Abstract
Verticillin A is a diketopiperazine compound which was previously isolated from Amanita flavorubescens Alk (containing parasitic fungi Hypomyces hyalines (Schw.) Tul.). Here, we initially found, by wound healing assay and Transwell assay in vitro, that verticillin A possesses an inhibitory effect against the migration and invasion of the human colon cancer cell. Subsequently, c-mesenchymal-epithelial transition factor (c-Met) was identified as a molecular target of verticillin A by screening key genes related to cell migration. Verticillin A-mediated c-Met suppression is at the transcriptional level. Further study demonstrated that verticillin A suppressed c-MET phosphorylation and decreased c-MET protein level. In addition, verticillin A inhibited the phosphorylation of c-MET downstream molecules including rat sarcoma (Ras)-associated factor (Raf), extracellular signal-regulated kinase (ERK), and protein kinase B (AKT). Overexpression of Erk partially reversed the verticillin A-mediated anti-metastasis action in the human colon cancer cell. More importantly, verticillin A also inhibited cancer cell metastasis in vivo. Thus, verticillin A can significantly inhibit the migration and invasion of colon cancer cells by targeting c-Met and inhibiting Ras/Raf/mitogen-activated extracellular signal-regulated kinase (MEK)/ERK signaling pathways. Therefore, we determined that verticillin A is a natural compound that can be further developed as an anti-metastatic drug in human cancers.
Keywords: Verticillin A, Colon cancer, Migration, Invasion, c-Mesenchymal-epithelial transition factor (c-MET)
1. Introduction
Colorectal cancer (CRC) is the third-most common type of cancer and the third leading cause of cancer-associated mortality worldwide (Lino-Silva et al., 2019). There are four main treatments for colon cancer patients: chemotherapy, radiotherapy, surgery, and targeted therapy (Engstrom, 2012). The cornerstone treatment is surgery. For early-stage cancers, surgery alone may cure the disease (Lino-Silva et al., 2019). Adjuvant chemotherapy following surgery is recommended (Printz, 2017; Oh et al., 2018). Targeted therapy is a personalized cancer therapy (Kou et al., 2017). However, more than 90% of cancer-related mortality is due to metastasis, which increases the difficulty of treatment (Budchart et al., 2017). Metastasis is a complicated process, involving cell adhesion, migration, and invasion. However, most steps in metastasis remain unclear (Christiano et al., 2000; Kou et al., 2017). Metastasis consists mainly of two steps: (1) physical translocation of a cancer cell to a distant organ; (2) development of the cancer cell into a metastatic lesion at that distant location (Fan et al., 2017). Once the formation of distant metastasis occurs, the mortality rate of CRC patients will increase significantly (Cohen et al., 2017). Therefore, there is an urgent need to develop chemotherapeutic agents to prevent metastasis effectively.
The c-mesenchymal-epithelial transition factor (c-MET) signaling system regulates many important cellular processes in development, cell function, and tissue homeostasis (Birchmeier et al., 2003). It has been reported to be related to the development and progression of many types of cancers (Mo and Liu, 2017). Recently, various studies have demonstrated that overexpression or genetic aberration of c-Met occurs in many cancers (Giordano et al., 1992; D'Amico et al., 2016; Arnold et al., 2017; Chiche et al., 2019), and c-Met overexpression was reported to be associated with CRC invasion and distant metastasis. Therefore, c-Met might be an important biomarker in CRC (Lee et al., 2018).
c-MET, the receptor tyrosine kinase encoded by the Met proto-oncogene, is a cell surface receptor (Organ and Tsao, 2011). The ligand of c-MET was identified as hepatocyte growth factor (HGF) (Ren et al., 2005). Upon binding to HGF, c-MET becomes phosphorylated, which recruits intracellular signaling molecules through a number of effector proteins to activate various downstream pathways, including the rat sarcoma (Ras)/extracellular signal-regulated kinase (ERK), phosphoinositide-3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), signal transducer and activator of transcription 3 (STAT3), focal adhesion kinase (FAK), and c-Jun N-terminal kinase (JNK) pathways (Birchmeier et al., 2003; Thayaparan et al., 2016; Xiang et al., 2017). Overexpression of c-Met is related to tumor growth and metastasis. Several inhibitors targeting c-Met or the downstream molecules are at present in preclinical studies or in clinical trial (Fodde et al., 2001; Chaffer and Weinberg, 2011; Sipos and Galamb, 2012; Zhang et al., 2019).
Verticillin A, a natural compound, isolated from the wild mushroom Amanita flavorubescens Alk, has been identified as a potent anticancer agent in vitro and in vivo (Liu et al., 2011; Zewdu et al., 2016). In a previous study, we demonstrated that verticillin A inhibited histone methyltransferases SUV39H1 and MLL1 to reduce H3K4me3 and H3K9me3 deposition at a series of apoptosis regulatory gene promoters to inhibit pancreatic cancer cell proliferation in vitro (Paschall et al., 2015; Lu et al., 2018). The aim of the present study is to explore whether verticillin A could inhibit cancer metastasis. Wound healing assay and Transwell assay were performed to assess the effect of verticillin A on migration and invasion of colon cancer cells in vitro. Western blotting, quantitative real-time polymerase chain reaction (qRT-PCR), RNA interference (RNAi) assay, and plasmid transient transfection were also used in the present work to elucidate its molecular mechanism.
2. Materials and methods
2.1. Cell lines
Colon cancer CT26, RKO, and DLD1 cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA), and were incubated in high-glucose Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/mL)/streptomycin (100 mg/mL) at 37 °C in an atmosphere of 5% CO2.
2.2. Reagents
Verticillin A was isolated from mushroom with purity of >99% as described previously (Liu et al., 2011). Antibodies against c-MET, phosphorylated (p)-MET (Y1234/1235), AKT, p-AKT, Ras, Ras-associated factor (Raf), p-Raf, steroid receptor coactivator (Src), and cellular myelocytomatosis viral oncogene (c-Myc) were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies against ERK, p-ERK, urokinase plasminogen activator (u-PA), and N-(4-hydroxyphenyl) retinamide (HPR)-conjugated secondary antibodies were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.3. Mice
BALB/c mice were purchased from the company SLAC experimental animal in Shanghai, China. Female mice aged four to six weeks were used.
2.4. Cell viability analysis
Cell viability was tested by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded at a density of 8000 cells per well in 96-well plates and allowed to adhere overnight. Cell viability was assessed 48 h after treatment with various concentrations of verticillin A, and absorbance was measured at 570 nm wavelength.
2.5. Wound healing assay
Cells were seeded into six-well plates to form a confluent monolayer after 24 h incubation. Intersecting scratches were made across the center of the well with a pipette tip and washed with phosphate-buffered saline (PBS) to remove the detached cells. Then cells were added with different doses of verticillin A. The recording sites of each well were marked, and representative images were taken at the marked sites using a microscope (Nikon, Japan). The distances traveled by the cells were determined by measuring the wound width.
2.6. Cell migration and invasion assays
The cell migratory ability was measured using Transwell chambers (Corning, NY, USA) with 8-μm pore-sized filter. Matrigel basement membrane matrix (Corning, NY, USA) was added to each well before cells were plated on the upper chamber and incubated at 37 °C for 30 min. Then cells with serum-free medium were suspended in the upper chamber of the Transwell insert. DMEM medium containing 10% FBS with verticillin A or/and HGF (40 ng/mL) was added to the lower chamber and incubated for 8–10 h at 37 °C in a 5% CO2 incubator. After that, the non=invading cells were gently removed from the upper chamber by a cotton-tipped swab. Invading cells in the lower chamber were fixed with 100% methanol and then stained with 1 g/L crystal violet, and counted in five random fields.
2.7. qRT-PCR
Total RNA was extracted using TRIzol (Invitrogen, CA, USA) for qRT-PCR analysis of gene expression. qRT-PCR reactions were performed in an ABI PRISM 7300 system (Applied Biosystems, CA, USA). The amplification of specific PCR products was detected using SYBR Green PCR Master Mix (Applied Biosystems, CA, USA). The used PCR primer sequences of human were shown in Table 1.
Table 1.
