Abstract
Introduction
We aim to determine whether racial/ethnic health disparities are a consequence of caregiving for persons with dementia and/or health status before becoming a caregiver.
Methods
Longitudinal data from the Health and Retirement Study (1998–2012) on 7859 Black, Hispanic, and White couples were analyzed for changes in physical and mental health with incident dementia of a spouse.
Results
Blacks and Hispanics, but not Whites, had poorer health before becoming caregivers for a spouse with dementia, than those who did not become caregivers. Spouse's dementia onset was associated with caregiver's higher odds of depressive disorder, with no racial/ethnic variation. Racial disparities in caregiver's health were attributed to health differences before caregiving, not differential health changes due to caregiving.
Discussion
Older Blacks and Hispanics with poor health are at increased risk of caregiving for a spouse with dementia. Protecting the health of persons supporting spouses with dementia requires understanding socioeconomic and cultural factors driving care provision.
Keywords: caregiving, dementia, depression, racial disparities
1. INTRODUCTION
In 2018, Americans provided 18.5 billion hours of unpaid care for 5.8 million people with Alzheimer's disease or related dementias, valued at $234 billion. 1 Even among those who receive paid care, half of their care hours are provided by unpaid family members. 2 Private and public insurers typically do not cover long‐term care; thus, as the population ages and the number of persons with dementia rises over the next several decades, the demand for informal care is expected to grow. 3
Prior research found caring for a person with dementia was associated with poor health. Across studies, dementia caregivers had higher prevalence of depression 4 than non‐caregivers 5 and non‐dementia caregivers. 6 Spousal dementia caregivers may be particularly vulnerable to negative health consequences because of their advanced age and because they are often the sole caregiver. 7 There is less consensus on the association between dementia caregiving and physical health than mental health. 8 Recent systematic reviews 9 , 10 and meta‐analyses 11 found that compared to non‐caregivers, dementia caregivers had higher cortisol levels, blood pressure, inflammation levels, and lower immune system functioning, but evidence on other biomarkers was mixed.
Research on racial differences in health among caregivers for persons with dementia is limited and findings across studies are inconclusive, with reports of both worse and better mental health outcomes for minorities than Whites, as well as no difference by racial/ethnicity. 6 , 12 , 13 Racially and ethnically diverse populations may respond differently when a spouse develops dementia and requires care. Cultural expectations about caregiving and familial responsibility may affect caregiving decisions and the perceived burden of providing care. 14 On the other hand, lack of financial resources, difficulty with literacy and language, low institutional knowledge, and other factors related to resources may affect caregiving decisions and contribute to poorer health. 14 , 15
Common limitations across studies of dementia caregivers’ health are the reliance on cross‐sectional data and non‐representative samples. 12 Differences in the health of caregivers compared to non‐caregivers, and differences across racial/ethnic groups, may be partially driven by their health prior to providing care and this is unobserved in cross‐sectional studies. Recently, a few studies on non‐dementia caregivers used longitudinal data and concluded that caregiving increased depression but did not examine caregivers for persons with dementia or racial/ethnic disparities. 16 , 17 Despite a growing literature on the health of dementia caregivers, there are no longitudinal studies on the mental and physical health of dementia caregivers nor have rigorous methods been applied to the study of racial/ethnic disparities in the health of dementia caregivers.
In this study, we used longitudinal data of a large, diverse, and nationally representative sample of couples from the Health and Retirement Study. We advanced the field of study in three primary ways. We improved our understanding of racial/ethnic disparities in health among dementia caregivers by analyzing the health status of Black, White, and Hispanic coupled persons prior to the onset of a spouse's dementia and separately for those who do and do not subsequently provide care. Second, we quantified changes in health associated with onset of dementia of a spouse for caregivers and non‐caregivers and by intensity of caregiving. Third, we tested for differential changes by race/ethnicity. Thus, we added to the literature estimates of the health effects of the onset of spouse's dementia for both caregivers and persons living with a spouse with dementia who were not caregivers, and quantified racial and ethnic differences. By using the longitudinal data and estimating changes over time, the estimated effects are independent of unobserved person‐specific, time‐invariant factors.
