Abstract
Understanding limb development not only gives insights into the outgrowth and differentiation of the limb, but also has clinical relevance. Limb development begins with two paired limb buds (forelimb and hindlimb buds), which are initially undifferentiated mesenchymal cells tipped with a thickening of the ectoderm, termed the apical ectodermal ridge (AER). As a transitional embryonic structure, the AER undergoes four stages and contributes to multiple axes of limb development through the coordination of signalling centres, feedback loops, and other cell activities by secretory signalling and the activation of gene expression. Within the scope of proximodistal patterning, it is understood that while fibroblast growth factors (FGFs) function sequentially over time as primary components of the AER signalling process, there is still no consensus on models that would explain proximodistal patterning itself. In anteroposterior patterning, the AER has a dual-direction regulation by which it promotes the sonic hedgehog (Shh) gene expression in the zone of polarizing activity (ZPA) for proliferation, and inhibits Shh expression in the anterior mesenchyme. In dorsoventral patterning, the AER activates Engrailed-1 (En1) expression, and thus represses Wnt family member 7a (Wnt7a) expression in the ventral ectoderm by the expression of Fgfs, Sp6/8, and bone morphogenetic protein (Bmp) genes. The AER also plays a vital role in shaping the individual digits, since levels of Fgf4/8 and Bmps expressed in the AER affect digit patterning by controlling apoptosis. In summary, the knowledge of crosstalk within AER among the three main axes is essential to understand limb growth and pattern formation, as the development of its areas proceeds simultaneously.
Keywords: Apical ectodermal ridge (AER), Limb development, Fibroblast growth factor (FGF), Zone of polarizing activity (ZPA)
1. Introduction
Studies on vertebrate limb development are of great significance for the understanding of pattern formation and morphogenesis in embryology (Casanova et al., 2011; Rodriguez-Leon et al., 2013). A good conception of limb development both includes the outgrowth and differentiation of the limb, and provides information of clinical relevance, such as reasons for congenital limb deficiencies, or clues about regeneration. Research to date has revealed that limb development begins with the formation of two paired limb buds (the forelimb and hindlimb buds), which are initially undifferentiated mesenchymal cells tipped with a thickening of the ectoderm, termed the apical ectodermal ridge (AER) (Fig. 1) (Irvine and Rauskolb, 2001; Towers and Tickle, 2009). It is believed that the AER is a signalling centre with a key role in regulating limb growth and development (Casanova et al., 2011; Mallick, 2013).
Fig. 1.
Forelimb bud (at the level of the heart in mouse embryo)
The green layer indicates the apical ectodermal ridge. AER: apical ectodermal ridge. Reprinted from Zeller et al. (2009), Copyright 2009, with permission from Springer Nature
The AER is a transitional embryonic structure that regulates gene expression involved with limb development in four stages through the secretion of signalling molecules (Mallick, 2013; Rodriguez-Leon et al., 2013). Thus, an understanding of AER development is essential for further research on limb growth. For the initiation of AER function, fibroblast growth factor 10 (FGF10) (encoded by Fgf10 gene) expressed from mesenchymal cells binds the epithelial FGF receptor 2b (FGFR2b) with heparin sulfate to activate Fgf8 in the AER precursor cells (Barrow et al., 2003; Itoh and Ohta, 2014). During maturation, Engrailed-1 (En1) is required to repress the expression of Wnt family member 7a (Wnt7a) to the dorsal ectoderm in order to form the dorsal–ventral boundary (Logan et al., 1997), while the upstream of En1 is regulated by bone morphogenetic protein (BMP) (Pizette et al., 2001). Furthermore, Casanova et al. (2011) discovered that AT-rich interaction domain 3b (Arid3b) participated in AER maturation. The maintenance stage is highly relevant to limb outgrowth and pattern formation, where several signalling pathways function within the AER through a positive feedback loop termed sonic hedgehog (SHH)-Gremlin 1 (GREM1)-FGF regulatory loop (Bouldin et al., 2010; Rodriguez-Leon et al., 2013). Following the disconnection of the SHH-GREM1-FGF regulatory loop, the regression stage of the AER occurs for size regulation (Scherz et al., 2004).
