<0.05). Kaplan–Meier analysis revealed patients receiving neoadjuvant chemotherapy had the best 5-year overall survival at 48.3% compared to adjuvant chemotherapy (42.6%) or no chemotherapy (37.8%) (p<0.001).
Conclusions
The increasing use of perioperative chemotherapy noted in prior studies has continued through 2015. Neoadjuvant chemotherapy appears to drive this increase while adjuvant chemotherapy utilization remains unchanged. Clinical and socioeconomic factors affect utilization of perioperative chemotherapy.
Keywords: Drug therapy, Neoplasm invasiveness, Survival, Urinary bladder neoplasms
INTRODUCTION
Urinary bladder cancer is the 6th most prevalent malignancy in the USA, comprising 5% of total cancer diagnoses, and 16,400 deaths in 2016. Roughly 20% to 30% of patients present with muscle invasive bladder cancer (MIBC) [1]. The standard of care for treating MIBC is considered to be radical cystectomy (RC) with bilateral pelvic lymph node dissection and consideration for neoadjuvant chemotherapy (NAC) in most clinical guidelines worldwide [2]. Furthermore, if patients do not receive NAC, published data supports the use of adjuvant chemotherapy (AC) for patients after RC if lymph nodes are found to be positive or the bladder tumor has advanced locally (pT3-4) [3].
In 2003, Level I evidence became available to support the use of cisplatin-based NAC in cT2-4aN0M0 MIBC [4]. Prior to the publication of such evidence supporting use of NAC in 2003, as few as 11% of patients with MIBC received NAC prior to RC [5]. Data from multiple studies support the findings of significant growth in the use of perioperative chemotherapy (POC) in treating MIBC from 2003 to 2010; however, several barriers to high quality bladder cancer treatment in underserved populations have been identified [6,7].
The primary goal of this study was to determine if the trends previously described have continued in a contemporary population (2011 to 2015) using data from the National Cancer Database (NCDB). We aimed to identify clinical and demographic factors that were predictive of NAC use. We hypothesized that NAC use would increase over the study period.
MATERIALS AND METHODS
The NCDB is a joint quality improvement project produced by the American Cancer Society and the American College of Surgeons. It is the largest clinical cancer registry in the world, capturing data from 30% of US hospitals and 70% of all patients newly diagnosed with cancer [8]. Categories of data collected include patient factors, tumor characteristics, staging details, adjuvant treatments, and outcomes, all of which is recorded using nationally standardized coding guidelines. All information is collected in a Health Insurance Portability and Accountability Act compliant manner. Data submitted to the NCDB undergoes extensive quality monitoring and validity reviews on an annual basis [9]. The data utilized in this study came from the publicly shared and deidentified NCDB data set, also known as the Participant User File [8]. Approval by the institutional review board was not necessary because no patient or hospital identifiers were analyzed. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for analytic or statistical methodology employed or the conclusions drawn from these data by the investigator.
The NCDB bladder cancer dataset was queried for patients without prior malignancy who were diagnosed with cT2-4N0M0 urothelial MIBC from 2011 to 2015 and underwent subsequent RC. Patients were excluded if metastatic disease was present prior to RC or if they had received prior radiation therapy. Only cases coded with urothelial cell carcinoma were included. All patients undergoing RC were divided into 3 cohorts: no POC, NAC, or AC. Patients who received both NAC and AC were excluded. All three groups were then compared in terms of basic, clinical and pathologic parameters. Demographic information analyzed included age, gender, race, income status, distance traveled to facility, facility type, geographic region, and insurance status. Of note, treatment facility type was categorized as low volume or high volume. Treatment facilities that accrued 500 or more newly diagnosed cancer cases per year were considered high-volume (including academic centers), whereas facilities with less than 500 were labeled low-volume. Regions where patients received care was divided into East, Central, and West location. Clinical information included Charlson Comorbidity Index (CCI) and clinical stage. Perioperative and survival parameters analyzed included time from diagnosis to surgery, unplanned 30-day readmission after surgery, length of hospital stay, pathologic staging, node positivity, presence of positive margins, length of follow-up, and mortality (overall, 30-, and 90-day). The chemotherapeutic agents and number of cycles are not described within the NCDB.