Understanding the health consequences of dementia for different racial and ethnic populations is increasingly important given the growing number of older Americans with dementia and the higher risk of dementia of Black and Hispanic persons than Whites. Findings will inform policy, health care, and other interventions aimed at supporting families living with dementia and reducing health disparities associated with dementia.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed extant literature using Google Scholar and other traditional sources. In addition, reference lists were consulted to identify relevant studies. The health and well‐being of family caregivers of persons with dementia have been studied for several years, but the literature has not been able to move beyond associations. Recent methodological advances in the literature on family caregivers broadly have not been applied to those caring for persons living with dementia. The most recent and relevant publications on caregivers of persons with dementia and transitions into/out of spousal caregiving are cited.
Interpretation: Our findings demonstrate that previously observed racial/ethnic disparities in health of dementia caregivers is due in part to health status prior to becoming a caregiver, rather than differential changes in health upon transitioning into caregiving.
Future directions: This study indicates that Hispanics and Blacks in poor health are more likely to become caregivers than Whites. Future research is needed to understand the economic, environmental, social, and cultural factors driving care provision, as well as the types of programs needed to support the health and well‐being of diverse dementia caregivers.
2. METHOD
2.1. Data and sample
We used eight waves (1998 to 2012) of biennial data from a nationally representative prospective cohort of U.S. adults aged 51 years and older, the Health and Retirement Study (HRS). Since 1992, the study has collected data on topics including health, cognition, family, employment, income, and wealth from respondents and their spouses following them until death and adding new cohorts over time to maintain population representation. It includes minority oversamples of African Americans and Hispanics and minority response rates at baseline and in follow‐ups have been equal to or better than that of majority Whites. 18 Spanish versions of questionnaires are administered by bilingual interviewers to Spanish‐speaking respondents. Participants are compensated about $80 and verbal informed consent is obtained from all respondents. Ethics approval was obtained from the Health Sciences and Behavioral Sciences Institutional Review Board at the University of Michigan.
Both individuals within the couples were included in our sample. We restricted our sample to White, Black, and Hispanic couples with one spouse aged 60 and older, living in the community or in nursing homes. We excluded couples with a spouse younger than 40 (n = 26). If an individual dropped out for the wave, the couple was excluded for that wave. Our sample included 7859 unique couples pooled across all waves for 61,738 person waves. We used self‐reported race and ethnicity to identify Whites, Blacks, and Hispanics. Among the unique couples: 79.6% are White, 11.2% are Black, and 9.2% are Hispanic.
2.2. Measures
2.2.1. Dementia
The classification of dementia is based on cognitive‐functioning scores that were validated by a diagnosis of dementia for a subset of HRS respondents in the Aging, Demographics, and Memory Study who had completed a neuropsychological assessment and had been given a diagnosis. 19 , 20 Following prior research and based on this validation, individuals were classified as having probable dementia (hereinafter “dementia”) based on a sum score of 0 to 6 (out of 27) of test items that evaluate memory and concentration and executive function (immediate and delayed word recall, counting down from 100 by sevens, counting down from 20). 20 , 21 Tests were administered to respondents using an adapted version of the Telephone Interview for Cognitive Status (TICS) at each wave. The measures were imputed when missing. 22 For the 8.7% of respondents who did not complete the cognitive assessment, dementia is determined using information provided by a proxy respondent, typically a spouse or other family member. 18 Probable dementia for those with a proxy interview is assigned for sum scores of 6 to 11 for the following: number of instrumental activities of daily living (IADLs; 0 to 5), interviewer impairment rating (0 = no cognitive limitations, 1 = some limitations, 2 = cognitive limitations), and proxy informant's rating of the respondent's memory (from 0 [excellent] to 4 [poor]).
To reduce measurement error in classifying dementia, we required one wave with a dementia classification and evidence of continued cognitive impairment in the subsequent survey wave (≈2 years later) or death. 23 , 24 Thereafter, an individual was classified as having dementia. We used three categories for 2‐year changes in dementia: no dementia, new dementia (no dementia in prior wave and dementia in current wave), and preexisting dementia (dementia in prior wave and dementia in current wave).