Vertebral limb growth and development proceeds in three main axes (Fig. 2), with the AER acting throughout this process. Based on classical manipulation experiments (Saunders, 1948), this structure was used to be considered to control proximodistal patterning. However, the ridge also influences the regulation of anteroposterior patterning, dorsoventral patterning, and the shaping of the limbs (Choi et al., 2012; Delgado and Torres, 2017). This review aims to summarise the specific roles of the AER in three main axes and in shaping the developing limbs, by conducting a comprehensive search in the database sources up to 2019: MEDLINE, Embase, Scopus, Google Scholar, Cochrane Library, and Web of Science databases. The search terms used were “apical ectodermal ridge,” “developing limb/limb development,” and “limb bud” for searching valuable information in the title, keywords, and abstract. No restrictions were placed on the type of articles, study design, language, or publication year. The searching results were manually reviewed to exclude irrelevant articles.
Fig. 2.
Limb axis and elements
Proximodistal axis: proximal to distal (stylopod to autopod); Anteroposterior axis: anterior to posterior (thumb (digit 1) to little finger (digit 5)); Dorsoventral axis: dorsal to ventral (back of the hand to the palm). Reprinted from Duboc and Logan (2009), Copyright 2009, with permission from Elsevier
2. Role of AER in proximodistal patterning
The primary function of the AER is a regulation of proximodistal patterning. A truncation of the limb skeleton may occur in any stage of limb development when the AER is removed in experiments (Saunders, 1948; Summerbell et al., 1973). The removal of the AER results in arrested limb development, with the achieved degree of development determined by the stage of development at which the AER is removed (Saunders, 1948; Tickle, 2003). These findings help us to better understand the mechanism of limb hypoplasia and truncation along the proximodistal axis in humans.
2.1. Current models for specifying positional information in proximodistal patterning
Several models have been proposed to explain proximodistal patterning, such as the progress zone (PZ) model (Summerbell et al., 1973), the early specification model (Dudley et al., 2002), and the two-signal gradient model (Tabin and Wolpert, 2007; Cooper et al., 2011). In the PZ model, positional information of the developing limb is from the PZ immediately beneath the AER (Summerbell et al., 1973; Wolpert, 2002). The PZ acts as a developmental clock suggesting that the length of time undifferentiated mesenchymal cells spend in the PZ specifies their positional value along the proximodistal axis (Wolpert, 2002; Tickle, 2003; Towers and Tickle, 2009). That is, positional values in the PZ model are produced when cells leave the zone (Tickle, 2003). However, the early specification model indicates that specific positional information is assigned upon the formation of the limb bud (Dudley et al., 2002; Tickle and Wolpert, 2002). The difference in positional values between these two models is illustrated in Fig. 3. As for the two-signal gradient model, it involves retinoic acid (RA) signalling as a proximal signal and FGFs as distal signals to regulate proximodistal patterning simultaneously (Mariani et al., 2008).
Fig. 3.
Comparison between the progress zone model and the early specification model
Left: progress zone (PZ) model; positional values along the proximodistal axis. The shaded region is the “PZ.” Right: early specification model; positional values are specified at the formation of the limb bud. Reprinted from Tickle (2003), Copyright 2003, with permission from Elsevier
Therefore, the PZ model and early specification model contradict each other. Moreover, studies in recent years have supplied evidence for the two-signal gradient model (Roselló-Díez et al., 2014). In fact, there is still no consensus about models among researchers, but each of these models contributes to the understanding of limb development and roles of the AER, in particular, the signals expressed in the AER. Thus, substantial evidence is still required to propose or support a valid model that credibly explains the mechanism of proximodistal patterning.
2.2. Molecular and genetic bases of proximodistal patterning
FGF signalling is instructive in proximodistal patterning, and has been considered as the primary component of the AER signalling (Mallick, 2013). Evidence for the function of FGFs in proximodistal patterning was supplied by an FGF-soaked bead experiment in chick wing buds. During the experiment, the AER was replaced by an FGF-soaked bead compared with one no-treatment group and one ridge-removal group without the FGF bead. Results showed that the FGF-soaked bead rescued the outgrowth and proximodistal patterning even with the AER removed (Niswander et al., 1993). Using mouse embryos, Moon and Capecchi (2000) tested the function of FGF signalling by the direct inactivation of Fgf genes (Fgf8) in the AER. As a result, bud outgrowth was reduced and truncation appeared. A similar study by Lewandoski et al. (2000) also supported these results. However, the inactivation of other Fgf genes does not affect limb development, except by the simultaneous inactivation of Fgf4 and Fgf8 (Martin, 1998; Moon et al., 2000; Sun et al., 2000; Towers and Tickle, 2009). The simultaneous absence of Fgf4 and Fgf8 in the AER at different stages of limb development eventually results in a lack of induction of limb development (Sun et al., 2002). Besides, both of these genes in the AER are involved in the maintenance of the undifferentiated zone adjacent to the AER.
