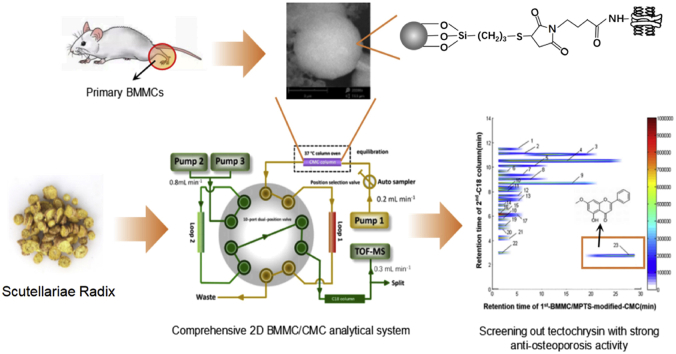
Abstract
Osteoporosis is a bone metabolic disease caused by the imbalance between osteoblasts and osteoclasts due to excess osteoclastogenesis, manifesting in the decrease of bone density and bone strength. Scutellariae Radix shows good anti-osteoporosis activity, but the effective component is still unclear. Cell membrane chromatography (CMC) is a biological affinity chromatography with membrane immobilized on a silica carrier as the stationary phase. It can realize a dynamical simulation of interactions between drugs and receptors on cell membrane, which is suitable for screening active compounds from complex systems. In this study, the components of Scutellariae Radix with potential anti-osteoporosis activity through inhibiting the differentiation from bone marrow mononuclear cells (BMMCs) to osteoclast were screened by a BMMC/CMC analytical system. Firstly, a new 3-mercaptopropyltrimethoxysilane (MPTS)-modified BMMC/CMC stationary phase was developed to realize covalent binding with cell membrane fractions. By investigating the retention time (tR) of the positive drug, the life span of the MPTS-modified CMC columns was significantly improved from 3 to 12 days. Secondly, 6 components of Scutellariae Radix were screened to show affinity to membrane receptors on BMMCs by a two-dimensional BMMC/CMC–TOFMS analytical system. Among them, tectochrysin demonstrated the best anti-osteoporosis effect in vitro, which has never been reported. We found that tectochrysin could inhibit the differentiation of BMMCs into osteoclasts induced by receptor activator of nuclear factor-κΒ ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) in a concentration-dependent manner in vitro. In vivo, it significantly reduced the loss of bone trabeculae in ovariectomized mice, and decreased the level of C-terminal cross-linking telopeptides of type 1 collagen (CTX-1), tartrate-resistant acid phosphatase 5b (TRAP-5b), interleukin 6 (IL-6) in serum. In conclusion, tectochrysin serves as a potential candidate in the treatment of osteoporosis. The proposed two-dimensional MPTS-modified BMMC/CMC-TOFMS analytical system shows the advantages of long-life span and fast recognition ability, which is very suitable for infrequent cell lines.
Key words: 3-Mercaptopropyltrimethoxysilane-modifiedsilica, Two-dimensional cell membrane chromatography, Scutellariae Radix, Anti-osteoporosis, Tectochrysin
Graphical abstract
To screen anti-osteoporosis components from Scutellariae Radix, 2D BMMC/CMC-TOFMS analytical system was established and the column life span was significantly prolonged by MPTS modification on silica stationary phase. Tectochrysin was screened out with good anti-osteoporosis activity for the first time.
1. Introduction
Osteoporosis is a systemic metabolic skeletal disease characterized by reduced bone mass and destruction of bone microstructure, which results in increased bone fragility and incidence of fracture1,2. Primary osteoporosis mostly occurs in menopausal women with a series of clinical symptoms, including pain and fracture, severely compromising the quality of life3. The pathogenesis of postmenopausal osteoporosis results from the excess osteoclastogenesis versus osteogenesis. To inhibit osteoclasts differentiation and functions remain an important strategy for osteoporosis treatment. Although various drugs are available, estrogen substitutes, e.g., diphosphates, calcitonin, and calcium preparations, are complicated with serious side effects such as breast cancer, osteonecrosis, and cardiovascular events4. Thus, developing safe and effective drugs for the treatment of osteoporosis is of great importance.
Traditional Chinese medicine (TCM) has been used to invigorate kidney and strengthen bone for a long history. People have been trying to find a breakthrough in the treatment of osteoporosis from TCM5,6. Scutellariae Radix is one of the most widely used TCM in clinical application and has been proved to have a wide range of pharmacological activities, mainly for bacteriostasis, antipyretic analgesia, and anti-cancer7. Recent studies found that Scutellariae Radix shows good anti-osteoporosis activity8, which could significantly prevent weightlessness induced osteoporosis and enhance the bone mineral density and bone microarchitecture9. Among which wogonin was reported to inhibit osteoclast differentiation by inhibiting the transfer and transcriptional activation of nuclear factor of activated T-cell 1 (NFATc1) from the cytoplasm to nucleus10. Baicalein activates mammalian target of rapamycin complex 1 (mTORC1) signaling pathways through protein kinases and transcription factors, such as P-4E/BP 1 and P-S6K1, to stimulate MT3T3-E1 cells to differentiate into osteoblasts11. However, there was no comprehensive mapping of the anti-osteoporosis components from Scutellariae Radix extracts. We deduced that other similar mother-nucleus compounds with a lower content in Scutellariae Radix could have even better anti-osteoporosis activity.
Cell membrane chromatography (CMC) is a biological affinity chromatography technique with cell membrane as a stationary phase. It has dual functions of chromatographic separation and characterization of active components12, which can be directly used to study the screening of active components from complex systems and the interaction between drugs and membrane receptors13. The better the retention behavior the compound has, the stronger it bonds with the receptors on membrane. Our group has developed a series of CMC models, which were used in the screening of more than 40 kinds of TCM or herbal medicines14. Primary bone marrow mononuclear cells (BMMCs) differentiate into osteoclasts under the stimulation of macrophage colony-stimulating factor (MCSF) and receptor activator of nuclear factor-κB ligand (RANKL) via receptor activator of nuclear factor-κB (RANK)15,16. Thus, in our study, the primary BMMC was selected as the target cell for screening anti-osteoporosis components from Scutellariae Radix.