Used PCR primer sequences of human
| Gene | Primer sequence |
| E-cadherin | Forward: 5'-GCTCACATTTCCCAACTC-3' Reverse: 5'-GTGGCAATGCGTTCTCTA-3' |
| c-Met | Forward: 5'-CTTAGCCAACCGAGAGAC-3' Reverse: 5'-GGGAAGGAGTGGTACAAC-3' |
| u-PA | Forward: 5'-AAAACCTCATCCTACACA-3' Reverse: 5'-ATCAGCTTCACAACAGTC-3' |
| RhoA | Forward: 5'-GCTGGGCAGGAAGATTAT-3' Reverse: 5'-GTGTGCTCATCATTCCGA-3' |
| RhoC | Forward: 5'-CTCATCGTCTTCAGCAA-3' Reverse: 5'-AGTGCTTCACCTCTGGGG-3' |
| β-actin | Forward: 5'-ACACCCCAGCCATGTACGTT-3' Reverse: 5'-TCACCGGAGTCCATCACGAT-3' |
2.8. Western blot analysis
Cells were lysed in a total lysis buffer. The cell lysates were separated on 12% gels and transferred to a polyvinylidene fluoride (PVDF) membrane (Milipore, Bellerica, MA, USA). The membrane was incubated in 0.05 g/mL skimmed milk. Blocked membranes were incubated with primary antibodies and secondary antibodies according to the manufacturers’ protocol. Immune complexes were detected with enhanced chemiluminescence (Pierce ECL, Thermo Scientific, MA, USA).
2.9. RNA interference assay
DLD1, RKO, and CT26 cells expressing small interfering RNA (siRNA) targeting c-Met or non-targeting siRNA were generated using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. The sequence is 5'-GCCCAACUACAGAAAUGGU-3' for human c-Met-specific siRNA and 5'-UAGCGACUAAACACAUC AAUU-3' for non-targeting siRNA. The sequence is 5'-GCCGCGTATGTCAGTAAAC-3' for mouse c-Met-specific siRNA and 5'-TTCTCCGAACGTGTCAC GT-3' for non-targeting siRNA. Then the migration and invasion capabilities of these cells were measured by Transwell assay.
2.10. Plasmid transient transfection
Plasmids pcDNA3.1(+)-Erk and negative control were provided by Icartab (Jiangsu, China). To overexpress Erk, cells were transfected with plasmids for 24 h using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s protocol. Later, cells were incubated with a different concentration of verticillin A for 24 h to determine the cell migration and invasion ability by Transwell assay.
2.11. In vivo study
Four to six weeks old BALB/c mice were inoculated with murine colon cancer cell CT26 through tail vein injection (1×105 cells/mouse). Three days later, the mice were randomly divided into two groups. Seven mice were used in each group. The experimental group was injected with verticillin A at a dosage of 1 mg/kg body weight. PBS was used as vehicle control. Injection was conducted every other day for 14 d. Then the mice were sacrificed. The lung tissues of the mice were taken out and fixed in formalin, and the number of tumors was counted. Body weight was measured to determine the toxicity.
2.12. Statistical analysis
In vitro data are presented as mean±standard deviation (SD) of three independent experiments. Statistical analysis was performed using one-way analysis of variance (ANOVA) and Student’s t-test. A P-value of <0.05 was considered as statistically significant.
3. 3 Results
3.1. Effect of verticillin A on survival of colon cancer cells
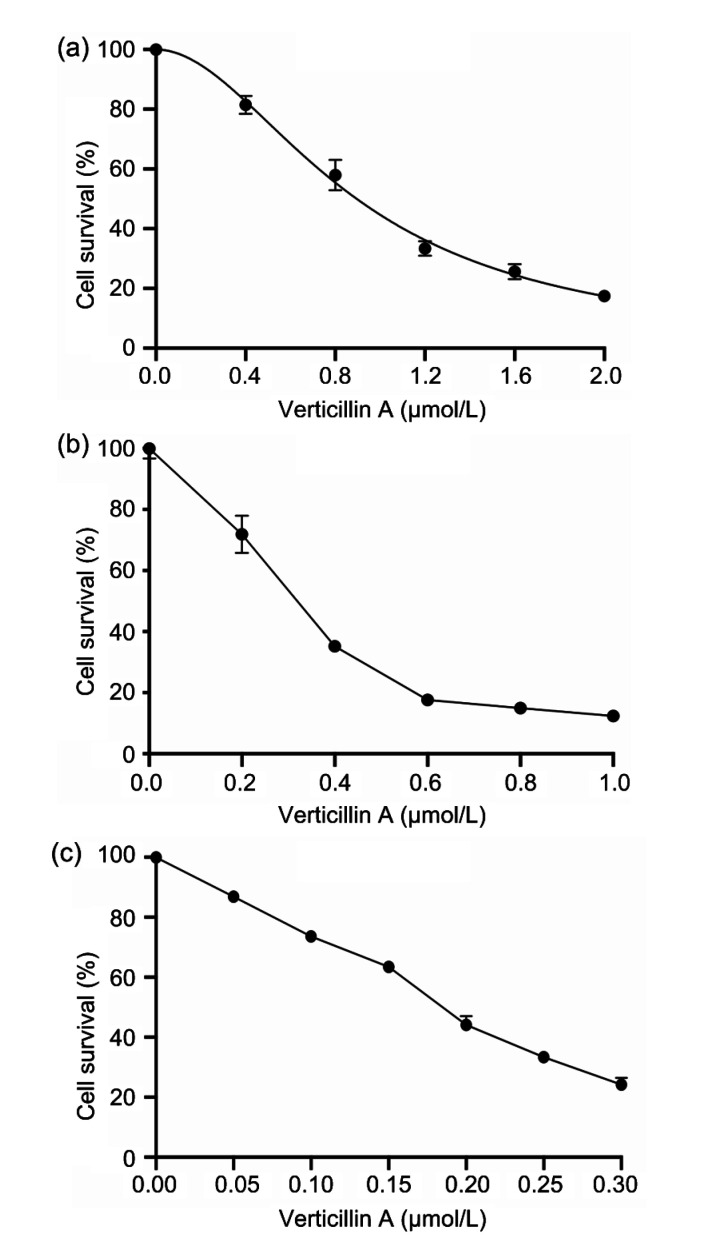
Human colon cancer cells DLD1 and RKO, and murine colon cancer cell CT26 were treated with different concentrations of verticillin A for 48 h and the survival rate was analyzed. The IC50 (half maximal inhibitory concentration) values of verticillin A for DLD1, RKO, and CT26 were 895.7, 306.5, and 177.2 nmol/L, respectively. The results demonstrated that verticillin A significantly suppressed colon cancer cell survival in a dose-dependent manner. The sensitivity to verticillin A varied with the different types of cells (Fig. 1).
Fig. 1.