Student's t-test was performed for continuous variables, and Fischer's exact or Pearson chi-square tests for categorical variables. Logistic regression analysis for any chemotherapy use included age, CCI, clinical stage, income, geographic region, and insurance status. These factors were chosen as they were significantly different between cohorts (p<0.05) on an unadjusted analysis, and these factors were available preoperatively to guide the decision for NAC. Other factors, including positive surgical margins or pathologic node positive disease, are not available to assist with NAC determination in the preoperative setting. As a sensitivity analysis, we included all preoperative factors regardless of significance. Cox regression analysis for all-cause mortality was conducted using age, CCI, cT stage, and chemotherapy use. These factors were chosen as they were felt to be important clinical determinants for choosing NAC. Kaplan–Meier survival analysis was also completed for No POC, NAC, and AC. We utilized IBM SPSS Statistics ver. 25.0 (IBM Corp., Armonk, NY, USA) for all analyses, with p-value of <0.05 denoting statistical significance. Our primary outcome was chemotherapy prevalence. Our secondary outcomes included predictors of receiving chemotherapy and overall survival stratified by POC use.
RESULTS
A total 7,101 patients were identified, and preoperative variables are noted in Table 1. The majority of patients were male (74.2%) and mean age of participants was 68.4 years. Three cohorts were created, those who did not receive POC prior to or after RC, those who received NAC prior to RC, and those who received AC after RC. The no POC cohort contained data from 3,413 patients, while the group receiving NAC contained data from 1,937 patients and 1,751 patients comprised the AC group. When compared across three groups, each was similar in regard to gender, facility type, geographic location, or race. Significantly more patients with CCI of 0 received POC while those with CCI of 2 or 3+ were significantly less likely to receive POC. For example, in the no POC cohort 64.8% had CS of 0, while in the NAC and AC cohorts the 72.6% and 70.5% had a CCI of 0, respectively. There was a higher proportion of cT2 stage in the no POC cohort, as compared to any chemotherapy group, with 82.0% of the no POC cohort staged as cT2 and about 75% of the NAC and AC cohorts staged cT2. Patients receiving any POC tended to have higher income status, as 29.0% of the no POC group was classified with yearly income of ≥$63,000, in contrast to the NAC and AC cohorts with 34.0% and 32.3% classified with ≥$63,000. Concordantly, patients receiving any POC had higher rates of private insurance compared to no POC (34.2% to 37.3% vs. 22.7%).
Table 1. Patient demographics and clinical tumor characteristics.
| Variable | All (n=7,101) | No POC (n=3,413) | NAC (n=1,937) | AC (n=1,751) | p-value |
|---|---|---|---|---|---|
| Mean age (y) | 68.4±10.0 | 71.3±9.9 | 65.4±9.4 | 66.1±9.2 | <0.001 |
| <50 | 307 (4.3) | 94 (2.8) | 125 (6.5) | 88 (5.0) | |
| 51–60 | 1,216 (17.1) | 411 (12.0) | 433 (22.4) | 372 (21.2) | |