For common CMC columns, the cell membrane and silica gel are connected by hydrophobic interaction, resulting in the membrane fractions easiness to fall off from stationary silica phase, and the life span is almost within 72 h17. The activity of the stationary phase in cell membrane chromatography directly affects the efficiency of the whole CMC system. Besides, the short life of the column will lead to significant consumption of raw materials, which limits the application of CMC models on the primary cells or other cells that are difficult to expand or obtain18. We previously reported a covalently modified 3-aminopropyltriethoxysilane (APTES) silica CMC stationary phase18,19. Through covalent bonding, the life of the column has been successfully extended to about 12 days, and the column efficiency has been significantly improved. However, glutaraldehyde was required as a bridge during this procedure, and a decrease on yield was caused by self-polymerization of glutaraldehyde20.
In this study, a 3-merraptopropyltrimethoxysilane (MPTS) modified silica gel was produced, to obtain an active ester group on the surface, which can covalently bind with the amino group on the surface of cell membrane21, 22, 23. Modified by MPTS showed the following advantages over APTES: (1) solve the problem of glutaraldehyde self-polymerization. (2) The reaction activity of mercapto-maleimide is higher than that of amino-aldehyde group24. (3) The reaction procedure was milder and faster. Next, a two-dimensional (2D) MPTS-modified BMMC/CMC system was developed for comprehensive screening of anti-osteoporosis components from Scutellariae Radix, which effectively saved the cell dosage of primary cells. The new MPTS-modified BMMC/CMC method shows suitable suitability for primary cells and can be expected for further applications.
2. Experimental section
2.1. Reagents and instruments
Silica gel (5 μm, 200 Å) was obtained from Qingdao Meigao Chemical Co., Ltd. (Qingdao, China) and was activated at 120 °C before use. Scutellariae Radix was purchased from Leiyunshang Pharmacy Co., Ltd. (Shanghai, China). Gefitinib (GFT), dexamethasone (DXMS), tectochrysin were purchased from Shanghai Yuanye Biotech Co., Ltd. (Shanghai, China) (purities≥98%). Dulbecco's modified Eagle's medium (DMEM), phosphate buffer saline (PBS) were purchased from Hyclone (Thermo Fisher, Waltham, MA, USA). Fetal bovine serum (FBS) was purchased from Gibco Life Technology Co. (Australia), penicillin streptomycin, and trypsin were purchased from Gibco-BRL Co. (Rockville, MD, USA). N,N-Dimethylformamide (DMF), 3-mercaptopropyltrimethoxysilane (MPTS), 3-aminopropyltriethoxysilane (APTES), N-(4-maleimide butyryl oxide) succinimide (GMBS), dimethyl sulfoxide (DMSO), HPLC-grade acetonitrile and MS-grade ammonia acetate were purchased from Sigma Co. (St. Louis, MO, USA). TRAP staining kit was purchased from Sigma–Aldrich, Co. (St. Louis, MO, USA).
2.2. Preparation of sample and standard solutions
Scutellariae Radix (100 g) was immersed in 1 L of 50% ethanol, heating reflux for 1.5 h. The decoction was filtered through four layers of gauze. The residue was extracted once more. The extracting solution was merged and condensed to a concentration of 1.0 g/mL. The decoction was stored at 4 °C for use12. GFT, DXMS, and tectochysin were separately dissolved to 50 mmol/L by DMSO to make a stock solution. A mixed standards solution of GFT and DXMS (1 mmol/L each) was prepared in methanol.
2.3. Synthesis of MPTS-modified stationary phase
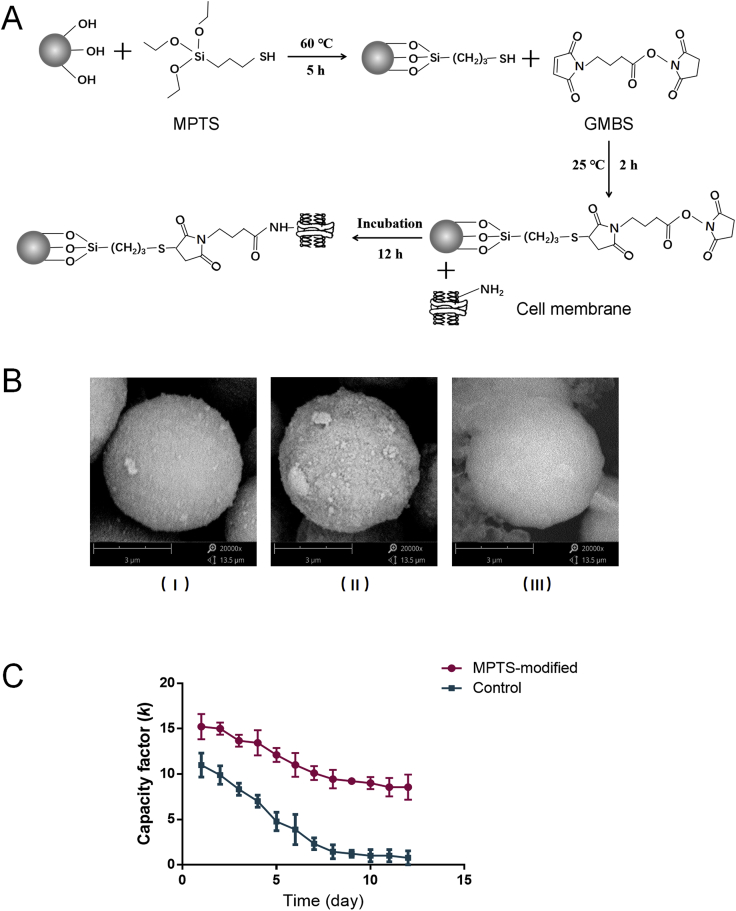
The reaction scheme is shown in Fig. 1A. Briefly, 1 mL MPTS was added to 1 g silica gel (5 μm, 200 Å) and stirred for 5 h in 100 mL DMF under the condition of 60 °C and nitrogen protection. Silica gel was bonded to MPTS to obtain sulfhydryl groups on the surface. Then the suspension was cleaned with DMF at 5000×g for three times. After that, the sediment was obtained and reacted with 5% GMBS in 500 mL DMSO solution for 2 h, so that free active ester groups were exposed on the surface of silica gel, which could covalently bind with the active amino groups on the surface of cell membrane. The sediment was cleaned with DMSO at 5000×g for three times and dried in the vacuum drying pump for 48 h.