Cytotoxicity of verticillin A in colon cancer cells
Human colon cancer cells DLD1 (a) and RKO (b), and murine colon cancer cell CT26 (c) were treated with different concentrations of verticillin A for 48 h and then analyzed by MTT assay to determine the sensitivity of different types of colon cells to verticillin A. Data are presented as mean±standard deviation (SD), n=3
3.2. Effects of verticillin A on colon cancer cell migration and invasion in vitro
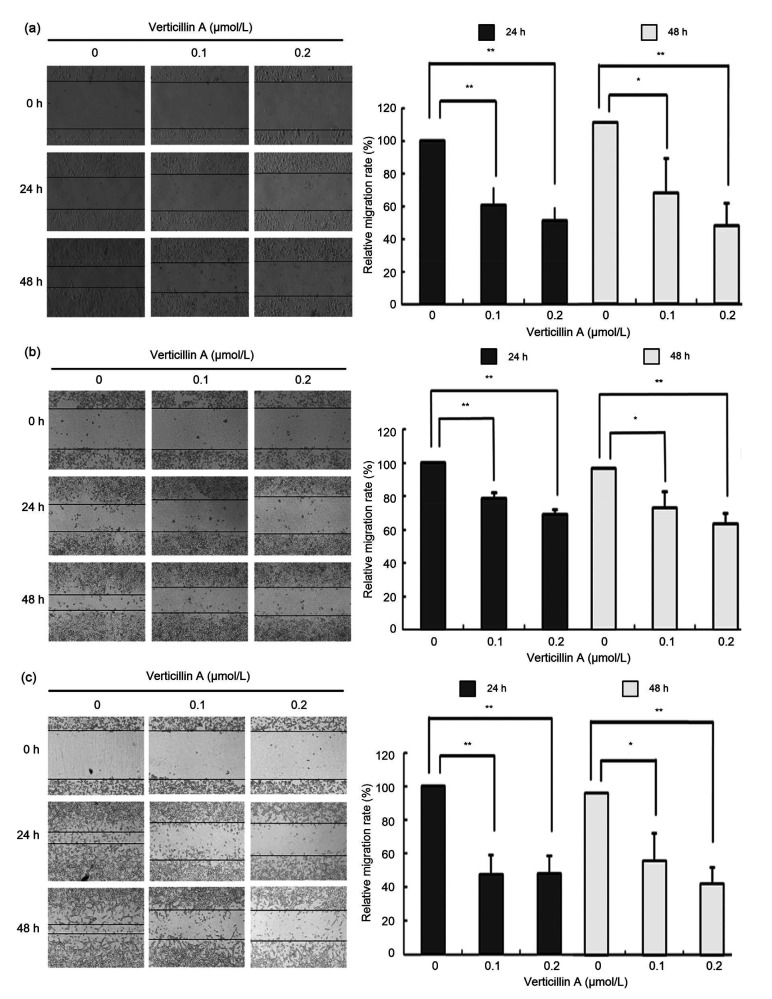
To examine the effects of verticillin A on cell migration and invasion, we first conducted a wound-healing assay in colon cancer cells DLD1, RKO, and CT26. We found that verticillin A treatment remarkably inhibited the ability of wound healing in DLD1, RKO, and CT26 cells, compared to the cells without verticillin A treatment. The inhibitory effect was about 50% after verticillin A treatment for 48 h in DLD1 and CT26. In addition, verticillin A significantly inhibited colon cancer cell migration in a dose-dependent manner (Fig. 2).
Fig. 2.
Effect of verticillin A on the migration of colon cancer cells in vitro
Verticillin A suppressed the migration of colon cancer cells in vitro. Colon cancer cells DLD1 (a), RKO (b), and CT26 (c) were treated with 0.1 and 0.2 μmol/L verticillin A for 24 and 48 h. Then the effect of verticillin A on the migratory ability of these tumor cells was analyzed by wound healing assay in vitro. Data are presented as mean±standard deviation (SD), n=3. * P<0.05, ** P<0.01
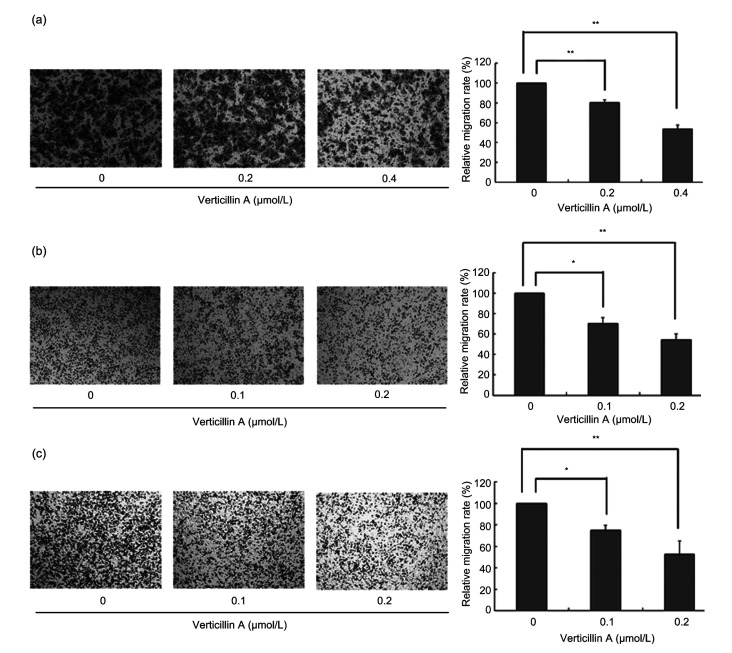
Through Transwell assay with Matrigel, we further showed that verticillin A significantly reduced colon cancer cell invasive ability dose-dependently. The numbers of all three cell lines that invaded through the membranes were fewer than half of those under untreated condition at higher concentrations (Fig. 3). Our data demonstrated that verticillin A dose-dependently inhibited colon cancer cell migration and invasion.
Fig. 3.
Effects of verticillin A on colon cancer cell migration and invasion
Verticillin A inhibited colon cancer cell migration and invasion in vitro. Through Transwell assay, colon cancer cells DLD1 (a), RKO (b), and CT26 (c) were treated with different concentrations of verticillin A for 24 h. Then, the number of trans-membrane cells to the lower chamber was detected. The relative migration rate was determined. Magnification: 10×10. Data are presented as mean±standard deviation (SD), n=3. * P<0.05, ** P<0.01
3.3. Effects of verticillin A-targeted c-Met on colon cancer cell migration and invasion
The above observation indicated that verticillin A significantly inhibited the migration and invasion of colon cancer cells DLD1, RKO, and CT26. In order to find out which metastasis-related genes were affected by verticillin A, qRT-PCR analysis was used to screen the metastasis-related gene expression.
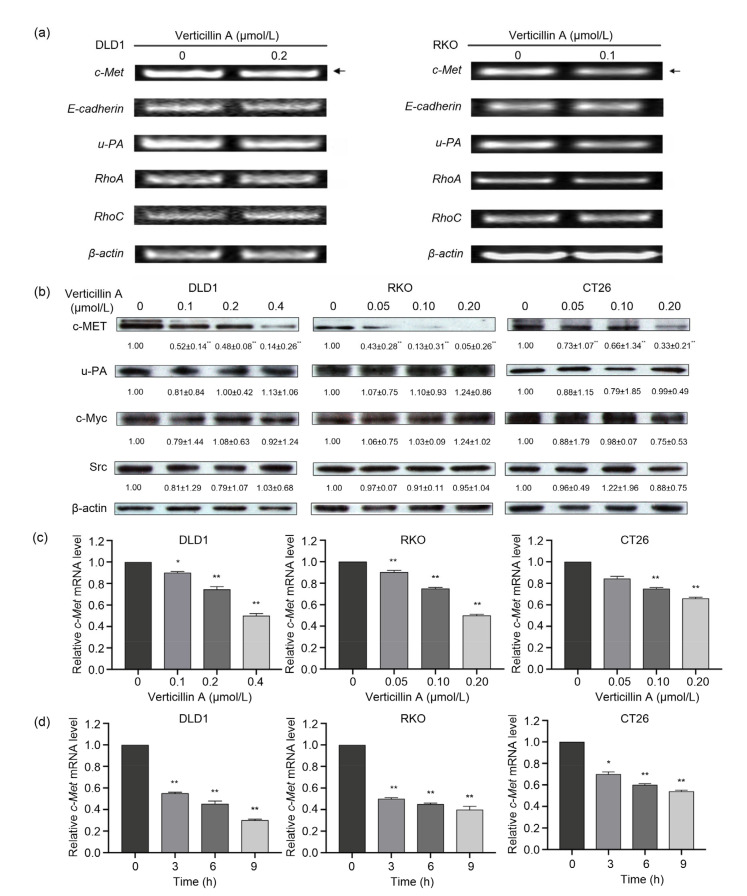
Human colon cancer cell lines DLD1 and RKO were treated with 0.2 and 0.1 μmol/L verticillin A for 24 h, respectively. Total RNA was extracted and analyzed by RT-PCR to detect the expression of key genes associated with tumor metastasis, including c-Met, E-cadherin, u-PA, RhoA, and RhoC. Among these genes, the expression of c-Met and u-PA was downregulated significantly while the others exhibited little change (Fig. 4a).
Fig. 4.