2.2.2. Caregiving
Spousal caregivers were identified by respondents who reported whether he/she received help for functional limitations, and, if so, who helped and how many help hours were provided per week at the current wave. 16 We classified spousal caregivers providing 20 hours or less and at least 1 hour per week as “low‐intensity” caregivers and greater than 20 hours as “high‐intensity” caregivers. Spouses who did not provide care were categorized as non‐caregivers. We used four categories to identify changes between non‐caregiver and caregiver states: transitioned out of caregiving (caregiver in prior wave and not a caregiver in current wave), low‐intensity caregiver (not a caregiver in prior wave and caregiver providing 20 hours or less in current wave), and high‐intensity caregiver (not a caregiver in prior wave and caregiver providing more than 20 hours in current wave). Caregivers were otherwise categorized as non‐caregiver (no caregiving in prior or current wave) or caregiver (caregiver in both prior and current waves). Caregivers for a spouse with dementia provided on average 49.3 weekly hours compared to 24.7 weekly hours for a spouse without dementia. Weekly hours among dementia caregivers were highly skewed: 69.2 at the 75th percentile and around‐the‐clock (24‐hour) care every day at the 90th percentile.
2.2.3. Depressive symptoms and self‐reported health
Depressive symptoms are based on the validated Center for Epidemiologic Studies Depression Scale (CES‐D), which is commonly used in research on caregivers. 25 The HRS CES‐D includes eight binary response questions about the respondent's mood in the last week: (1) “Much of the time during the past week, you felt depressed”; (2) “Much of the time during the past week, you felt that everything you did was an effort”; (3) “Your sleep was restless”; (4) “You were happy”; (5) “You felt lonely”; (6) “You enjoyed life”; (7) “You felt sad”; and (8) “You could not get going.” Clinical significance is defined as a score of four or greater out of a maximum score of eight. 26 We created an indicator variable equal to one for number of depressive symptoms greater than or equal to four and zero otherwise. Self‐reported health status, a measure correlated with mortality across racial/ethnic groups, 18 , 27 is a five‐point scale (poor, fair, good, very good, excellent) and is converted to an indicator variable equal to one for self‐reports of fair or poor health and zero otherwise. Onset for both variables is equal to one if poor health status/depressive disorder equaled zero in the prior wave and one in the current wave.
2.3. Analytical approach
We first quantified prevalence of dementia and caregiving among the 7859 unique couples over the 14‐year study period. We next examined the prevalence of depressive disorder and poor self‐reported health among respondents before and after onset of a spouse's dementia separately for respondents who became caregivers and those who did not after a spouse's dementia onset and by race/ethnicity. Third, we used a multi‐level logistic regression model to estimate the effect of onset of dementia of a spouse and change in caregiving hours on incident depressive disorder (n = 47,873 person waves) and onset of poor self‐reported health (n = 44,295 person waves). The feature that distinguishes this model from an ordinary regression model is the presence of two random intercepts (couple and individual) and unstructured variance to account for the nested nature of our longitudinal data—individuals were nested in couples and observed in multiple waves. We estimated models of changes in health outcomes and predictors of interest (spouse dementia onset and caregiving) to isolate these relationships from unobservable characteristics fixed over time (eg, culture and norms). Finally, we tested for interaction effects of race/ethnicity and our predictors of interest. In all models, we included controls for characteristics of persons and households including age, education, race/ethnicity, sex, household wealth quartiles, and study year.
3. RESULTS
Table 1 shows dementia and caregiving rates for 7859 unique couples over the 14‐year study period. Among couples, 17.1% had a spouse who had dementia or who acquired dementia over the study period. Prevalence rates of dementia among couples over the study period was higher for Blacks (27.0%) and Hispanics (25.0%) than Whites (14.7%). Among couples with a spouse with dementia over the study period, 87.0% of Whites, 71.4% of Blacks, and 76.2% of Hispanics had a spousal caregiver. Among couples without a spouse with dementia, the rates of caregiving were not statistically different for Whites (38.7%), Blacks (41.0%), and Hispanics (40.0%).
TABLE 1.