| 61–70 | 2,401 (33.8) | 947 (27.7) | 763 (39.4) | 691 (39.5) | |
| >70 | 3,177 (44.7) | 1,961 (57.5) | 616 (31.8) | 600 (34.3) | |
| Male (sex) | 5,269 (74.2) | 2,496 (73.1) | 1,448 (74.8) | 1,325 (75.7) | 0.115 |
| Race | 0.544 | ||||
| White | 6,479 (91.2) | 3,119 (91.4) | 1,751 (90.4) | 1,609 (91.9) | |
| Black | 418 (5.9) | 195 (5.7) | 128 (6.6) | 95 (5.4) | |
| Other | 204 (2.9) | 99 (2.9) | 58 (3.0) | 47 (2.7) | |
| CCI | <0.001 | ||||
| 0 | 4,852 (68.3) | 2,211 (64.8) | 1,406 (72.6) | 1,235 (70.5) | |
| 1 | 1,644 (23.2) | 845 (24.8) | 407 (21.0) | 392 (22.4) | |
| 2 | 466 (6.6) | 273 (8.0) | 98 (5.1) | 95 (5.4) | |
| 3+ | 139 (2.0) | 84 (2.5) | 26 (1.3) | 29 (1.7) | |
| cT Stage | <0.001 | ||||
| 2 | 5,585 (78.7) | 2,800 (82.0) | 1,465 (75.6) | 1,320 (75.4) | |
| 3 | 796 (11.2) | 328 (9.6) | 246 (12.7) | 222 (12.7) | |
| 4 | 720 (10.1) | 285 (8.4) | 226 (11.7) | 209 (11.9) | |
| Income status | 0.005 | ||||
| <$38,000 | 1,071 (15.1) | 548 (16.1) | 274 (14.1) | 249 (14.2) | |
| $38,000–47,999 | 1,798 (25.3) | 903 (26.5) | 463 (23.9) | 432 (24.7) | |
| $48,000–62,999 | 1,995 (28.1) | 958 (28.2) | 538 (27.8) | 499 (28.5) | |
| ≥$63,000 | 2,214 (31.2) | 991 (29.0) | 658 (34.0) | 565 (32.3) | |
| Unknown | 23 | 13 | 4 | 6 | |
| Facility type | 0.825 | ||||
| High volume | 6,054 (85.3) | 2,919 (85.5) | 1,647 (85.0) | 1,488 (85.0) | |
| Low volume | 1,047 (14.7) | 494 (14.5) | 290 (15.0) | 263 (15.0) | |
| Geographic location | 0.142 | ||||
| Eastern | 2,826 (100) | 1,314 (46.5) | 786 (27.8) | 726 (25.7) | |
| Central | 3,075 (100) | 1,515 (49.3) | 835 (27.2) | 725 (23.6) | |
| Western | 1,162 (100) | 570 (49.1) | 298 (25.6) | 294 (25.3) | |
| Insurance status | <0.001 | ||||
| Uninsured | 145 (2.0) | 67 (2.0) | 37 (1.9) | 41 (2.3) | |
| Private | 2,096 (29.5) | 776 (22.7) | 722 (37.3) | 598 (34.2) | |
| Medicaid | 359 (5.1) | 146 (4.3) | 111 (5.7) | 102 (5.8) | |
| Medicare | 4,329 (61.0) | 2,365 (69.3) | 1,004 (51.8) | 960 (54.8) | |
| Other govt | 79 (1.1) | 27 (0.8) | 29 (1.5) | 23 (1.3) | |
| Unknown | 93 (1.3) | 32 (0.9) | 34 (1.8) | 27 (1.5) |
Values are presented as mean±standard deviation, number (%), or number only.
POC, perioperative chemotherapy; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy; CCI, Charlson Comorbidity Index; Govt, government.
Table 2 highlights perioperative and survival outcomes. Hospital stay following surgery was significantly longer in those who received POC versus those who did not (p<0.001). As expected, those with positive nodes (pN+) were most likely to receive AC (39.4%) when compared to patients receiving NAC or no POC at all (23.2% vs. 22.4%, p<0.001). Length of follow-up in months was significantly shorter in those who did not receive POC (25.5 months) compared to 28.9 months in NAC patients and 28.4 months in AC patients. Post-RC 30-day mortality was highest for those who did not receive POC at 3.7%, as opposed to NAC patients (1.6%) or AC users (0.7%) (p<0.001). Mortality within 90 days of treatment was most likely in those who did not receive POC treatment (9.8%), while it was 5.7% in NAC group and 2.8% in AC group (p<0.001).
Table 2. Perioperative and survival outcomes.