Effect of verticillin A-targeted c-Met on colon cancer cell migration
Verticillin A targeted c-Met to suppress colon cancer cell migration. (a) Human colon cancer cell lines DLD1 and RKO were treated with 0.2 or 0.1 μmol/L verticillin A for 24 h, respectively. Total RNA was extracted and analyzed by RT-PCR to detect the expression of key genes correlated to tumor metastasis. (b) Western blot analysis of verticillin A on the metastasis-associated protein expression of colon cancer cells. Colon cancer cells DLD1, RKO, and CT26 were treated with different concentrations of verticillin A for 48 h, and then proteins were extracted. The expression of metastasis-associated proteins was analyzed by Western blot. β-Actin was used as loading control. Densitometric analysis of the protein bands was done using ImageJ (National Institutes of Health (NIH)). (c) Colon cancer cells DLD1, RKO, and CT26 were treated with different concentrations of verticillin A for 24 h, and total RNA was extracted and analyzed by RT-PCR to detect the level of c-Met mRNA. (d) DLD1, RKO, and CT26 were treated with verticillin A for 0, 3, 6, and 9 h, and total RNA was extracted and analyzed by RT-PCR to detect the level of c-Met mRNA. Data are presented as mean±standard deviation (SD), n=3. * P<0.05, ** P<0.01, vs. control (0 μmol/L verticillin A or 0 h)
Next, we analyzed the influence of verticillin A on the metastasis-associated protein levels in colon cancer cells by western blotting, and found that verticillin A selectively decreased c-MET protein level in a dose-dependent manner in DLD1, RKO, and CT26 cells, suggesting that c-Met might be the molecular target of verticillin A (Fig. 4b).
To determine whether the verticillin A-mediated suppression of c-Met is at the transcriptional level, qRT-PCR was used to determine the effects of verticillin A on the messenger RNA (mRNA) level of c-Met. The results showed that verticillin A decreased c-Met mRNA level in a dose-and time-dependent manner (Figs. 4c and 4d). Three hours after verticillin A treatment, c-Met mRNA levels were decreased by nearly 50% in DLD and RKO cells. These observations suggest that verticillin A-mediated c-Met suppression is likely at the transcriptional level.
3.4. Effects of c-Met on cell migration and invasion in colon cancer cells
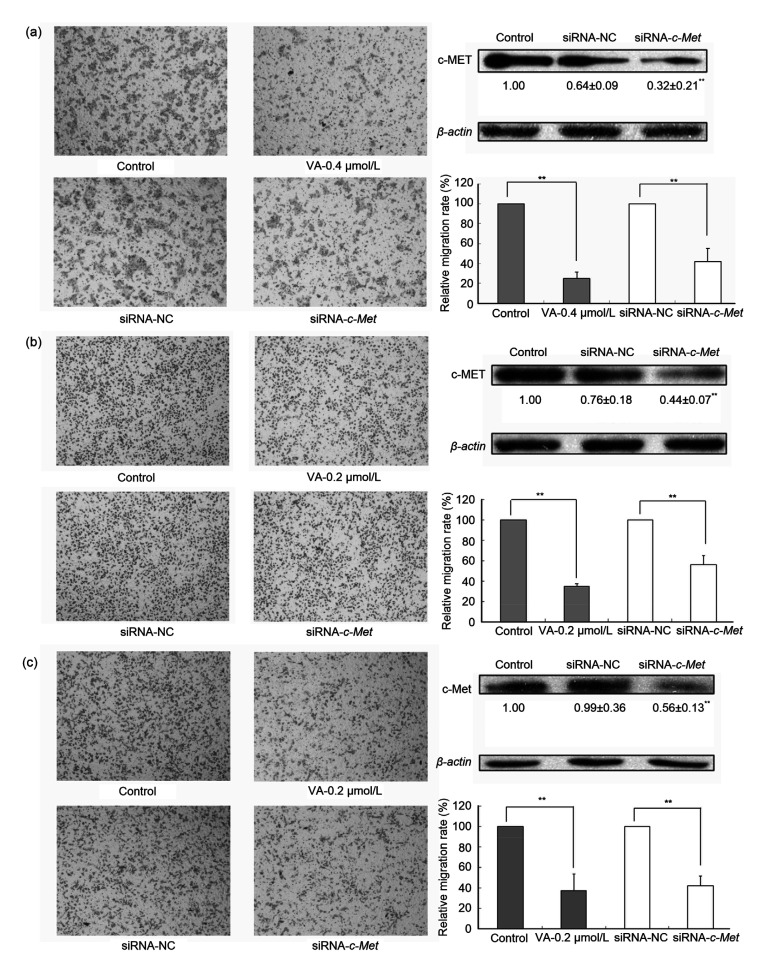
In order to further understand the action of c-Met in colon cancer progression, we silenced the c-Met expression in DLD1, RKO, and CT26 via siRNA-mediated knockdown. We confirmed the efficiency of gene silencing by western blot analysis and observed a 50%–70% decrease in c-MET protein levels in DLD1, RKO, and CT26 (Fig. 5). We then examined the effects of c-Met and verticillin A on the migratory and invasive abilities of colon cancer cells. Our data demonstrated that the silencing of c-Met expression partially reduced migration and invasion, suggesting that c-Met may play an important role in regulating colon cancer cell migration and invasion and is at least one of the molecular targets of verticillin A (Fig. 5).
Fig. 5.
Effects of c-Met on cell migration and invasion in colon cancer cells
c-Met enhanced cell migration and invasion in colon cancer cells. Silencing c-Met expression via c-Met-specific siRNA reversed the migration and invasion of colon cancer cells. Colon cancer cells DLD1 (a), RKO (b), and CT26 (c) were transfected with either small interfering RNA (siRNA)-negative control (NC) or siRNA-c-Met and incubated for 24 h. The silencing efficiency is confirmed by western blot assay. Another group of silencing cells was treated with verticillin A (VA) for 24 h. Then, Transwell assay was used to detect the trans-membrane migration ability of these cells. Densitometric analysis of the protein bands was done using ImageJ (National Institutes of Health (NIH)). Magnification: 10×10. Data are presented as mean±standard deviation (SD), n=3. ** P<0.01
3.5. Effects of verticillin A on HGF-induced c-MET phosphorylation and cell migration and invasion
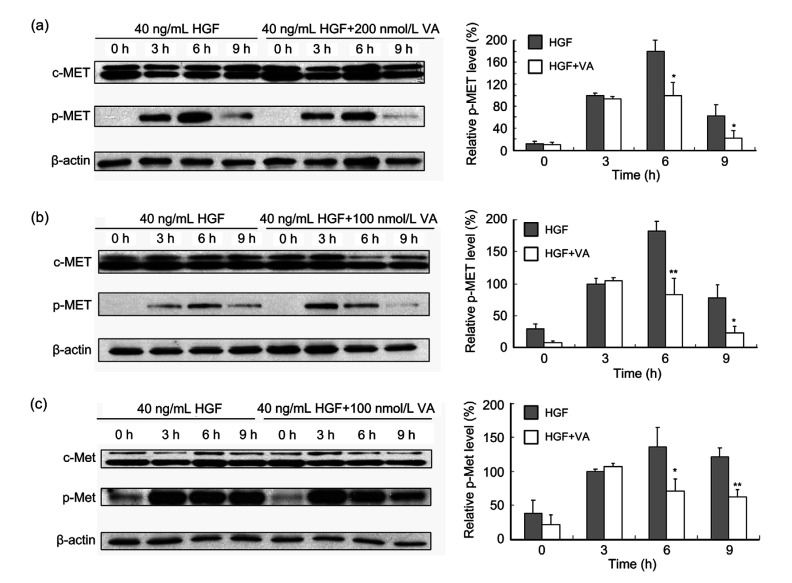
HGF/c-MET pathway has multifunctional effects on various cell types (Saigusa et al., 2012; Mo and Liu, 2017; Parizadeh et al., 2019). Abnormal activation of the HGF/c-MET signaling pathways is reported to be associated with cell motility, migration, and invasion in several tumor types (Giordano et al., 1992; Gholamin et al., 2014; Glodde et al., 2017). Therefore, we investigated whether verticillin A affected the HGF/c-MET signaling pathway. Colon cancer cells DLD1, RKO, and CT26 were treated with 40 ng/mL HGF alone or co-treated with 40 ng/mL HGF and 200 nmol/L verticillin A for 0, 3, 6, and 9 h. Then, the proteins were extracted to conduct western blot analysis. As shown in Fig. 6, stimulation with HGF significantly increased c-MET phosphorylation. Three hours after HGF treatment, c-MET has been activated and p-MET reached the maximum at 6 h in all three cell lines. Interestingly, HGF-induced c-MET phosphorylation at tyrosine residues 1234/1235 was significantly attenuated by verticillin A treatment. At 6 h after HGF treatment, the inhibitory effect was above 50% in all three cell lines (Fig. 6).
Fig. 6.