Description of sample of unique couples over study waves, 1998–2012
| Respondent dementia (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 6519) | Yes (n = 1340) | ||||||||
| Characteristic in % | Total (n = 7859) | Spouse is not caregiver (n = 3977) | Spouse is caregiver (n = 2542) | Spouse is not caregiver (n = 230) | Spouse is caregiver (n = 1110) | ||||
| Spouse Race/Ethnicity (row %) | |||||||||
| White | 6256 (79.6) | 3273 | (61.3) | 2062 | (38.7) | 119 | (12.9) | 802 | (87.1) |
| Black | 880 (11.2) | 379 | (59.0) | 263 | (41.0) | 68 | (28.6) | 170 | (71.4) |
| Hispanics | 723 (9.2) | 325 | (60.0) | 217 | (40.0) | 43 | (23.8) | 138 | (76.2) |
| Spouse mean age (SD) | 74.2 (9.1) | 72.2 | (8.6) | 74.9 | (9.1) | 77.2 | (9.7) | 79.3 | (8.7) |
Notes: Sample of HRS respondents and their spouses 1998 to 2012. Sample restricted to one observation per couple for descriptive sample of unique couples. Caregiver is defined as any hours >0 in at least one wave during the study period. Spouse is of the respondent who has or will acquire dementia over the study period. Unweighted row percentages included.
Abbreviation: SD, standard deviation.
Figure 1 shows the prevalence of each health outcome measured one wave (about 2 years) before the onset of dementia (t = ‐2), at the wave when incident dementia of his/her spouse occurred (t = 0); and at the subsequent wave, about 2 years later (t = 2) by race/ethnicity and separately for those who become spousal caregivers (cg) and those who do not (nocg) after onset of a spouse's dementia. Among Blacks and Hispanics, 2 years before the onset of a spouse's dementia, there were higher rates of depressive disorder among those who became caregivers (19.4% B, 33.9% H) than those who did not become caregivers (12.6% B, 28.6% H). Rates increased to 25.3% and 36.4% for Black and Hispanic caregivers, respectively, with the onset of a spouse's dementia. Among Whites, prevalence of depressive disorder is similar for future caregivers (14.7%) and other non‐caregiver spouses (16.3%) 2 years before a spouse's onset of dementia, and rates increase over time for caregivers to 22.9% at onset (t = 0) and 27.7% 2 years after a spouse's onset of dementia, more than for non‐caregivers (16.6% at onset, 18.9% 2 years after dementia onset).
FIGURE 1.
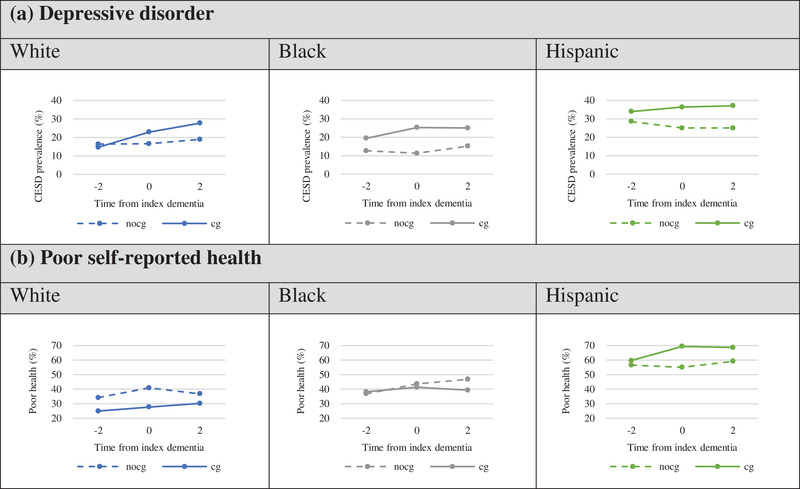
Prevalence of depressive disorder and poor self‐reported health before (t = –2), at (t = 0), and after (t = 2) onset of spousal dementia by race/ethnicity and caregiving status at t = 0 or t = 2. Note: Prevalence of depressive disorder and poor health by race/ethnicity among couples with incident dementia of a spouse over the study period 1998–2012. Prevalence rates are shown separately and by a solid line for those who become caregivers (cg) and a dashed line for those who do not become caregivers (nocg) after onset of a spouse's dementia
Black spouses who become caregivers are slightly more likely to report fair or poor health (38.2%) than non‐caregivers (36.8%) before the onset of a spouse's dementia and show no increase in prevalence over time after taking on caregiving tasks. Among Whites, future spousal caregivers are less likely to be in poor health (25.0%) than non‐caregivers (34.2%); however, rates rise among caregivers to 30.2% 2 years after onset of a spouse's dementia. Among Hispanics, future spousal caregivers have only slightly higher rates of poor health (59.7%) compared to non‐caregivers (56.5%), and rates increase for caregivers with the onset of a spouse's dementia (69.4%).