| Variable | All (n=7,101) | No POC (n=3,413) | NAC (n=1,937) | AC (n=1,751) | p-value |
|---|---|---|---|---|---|
| Days from diagnosis to cystectomy | 97.4±70.0 | 59.6±43.8 | 160.0±56.6 | 101.8±74.0 | <0.001 |
| Unplanned 30 day readmission after surgery | 641 (9.0) | 313 (9.2) | 184 (9.5) | 144 (8.2) | 0.370 |
| Hospital stay | 8.9±7.9 | 9.7±8.6 | 8.3±7.2 | 8.0±7.2 | <0.001 |
| pT stage | <0.001 | ||||
| <2 | 970 (13.7) | 190 (5.6) | 533 (27.5) | 247 (14.1) | |
| 2 | 1,735 (24.4) | 1,054 (30.9) | 407 (21.0) | 274 (15.6) | |
| 3 | 2,729 (38.4) | 1,420 (41.6) | 570 (29.4) | 739 (42.2) | |
| 4 | 1,290 (18.2) | 616 (18.0) | 299 (15.4) | 375 (21.4) | |
| Unknown | 377 (5.3) | 133 (3.9) | 128 (6.6) | 116 (6.6) | |
| Node positive | 1,906 (26.8) | 766 (22.4) | 450 (23.2) | 690 (39.4) | <0.001 |
| Positive margins | 883 (12.4) | 433 (12.7) | 179 (9.2) | 271 (15.5) | <0.001 |
| Length of follow-up (mo) | 27.1±17.2 | 25.5±18.0 | 28.9±16.3 | 28.4±15.9 | <0.001 |
| Mortality (all pts) | 2,620 (36.9) | 1,384 (40.6) | 597 (30.8) | 639 (36.5) | <0.001 |
| Within 30 days of treatment | 135 (1.9) | 102 (3.0) | 23 (1.2) | 10 (0.6) | <0.001 |
| Within 90 days of treatment | 396 (5.6) | 274 (8.0) | 83 (4.3) | 39 (2.2) | <0.001 |
Values are presented as mean±standard deviation or number (%)
POC, perioperative chemotherapy; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy.
On logistic regression analysis for receiving any POC, we analyzed preoperative factors found to be significantly different on unadjusted analysis (Table 3). This included age, CCI, clinical stage, income, and insurance type. Of these variables, age group greater than 60 (odds ratio [OR], 0.71 to 0.29) and CCI (OR, 0.59 to 0.85) were associated with decreased odds of receiving POC. Conversely, increasing clinical stage, increasing income, and insurance status were independently associated with increased odds of receiving POC. Those with cT3 (OR, 1.44) and cT4 (OR, 1.49) were at increasing odds, compared to cT2. Yearly income >$48,000 (OR, 1.22 to 1.36) was associated with increased odds, while Medicaid (OR, 1.29) and Medicare (OR, 1.36) were associated with decreased odds of receiving any POC. A sensitivity analysis including all preoperative factors, regardless of significance, was performed. Similar findings were noted (Supplementary Table 1).
Table 3. Logistic regression for any chemotherapy use.
| Variable | OR | 95% CI low | 95% CI high | p-value |
|---|---|---|---|---|
| Age group (<50 ref.) (y) | ||||
| 51–60 | 0.858 | 0.653 | 1.128 | 0.273 |
| 61–70 | 0.703 | 0.538 | 0.919 | 0.010 |
| >70 | 0.292 | 0.222 | 0.384 | <0.001 |
| CCI (0 ref.) | ||||
| CCI 1 | 0.853 | 0.759 | 0.959 | 0.008 |
| CCI 2 | 0.649 | 0.531 | 0.793 | <0.001 |
| CCI 3+ | 0.594 | 0.416 | 0.848 | 0.004 |
| cT stage (cT2 ref.) | ||||
| cT3 | 1.440 | 1.232 | 1.684 | <0.001 |
| cT4 | 1.488 | 1.263 | 1.754 | <0.001 |
| Income (<$38,000 ref.) | ||||
| $38,000–47,999 | 1.080 | 0.922 | 1.264 | 0.342 |
| $48,000–62,999 | 1.217 | 1.041 | 1.422 | 0.014 |
| ≥$63,000 | 1.364 | 1.169 | 1.592 | <0.001 |
| Insurance (uninsured ref.) | ||||
| Private | 1.581 | 1.118 | 2.234 | 0.010 |
| Medicaid | 1.290 | 0.868 | 1.916 | 0.207 |
| Medicare | 1.356 | 0.954 | 1.927 | 0.089 |
| Other govt or unknown | 2.419 | 1.513 | 3.866 | <0.001 |
OR, odds ratio; CI, confidence interval; Ref., reference; CCI, Charlson Comorbidity Index; Govt, government.