Effect of verticillin A on c-MET phosphorylation
Western blot analysis showed that verticillin A (VA) inhibited c-MET phosphorylation activated by hepatocyte growth factor (HGF) in colon cancer cells. DLD1 (a), RKO (b), and CT26 (c) were treated with 40 ng/mL HGF or co-treated with 40 ng/mL HGF and VA for 0, 3, 6, and 9 h, and then proteins were extracted. Western blot analysis showed the expression of c-MET and p-MET, and β-actin was used as loading control. Densitometric analysis of the protein bands was done using ImageJ (National Institutes of Health (NIH)). Data are presented as mean±standard deviation (SD), n=3. * P<0.05, ** P<0.01, vs. HGF
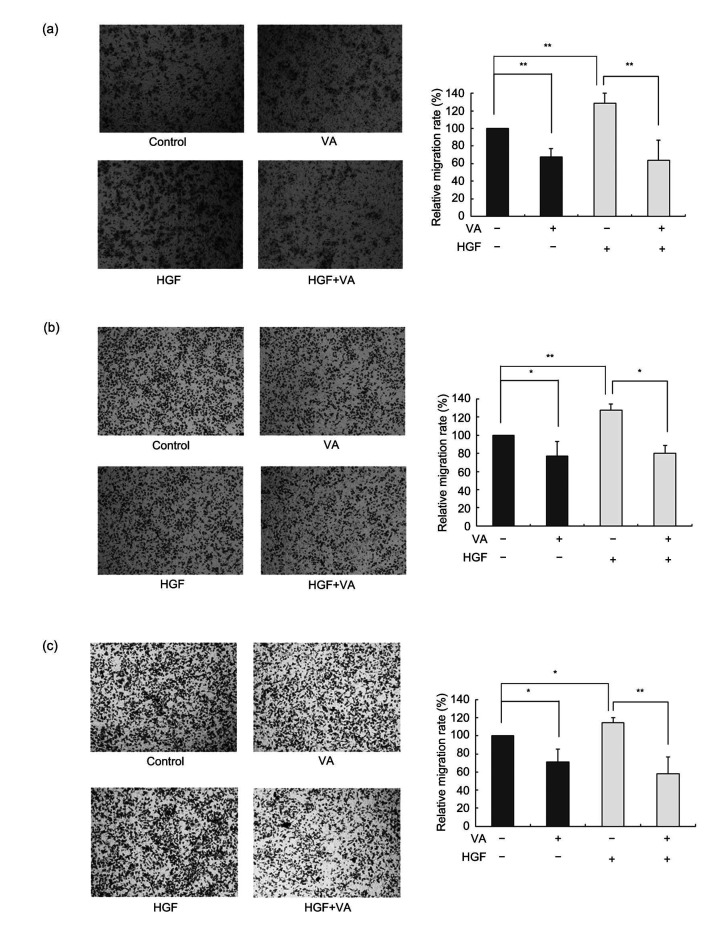
Using Transwell assay to detect the HGF-stimulated migration and invasion of colon cancer cells indicated that HGF treatment increased the trans-membrane migratory ability compared to non-HGF-treated cells. After 24-h treatment with HGF, the number of cells that migrated through the membranes was 20% more than that without treatment. However, verticillin A could also suppress this invasive process and the inhibitory effect was about 50% (Fig. 7). The data indicated that verticillin A could attenuate c-MET phosphorylation stimulated by HGF and suppress HGF-induced colon cancer cell migration and invasion.
Fig. 7.
Effect of verticillin A on HGF-stimulated colon cancer cell migration
Verticillin A (VA) suppressed hepatocyte growth factor (HGF)-stimulated colon cancer cell migration. Colon cancer cells DLD1 (a), RKO (b), and CT26 (c) were treated with 40 ng/mL HGF or co-treated with 40 ng/mL HGF and 200 nmol/L VA for 24 h and then the trans-membrane migration ability of these cells was detected using Transwell assay. Magnification: 10×10. Data are presented as mean±standard deviation (SD), n=3. * P<0.05, ** P<0.01
3.6. Effect of verticillin A on the activation of c-MET downstream molecules
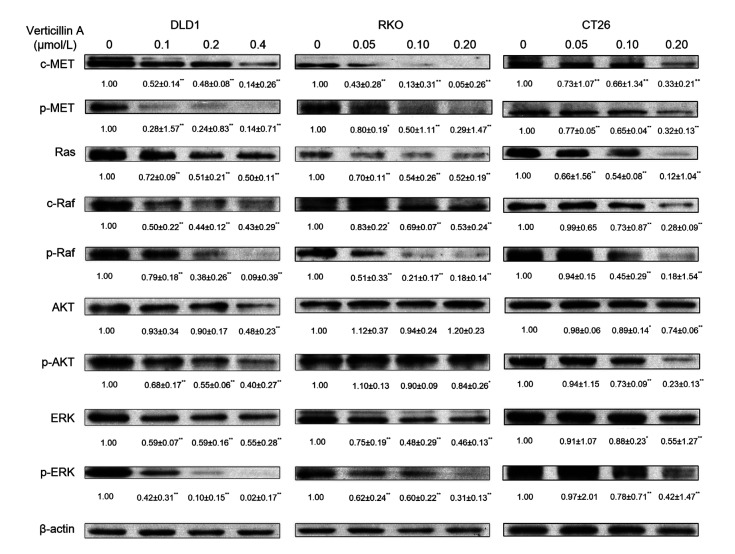
c-MET activation in general results in the activation of its downstream signal cascades, including the Ras/ERK and PI3K/AKT pathways. These pathways are crucial for a variety of cellular responses including cell survival, migration, and invasion (Organ and Tsao, 2011; Xiang et al., 2017). However, the relationship between c-MET expression and activation of AKT and ERK in colon cancer cells has not been fully elucidated. To examine whether verticillin A affected the c-MET downstream molecules, DLD1, RKO, and CT26 cells were incubated with different concentrations of verticillin A for 48 h and the whole-cell lysates were produced for western blot analysis. As shown in Fig. 8, treatment of three colon cancer cells with verticillin A dose-dependently suppressed c-MET protein expression and decreased its basal activation. At the same time, we also found that the expression of several other proteins affecting the cell invasion capability downstream of c-MET was also reduced, including that of Ras and ERK. The phosphorylation of both AKT and ERK was significantly inhibited after verticillin A treatment in a dose-dependent manner (Fig. 8). These results suggested that verticillin A suppressed c-MET protein expression and the basal activation of ERK and AKT in colon cancer cells at the same time.
Fig. 8.