3.1. Onset of depressive disorder
The odds of acquiring depressive disorder are 2.05 (P < .001) times higher for a person whose spouse acquires dementia compared to a person whose spouse does not have dementia, and 1.95 (P < .001) times higher for a person whose spouse has had dementia for 2 years or more (preexisting) compared to a person whose spouse does not have dementia (Table 2, Model 1). Hispanics have higher rates of depressive disorder than Whites (odds ratio [OR] 1.74, P < .001), with no statistical difference between Blacks and Whites after adjustments for age, sex, wealth, and education. In Model 2, the addition of spousal caregiver status attenuates the estimated effects on onset of depressive disorder of incident dementia (OR = 1.66, P < .001) and preexisting dementia (OR = 1.60, P < .001) of a spouse. In addition, the odds of acquiring depressive disorder are 1.51 (P < .001) times higher for a person who takes on low‐intensity caregiving for a spouse compared to a person who is not caring for their spouse, and 1.79 (P < .001) times higher for a person who takes on high‐intensity caregiving for a spouse, compared to a person who is not a caregiver. Although level differences exist in the odds of acquiring depressive disorder by race, tests for interaction effects of race and caregiving are not statistically significant, as illustrated in Figure 2. Figure 2 shows the probability of acquiring depressive disorder by race/ethnicity (panels a, b) based on estimates from Model 2 (Table 2). It shows the highest risk of depressive disorder onset is for high‐intensity caregivers (panel c).
TABLE 2.
Odds ratios for onset of depressive disorder and poor health associated with incident spousal dementia and take‐up of caregiving
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Depressive disorder | Poor health | |||
| Odds ratio (SE) | Odds ratio (SE) | Odds ratio (SE) | Odds ratio (SE) | |
| Race (ref: White) | ||||
| Black | 1.109 (0.105) | 1.124 (0.106) | 1.350*** (0.118) | 1.351** (0.118) |
| Hispanic | 1.740*** (0.166) | 1.775*** (0.169) | 2.162*** (0.204) | 2.163*** (0.204) |
| Dementia (ref: no dementia) | ||||
| New dementia | 2.049*** (0.255) | 1.664*** (0.211) | 0.931 (0.120) | 0.939 (0.125) |
| Preexisting | 1.946*** (0.229) | 1.604*** (0.191) | 1.138 (0.138) | 1.150 (0.144) |
| Caregiving (ref: non‐caregiver) | ||||
| Transition out | 1.419** (0.180) | 1.034 (0.127) | ||
| Caregiving hours ≤ 20 | 1.509*** (0.129) | 1.084 (0.090) | ||
| Caregiving hours > 20 | 1.794*** (0.196) | 0.913 (0.099) | ||
Notes: Three‐level logistic regression with two random intercepts. Study waves 1998 to 2012 (n = 47,873 person waves [depression]; n = 44,295 person waves [poor health]). Controls include potential caregiver's age (<65 [reference group (ref)], 65–69, 70–74, 75–79, 80–84, 85+), education (less than high school, high school [ref), college and above), sex (male [ref], female), household wealth quartiles (first quartile [ref]), and study year.
P < .05.
P < .01.
P < .001.
Abbreviation: SE, standard error.
FIGURE 2.