The AER produces a large number of FGF proteins encoded by Fgf4, Fgf8, Fgf9, and Fgf17 (in a mouse embryo), or Fgf19 (in a chick embryo), which function at different stages of limb development (Fig. 4) (Tickle, 2003; Rodriguez-Leon et al., 2013). The gene Fgf8 participates in regulating the outgrowth and development throughout proximodistal patterning, while other Fgf genes are activated at specific stages. The expression of Fgf4 and Fgf17 lasts until the beginning of digit patterning (Niswander and Martin, 1992), while the expression of Fgf9 still persists in the later stage of digit patterning (Hajihosseini and Heath, 2002).
Fig. 4.
FGF signalling of AER functions sequentially over time, not showing FGF19
P-D: proximal-distal; LPM: lateral plate mesoderm; AER: apical ectodermal ridge; FGF: fibroblast growth factor. Reprinted from Tickle (2003), Copyright 2003, with permission from Elsevier
There are also other molecules and genes regulating proximodistal patterning, such as RA signalling (Yashiro et al., 2004), T-box transcription factor 5 (Tbx5) and Tbx4 (Nishimoto et al., 2015), myeloid ecotropic viral integration site (Meis) (Mercader et al., 1999, 2000), Homeobox A11 (Hoxa11) and Hoxa13 (Nelson et al., 1996; Mercader et al., 2009), and Shh (Laufer et al., 1994; Niswander et al., 1994). The disruption of RA signalling through the deletion of retinaldehyde dehydrogenase 2 (Raldh2) leads to forelimb abnormalities (Niederreither et al., 2002). Moreover, RA signalling controls the expression of Tbx5 serving the forelimb and Tbx4 serving the hindlimb with the expression of Hox genes and β-catenin, and later through an Fgf10-AER-Fgf8 feed-forward mechanism to regulate limb growth and development (Fig. 5) (Nishimoto et al., 2015). Through the feed-forward loop, Fgf10 is regulated by the RA both directly and indirectly through Tbx genes. The corresponding genes encode MEIS1/2, HOXA11, and HOXA13 proteins as proximodistal markers in the stylopod, zeugopod, and autopod, respectively (Fig. 6) (Roselló-Díez et al., 2014). The Shh gene, expressed in the zone of polarizing activity (ZPA), primarily regulates anteroposterior patterning. However, the expression of Fgf4 requires SHH signalling through the SHH-FGH feedback loop to regulate proximodistal patterning (Fig. 7) (Pownall and Isaacs, 2010).
Fig. 5.
Feed-forward mechanism
The factors retinoic acid (RA)/β-catenin/Homeobox (Hox) cooperatively activate T-box transcription factor 5 (Tbx5) in the forelimb and Tbx4 in the hindlimb. Genes fibroblast growth factor 10 (Fgf10) and Fgf8 are regulated by RA both directly, and indirectly with Tbx. PZ: progress zone; AER: apical ectodermal ridge
Fig. 6.
Expression of Meis, Hoxa11, and Hoxa13 in proximodistal patterning
PD: programmed death; Meis: myeloid ecotropic viral integration site; Hoxa: Homeobox A. Reprinted from Roselló-Díez et al. (2014), Copyright 2014, with permission from the Company of Biologists Ltd.
Fig. 7.
SHH-FGF feedback loop
ZPA: zone of polarizing activity; SHH: sonic hedgehog; AER: apical ectodermal ridge; FGF: fibroblast growth factor
2.3. Roles of FGF10 signalling in pre-AER and post-AER induction
The factor FGF10, as a paracrine FGF, plays different roles in pre-and post-AER phases along with limb development. During pre-AER induction, Fgf10 functions in the lateral plate mesoderm to initiate the formation of AER through Fgf10/AER-Fgfr2b signalling, and then establishes the Fgf10-AER-Fgf8 feed-forward loop (Sekine et al., 1999; Danopoulos et al., 2013). Moreover, a new function of FGF10 found in a study on FGF10 null embryos is that FGF10 involves the regulation of epithelial to mesenchymal transition, thus allowing the formation of the progenitors of the limb (Gros and Tabin, 2014; Jin et al., 2019). It remains unclear, however, whether epithelial to mesenchymal transition is the direct consequence of Fgf10 expression. During post-AER induction, Fgf10 is responsible for maintaining the integrity of AER and FGFR2b signalling by promoting Fgf8 and Wnt3a expression in the AER, which in turn maintain Fgf10 expression in the PZ to initiate the amplification of the progenitors of the limb (Sekine et al., 1999; Mariani et al., 2008; Jin et al., 2019).