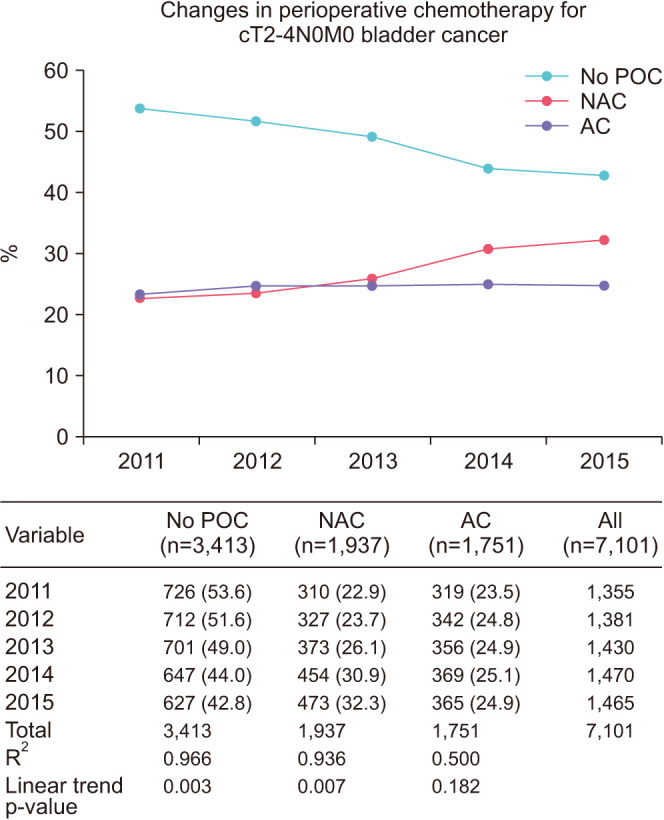
Trends of POC are graphically displayed in Fig. 1. The overall use of POC increased from 46.4% in 2011 to 57.2% in 2015 (p=0.003). NAC use increased from 22.9% to 32.3% (p=0.007), while AC use experienced no significant change (23.5% to 24.9%, p=0.182). Linear regression was performed and denoted by R2. Linear association was noted for increased use of NAC (R2=0.97) and correlated with a decrease in the use of no POC (R2=0.94); however, a linear association was not seen for AC use (R2=0.5).
Fig. 1. Stage presentation over time. POC, perioperative chemotherapy; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy.
Kaplan–Meier analysis for 5-year overall survival was performed. Analysis was revealed patients receiving NAC had the best 5-year overall survival at 48.3% compared to AC (42.6%) or no chemotherapy (37.8%) (log-rank p<0.001; Fig. 2). Cox regression for all-cause mortality was performed including factors that were assumed to be clinically important when determining use of chemotherapy prior to surgery (Table 4). Age, CCI, cT stage, and chemotherapy use were included, and the results indicate NAC and AC are both associated with survival benefit (hazard ratio, 0.78 and 0.87, respectively).
Fig. 2. Kaplan–Meier overall survival. POC, perioperative chemotherapy; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy; OS, overall survival; SD, standard deviation; SE, standard error.
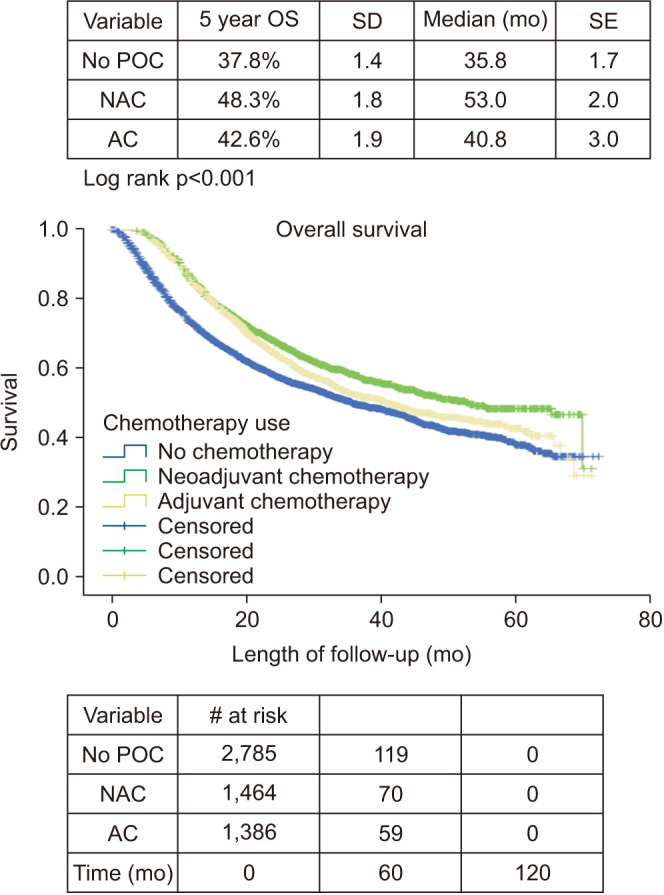
Table 4. Cox regression for all-cause mortality.