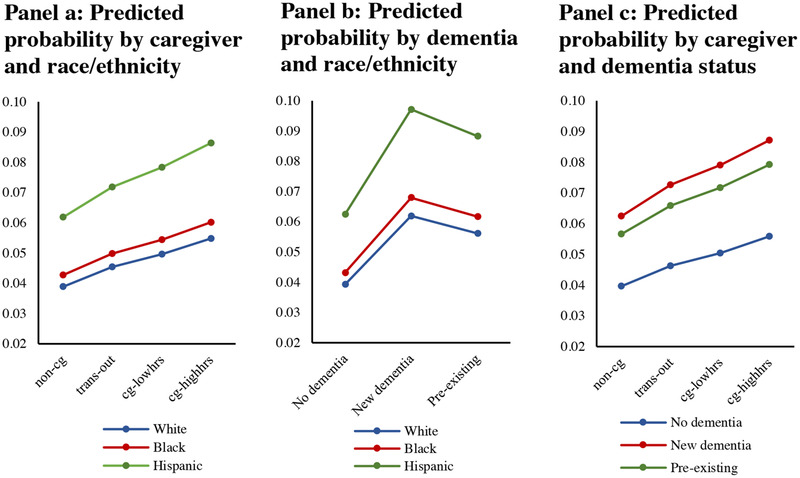
Predicted probability of onset of depressive disorder by race, dementia, and caregiving. Note: Predictions from multivariate logistic regression with random‐intercept, robust variance estimators (Table 2, model 2), study waves 1998–2012 (n = 47,873 person waves. Adjusted for factors: race/ethnicity, dementia, caregiving, sex, age, education, household wealth, survey year
3.2. Onset of poor health
The odds of change to poor self‐reported health from good, very good, or excellent health status are 0.93 and not statistically different for a person whose spouse acquires dementia compared to a person whose spouse does not, and 1.14 and not statistically different for a person whose spouse has had dementia for 2 years or more (preexisting), compared to a person whose spouse does not have dementia (Table 2, Model 3). Hispanics (OR = 1.87, P < .001) and Blacks (OR = 1.29, P < .001) have higher rates of onset of poor health than Whites. The effect of dementia remains statistically insignificant with the addition of spousal caregiver status (Model 4). The odds of change to poor self‐reported health from good, very good, or excellent health are no different for a person who takes on low‐ or high‐intensity caregiving for a spouse compared to a person who is not a caregiver. Tests for interaction effects of race and caregiving are not statistically significant.
4. DISCUSSION AND IMPLICATIONS
This study analyzed how the health of Black, Hispanic, and White caregivers and non‐caregivers is impacted by the onset of dementia of his/her spouse. We found caregiving for a spouse with dementia is common. Eighty‐three percent of coupled persons with dementia had a spousal caregiver who, on average, provided 49.3 hours of care per week. Poor mental and physical health is common for spouses of persons with dementia, and this is higher among Blacks and Hispanics than Whites. Blacks and Hispanics who became caregivers after onset of dementia of his/her spouse had poorer mental health before becoming caregivers than persons who did not become caregivers. This was not the case among White persons—they had lower levels of depressive disorder and poor self‐reported health before onset of a spouse's dementia than Hispanics and Blacks and the level of depressive disorder was the same for White persons who did and did not become caregivers for their spouses with dementia. The level of poor self‐reported health was lower among White persons who would become caregivers relative to non‐caregivers. An important area for future study is improving understanding of the environmental, social, economic, and cultural factors driving care provision among racial and ethnic minorities with poor health.
Consistent with other studies, we found that transitioning into caregiving and caregiving intensity was associated with higher rates of depressive disorder. 6 , 16 , 28 , 29 The rate of change, however, was the same for Blacks, Hispanics, and Whites. In addition, we found higher rates of onset of depressive symptoms among Whites, Blacks, and Hispanics with a spouse with newly acquired dementia independent of take‐up of caregiving tasks. Spouses of persons with dementia, regardless of whether they are caregivers, require clinical attention to their mental health. Our results differ from the few studies that examined racial/ethnic differences in health of caregivers. We found White spousal caregivers were equally (not more or less) likely 6 , 13 , 30 as Hispanics and Blacks to have negative health effects. We added to the literature by demonstrating that racial differences in initial selection into caring may account for differences found later in the caring cycle.
Unlike some previous studies, we found no association of dementia on self‐reported physical health, 8 , 31 , 32 and this was consistent for all racial and ethnic groups. The follow‐up time available in our data may not be sufficient to capture a change in this global measure of health. Reporting differences in self‐reported health status by race/ethnicity may affect our ability to detect disparities, as specific racial/ethnic groups may differently weigh health dimensions. 26 Reporting differences by race in some health domains have been reported. 33
Using longitudinal data, we measured changes in health status associated with changes in a spouse's health (ie, onset of dementia) and changes in caregiving (ie, take‐up or not). Thus, the estimates adjusted for selection into spousal caregiving based on unobservable differences that were fixed over time and for differences in baseline health. Prior estimates based on cross‐sectional data 31 may have been biased upward. To test this, we estimated models based on levels (ie, prevalence) of depressive symptoms and levels of self‐reported poor health and found larger estimated effects, about 15% higher for depressive symptoms and statistically significant increases in self‐reported poor health.
