Abstract
Depression is often associated with dysfunction in the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS). Individuals with autism spectrum disorder (ASD) may experience physiological dysregulation and psychological comorbidities; however, the extent to which the interactions between these systems predict internalizing symptoms in ASD has not been investigated. The study examined interactions with the HPA axis and ANS in 10–13-year-old children with ASD (n=41) and typical development (TD; n=46). The interrelated systems uniquely contributed to depressive symptoms in ASD above and beyond any system in isolation. A reciprocal, parasympathetic-dominant ANS was related to fewer affective symptoms in ASD. Findings highlight the importance of examining arousal across multiple systems to more precisely identify profiles associated with maladaptive psychiatric outcomes in ASD.
Keywords: Autism, Hypothalamic Pituitary Adrenal Axis, Autonomic Nervous System, Depression, Heart Rate Variability
Depression is one of the most common mental health conditions worldwide, with recent estimates from the National Institute of Mental Health approximating 7.2% of adults and a staggering 14.4% of adolescents in the United States reporting a major depressive episode in the past 12-months (SAMHSA, 2019). Investigations into potential biological mechanisms of depression have identified physiological arousal systems as possible underlying factors. Two primary arousal systems, the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS), may substantially influence psychological functioning, leading to significant behavioral consequences. Moreover, though they are independent systems, the HPA axis and ANS are interrelated with considerable neural overlap, which serves to maintain an appropriate response to environmental demands and to adaptively regulate behavior and emotion based upon the current context. Vulnerable populations, including individuals with autism spectrum disorder (ASD), are at an especially increased risk of being diagnosed with a depressive disorder in their lifetime, with comorbid rates of depression recently reported at 20.2% for adolescents with ASD (Greenlee, Mosley, Shui, Veenstra-VanderWeele, & Gotham, 2016), with rates increasing to 40% by adulthood (Hudson, Hall, & Harkness, 2019). Given the implication of the HPA and ANS systems in depression in the general population, these systems may also contribute to the complex association between ASD symptomatology and high prevalence of internalizing disorders. However, continued investigations into the interactions of these systems in ASD are warranted.
The HPA axis is the primary neuroendocrine stress system that releases the human glucocorticoid, cortisol, from the adrenal glands (Herman & Cullinan, 1997). The cascade is initiated by release of corticotropin-releasing hormone (CRH) from the hypothalamus, followed by adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary to ultimately signal the release of cortisol. Secretion is modulated by three main functions of the HPA axis—diurnal regulation, response to stress, and negative feedback to restore basal levels. Limbic forebrain brain regions, including the prefrontal cortex, amygdala, and hippocampus, also modulate regulation by initiating or inhibiting the HPA axis cascade (Herman & Cullinan, 1997).
The autonomic nervous system (ANS) of the peripheral nervous system is divided into two branches—the sympathetic (SNS) and parasympathetic (PNS) nervous systems. The two branches act in a relatively reciprocal fashion, with the PNS defined as the ‘rest and digest’ branch, lowering heart rate, blood pressure, and increasing digestion, among other functions. In contrast, the SNS mediates the more metabolically demanding ‘fight or flight’ response, such as increased heart rate, respiration, and blood pressure. The ANS has been proposed as a primary behavioral regulator, with the balance between the PNS and the SNS being crucial in mediating that regulation (Berntson, Norman, Hawkley, & Cacioppo, 2008). Similar to regulation of the HPA axis, centrally mediated brain regions such as the prefrontal cortex and amygdala send downstream signals to the ANS brainstem output regions, thereby providing a top-down influence on autonomic control (Benarroch, 1993; 2004).
A number of studies have cited atypical levels of salivary cortisol, a reliable measure of HPA axis function (Kirschbaum & Hellhammer, 1989), in depressed patients relative to healthy controls (see Stetler & Miller, 2011 for review). For example, in a large cohort of adults, those with current or remitted major depressive disorder showed evidence of hypercortisolism, such as elevated evening cortisol or cortisol awakening response (Vreeburg et al., 2009). Several studies in children and adolescents have also reported elevated cortisol, especially in the evening, in those with clinically-significant levels of depressive symptoms or with a depression diagnosis (Dahl et al., 1991; Van den Bergh & Van Calster, 2009; Van den Bergh, Van Calster, Pinna Puissant, & Van Huffel, 2008). However, others have reported atypically low cortisol (hypocortisolism) in depressive disorders, especially in chronic, severe cases (e.g. Badanes, Watamura, & Hankin, 2011; Bremmer et al., 2007; Heim, Ehlert, & Hellhammer, 2000). Similarly, reduced parasympathetic tone (e.g. Rottenberg, Clift, Bolden, & Salomon, 2007; Thayer, Smith, Rossy, Sollers, & Friedman, 1998; Yaptangco, Crowell, Baucom, Bride, & Hansen, 2015) and/or sympathetic predominance (Schumann, Andrack, & Bär, 2017) may distinguish depressed individuals from healthy controls. Nonetheless, others report no relation between autonomic function and mood (Byrne et al., 2010); however, prevailing opinion remains that a dynamic physiological system capable of reacting and adapting to environmental demands is critical to psychological health (Friedman, 2007).