Effect of verticillin A on the activation of c-MET downstream molecules
Verticillin A suppressed the activation of c-MET downstream molecules. Western blot analysis showed protein levels in downstream signaling of c-MET regulated by verticillin A. Colon cancer cells DLD1, RKO, and CT26 were treated with different concentrations of verticillin A for 48 h and then proteins were extracted. Western blot analyzed the key protein levels that were regulated downstream of c-MET signaling, and β-actin was used as loading control. Densitometric analysis of the protein bands was done using ImageJ (National Institutes of Health (NIH)). Data are presented as mean±standard deviation (SD), n=3. * P<0.05, ** P<0.01, vs. control (0 μmol/L verticillin A)
3.7. Verticillin A-mediated anti-metastatic effects reversed by Erk overexpression in part
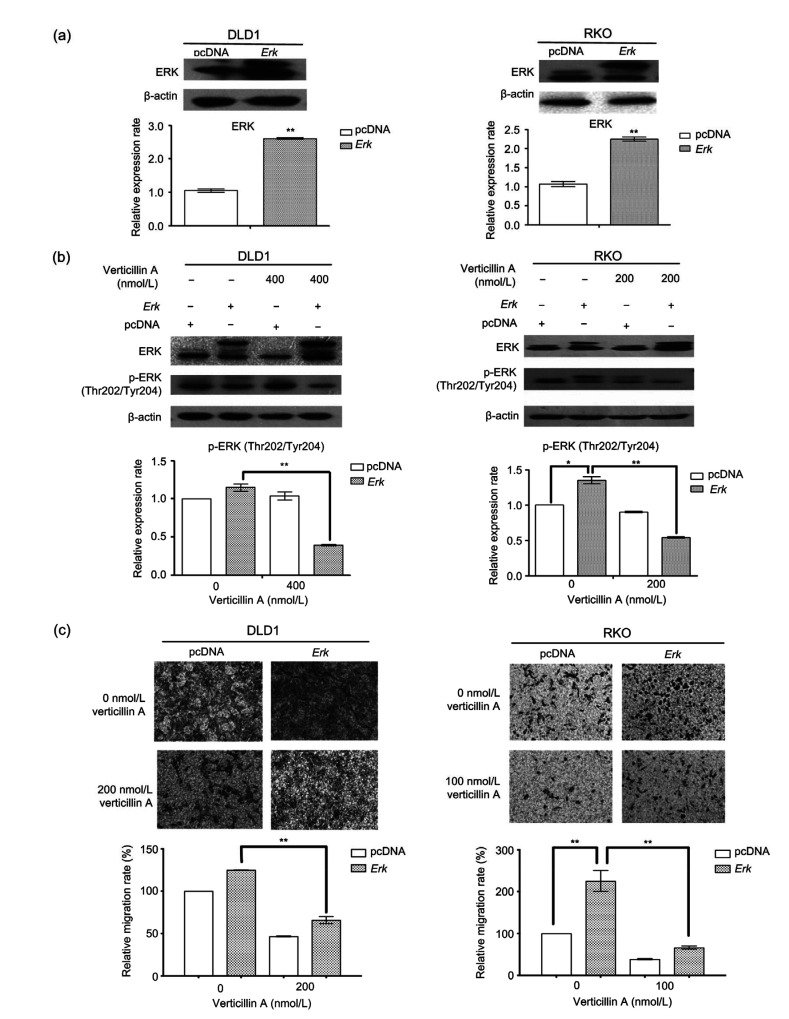
To determine whether Ras/ERK signaling contributed to the inhibitory effect of verticillin A on cell migration, we tested whether the overexpression of Erk reversed the action of verticillin A. DLD1 and RKO cells were transiently transfected with either an Erk-expressing construct or an empty vector. ERK levels elevated about 2-fold in DLD1 and RKO cells after 24-h transfection with Erk-expressing vector (Fig. 9a). In addition, the expression of p-ERK (Thr202/Tyr204) was also increased in both Erk-overexpressing cells (Fig. 9b). Consistent with the elevation of p-ERK protein level, both DLD1 and RKO cells that were transfected with Erk constructs showed a remarkable increase in their migratory capability as compared to the cells with an empty vector. The verticillin A-mediated cell migratory inhibition was partially reversed from 45% to 24% in Erk-overexpressing RKO cells and from 49% to 47% in DLD cells (Fig. 9c). These observations demonstrated that inhibition of Erk activation is evident in Erk-transfected cells after 24-h verticillin A treatment and verticillin A-mediated anti-metastatic effects were reversed by Erk overexpression in part in colon cancer cells.
Fig. 9.
Effect of Erk on the migration–inhibition of verticillin A
Overexpression of Erk partially reversed the migration–inhibition of verticillin A. (a) DLD1 and RKO cells were transiently transfected with an Erk-expressing construct or an empty vector for 24 h. Cells were analyzed by western blot for the expression level of ERK. The protein levels were quantified and presented in the bottom panels. (b) The transfected colon cancer cells were treated with verticillin A at 400 nmol/L (for DLD1 cells) or 200 nmol/L (for RKO cells) for 24 h, and analyzed by western blot for ERK and p-ERK protein levels. The protein levels were quantified and presented in the bottom panels. (c) The transfected colon cancer cells were seeded onto the upper chamber and treated with verticillin A for 24 h. Cell migratory behaviors were analyzed by migration chamber assay. The relative quantitative determination of migrated cells was calculated with five fields counted per experiment. Magnification: 10×10. Data are presented as mean±standard deviation (SD) from three independent experiments. * P<0.05, ** P<0.01
3.8. Effect of verticillin A on colon cancer cell lung metastasis
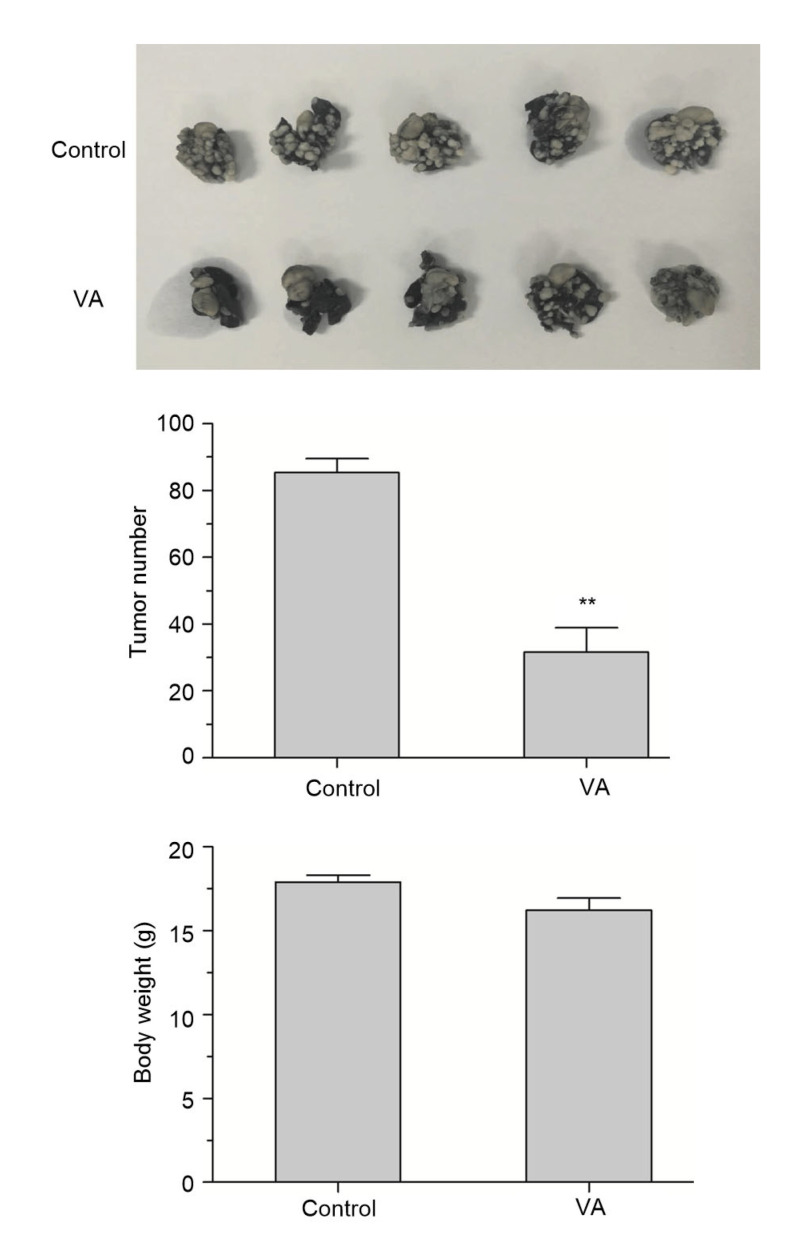
To determine whether the observation that verticillin A effectively inhibits colon cancer cell migration in vitro can be extended to cancer cell migration suppression in vivo, the murine colon carcinoma cell CT26 was injected into BALB/c mice. The experimental group was injected with verticillin A at a dosage of 1 mg/kg body weight. PBS was used as negative control. As expected, compared with negative control, mice in the group injected with verticillin A have less metastasis on their lungs, and there was no significant change in their body weights, suggesting that verticillin A at this dose may have little toxicity to mice. Therefore, our data indicated that verticillin A can inhibit colon cancer cell lung metastasis in vivo (Fig. 10).
Fig. 10.