Figure 1.
(A) Synthesis of MPTS-modified silica gel and its reaction with the cell membrane. (B) Actual micrograph of (Ⅰ) silica gel; (Ⅱ) MPTS-modified silica gel; (Ⅲ) MPTS-modified silica gel bonded with BMMC cell membrane. Magnification of the electron microscope is 20,000 times. (C) Capacity factor (k) of GFT on control columns and MPTS-modified columns prepared with BMMC cells within 12 days (n = 6).
2.4. 2D MPTS-modified BMMC/CMC analysis
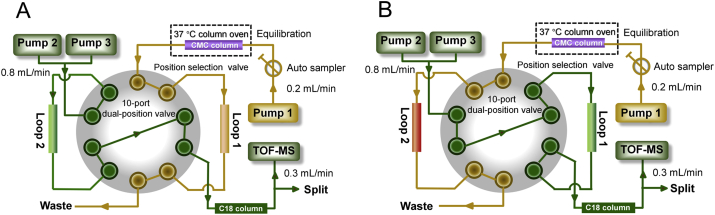
BMMCs were obtained from the femoral bone marrow of C57BL/6 mice (Shanghai Slack Co., Shanghai, China) at 8 weeks of age and was cultured with α-MEM medium added 10% fetal bovine serum and a mixture of penicillin (100 U/mL) and streptomycin (100 mg/mL), at 37 °C and 5% CO2. The MPTS-modified BMMC/CMC columns (10 mm × 2 mm i.d., 5 μm) were prepared according to our reported method previously25 by using MPTS-modified silica gels. Afterward, MPTS-modified comprehensive BMMC/CMC system was performed on an Agilent 1200 series HPLC system consisting of an autosampler that controlled by Agilent MassHunter Workstation (Agilent Technologies, Palo Alto, CA, USA), a binary (Pump 2 and Pump 3) solvent delivery system, a thermostatically controlled column apartment and an online degasser. The BMMC/CMC column was applied as the first-dimensional column, with 10 mmol/L ammonia acetate as the mobile phase and delivered at 0.2 mL/min. For the second dimension separation, A XBridge TM C18 column (100 mm × 3.0 mm i.d., 3.5 μm, Waters, Ireland) was used, and the mobile phase was composed of solvent A (0.1% formic acid) and solvent B (acetonitrile) at 0.8 mL/min by a linear gradient elution program: 0–5 min, from 10% B to 60% B; 5.01–10 min, 60% B; 10.01–13 min, 10% B. A 10-port dual-position valve (MXP9960-000, Rheodyne, Rohnert Park, CA, USA) equipped with two 500 μL sampling loops was applied to realize the switch between the two dimensions. The synchronization of the two dimensions was ensured by using a home-written program in Visual Basic 6.0 (Microsoft, Redmond, WA, USA). The eluent from the second-dimensional column was introduced to a 6220 TOF mass (Agilent Technologies, Palo Alto, CA, USA) equipped with an electrospray ionization interface, and signals were collected by the Agilent MassHunter Workstation (Agilent Technologies). All detailed operations of the 2D system were stated in our group's previous research13,26.
2.5. In vitro osteoclastogenesis assay
BMMCs were obtained from the femoral bone marrow of C57BL/6 mice (Shanghai Slack Co.) at 8 weeks of age. Six to 8 h later, suspended cells were collected and re-suspended with DMEM medium added MCSF (50 ng/mL). Seventy-two hours later, cells were digested and then cultured on 96-well plates (2.5 × 103 cells/well), divided into a control group and three groups treated with tectochrysin (25, 50, or 100 μmol/L). All groups were cultured with DMEM medium added RANKL (50 ng/mL). On day 7, cells were stained using a TRAP staining kit (Sigma–Aldrich) according to the manufacturer's protocols. Cells with three or more nucleus were regarded as osteoclast cells and counted.
2.6. In vivo experiments
2.6.1. Animals and experimental design
C57BL/6 female mice (Shanghai Slack Co.) of 8 weeks age were kept in a ventilated general-grade animal room at 24–28 °C. All procedures were complied with the guidelines of the Ethics Committee on Animal Experiments of the Second Military Medical University. The mice were randomly assigned to 3 groups (n = 5/group), including sham-treated group (Sham), ovariectomized (OVX) mice treated with normal saline, and OVX mice treated with tectochrysin dissolved in DMSO. After pre-experiment, we confirmed that the dosage of tectochrysin in mice was 5 mg/kg/day. All mice were anesthetized with 5% chloral hydrate and operated aseptically. Then, the operation area was sterilized with 75% alcohol. Small incisions were made on the back skin and peritoneum to expose the abdominal cavity and find out the bilateral ovaries. Bilateral ovaries of OVX group and tectochrysin treatment group mice, with the same volume of fat mass of sham operation group, were removed. After the operation, the skin incision was sutured with 5–0 non-absorbable suture. After that, mice were allowed to recover for 24 h. From the second postoperative day, 5 mg/kg/day of tectochrysin or normal saline was given by intraperitoneal injection. After administration for 6 weeks, all mice were anesthetized with chloral hydrate, and the femur and arterial blood were obtained.