Collectively, the FGF family emanating from the AER regulates proximodistal patterning directly with different functions in corresponding stages of limb outgrowth and development. Meanwhile, newer “players” are being found that participate in the proximodistal patterning via specific feedback loops.
3. Role of AER in anteroposterior patterning
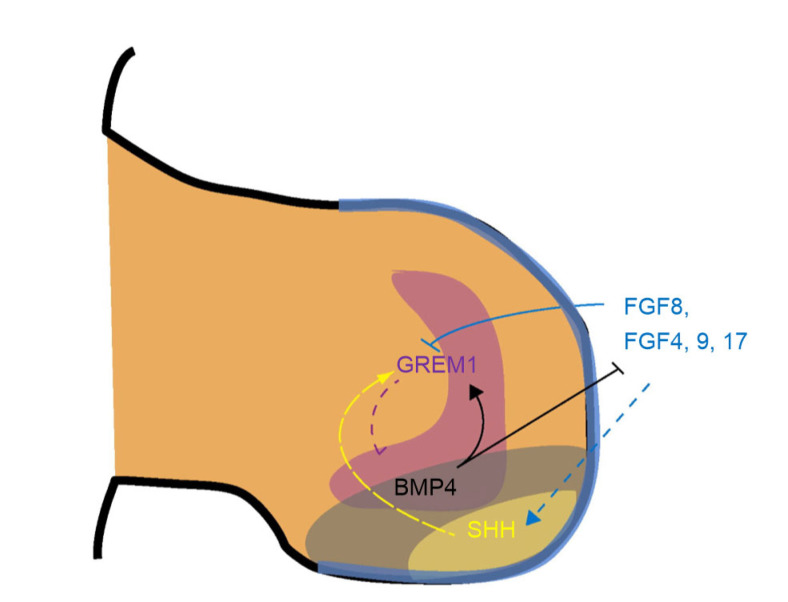
The main pathway where the AER participates in regulating anteroposterior patterning is the SHH-GREM1-FGF regulatory loop. As mentioned in the Introduction section, this feedback loop is essential for the maintenance stage of the AER. The gene Shh mediates anteroposterior patterning, and only exists in the posterior area of the limb bud, which is the so-called PA (Duboc and Logan, 2009). Within the SHH-GREM1-FGF regulatory loop (Fig. 8), the expression of Fgf genes, including Fgf4, Fgf8, Fgf9, and Fgf17, is required for the maintenance of SHH signalling. This loop is positively mediated by Grem1 as an antagonist of Bmp4, which is responsible for a negative regulation of Fgf expression in the AER (Duboc and Logan, 2009).
Fig. 8.
SHH-GREM1-FGF regulatory loop
SHH: sonic hedgehog; GREM1: Gremlin 1; FGF: fibroblast growth factor; BMP: bone morphogenetic protein. Reprinted from Duboc and Logan (2009), Copyright 2009, with permission from Elsevier
Additionally, FGFs regulate E26 transformation-specific (ETS) variant 4 (Etv4) and Etv5 of the ETS transcription factors, which are involved with restricting the expression of Shh in the anterior mesenchyme (Zhang et al., 2009). Authors of the same study found that the inactivation of Fgf genes in the AER or FGFRs leads to reduction of Etv4 and Etv5 expression. Furthermore, the ectopic expression of Shh gene occurs in the anterior mesenchyme and extra digits appear as Etv4 and Etv5 are inactivated (Fig. 9). These results are also supported by Lettice et al. (2012).
Fig. 9.
Extra digits exhibited in the hindlimb
The asterisk indicates the extra digit. Tcre: transcervical resection of endometrium; Etv: E26 transformation-specific (ETS) variant; mt: metatarsal. Reprinted from Zhang et al. (2009), Copyright 2009, with permission from Elsevier
Thus, the AER not only promotes Shh expression in ZPA for proliferation, but also inhibits Shh expression in the anterior mesenchyme. Anteroposterior patterning is regulated through the dual-direction regulatory loop established between signals from the ZPA (SHH) and AER (FGFs).