Effect of verticillin A on colon cancer lung metastasis
Verticillin A (VA) suppressed colon cancer lung metastasis in vivo. Murine colon cancer CT26 cells were injected into BALB/c mice (1×105 cells/mouse). Three days later, mice were treated with 0.2 mL PBS or 1 mg/kg VA every 2 d for 14 d. The mice were sacrificed and the lung tissues were removed 21 d later. The lung tissues were fixed in formalin and photos were taken (upper panel). The number of tumors on the lung tissues and average weight of each group of mice are shown. Data are presented as mean±standard error of the mean (SEM), n=5. ** P<0.01, vs. control
4. Discussion
c-MET receptor has recently been suggested as a therapeutic target for gastrointestinal cancer metastasis (Mihailidou et al., 2017). Cells overexpressing c-Met showed high invasion and migratory potentials (Gardner et al., 2014). Many types of carcinoma have also been found to express or overexpress c-Met. According to the statistics, c-MET is overexpressed in approximately 60%–70% colon cancer patients’ tumor tissues (Jia et al., 2016). Studies indicated that the overexpression of c-MET in gastrointestinal cancers such as pancreatic, gastric, and colon cancers is associated with tumor growth, progression, and metastasis (Huang et al., 2018; Kim et al., 2018). Therefore, c-MET is an important regulator of metastasis formation and the HGF/MET pathway is a promising target for anti-metastasis therapy. In the present study, we first silenced the expression of c-Met via gene-specific siRNA or activation of c-MET by HGF in colon cancer cells to investigate the cells’ migratory ability. As expected, we found that downregulation of c-Met expression reduced the migratory ability of colon cancer cells (Fig. 5). Activation of c-MET by HGF treatment led to enhanced migratory ability of colon cancer cells (Figs. 6 and 7).
Our results indicated that c-Met is a key regulator of metastasis formation in colon cancer. Silencing of c-Met expression or suppression of c-MET activation could inhibit metastasis in colon cancer.
Verticillin A has been reported to be a potent apoptosis sensitizer in human colon cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or factor-associated suicide (FAS) in vitro at nanomolar concentrations (Paschall et al., 2015). Recently, verticillin A was also found to be a sensitizer to metastatic human CRC cells resistant to 5-fluorouracil (5-FU) which was normally used as a chemotherapeutic drug for patients with metastatic CRC (Paschall et al., 2015).
In our present study, we first found that verticillin A was an effective tumor metastasis suppressor both in vitro and in vivo in colon cancer (Figs. 2, 3, and 10). Subsequently, we observed that c-MET protein levels were reduced in all three colon cancer cells after dose-dependent verticillin A stimulation (Fig. 4b), suggesting that verticillin A-induced c-MET receptor reduction is a general phenomenon in colon cancer. We further demonstrated that verticillin A also affected the mRNA expression levels of c-Met in both time-and dose-dependent manners in all three colon cancer cells. This indicated that verticillin A inhibited c-Met expression at the transcriptional level (Figs. 4c and 4d). Therefore, verticillin A-mediated metastasis suppression in colon cancer cells was mainly due to c-Met inhibition. Furthermore, verticillin A also suppressed HGF-stimulated colon cancer cell migration and invasion (Fig. 7), indicating that this inhibitory effect is also related to the inhibition of HGF/c-MET signaling.
c-MET activation will enable the activation of several downstream signaling modulators simultaneously. These pathways include the Ras/ERK, PI3K/AKT/mTOR, phosphatidylinositol 3,4,5-trisphosphate (PIP3)/protein kinase C (PKC), STAT3/JNK, and FAK pathways. Ras/ERK can regulate a large number of genes, including those involved in cell survival, cell motility, and metastasis (Pan et al., 2018; Du et al., 2019; Kurtzeborn et al., 2019; Pereira et al., 2019). Therefore, this pathway is a key pathway to target for preventing CRC metastasis. PI3K/AKT is mainly responsible for cell survival (Wei et al., 2017). PIP3/ PKC can negatively regulate c-MET receptor phosphorylation and activity (Kiselev et al., 2015). STAT3/Janus kinase (JAK) (Mao et al., 2018) can regulate transformation. FAK can lead to cell migration and the promotion of anchorage independent growth (Hui et al., 2009). In our present studies, we found that besides c-MET protein inhibition, verticillin A could also down-regulate the phosphorylation of c-MET and several other proteins affecting the cell invasion capability downstream of c-MET including Ras and ERK. The phosphorylation of Raf, ERK, and AKT was also reduced simultaneously as shown in Fig. 8. Verticillin A dose-dependently reduced the phosphorylation of Raf, AKT, and ERK, indicating that suppression of the Ras/ERK pathway may also be the target of the anti-metastatic action of verticillin A. We further validated that overexpression of Erk will enhance the migratory ability of colon cancer cells and verticillin A-mediated anti-metastasis effects were partially reversed by Erk overexpression (Fig. 9). These results further demonstrated that the Ras/EKR pathway may contribute to the verticillin A-mediated colon cancer cells’ migratory inhibition. Since the PI3K/AKT, STAT, and FAK pathways are also downstream of c-MET, they may also regulate the migratory and inhibitory effect of verticillin A in colon cancers. Indeed, overexpression of Erk only partially reversed verticillin A-mediated inhibitory effects on colon cancer cell migration; however, there is no demonstration that this depends on c-Met targeting (Fig. 9c). Further investigations are required to determine whether the other pathways downstream of c-MET can modulate verticillin A-mediated anti-metastasis in colon cancer cells.
Finally, verticillin A inhibited lung metastasis of colon cancer cells in an in vivo experimental metastasis model (Fig. 10). The results are interesting and of potential translational importance.
Although the underlying mechanism of verticillin A anti-metastasis remains to be fully elucidated, our present studies strongly indicated that verticillin A may be an effective natural compound that needs to be further developed, in order to be used as an anti-metastatic drug for human cancers.
In summary, our current studies showed that verticillin A suppresses colon cancer migration and invasion. The inhibition of c-Met expression is at the transcriptional level and Ras/ERK signaling pathway may contribute to the anti-metastasis action of verticillin A in colon cancer.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY20H160039), the National Natural Science Foundation of China (No. 31570811), and the Siyuan Foundation, Hongkong, China
Contributors: Fei-yan LIU designed study and analyzed data; Fei-yan LIU and Jing-xin LU wrote the manuscript; Qian-qian LIU and Xue-li ZENG performed experiments; Yue-lin GUAN performed plasmid transient transfection; Kai TU provided in vivo study. All authors have read and approved the final version of the manuscript, and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Qian-qian LIU, Xue-li ZENG, Yue-lin GUAN, Jing-xin LU, Kai TU, and Fei-yan LIU declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Arnold L, Enders J, Thomas SM. Activated HGF-c-Met axis in head and neck cancer. Cancers. 2017;9(12):169. doi: 10.3390/cancers9120169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 3.Budchart P, Khamwut A, Sinthuvanich C, et al. Partially purified Gloriosa superba peptides inhibit colon cancer cell viability by inducing apoptosis through p53 upregulation. Am J Med Sci. 2017;354(4):423–429. doi: 10.1016/j.amjms.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 5.Chiche A, Di-Cicco A, Sesma-Sanz L, et al. p53 controls the plasticity of mammary luminal progenitor cells downstream of Met signaling. Breast Cancer Res, 21:13. 2019 doi: 10.1186/s13058-019-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christiano AP, Yoshida BA, Dubauskas Z, et al. Development of markers of prostate cancer metastasis: review and perspective. Urol Oncol. 2000;5(5):217–223. doi: 10.1016/S1078-1439(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SA, Yu M, Baker K, et al. The CpG island methylator phenotype is concordant between primary colorectal carcinoma and matched distant metastases. Clin Epigenetics, 9:46. 2017 doi: 10.1186/s13148-017-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Amico L, Belisario D, Migliardi G, et al. c-MET inhibition blocks bone metastasis development induced by renal cancer stem cells. Oncotarget. 2016;7(29):45525–45537. doi: 10.18632/oncotarget.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du PC, Liang HB, Fu XW, et al. SLC25A22 promotes proliferation and metastasis by activating MAPK/ERK pathway in gallbladder cancer. Cancer Cell Int, 19:33. 2019 doi: 10.1186/s12935-019-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engstrom PF. Ten years of progress in colon cancer therapy. J Natl Compr Canc Netw. 2012;10(5):574–576. doi: 10.6004/jnccn.2012.0058. [DOI] [PubMed] [Google Scholar]