2.6.2. Bone histomorphometric analysis
Bone histomorphometric analyses were performed as previously described27. Briefly, femurs were fixed and decalcified for two weeks. Sections (4-μm thickness) were then prepared with a microtome and stained with hematoxylin and eosin (HE).
2.6.3. Microcomputed tomography analysis
Microcomputed tomography (Micro-CT, Skyscan, Antwerp, Belgium) analyses were performed using 80 kV, 124 μA, and 8 μm resolution. Structural parameters including the bone mineral density (BMD), bone volume/total volume (BV/TV), bone surface area/total volume (BS/TV), and trabecular number (Tb.N) for the metaphyseal region of the proximal tibiae were analyzed.
2.6.4. Serum biochemistry
The blood was collected via fundus venous plexus under anesthesia. Then serum was collected. The levels of interleukin 6 (IL-6), C-terminal cross-linking telopeptides of type 1 collagen (CTX-1), tartrate-resistant acid phosphatase 5b (TRAP-5b) and osteocalcin (OCN) in the serum were measured using ELISA kits (Anogen, Mississauga, ON, Canada) following the manufacturer's instructions.
3. Results and discussion
3.1. Reproducibility and life span of MPTS-modified CMC columns
Because of the problem that cell membrane was easy to fall off from silica gel, the life span of traditional columns was only within 72 h17, which has greatly prevented the CMC from being widely applied. BMMCs used in this research were primary cells obtained from the femoral bone marrow of mice, so large number of cells bring tedious operation and high cost. In our previous research, a post-preparation treatment by paraformaldehyde was used and successfully prolonged the life span to about six days. However, this method might influence the bioactivity of the cell membrane, and meanwhile, the procedure added the difficulty of column preparation18. Based on this, chemical modification on silica gel was developed. Firstly, APTES was adopted and successfully extended the column span to about 12 days18. In this research, MPTS was selected as the new material for the modification on silica gel. Compared to APTES, the reaction condition was milder, the reaction time was controlled to less than 7 h, and the self-polymerization of glutaraldehyde in reaction procedure was avoided. Furthermore, the reaction activity of mercapto with the maleamide group is higher than that of the amino group and aldehyde group24,28. As shown in Supporting Information Fig. S1, the immobilized membrane protein contents on 40 mg MPTS, APTES and non-modified silica gel were 443.33 ± 16.50, 398.01 ± 38.18 and 289.67 ± 9.43 μg, respectively, indicating that MPTS-modified silica gel has the best efficiency of bonding cell membrane proteins.
Scanning electron microscope was used to characterize the surface of silica gel with and without MPTS-modification, as well as the effect of bonded with BMMC cell membrane (Fig. 1B). Successful surface modification of silica gel was observed, and the ability to bond with cell membrane was ideal. The retention peak time (tR) of positive drug GFT was chosen as an evaluation to examine the life span and reproducibility of MPTS-modified BMMC columns. A total of 3.5 × 107 BMMCs were obtained to prepare BMMC cell membrane columns made from the MPTS-modified and non-modified silica gel (n = 6). The capacity factor k was then defined to assess the membrane affinity of different compounds, which is descripted as Eq. (1):
| (1) |
where t0 is the dead time determined by the non-retained compound. k of GFT was calculated for 12 days with six injections for each day. As shown in Fig. 1C, k of GFT on control silica gel columns decreased rapidly in the first five days and was nearly 0 on 8th day. While on MPTS-modified columns, average of k of six columns was 8.3 in 12th day. The results showed that after covalent modification by MPTS, the binding ability and stability between silica gel and cell membranes was greatly enhanced, successfully prolonging the column life span to at least 12 days. Besides, the reproducibility was enhanced from RSD 20% to less than 10% for the first three days when the column efficiency falls most18.
3.2. Application of the MPTS-modified BMMC/CMC system
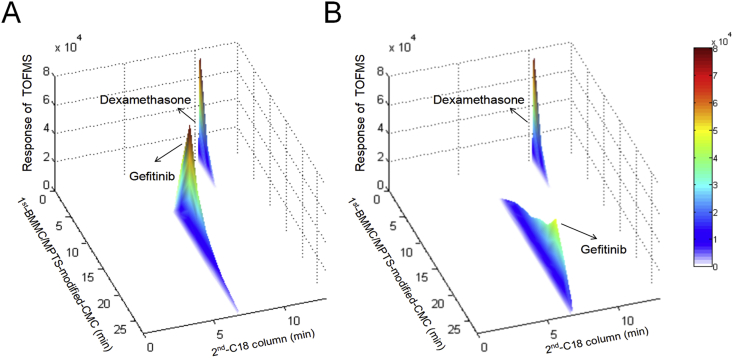
Firstly, the selectivity and efficiency of the BMMC/CMC system were evaluated. The brief scheme of 2D MPTS-modified BMMC/CMC/C18 column/TOFMS system was shown as Fig. 2. GFT (epidermal growth factor receptor antagonist) was selected as a positive drug, and DXMS (hormones) was selected as a negative drug to investigate the selectivity and efficiency of screening system, for epidermal growth factor receptor, was expressed on BMMC membrane 29,30. The retention plots of GFT and DXMS, respectively on BMMC/CMC columns and MPTS-modified BMMC/CMC columns were shown in Fig. 3A and B. GFT showed strong retention behavior on both non-modified and modified columns, but peak time on MPTS-modified columns was significantly longer than that on regular columns, while DXMS was barely retained on this CMC model, reaching the peak at about 2 min. It indicates the selectivity, availability, and higher efficiency of MPTS-modified BMMC/CMC system.