4. Role of AER in dorsoventral patterning
Dorsoventral patterning is regulated by the interaction between the AER and the ectoderm (dorsal and ventral regions). During normal limb development, the Wnt7a gene is expressed in the dorsal ectoderm, while En1 expression is only detected in the ventral ectoderm (Delgado and Torres, 2017). Findings increasingly support that the Fgf8, Sp6/8, and Bmp2/4/7 expressed in the AER have crucial roles in dorsoventral patterning (Fig. 10) (Fernandez-Teran and Ros, 2008; Haro et al., 2014). The genes Fgfs, Sp6/8, and Bmps activate the expression of En1, which in turn acts by restricting the expression of Wnt7a in the ventral ectoderm (Loomis et al., 1996). Conversely, Wnt7a expression in the dorsal ectoderm induces LIM homeodomain factor 1b (Lmx1b), and thus it triggers dorsal differentiation.
Fig. 10.
Interaction between the AER and the ectoderm
AER: apical ectodermal ridge; Shh: sonic hedgehog; Wnt7a: Wnt family member 7a; En1: Engrailed-1; Lmx1b: LIM homeodomain factor 1b; Bmp: bone morphogenetic protein; Fgf: fibroblast growth factor. Reprinted from Delgado and Torres (2017), Copyright 2017, with permission from Elsevier
Furthermore, Bmps, Wnt7a, and En1 give feedback to the AER through the SHH-FGF loop, that is, these factors are also crucial to the initiation, maturation, and maintenance of the AER as mentioned. In this way, the location of the AER at the boundary between the ventral and dorsal ectoderms can be partly explained.
5. Role of AER in shaping the developing limb
Levels of the FGF4 and FGF8 proteins expressed in the AER affect the patterning of individual digits, and are regulated by Bmps in the AER (Fig. 11).
Fig. 11.
Interaction of FGFs and BMPs in shaping individual digits (mouse embryo)
(a) During normal development, bone morphogenetic protein (BMP) receptor 1a (BMPR1a) downregulates fibroblast growth factor 8 (Fgf8) expression to activate apoptosis in the interdigit zone. (b) After removal of Bmpr1a, the expansion of Fgf4/8 expression causes abnormal cell survival, and thus webbed digits appear. Reprinted from Pajni-Underwood et al. (2007), Copyright 2007, with permission from the Company of Biologists Ltd.
When the gene BMP receptor 1a (Bmpr1a) is inactivated, expression of Fgf4 and Fgf8 in the AER is upregulated, causing webbed digits and less cell death in the interdigit zone (Pajni-Underwood et al., 2007). This suggests that Fgf4 and Fgf8 can prevent apoptosis in the interdigital region. Additionally, the removal of Bmp2/4 from the AER also results in increased expression of Fgf4 and Fgf8 with the same results. Furthermore, a study by Choi et al. (2012) including the removal of BMP ligands (Bmp2/4/7) from the AER reported polydactyly and webbed digits. Hence, Fgf4/8 and Bmps from the AER are responsible for shaping developing digits.
6. Signalling interactions across different signalling centres during limb development
As explained previously, the AER, PZ, and ZPA, seen as three signalling centres besides the mesenchyme, regulate almost all phases of limb development simultaneously. Meanwhile, four AER compartments interact with each other by respective signalling pathways during the process. Jin et al. (2019) linked signalling to these four compartments with effects of Fgf10 (Fig. 12), clearly showing pathways of RA/Hox/β-catenin signalling to Tbx4/Tbx5, Fgf10-AER-Fgf8 feedback loop, SHH-GREM1-FGF regulatory loop, and repression of FGFs on Shh expression, as mentioned above. Additionally, Fgf10 express is stabilized by Wnt2b (forelimb) and Wnt8c (hindlimb), and Fgf8 expression is stabilized by Wnt3a (Kawakami et al., 2001). Furthermore, BMP signalling also upregulates Tbx expression, except for initiating Grem1 expression (Rodriguez-Esteban et al., 1999; Sheeba and Logan, 2017). Reciprocal interactions form a basic loop to implement a regulation by signalling centres for the outgrowth and patterning of the limb.
Fig. 12.