| Variable | HR | 95% CI low | 95% CI high | p-value |
|---|---|---|---|---|
| Age | 1.021 | 1.017 | 1.025 | <0.001 |
| CCI (0 ref.) | ||||
| CCI 1 | 1.146 | 1.048 | 1.254 | 0.003 |
| CCI 2 | 1.343 | 1.158 | 1.559 | <0.001 |
| CCI 3+ | 1.599 | 1.219 | 2.097 | 0.001 |
| cT stage (cT2 ref.) | ||||
| cT3 | 1.519 | 1.356 | 1.702 | <0.001 |
| cT4 | 1.706 | 1.519 | 1.916 | <0.001 |
| Chemotherapy (no chemo ref) | ||||
| NAC | 0.776 | 0.703 | 0.857 | <0.001 |
| AC | 0.865 | 0.786 | 0.952 | 0.003 |
HR, hazard ratio; CI, confidence interval; ref., reference; CCI, Charlson Comorbidity Index; NAC, neoadjuvant chemotherapy; AC, adjuvant chemotherapy.
DISCUSSION
Our review of the NCDB from 2011 to 2015 shows that trends in POC have continued to rise, driven primarily by the increase in NAC. Clinical factors such as increasing age and CCI were negative-predictors of receiving POC, while increasing clinical stage positively predicted POC. Additionally, non-clinical socioeconomic factors such as income status and insurance type were independently associated with utilization of POC. Furthermore, overall survival was best in the NAC group. Although rates of chemotherapy utilization continue to increase in the management of MIBC, significant clinical and non-clinical barriers may prevent at-risk populations from consideration of gold standard treatment.
Our analysis reveals increased utilization of POC throughout the study period. In a similar analysis from 2006 to 2010, NAC recipients more than doubled [7]. During this study period (2011 to 2015), there was an absolute increase in NAC recipients of 9.4%, equating to a relative increase of 41%. To providers, this appears to be an encouraging sign. The underlying factors that have led to increased utilization are not discerned from this analysis and deserve further research. Our identification of low income and certain insurance types having decreased odds of receiving chemotherapy may indicate socioeconomic disparities at play in this treatment decision. As different treatment modalities such as neoadjuvant immunotherapy or biomarker-based decision-making progress, the trend for increased NAC may develop further [10].
As previously mentioned, NAC is the main driver behind overall POC growth while AC did not see a significant change in utilization. AC use may lag for multiple reasons. For one, when compared to NAC there is a lack of adequate published literature, largely due to study limitations and small sample sizes [11,12]. Furthermore, there may be certain clinical factors, unmeasured in our analysis, that affect this treatment decision. Issues such as baseline renal function, comorbidity, or patient preference may affect patient and provider decision-making regarding the use of NAC. Our Kaplan-Meier analysis and Cox regression suggest there may be a survival benefit associated with AC use. Although selection bias may affect the interpretation of our survival analysis, the findings should encourage providers to consider AC use with high-risk pathology. Taken altogether, NAC remains the preferred option from an oncologic perspective and appears to correlate with the greatest survival advantage [2].
Clinical factors such as increasing age and CCI had a negative association with POC reception. Bladder cancer is recognized as a disease of the elderly [13], which is consistent with our study population mean age of 68.4 years. Increasing age continues to be associated with decreased likelihood of POC receipt, which is supported by findings in similar studies examining prior years [7]; despite multiple studies demonstrating equal benefit of NAC in elderly compared younger counterparts [14,15]. The disparity has led to efforts to individualize treatment based on functional status rather than chronological age; however, significant progress needs to be made to prevent the aging population from continued undertreatment [16]. Patients with increased comorbidities, as evidenced by CCI, are less likely to receive NAC, presumably because of concerns about treatment tolerability. However, previous literature suggests there is no increased risk of perioperative morbidity in those who are well-selected to receive NAC [17]. Greater age and comorbidity have been associated with both reduced overall survival and cancer-specific survival, which may in part be due to undertreatment with POC [12].