Figure 2.
Brief scheme of 2D MPTS-modified BMMC/CMC/C18 column/TOFMS system. (A) Ten-port dual position valve was at position 1. CMC column 1 was equilibrated, and the 1st fraction was collected in loop 1. (B) Ten-port dual position valve was switched to position 2. The 1st fraction was being analyzed by a C18 column combined with TOFMS while the 2nd fraction was collected in loop 2.
Figure 3.
Typical 3D plot of mixed standards obtained by (A) control BMMC/CMC/C18 column/TOFMS system and (B) MPTS-modified BMMC/CMC/C18 column/TOFMS system.
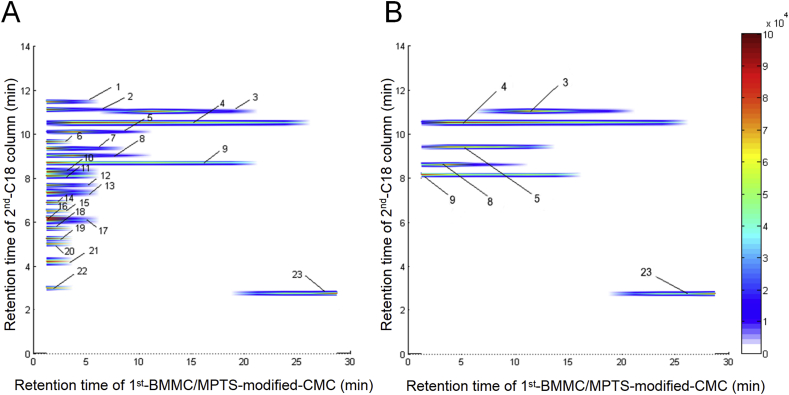
Then, comprehensive 2D MPTS-modified BMMC/CMC system was applied to screening the potential active components of anti-osteoporosis from Scutellariae Radix. Detailed identification results of components of Scutellariae Radix analyzed by C18 column-TOF/MS was shown in Supporting Information Table S1. Totally, six components with retention behavior on BMMC were screened from Scutellariae Radix (Fig. 4A and B). By matching with the chemical constituent database of Scutellariae Radix and the validation of standard samples, the six compounds were identified as chrysin, baicalein, wogonin, oroxylin A, tectochrysin, and baicalein-7-O-β-d-glucoside. Specific retention behavior information was shown in Table 1. Through literature research, except tectochrysin, the other 5 compounds all have been input to anti-osteoporosis research and reported to have a certain degree of inhibition on osteoporosis11,12,31. The results above further validate the validity and accuracy of the 2D BMMC/CMC system in screening potential active components of Scutellariae Radix. Among these 6 compounds, tectochrysin has never been reported to be used in anti-osteoporosis research, while it showed the most definite affinity with BMMC cell membrane, which indicated it could tightly bind with receptors on the cell membrane and most likely to be active. Therefore, in this study, we focus on the anti-osteoporosis activity of tectochrysin both in vitro and in vivo.
Figure 4.
(A) 2D plots of compounds of Scutellariae Radix extracts obtained by the 2D MPTS-modified BMMC/CMC/C18 column/TOFMS system. (B) 2D plots of standards of potentially active compounds obtained by the 2D MPTS-modified BMMC/CMC/C18 column/TOFMS system.
Table 1.
Potentially active components in Scutellariae Radix characterized by the MPTS-modified BMMC/CMC/C18/TOFMS analysis system.
| Comp. No. | Identification | tR (C18, min) | tR/k (BMMC-CMC, min) | [M+H]+ (m/z) |
Error (ppm) | Abund. match (%) | Formula | |
|---|---|---|---|---|---|---|---|---|
| Exact | Accurate | |||||||
| 3 | Chrysin | 11.3 | 12.5/9.42 | 255.0652 | 255.0653 | 0.2 | 99.26 | C15H10O4 |
| 4 | Baicalein | 10.8 | 7.5/5.25 | 271.0601 | 271.0598 | −0.15 | 99.88 | C15H10O5 |
| 5 | Baicalein-7-O-β-d-glucopyranoside | 9.4 | 5/3.17 | 433.1129 | 433.1128 | −0.32 | 99.87 | C21H20O10 |
| 8 | Wogonin | 8.8 | 5/3.17 | 285.0757 | 285.0754 | −0.9 | 98.95 | C16H12O5 |
| 9 | Oroxylin A | 8.1 | 5/3.17 | 285.0757 | 285.0751 | −2.32 | 98.28 | C16H12O5 |
| 23 | Tectochrysin | 2.2 | 30/24 | 269.0808 | 269.0808 | −0.33 | 99.88 | C16H12O4 |
3.3. Tectochrysin inhibits osteoclastogenesis in vitro
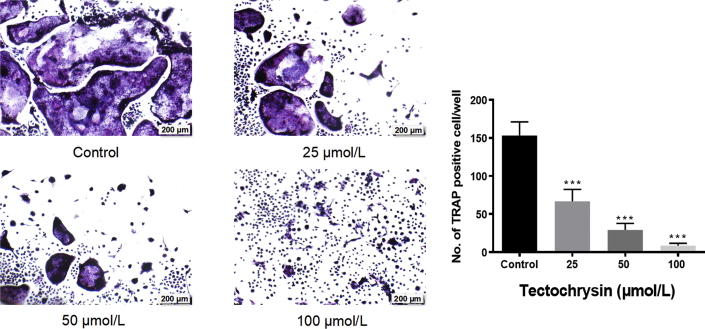
To examine the effects of tectochrysin on osteoclastogenesis, BMMCs were treated with RANKL and MCSF in the presence of 25, 50, and 100 μmol/L tectochrysin. The selection of dose of tectochrysin was according to the cytotoxicity experiments (Fig. S1). Compared to the control group, tectochrysin dose-dependently reduced the number of osteoclasts (P < 0.05) (Fig. 5A and B). It suggested that tectochrysin dose-dependently suppress osteoclasts differentiation in vitro32.