Our analysis has limitations. Analysis of changes in health between survey waves may not reflect a long‐term trend. 34 Minority sample sizes were smaller than those for Whites, which may limit our ability to detect racial and ethnic differences. Racial and ethnic differences in reporting of health status and depressive symptoms may exist; however, these were studied with respect to health levels, and were not the changes that we studied here. 35 Our analytical approach relied on changes in health, dementia, and caregiving for estimated effects independent of unobserved factors fixed over time, but not unobserved factors changing over time and correlated with caregiving. In contrast to caregiving, timing of onset of dementia of a spouse may be largely unexpected, and we found an independent and negative effect of it on mental health.
Dementia imposes significant medical and long‐term care costs. Less often considered and quantified is spillover burden of dementia on the health of family and caregivers. Programs aimed at promoting the mental and physical health of dementia caregivers should consider racial/ethnic differences in health prior and should also take into account the health impacts on non‐caregiver spouses. An important area for future study is improving understanding of the environmental, social, economic, and cultural factors driving care provision among racial and ethnic minorities with poor health. Respite care and counseling services for persons caring for a spouse with dementia may reduce medical costs of the caregiver and furthermore, delay nursing home use of the care recipient and yet research on these and other interventions is limited. 36 In the absence of any current treatments to delay, prevent, or slow the progression of dementia, research‐informed clinical care and policies can address the myriad effects of dementia on patients and their families and society, and reduce the burden of disease now.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Chen C, Thunell J, Zissimopoulos J. Changes in physical and mental health of Black, Hispanic, and White caregivers and non‐caregivers associated with onset of spousal dementia. Alzheimer's Dement. 2020;6:e12082 10.1002/trc2.12082
Footnotes
We ran Models 2 (depressive disorder onset) and 4 (poor health onset) with an interaction between the race/ethnicity and caregiving status variables. The interaction terms were not statistically significant. In a postestimation test of joint significance of the interaction (likelihood ratio), we found no statistical significance.
REFERENCES
- 1. Gaugler J, James B, Johnson T, Marin A, Weuve J. 2019 Alzheimer's disease facts and figures.(Report). Alzheimers Dement. 2019;15(3):321. [Google Scholar]
- 2. Reckrey JM, Morrison RS, Boerner K, et al. Living in the community with dementia: who receives paid care?. J Am Geriatr Soc. 2020;68(1):186‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zissimopoulos J, Crimmins E, Clair PS. The value of delaying Alzheimer's disease onset. Forum Health Econ Policy. 2015;18(1):25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12(3):240‐249. [PubMed] [Google Scholar]
- 5. Kiecolt‐Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991;53(4):345‐362. [DOI] [PubMed] [Google Scholar]
- 6. Schoenmakers B, Buntinx F, Delepeleire J. Factors determining the impact of care‐giving on caregivers of elderly patients with dementia. A systematic literature review. Maturitas. 2010;66(2):191‐200. [DOI] [PubMed] [Google Scholar]
- 7. National Alliance for Caregiving . Caregiving in the US. AARP; Bethesda, MD: The National Alliance for Caregiving; 2005. [Google Scholar]
- 8. Schulz R, O'Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35(6):771‐791. [DOI] [PubMed] [Google Scholar]
- 9. Allen AP, Curran EA, Á Duggan, et al. A systematic review of the psychobiological burden of informal caregiving for patients with dementia: focus on cognitive and biological markers of chronic stress. Neurosci Biobehav Rev. 2017;73:123‐164. [DOI] [PubMed] [Google Scholar]