- 11.Fan JX, Zheng DW, Rong L, et al. Targeting epithelial-mesenchymal transition: metal organic network nano-complexes for preventing tumor metastasis. Biomaterials. 2017;139:116–126. doi: 10.1016/j.biomaterials.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Fodde R, Smits R, Clevers H. APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55–67. doi: 10.1038/35094067. [DOI] [PubMed] [Google Scholar]
- 13.Gardner FP, Serie DJ, Salomao DR, et al. c-MET expression in primary and liver metastases in uveal melanoma. Melanoma Res. 2014;24(6):617–620. doi: 10.1097/CMR.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 14.Gholamin S, Fiuji H, Maftouh M, et al. Targeting c-MET/HGF signaling pathway in upper gastrointestinal cancers: rationale and progress. Curr Drug Targets. 2014;15(14):1302–1311. doi: 10.2174/1389450115666141107105456. [DOI] [PubMed] [Google Scholar]
- 15.Giordano S, di Renzo MF, Olivero M, et al. The c-met/HGF receptor in human tumours. Eur J Cancer Prev. 1992;1(6):45–50. doi: 10.1097/00008469-199210003-00007. [DOI] [PubMed] [Google Scholar]
- 16.Glodde N, Bald T, van den Boorn-Konijnenberg D, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity. 2017;47(4):789–802e9. doi: 10.1016/j.immuni.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Huang KH, Sung IC, Fang WL, et al. Correlation between HGF/c-Met and Notch1 signaling pathways in human gastric cancer cells. Oncol Rep. 2018;40(1):294–302. doi: 10.3892/or.2018.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui AY, Meens JA, Schick C, et al. Src and FAK mediate cell-matrix adhesion-dependent activation of Met during transformation of breast epithelial cells. J Cell Biochem. 2009;107(6):1168–1181. doi: 10.1002/jcb.22219. [DOI] [PubMed] [Google Scholar]
- 19.Jia YT, Dai GY, Wang JX, et al. c-MET inhibition enhances the response of the colorectal cancer cells to irradiation in vitro and in vivo. Oncol Lett. 2016;11(4):2879–2885. doi: 10.3892/ol.2016.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HS, Chon HJ, Kim H, et al. MET in gastric cancer with liver metastasis: the relationship between MET amplification and Met overexpression in primary stomach tumors and liver metastasis. J Surg Oncol. 2018;117(8):1679–1686. doi: 10.1002/jso.25097. [DOI] [PubMed] [Google Scholar]
- 21.Kiselev VY, Juvin V, Malek M, et al. Perturbations of PIP3 signalling trigger a global remodelling of mRNA landscape and reveal a transcriptional feedback loop. Nucleic Acids Res. 2015;43(20):9663–9679. doi: 10.1093/nar/gkv1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kou LF, Yao Q, Sivaprakasam S, et al. Dual targeting of L-carnitine-conjugated nanoparticles to OCTN2 and ATB0,+ to deliver chemotherapeutic agents for colon cancer therapy. Drug Deliv. 2017;24(1):1338–1349. doi: 10.1080/10717544.2017.1377316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtzeborn K, Kwon HN, Kuure S. MAPK/ERK signaling in regulation of renal differentiation. Int J Mol Sci. 2019;20(7):1779. doi: 10.3390/ijms20071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Lee J, Park SH, et al. c-MET overexpression in colorectal cancer: a poor prognostic factor for survival. Clin Colorectal Cancer. 2018;17(3):165–169. doi: 10.1016/j.clcc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Lino-Silva LS, Guzmán-López JC, Zepeda-Najar C, et al. Overall survival of patients with colon cancer and a prolonged time to surgery. J Surg Oncol. 2019;119(4):503–509. doi: 10.1002/jso.25354. [DOI] [PubMed] [Google Scholar]
- 26.Liu FY, Liu QQ, Yang DF, et al. Verticillin A overcomes apoptosis resistance in human colon carcinoma through DNA methylation-dependent upregulation of BNIP3. Cancer Res. 2011;71(21):6807–6816. doi: 10.1158/0008-5472.CAN-11-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu CW, Yang DF, Sabbatini ME, et al. Contrasting roles of H3K4me3 and H3K9me3 in regulation of apoptosis and gemcitabine resistance in human pancreatic cancer cells. BMC Cancer, 18:149. 2018 doi: 10.1186/s12885-018-4061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao D, Feng L, Gong H. The antitumor and immunomodulatory effect of Yanghe decoction in breast cancer is related to the modulation of the JAK/STAT signaling pathway. Evid Based Complement Alternat Med, 2018: 8460526. 2018 doi: 10.1155/2018/8460526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihailidou C, Karamouzis MV, Schizas D, et al. Co-targeting c-Met and DNA double-strand breaks (DSBs): therapeutic strategies in BRCA-mutated gastric carcinomas. Biochimie. 2017;142:135–143. doi: 10.1016/j.biochi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Mo HN, Liu P. Targeting MET in cancer therapy. Chronic Dis Transl Med. 2017;3(3):148–153. doi: 10.1016/j.cdtm.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh BY, Park YA, Huh JW, et al. Prognostic impact of tumor-budding grade in stages 1–3 colon cancer: a retrospective cohort study. Ann Surg Oncol. 2018;25(1):204–211. doi: 10.1245/s10434-017-6135-5. [DOI] [PubMed] [Google Scholar]
- 32.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1_Suppl):S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Y, Cheung ST, Tong JHM, et al. Granulin epithelin precursor promotes colorectal carcinogenesis by activating MARK/ERK pathway. J Transl Med, 16:150. 2018 doi: 10.1186/s12967-018-1530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parizadeh SM, Jafarzadeh-Esfehani R, Fazilat-Panah D, et al. The potential therapeutic and prognostic impacts of the c-MET/HGF signaling pathway in colorectal cancer. IUBMB Life. 2019;71(7):802–811. doi: 10.1002/iub.2063. [DOI] [PubMed] [Google Scholar]
- 35.Paschall AV, Yang DF, Lu CW, et al. H3K9 trimethylation silences Fas expression to confer colon carcinoma immune escape and 5-fluorouracil chemoresistance. J Immunol. 2015;195(4):1868–1882. doi: 10.4049/jimmunol.1402243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira SS, Monteiro MP, Costa MM, et al. MAPK/ERK pathway inhibition is a promising treatment target for adrenocortical tumors. J Cell Biochem. 2019;120(1):894–906. doi: 10.1002/jcb.27451. [DOI] [PubMed] [Google Scholar]
- 37.Printz C. Some stage III colon cancer patients may need only half of the standard chemotherapy course. Cancer. 2017;123(20):3871. doi: 10.1002/cncr.31042. [DOI] [PubMed] [Google Scholar]
- 38.Ren Y, Cao B, Law S, et al. Hepatocyte growth factor promotes cancer cell migration and angiogenic factors expression: a prognostic marker of human esophageal squamous cell carcinomas. Clin Cancer Res. 2005;11(17):6190–6197. doi: 10.1158/1078-0432.CCR-04-2553. [DOI] [PubMed] [Google Scholar]
- 39.Saigusa S, Toiyama Y, Tanaka K, et al. Inhibition of HGF/cMET expression prevents distant recurrence of rectal cancer after preoperative chemoradiotherapy. Int J Oncol. 2012;40(2):583–591. doi: 10.3892/ijo.2011.1200. [DOI] [PubMed] [Google Scholar]
- 40.Sipos F, Galamb O. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions in the colon. World J Gastroenterol. 2012;18(7):601–608. doi: 10.3748/wjg.v18.i7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thayaparan T, Spicer JF, Maher J. The role of the HGF/Met axis in mesothelioma. Biochem Soc Trans. 2016;44(2):363–370. doi: 10.1042/BST20150252. [DOI] [PubMed] [Google Scholar]
- 42.Wei DY, Geng F, Liang SM, et al. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci Rep. 2017;37(2):BSR20160470. doi: 10.1042/BSR20160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang C, Chen JX, Fu PF. HGF/Met signaling in cancer invasion: the impact on cytoskeleton remodeling. Cancers. 2017;9(5):44. doi: 10.3390/cancers9050044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zewdu A, Lopez G, Braggio D, et al. Verticillin A inhibits leiomyosarcoma and malignant peripheral nerve sheath tumor growth via induction of apoptosis. Clin Exp Pharmacol. 2016;6(6):221. doi: 10.4172/2161-1459.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang QW, Ye ZD, Shi L. c-Met kinase inhibitors: an update patent review (2014–2017) Expert Opin Ther Pat. 2019;29(1):25–41. doi: 10.1080/13543776.2019.1552261. [DOI] [PubMed] [Google Scholar]