Figure 5.
Tectochrysin inhibits osteoclastogenesis in vitro. (A) Formation of TRAP-positive cells from BMMC cells (B) Quantification of osteoclasts. Results shown are means ± SD, n = 5; ∗∗∗P < 0.001 vs. control group.
3.4. Tectochrysin reduced ovariectomy-induced bone loss in vivo
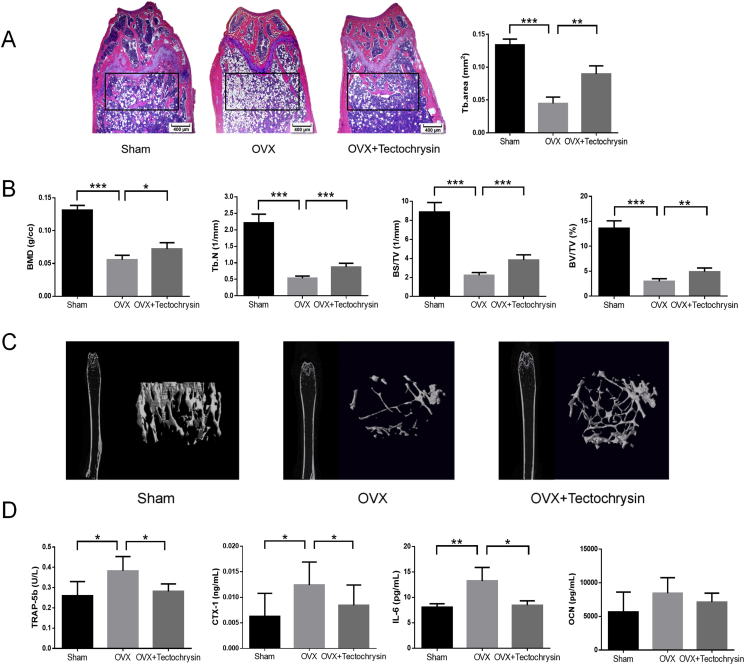
We evaluated the effects of tectochrysin on ovariectomy-induced bone loss. The trabeculae in the sham group were dense and uniform, while in OVX mice, the trabeculae were sparse and disordered (Fig. 6A). After six weeks of treatment with tectochrysin, the number of trabeculae was preserved, and the bone microstructure destruction was significantly ameliorated33. Compared with the model group, the area of trabecular bone in tectochrysin treatment group was significantly increased (P < 0.05). The findings were corroborated by Micro-CT analysis (Fig. 6B and C). The trabecular BV/TV, BS/TV, Tb.N, and BMD were decreased in OVX mice and preserved by treatment with tectochrysin relative to the OVX mice (P < 0.05).
Figure 6.
Tectochrysin inhibits ovariectomy-induced bone loss in vivo. Dosage of tectochrysin was 5 mg/kg/day. (A) Representative H&E staining of femoral sections and difference of trabecular area from each group 6 weeks after OVX operation. (B) Tb.N, BS/TV, BV/TV, and BMD were analyzed. (C) Representative microcomputed tomography sections of the femur from sham-treated OVX mice and OVX + tectochrysin mice. (D) BMD, IL-6, TRAP-5b, and OCN were examined in the serum. Results shown are means ± SD, n = 5; ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 vs. OVX operation group.
We examined the serum level of CTX-1, TRAP-5b, OCN, and IL-6 (Fig. 6D). The serum levels of CTX-1, TRAP-5b, and IL-6 in the OVX group were significantly higher than the sham group, indicating that OVX mice showed active bone resorption34, 35, 36. After 6 weeks of treatment with tectochrysin, the levels of CTX-1, TRAP-5b, and IL-6 in the serum of mice were decreased (P < 0.05), but OCN was not altered significantly (P > 0.05). The decline of bone resorption biomarkers indicates that tectochrysin inhibits bone resorption in vivo37.
4. Conclusions
A comprehensive 2D BMMC/CMC-TOFMS analysis system was established to screen anti-osteoporosis compounds from Scutellariae Radix, while short column life span and low reproducibility substantially prevented CMC from being widely applied to primary cells for the properties of hard to obtain and cannot be expanded. Point to this, MPTS was used to bind the cell membrane to silica gel by covalent bond instead of hydrophobic interaction. Through this strategy, the life span of the CMC column was successfully prolonged from 3 to 12 days. On this basis, a 2D MPTS-modified BMMC/CMC-TOFMS system was established, and tectochrysin was screened out having a potential activity of anti-osteoporosis. Follow-up experiments confirmed that in vitro, tectochrysin could inhibit the differentiation of BMMCs into osteoclasts in a concentration-dependent manner from 20 to 100 μmol/L. In vivo, tectochrysin could relieve the symptoms of osteoporosis by significantly improving the loss of bone trabeculae in OVX mice, decreasing the serum level of bone resorption conversion factor CTX-1 and TRAP-5b, as well as IL-6. These results show that tectochrysin has a significant anti-osteoporosis effect, and the primary mechanism is to inhibit the formation of osteoclasts, thereby alleviating the imbalance between osteogenesis and osteoclasts in vivo. Thus, the application of 2D MPTS-modified CMC system realized the screening of anti-osteoporosis pharmacodynamics of Scutellariae Radix. Not only that, the strategy of MPTS-modified silica gel CMC can be expanded the application arrange to other infrequent cell lines to realize screening of pharmacodynamics from complex chemical samples.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.81973291 to Xiaofei Chen, 81573396 to Yifeng Chai, 81973275 to Yongfang Yuan, 81703674 to Yue Liu, 81703779 to Rong Wang, 81803815 to Shaozhan Wang and 81871099 to Xiao Chen), Fund of Shanghai Science and Technology Committee, China (19QA1411500 to Xiaofei Chen, 17401900800 to Yongfang Yuan and 17YF1424700 to Yue Liu).