- 10. Fonareva I, Oken BS. Physiological and functional consequences of caregiving for relatives with dementia. Int Psychogeriatr. 2014;26(5):725‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roth DL, Sheehan OC, Haley WE, Jenny NS, Cushman M, Walston JD. Is family caregiving associated with inflammation or compromised immunity? A meta‐analysis. Gerontologist. 2019;59(5):e521‐e534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson B, Tatangelo G, McCabe M. Depression and anxiety among partner and offspring carers of people with dementia: a systematic review. Gerontologist. 2018;59(5):e597‐610. [DOI] [PubMed] [Google Scholar]
- 13. Covinsky KE, Newcomer R, Fox P, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. J Gen Intern Med. 2003;18(12):1006‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grady PA, Rosenbaum LM. The science of caregiver health. J Nurs Scholarsh. 2015;47(3):197‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583‐594. [DOI] [PubMed] [Google Scholar]
- 16. Dunkle RE, Feld S, Lehning AJ, Kim H, Shen H‐W, Kim MH. Does becoming an ADL spousal caregiver increase the caregiver's depressive symptoms?. Res Aging. 2014;36(6):655‐682. [DOI] [PubMed] [Google Scholar]
- 17. Kaufman JE, Lee Y, Vaughon W, Unuigbe A, Gallo WT. Depression associated with transitions into and out of spousal caregiving. Int J Aging Hum Dev. 2018;88(2):127‐149. [DOI] [PubMed] [Google Scholar]
- 18. Ofstedal MB, Fisher GG, , & . Documentation of Cognitive Functioning Measures in the Health and Retirement Study. Ann Arbor, MI: University of Michigan; 2005. http://hrsonline.isr.umich.edu/sitedocs/userg/dr‐006.pdf. [Google Scholar]
- 19. Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol B‐Psychol. 2011;66:162‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i162‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fisher G, Hassan H, Rodgers W, Weir D. Health and retirement study imputation of cognitive functioning measures: 1992‐2010. J Occup Health Psychol. 2013;19:231‐242. [Google Scholar]
- 23. Freedman VA, Kasper JD, Spillman BC, Plassman BL. Short‐term changes in the prevalence of probable dementia: an analysis of the 2011‐2015 National Health and Aging Trends Study. J Gerontol B. 2018;73(suppl_1):S48‐S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zissimopoulos JM, Tysinger BC. St. Clair PA, Crimmins EM. The impact of changes in population health and mortality on future prevalence of Alzheimer's disease and other dementias in the United States. J Gerontol B. 2018;73(suppl_1):S38‐S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385‐401. [Google Scholar]
- 26. Steffick DE. Documentation of Affective Functioning Measures in The Health and Retirement Study. Ann Arbor, MI: University of Michigan; 2000. [Google Scholar]
- 27. McGee DL, Liao Y, Cao G, Cooper RS. Self‐reported health status and mortality in a multiethnic US cohort. Am J Epidemiol. 1999;149(1):41‐46. [DOI] [PubMed] [Google Scholar]
- 28. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(10):8. [DOI] [PubMed] [Google Scholar]
- 29. Kiecolt‐Glaser JK, Marucha PT, Mercado A, Malarkey WB, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346(8984):1194‐1196. [DOI] [PubMed] [Google Scholar]
- 30. Roth DL, Dilworth‐Anderson P, Huang J, Gross AL, Gitlin LN. Positive aspects of family caregiving for dementia: differential item functioning by race. J Gerontol B Psychol Sci Soc Sci. 2015;70(6):813‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bremer P, Cabrera E, Leino‐Kilpi H, et al. Informal dementia care: consequences for caregivers' health and health care use in 8 European countries. Health Policy. 2015;119(11):12. [DOI] [PubMed] [Google Scholar]
- 32. Ory MG, Hoffman RR, III , Yee JL, Tennstedt S, Schulz R. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39(2):177‐186. [DOI] [PubMed] [Google Scholar]
- 33. Dowd JB, Todd M. Does self‐reported health bias the measurement of health inequalities in US adults? Evidence using anchoring vignettes from the Health and Retirement Study. J Gerontol B Psychol Sci Soc Sci. 2011;66(4):478‐489. [DOI] [PubMed] [Google Scholar]
- 34. Freedman VA, Spillman BC, Andreski PM, et al. Trends in late‐life activity limitations in the United States: an update from five national surveys. Demography. 2013;50(2):661‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Idler EL, Benyamini Y. Self‐rated health and mortality: a review of twenty‐seven community studies. J Health Soc Behav. 1997;38(1):21‐37. [PubMed] [Google Scholar]
- 36. Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects. Int Psychogeriatr. 2006;18(4):577‐595. [DOI] [PubMed] [Google Scholar]