Clinical staging has long been one of the best predictors of outcome survival and was found to be a predictor of POC use [18]. One study found advanced tumor stage to be a predictor of NAC use specifically, with the majority of urologists polled indicating that tumor stage >T3-4a to be the best indication for NAC application [19]. We note similar findings in our analysis, as POC was increasingly utilized with cT3 and cT4 disease. While increasing yearly POC use is suggestive of increased guideline compliance, non-adherence is still prevalent [19].
Non-clinical factors such as income and insurance continue to have predictive value in determining who receives POC. These findings are consistent with previous publications using NCDB data which have not only suggested difference in treatment among insurance type and income, but also minority race and hospital type [7,20]. Unfortunately, socioeconomic variables may account for variance in receiving RC in approximately one-fifth of patients [21]. Data analysis from 2006 to 2010 suggested increased likelihood of POC use in patients making >$35,000 annually [7]; however, that seems to have changed during the current study period. An increase up to >$48,000 annually and private insurance are associated with increased likelihood of POC use, which suggests social factors may affect the treatment planning of more patients in years to come. From 2006 to 2010, patients in the Northeastern United States were more likely to receive POC than those in other regions [7]; however, our analysis suggests that from 2011 to 2015, there is no significant difference in POC usage based on geographic location. This suggests that in recent years POC use has increased in regions where it was less readily adopted in the past. While it is important to note that patient factors have a significant role in treatment options, both physician and facility factors can contribute to the final treatment selected [22]. The intricacy of this relationship is not completely elucidated from our analysis but is deserving of further evaluation.
A shorter length of stay in the hospital following RC was associated with those who received POC, and the rate of unplanned readmission within 30 days was similar between all cohorts. Similar to previous literature, this suggests that the use of POC in well selected patients does not increase hospital stay or complications [17]. As expected, NAC therapy was associated with the best long-term outcome survival. This finding is congruent with current guidelines [23]. When comparing 5-year overall survival of all three groups, those who received NAC had a 12.5% difference favoring improved overall survival compared to AC group and a 24.4% difference when compared to the no chemotherapy group.
Limitations to this study do exist. A retrospective study design utilizing a national database with limited data points leads to inherent selection bias [24]. Although the study sample is large in nature, data is derived solely from hospitals participating in the NCDB, allowing for sample bias [7]. Additionally, the decision points regarding the use of chemotherapy are not discerned from a large retrospective review. The details of comorbid conditions, renal function, and patient/provider preferences are not known in this dataset. Furthermore, the specifics of chemotherapy regimens, including chemotherapy agents and cycles, are not provided within the NCDB.
CONCLUSIONS
The increasing trend of POC usage noted in prior studies has continued through 2015. NAC appears to drive this increase while AC utilization remains unchanged. Clinical factors, including tumor stage, age, and comorbidity, as well as socioeconomic factors, including income and insurance status, affect utilization of POC.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Coleman McFerrin and Zachary Hamilton.
- Data acquisition: Zachary Hamilton.
- Statistical analysis: Zachary Hamilton.
- Data analysis and interpretation: Coleman McFerrin and Facundo Davaro, Allison May, Syed Raza, Sameer Siddiqui, and Zachary Hamilton.
- Drafting of the manuscript: Coleman McFerrin and Facundo Davaro.
- Critical revision of the manuscript: Allison May, Syed Raza, Sameer Siddiqui, and Zachary Hamilton.
- Approval of the final manuscript: Coleman McFerrin, Facundo Davaro, Allison May, Syed Raza, Sameer Siddiqui, and Zachary Hamilton.
SUPPLEMENTARY MATERIAL
Supplementary material can be found via https://doi.org/10.4111/icu.20200132.