Footnotes
Peer review under responsibility of Institute of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.01.019.
Contributor Information
Jiacan Su, Email: drsujiacan@163.com.
Yongfang Yuan, Email: nmxyyf@126.com.
Xiaofei Chen, Email: xfchen2010@163.com.
Author contributions
Yanqiu Gu: investigation, validation, data curation, writing—original draft. Xiao Chen: investigation and project administration. Yao Wang: investigation and data curation. Leyi Zheng: validation and formal analysis. Xiaoqun Li: resources. Yue Liu, Rong Wang and Shaozhan Wang: funding acquisition. Shengnan Li: visualization. Yifeng Chai and Jiacan Su: supervision, writing—review & editing. Xiaofei Chen and Yongfang Yuan: conceptualization, methodology, software, writing—review & editing.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lane N.E. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. 2006;194:S3–S11. doi: 10.1016/j.ajog.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 2.Black D.M., Rosen C.J. Clinical practice. postmenopausal osteoporosis. N Engl J Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H., Zhao N., Zheng P., Xu X., Liu M., Luo D. Prevention and treatment of osteoporosis using Chinese medicinal plants: special emphasis on mechanisms of immune modulation. J Immunol Res. 2018;2018:6345857. doi: 10.1155/2018/6345857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreopoulou P., Bokckman R.S. Management of postmenopausal osteoporosis. Annu Rev Med. 2015;66:329–342. doi: 10.1146/annurev-med-070313-022841. [DOI] [PubMed] [Google Scholar]
- 5.Zhang N.D., Han T., Huang B.K., Rahman K., Jiang Y.P., Xu H.T. Traditional Chinese medicine formulas for the treatment of osteoporosis: implication for antiosteoporotic drug discovery. J Ethnopharmacol. 2016;189:61–80. doi: 10.1016/j.jep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Jin Y.X., Wu P., Mao Y.F., Wang B., Zhang J.F., Chen W.L. Chinese herbal medicine for osteoporosis: a meta-analysis of randomized controlled trials. J Clin Densitom. 2017;20:516–525. doi: 10.1016/j.jocd.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Cheng C.S., Chen J., Tan H.Y., Wang N., Chen Z., Feng Y. Scutellaria baicalensis and cancer treatment: recent progress and perspectives in biomedical and clinical studies. Am J Chin Med. 2018;46:25–54. doi: 10.1142/S0192415X18500027. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G., Li C., Niu Y., Yu Q., Chen Y., Liu E. Osteoprotective effect of Radix Scutellariae in female hindlimb-suspended Sprague–Dawley rats and the osteogenic differentiation effect of its major constituent. Molecules. 2017;22 doi: 10.3390/molecules22071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C.R., Zhang G.W., Niu Y.B., Pan Y.L., Zhai Y.K., Mei Q.B. Antiosteoporosis effect of Radix Scutellariae extract on density and microstructure of long bones in tail-suspended Sprague–Dawley rats. Evid Base Compl Alternative Med. 2013;2013:753703. doi: 10.1155/2013/753703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng X., Yang L., Zhang C., Qin H., Liang Q. Wogonin inhibits osteoclast differentiation by inhibiting NFATc1 translocation into the nucleus. Exp Ther Med. 2015;10:1066–1070. doi: 10.3892/etm.2015.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S.F., Tang J.J., Chen J., Zhang P., Wang T., Chen T.Y. Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des Dev Ther. 2015;9:5169–5183. doi: 10.2147/DDDT.S81578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu Y., Chen X., Wang R., Wang S., Wang X., Zheng L. Comparative two-dimensional HepG2 and L02/cell membrane chromatography/C18/time-of-flight mass spectrometry for screening selective anti-hepatoma components from Scutellariae Radix. J Pharmaceut Biomed Anal. 2019;164:550–556. doi: 10.1016/j.jpba.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Cao Y., Zhang H., Zhu Z., Liu M., Liu H. Comparative normal/failing rat myocardium cell membrane chromatographic analysis system for screening specific components that counteract doxorubicin-induced heart failure from Acontium carmichaeli. Anal Chem. 2014;86:4748–4757. doi: 10.1021/ac500287e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Chen X., Gu Y., Cao Y., Yuan Y., Hong Z. Progress of cell membrane chromatography and its application in screening active ingredients of traditional Chinese medicine. Chin J Anal Chem. 2018;46:1695–1702. [Google Scholar]
- 15.Chen X., Zhi X., Pan P., Cui J., Cao L., Weng W. Matrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. Faseb J. 2017;31:4855–4865. doi: 10.1096/fj.201700316R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanzaki H., Shinohara F., Itohiya K., Yamaguchi Y., Katsumata Y., Matsuzawa M. RANKL induces Bach1 nuclear import and attenuates Nrf2-mediated antioxidant enzymes, thereby augmenting intracellular reactive oxygen species signaling and osteoclastogenesis in mice. Faseb J. 2017;31:781–792. doi: 10.1096/fj.201600826R. [DOI] [PubMed] [Google Scholar]
- 17.Chen X., Cao Y., Lv D., Zhu Z., Zhang J., Chai Y. Comprehensive two-dimensional HepG2/cell membrane chromatography/monolithic column/time-of-flight mass spectrometry system for screening anti-tumor components from herbal medicines. J Chromatogr A. 2012;1242:67–74. doi: 10.1016/j.chroma.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 18.Ding X., Cao Y., Yuan Y., Gong Z., Liu Y., Zhao L. Development of APTES-decorated HepG2 cancer stem cell membrane chromatography for screening active components from Salvia miltiorrhiza. Anal Chem. 2016;88:12081–12089. doi: 10.1021/acs.analchem.6b02709. [DOI] [PubMed] [Google Scholar]