Compositions of the limb bud and its signalling interactions
The limb bud consists of mesenchyme, progress zone (PZ), zone of polarizing activity (ZPA), and apical ectodermal ridge (AER) from proximal to distal. The closed loop regulating the outgrowth and development of the limb bud includes retinoic acid (RA)/homeobox (Hox)/β-catenin signalling, a fibroblast growth factor 10 (Fgf10)-AER-Fgf8 feedback loop, and a sonic hedgehog (SHH)-Gremlin 1 (GREM1)-FGF regulatory loop. Tbx: T-box transcription factor; Fgfr2b: FGF receptor 2b; Etv4: E26 transformation-specific (ETS) variant 4; Bmp: bone morphogenetic protein
7. Conclusions
The AER contributes to multiple axes of limb development through the coordination of signalling centres, feedback loops, and other cellular activities. The principal function of the AER is the regulation of proximodistal patterning. This is achieved directly through FGFs, while the specific model for explaining proximodistal patterning remains unclear. During anteroposterior patterning, the AER acts as a dual-direction regulator in which it promotes Shh expression in ZPA for proliferation, and inhibits Shh expression in the anterior mesenchyme. In dorsoventral patterning, the AER activates En1 expression, and thus it suppresses Wnt7a expression in the ventral ectoderm by the expression of Fgfs, Sp6/8, and Bmps. It also plays a vital role in shaping the individual digits, as the levels of Fgf4/8 and Bmps expressed in the AER affect digit patterning by controlling apoptosis. Consequently, it is important to understand the crosstalk among the three main axes of AER to accurately describe limb growth and pattern formation, as different processes occur simultaneously in limb development.
Footnotes
Project supported by the Key Research and Development Project of Shandong Province (No. 2017G006043), China
Contributors: Guo-hao LIN performed the literature research, wrote and edited the manuscript. Lan ZHANG provided expert comments, edited and revised the manuscript. Both authors have read and approved the final manuscript.
Compliance with ethics guidelines: Guo-hao LIN and Lan ZHANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by either of the authors.
References
- 1.Barrow JR, Thomas KR, Boussadia-Zahui O, et al. Ectodermal Wnt3/β-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17(3):394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouldin CM, Gritli-Linde A, Ahn S, et al. Shh pathway activation is present and required within the vertebrate limb bud apical ectodermal ridge for normal autopod patterning. Proc Natl Acad Sci USA. 2010;107(12):5489–5494. doi: 10.1073/pnas.0912818107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casanova JC, Uribe V, Badia-Careaga C, et al. Apical ectodermal ridge morphogenesis in limb development is controlled by Arid3b-mediated regulation of cell movements. Development. 2011;138(6):1195–1205. doi: 10.1242/dev.057570. [DOI] [PubMed] [Google Scholar]
- 4.Choi KS, Lee C, Maatouk DM, et al. Bmp2, Bmp4 and Bmp7 are co-required in the mouse AER for normal digit patterning but not limb outgrowth. PLoS ONE. 2012;7(5):e37826. doi: 10.1371/journal.pone.0037826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper KL, Hu JKH, Ten Berge D, et al. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science. 2011;332(6033):1083–1086. doi: 10.1126/science.1199499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danopoulos S, Parsa S, al Alam D, et al. Transient inhibition of FGFR2b-ligands signaling leads to irreversible loss of cellular β-catenin organization and signaling in AER during mouse limb development. PLoS ONE. 2013;8(10):e76248. doi: 10.1371/journal.pone.0076248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado I, Torres M. Coordination of limb development by crosstalk among axial patterning pathways. Dev Biol. 2017;429(2):382–386. doi: 10.1016/j.ydbio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Duboc V, Logan MPO. Building limb morphology through integration of signalling modules. Curr Opin Genet Dev. 2009;19(5):497–503. doi: 10.1016/j.gde.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Dudley AT, Ros MA, Tabin CJ. A re-examination of proximodistal patterning during vertebrate limb development. Nature. 2002;418(6897):539–544. doi: 10.1038/nature00945. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Teran M, Ros MA. The apical ectodermal ridge: morphological aspects and signaling pathways. Int J Dev Biol. 2008;52(7):857–871. doi: 10.1387/ijdb.072416mf. [DOI] [PubMed] [Google Scholar]