Logistic regression for any chemotherapy use, all factors included
References
- 1.DeGeorge KC, Holt HR, Hodges SC. Bladder cancer: diagnosis and treatment. Am Fam Physician. 2017;96:507–514. [PubMed] [Google Scholar]
- 2.Pradère B, Thibault C, Vetterlein MW, Leow JJ, Peyronnet B, Rouprêt M, et al. Peri-operative chemotherapy for muscle-invasive bladder cancer: status-quo in 2017. Transl Androl Urol. 2017;6:1049–1059. doi: 10.21037/tau.2017.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol. 2013;63:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 5.Porter MP, Kerrigan MC, Donato BM, Ramsey SD. Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol. 2011;29:252–258. doi: 10.1016/j.urolonc.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185:72–78. doi: 10.1016/j.juro.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Reardon ZD, Patel SG, Zaid HB, Stimson CJ, Resnick MJ, Keegan KA, et al. Trends in the use of perioperative chemotherapy for localized and locally advanced muscle-invasive bladder cancer: a sign of changing tides. Eur Urol. 2015;67:165–170. doi: 10.1016/j.eururo.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722–1728. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans TJN, Voskuilen CS, van der Heijden MS, Schmitz-Dräger BJ, Kassouf W, Seiler R, et al. Neoadjuvant treatment for muscle-invasive bladder cancer: the past, the present, and the future. Urol Oncol. 2017;36:413–422. doi: 10.1016/j.urolonc.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Adjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and network meta-analysis of randomized clinical trials. Oncotarget. 2017;8:81204–81214. doi: 10.18632/oncotarget.20979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth CM, Siemens DR, Li G, Peng Y, Tannock IF, Kong W, et al. Perioperative chemotherapy for muscle-invasive bladder cancer: a population-based outcomes study. Cancer. 2014;120:1630–1638. doi: 10.1002/cncr.28510. [DOI] [PubMed] [Google Scholar]
- 13.Korpics MC, Block AM, Martin B, Hentz C, Gaynor ER, Henry E, et al. Concurrent chemotherapy is associated with improved survival in elderly patients with bladder cancer undergoing radiotherapy. Cancer. 2017;123:3524–3531. doi: 10.1002/cncr.30719. [DOI] [PubMed] [Google Scholar]
- 14.Chau C, Wheater M, Geldart T, Crabb SJ. Clinical outcomes following neoadjuvant cisplatin-based chemotherapy for bladder cancer in elderly compared with younger patients. Eur J Cancer Care (Engl) 2015;24:155–162. doi: 10.1111/ecc.12282. [DOI] [PubMed] [Google Scholar]
- 15.Bamias A, Efstathiou E, Moulopoulos LA, Gika D, Hamilos G, Zorzou MP, et al. The outcome of elderly patients with advanced urothelial carcinoma after platinum-based combination chemotherapy. Ann Oncol. 2005;16:307–313. doi: 10.1093/annonc/mdi039. [DOI] [PubMed] [Google Scholar]
- 16.Guancial EA, Roussel B, Bergsma DP, Bylund KC, Sahasrabudhe D, Messing E, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015;10:939–949. doi: 10.2147/CIA.S74322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson DC, Nielsen ME, Matthews J, Woods ME, Wallen EM, Pruthi RS, et al. Neoadjuvant chemotherapy for bladder cancer does not increase risk of perioperative morbidity. BJU Int. 2014;114:221–228. doi: 10.1111/bju.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youssef RF, Lotan Y. Predictors of outcome of non-muscle-invasive and muscle-invasive bladder cancer. ScientificWorldJournal. 2011;11:369–381. doi: 10.1100/tsw.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martini T, Gilfrich C, Mayr R, Burger M, Pycha A, Aziz A, et al. The use of neoadjuvant chemotherapy in patients with urothelial carcinoma of the bladder: current practice among clinicians. Clin Genitourin Cancer. 2017;15:356–362. doi: 10.1016/j.clgc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of potentially curative therapies for muscleinvasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol. 2013;63:823–829. doi: 10.1016/j.eururo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802–811. doi: 10.1093/jnci/djq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer ES, Smith AB. Exploring the 3 A's of cystectomy access to care for muscle-invasive bladder cancer. Urol Oncol. 2015;33:105–107. doi: 10.1016/j.urolonc.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Georgiou CL, Pezaro C, Sengupta S. Putting guidelines into practice: has the era of perioperative chemotherapy arrived? Transl Androl Urol. 2018;7(Suppl 2):S255–S257. doi: 10.21037/tau.2018.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaid HB, Patel SG, Stimson CJ, Resnick MJ, Cookson MS, Barocas DA, et al. Trends in the utilization of neoadjuvant chemotherapy in muscle-invasive bladder cancer: results from the National Cancer Database. Urology. 2014;83:75–80. doi: 10.1016/j.urology.2013.07.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Logistic regression for any chemotherapy use, all factors included