- 19.Wang X.Y., Ding X., Yuan Y.F., Zheng L.Y., Cao Y., Zhu Z.Y. Comprehensive two-dimensional APTES-decorated MCF7-cell membrane chromatographic system for characterizing potential anti-breast-cancer components from yuanhu-baizhi herbal medicine pair. J Food Drug Anal. 2018;26:823–833. doi: 10.1016/j.jfda.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gowda S.G.B., Nakahashi A., Yamane K., Nakahashi S., Murai Y., Siddegowda A.K.C. Facile chemoselective strategy toward capturing sphingoid bases by a unique glutaraldehyde-functionalized resin. ACS Omega. 2018;3:753–759. doi: 10.1021/acsomega.7b01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng F., Hu B. MPTS-silica coated capillary microextraction on line hyphenated with inductively coupled plasma atomic emission spectrometry for the determination of Cu, Hg and Pb in biological samples. Talanta. 2007;73:372–379. doi: 10.1016/j.talanta.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 22.Samanta D., Sarkar A. Immobilization of bio-macromolecules on self-assembled monolayers: methods and sensor applications. Chem Soc Rev. 2011;40:2567–2592. doi: 10.1039/c0cs00056f. [DOI] [PubMed] [Google Scholar]
- 23.De Juan-Franco E., Caruz A., Pedrajas J.R., Lechuga L.M. Site-directed antibody immobilization using a protein A-gold binding domain fusion protein for enhanced SPR immunosensing. Analyst. 2013;138:2023–2031. doi: 10.1039/c3an36498d. [DOI] [PubMed] [Google Scholar]
- 24.Zhu J., Nguyen T., Pei R., Stojanovic M., Lin Q. Specific capture and temperature-mediated release of cells in an aptamer-based microfluidic device. Lab Chip. 2012;12:3504–3513. doi: 10.1039/c2lc40411g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding X., Chen X., Cao Y., Jia D., Wang D., Zhu Z. Quality improvements of cell membrane chromatographic column. J Chromatogr A. 2014;1359:330–335. doi: 10.1016/j.chroma.2014.07.071. [DOI] [PubMed] [Google Scholar]
- 26.Zheng L., Chen S., Cao Y., Zhao L., Gao Y., Ding X. Combination of comprehensive two-dimensional prostate cancer cell membrane chromatographic system and network pharmacology for characterizing membrane binding active components from Radix et Rhizoma Rhei and their targets. J Chromatogr A. 2018;1564:145–154. doi: 10.1016/j.chroma.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Zhi X., Cao L., Weng W., Pan P., Hu H. Matrine derivate MASM uncovers a novel function for ribosomal protein S5 in osteoclastogenesis and postmenopausal osteoporosis. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Corso C.D., Dickherber A., Hunt W.D. An investigation of antibody immobilization methods employing organosilanes on planar ZnO surfaces for biosensor applications. Biosens Bioelectron. 2008;24:811–817. doi: 10.1016/j.bios.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Maletz K., Kufer P., Mack M., Raum T., Pantel K., Riethmuller G. Bispecific single-chain antibodies as effective tools for eliminating epithelial cancer cells from human stem cell preparations by redirected cell cytotoxicity. Int J Canc. 2001;93:409–416. doi: 10.1002/ijc.1348. [DOI] [PubMed] [Google Scholar]
- 30.Jia D., Chen X., Cao Y., Wu X., Ding X., Zhang H. On-line comprehensive two-dimensional HepG2 cell membrane chromatographic analysis system for charactering anti-hepatoma components from rat serum after oral administration of Radix Scutellariae: a strategy for rapid screening active compounds in vivo. J Pharmaceut Biomed Anal. 2016;118:27–33. doi: 10.1016/j.jpba.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Zeng W., Yan Y., Zhang F., Zhang C., Liang W. Chrysin promotes osteogenic differentiation via ERK/MAPK activation. Protein Cell. 2013;4:539–547. doi: 10.1007/s13238-013-3003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin Z., Jin C., Chao L., Zheng Z., Liehu C., Panpan P. A matrine derivative M54 suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by targeting ribosomal protein S5. Front Pharmacol. 2018;9:22. doi: 10.3389/fphar.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Zhi X., Wang J., Su J. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2018;6:34. doi: 10.1038/s41413-018-0035-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shetty S., Kapoor N., Bondu J.D., Thomas N., Paul T.V. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20:846–852. doi: 10.4103/2230-8210.192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaitseva O.V., Shandrenko S.G., Veliky M.M. Biochemical markers of bone collagen type I metabolism. Ukrainian Biochem J. 2015;87:21–32. doi: 10.15407/ubj87.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Eick G.N., Devlin M.J., Cepon-Robins T.J., Kowal P., Sugiyama L.S., Snodgrass J.J. A dried blood spot-based method to measure levels of tartrate-resistant acid phosphatase 5b (TRACP-5b), a marker of bone resorption. Am J Hum Biol. 2019;31 doi: 10.1002/ajhb.23240. [DOI] [PubMed] [Google Scholar]
- 37.Chen X., Zhi X., Yin Z., Li X., Qin L., Qiu Z. 18β-Glycyrrhetinic acid inhibits osteoclastogenesis in vivo and in vitro by blocking RANKL-mediated RANK-TRAF6 interactions and NF-κB and MAPK signaling pathways. Front Pharmacol. 2018;9:647. doi: 10.3389/fphar.2018.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.