- 11.Gros J, Tabin CJ. Vertebrate limb bud formation is initiated by localized epithelial-to-mesenchymal transition. Science. 2014;343(6176):1253–1256. doi: 10.1126/science.1248228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajihosseini MK, Heath JK. Expression patterns of fibroblast growth factors-18 and -20 in mouse embryos is suggestive of novel roles in calvarial and limb development. Mech Dev. 2002;113(1):79–83. doi: 10.1016/S0925-4773(01)00656-6. [DOI] [PubMed] [Google Scholar]
- 13.Haro E, Delgado I, Junco M, et al. Sp6 and Sp8 transcription factors control AER formation and dorsal-ventral patterning in limb development. PLoS Genet. 2014;10(8):e1004468. doi: 10.1371/journal.pgen.1004468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irvine KD, Rauskolb C. Boundaries in development: formation and function. Annu Rev Cell Dev Biol. 2001;17:189–214. doi: 10.1146/annurev.cellbio.17.1.189. [DOI] [PubMed] [Google Scholar]
- 15.Itoh N, Ohta H. Fgf10: a paracrine-signaling molecule in development, disease, and regenerative medicine. Curr Mol Med. 2014;14(4):504–509. doi: 10.2174/1566524014666140414204829. [DOI] [PubMed] [Google Scholar]
- 16.Jin LB, Wu J, Bellusci S, et al. Fibroblast growth factor 10 and vertebrate limb development. Front Genet, 9:705. 2019 doi: 10.3389/fgene.2018.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami Y, Capdevila J, Büscher D, et al. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;104(6):891–900. doi: 10.1016/S0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 18.Laufer E, Nelson CE, Johnson RL, et al. Sonic hedgehog and Fgf-4 act through a signaling cascade and feedback loop to integrate growth and patterning of the developing limb bud. Cell. 1994;79(6):993–1003. doi: 10.1016/0092-8674(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 19.Lettice LA, Williamson I, Wiltshire JH, et al. Opposing functions of the ETS factor family define Shh spatial expression in limb buds and underlie polydactyly. Dev Cell. 2012;22(2):459–467. doi: 10.1016/j.devcel.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26(4):460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- 21.Logan C, Hornbruch A, Campbell I, et al. The role of Engrailed in establishing the dorsoventral axis of the chick limb. Development. 1997;124(12):2317–2324. doi: 10.1242/dev.124.12.2317. [DOI] [PubMed] [Google Scholar]
- 22.Loomis CA, Harris E, Michaud J, et al. The mouse Engrailed-1 gene and ventral limb patterning. Nature. 1996;382(6589):360–363. doi: 10.1038/382360a0. [DOI] [PubMed] [Google Scholar]
- 23.Mallick A. The function of apical ectodermal ridge in the formation of limb. Bangladesh J Sci Res. 2013;26(1-2):95–99. doi: 10.3329/bjsr.v26i1-2.20237. [DOI] [Google Scholar]
- 24.Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal–distal patterning. Nature. 2008;453(7193):401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin GR. The roles of FGFs in the early development of vertebrate limbs. Genes Dev. 1998;12(11):1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- 26.Mercader N, Leonardo E, Azpiazu N, et al. Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature. 1999;402(6760):425–429. doi: 10.1038/46580. [DOI] [PubMed] [Google Scholar]
- 27.Mercader N, Leonardo E, Piedra ME, et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127(18):3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- 28.Mercader N, Selleri L, Criado LM, et al. Ectopic Meis1 expression in the mouse limb bud alters P-D patterning in a Pbx1-independent manner. Int J Dev Biol. 2009;53(8-10):1483–1494. doi: 10.1387/ijdb.072430nm. [DOI] [PubMed] [Google Scholar]
- 29.Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26(4):455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon AM, Boulet AM, Capecchi MR. Normal limb development in conditional mutants of Fgf4. Development. 2000;127(5):989–996. doi: 10.1242/dev.127.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson CE, Morgan BA, Burke AC, et al. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122(5):1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- 32.Niederreither K, Fraulob V, Garnier JM, et al. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech Dev. 2002;110(1-2):165–171. doi: 10.1016/S0925-4773(01)00561-5. [DOI] [PubMed] [Google Scholar]
- 33.Nishimoto S, Wilde SM, Wood S, et al. RA acts in a coherent feed-forward mechanism with Tbx5 to control limb bud induction and initiation. Cell Rep. 2015;12(5):879–891. doi: 10.1016/j.celrep.2015.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114(3):755–768. doi: 10.1242/dev.114.3.755. [DOI] [PubMed] [Google Scholar]
- 35.Niswander L, Tickle C, Vogel A, et al. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75(3):579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- 36.Niswander L, Jeffrey S, Martin GR, et al. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994;371(6498):609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- 37.Pajni-Underwood S, Wilson CP, Elder C, et al. BMP signals control limb bud interdigital programmed cell death by regulating FGF signaling. Development. 2007;134(12):2359–2368. doi: 10.1242/dev.001677. [DOI] [PubMed] [Google Scholar]
- 38.Pizette S, Abate-Shen C, Niswander L. BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development. 2001;128(22):4463–4474. doi: 10.1242/dev.128.22.4463. [DOI] [PubMed] [Google Scholar]
- 39.Pownall ME, Isaacs HV. FGF Signalling in Vertebrate Development. Morgan and Claypool Life Sciences, San Rafael, USA; 2010. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Esteban C, Tsukui T, Yonei S, et al. The T-box genes Tbx4 and Tbx5 regulate limb outgrowth and identity. Nature. 1999;398(6730):814–818. doi: 10.1038/19769. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Leon J, Tomas AR, Johnson A, et al. Recent advances in the study of limb development: the emergence and function of the apical ectodermal ridge. In: Reyes D Casales A., editor. Embryo Development: Stages, Mechanisms and Clinical Outcomes. Nova Science Publishers Inc., New York; 2013. pp. 77–112. [PubMed] [Google Scholar]
- 42.Roselló-Díez A, Arques CG, Delgado I, et al. Diffusible signals and epigenetic timing cooperate in late proximo-distal limb patterning. Development. 2014;141(7):1534–1543. doi: 10.1242/dev.106831. [DOI] [PubMed] [Google Scholar]
- 43.Saunders JW., Jr The proximo‐distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108(3):363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- 44.Scherz PJ, Harfe BD, McMahon AP, et al. The limb bud Shh-Fgf feedback loop is terminated by expansion of former ZPA cells. Science. 2004;305(5682):396–399. doi: 10.1126/science.1096966. [DOI] [PubMed] [Google Scholar]
- 45.Sekine K, Ohuchi H, Fujiwara M, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21(1):138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 46.Sheeba CJ, Logan MPO. The roles of T-box genes in vertebrate limb development. Curr Top Dev Biol. 2017;122:355–381. doi: 10.1016/bs.ctdb.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Summerbell D, Lewis JH, Wolpert L. Positional information in chick limb morphogenesis. Nature. 1973;244(5417):492–496. doi: 10.1038/244492a0. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Lewandoski M, Meyers EN, et al. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25(1):83–86. doi: 10.1038/75644. [DOI] [PubMed] [Google Scholar]
- 49.Sun X, Mariani FV, Martin GR. Functions of FGF signalling from the apical ectodermal ridge in limb development. Nature. 2002;418(6897):501–508. doi: 10.1038/nature00902. [DOI] [PubMed] [Google Scholar]
- 50.Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007;21(12):1433–1442. doi: 10.1101/gad.1547407. [DOI] [PubMed] [Google Scholar]
- 51.Tickle C. Patterning systems–from one end of the limb to the other. Dev Cell. 2003;4(4):449–458. doi: 10.1016/S1534-5807(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 52.Tickle C, Wolpert L. The progress zone–alive or dead? Nat Cell Biol. 2002;4(9):E216–E217. doi: 10.1038/ncb0902-e216. [DOI] [PubMed] [Google Scholar]
- 53.Towers M, Tickle C. Growing models of vertebrate limb development. Development. 2009;136(2):179–190. doi: 10.1242/dev.024158. [DOI] [PubMed] [Google Scholar]
- 54.Wolpert L. Limb patterning: reports of model’s death exaggerated. Curr Biol. 2002;12(18):R628–R630. doi: 10.1016/S0960-9822(02)01137-5. [DOI] [PubMed] [Google Scholar]
- 55.Yashiro K, Zhao XL, Uehara M, et al. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell. 2004;6(3):411–422. doi: 10.1016/S1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 56.Zeller R, López-Ríos J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10(12):845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z, Verheyden JM, Hassell JA, et al. FGF-regulated Etv genes are essential for repressing Shh expression in mouse limb buds. Dev Cell. 2009;16(4):607–613. doi: 10.1016/j.devcel.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]