While the HPA axis and ANS are independent systems, they share substantial neural overlap, especially at the level of frontolimbic regions involved in top-down regulation of physiological output. As both have individually been implicated in psychiatric conditions, identifying interactions between the systems and the patterns of multiple physiological responses likely improves identification of risk factors associated with psychiatric conditions such as depression (Bauer, Quas, & Boyce, 2002). For example, during periods of rest, the prefrontal cortex maintains tonic inhibition over sympathoexcitatory circuitry (e.g., HPA axis, SNS) via inhibition of the amygdala and downstream projections to the nucleus tractus solitarius (NTS) and paraventricular nucleus (PVN) of the hypothalamus, key regions involved in ANS and HPA signaling (e.g. Benarroch, 1993; Thayer & Brosschot, 2005; Ulrich-Lai & Herman, 2009). Ultimately, the PNS is upregulated, promoting a calm visceral state while inhibiting the excitatory SNS and HPA systems (e.g. Friedman, 2007; Porges, 2007). If the prefrontal cortex is hypoactive, however, such as in depression (e.g. Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Koenigs & Grafman, 2009), the amygdala is disinhibited, leading to SNS and HPA activation, as well as PNS suppression (e.g. Thayer & Lane, 2009). Therefore, if there is dysfunction anywhere within these interrelated systems, the entire system could be altered, and different patterns of activation between systems will likely differentially effect behavioral outcomes (Bauer et al., 2002).
In a study of second- and third-grade children with typical development, high baseline parasympathetic regulation was shown to be protective against internalizing symptoms, even in the presence of elevated basal cortisol, whereas those with low PNS regulation and high HPA activity had highest levels of anxiety (El-Sheikh, Arsiwalla, Hinnant, & Erath, 2011). Other recent research in typically developing school-aged children has found that symmetrical hyperarousal of the SNS and HPA axis is correlated with increased internalizing and externalizing behaviors (El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008) or perceived stress (Rotenberg & McGrath, 2016), which supports an ‘additive’ model of arousal (Bauer et al., 2002). Nevertheless, research regarding interactions between the ANS and HPA axis remains relatively scarce, despite the unique advantages such investigations may provide in defining more precise at-risk physiological profiles. Therefore, identifying disruption within these systems could have critical clinical implications, especially for populations shown to be more susceptible to depression or anxiety.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in two core diagnostic domains—social communication and restricted, repetitive behaviors and interests (American Psychiatric Association, 2013). Of significant concern, many individuals with ASD are diagnosed with a comorbid internalizing disorder, with depression rates surpassing those of the global population (Mayes, Calhoun, Murray, Ahuja, & Smith, 2011). Moreover, individuals with ASD appear more likely to experience dysfunction within the HPA axis and/or ANS relative to typically developing (TD) peers, both at rest and during social and non-social stressors (see Benevides & Lane, 2013; Taylor & Corbett, 2014 for review). For example, youth with ASD have shown greater variability of the HPA axis (Corbett, Mendoza, Abdullah, Wegelin, & Levine, 2006; Corbett, Mendoza, Wegelin, Carmean, & Levine, 2008; Corbett, Schupp, Levine, & Mendoza, 2009), as well as elevated cortisol in the evening (Corbett et al., 2009; Muscatello & Corbett, 2017; Tomarken, Han, & Corbett, 2015). Parasympathetic regulation is often reduced in individuals with ASD relative to TD peers (Bal et al., 2010; Ming, Julu, Brimacombe, Connor, & Daniels, 2005; Vaughan Van Hecke et al., 2009), and this blunting is further associated with social difficulties (Edmiston, Jones, & Corbett, 2016; Patriquin, Scarpa, Friedman, & Porges, 2013; Vaughan Van Hecke et al., 2009). However, there is likely substantial heterogeneity in physiological function and dysfunction, as others have found no differences between individuals with ASD or TD in resting state autonomic functioning (Edmiston, Muscatello, & Corbett, 2017; Kushki, Brian, Dupuis, & Anagnostou, 2014; T. P. Levine et al., 2012; Neuhaus, Bernier, & Beauchaine, 2016; Watson, Roberts, Baranek, Mandulak, & Dalton, 2012) nor do they tend to differ in basal afternoon cortisol levels (e.g. Corbett et al., 2006; 2008; Tomarken et al., 2015). A summary of previous research is provided in Table 1.
Table 1.
Studies of Baseline HPA axis and ANS regulation in ASD
| Study | Methods | Sample | Primary Results |
|---|---|---|---|
| HPA Axis | |||
| Corbett et al., 2006 | Salivary Cortisol | 12ASD + 10TD; 6–11 yrs | No differences in cortisol levels; ASD group had more variable circadian rhythm. |
| Corbett et al., 2008 | Salivary Cortisol | 22ASD + 22TD; 6–12 yrs | ASD more variable and had elevated evening cortisol. |
| Corbett et al., 2009 | Salivary Cortisol | 22ASD + 22TD; 6–12 yrs | Elevated evening cortisol associated with increased stress from changes throughout the day in ASD. |
| Kidd et al., 2012 | Salivary Cortisol and α-amylase | 26ASD + 26TD; 2–5 yrs | No differences in diurnal rhythm/slope of cortisol (HPA axis) or α-amylase; ASD group more variable. |
| Muscatello et al, 2018 | Salivary Cortisol | 64ASD + 49TD; 7–17 yrs | Pubertal development and age associated with blunted diurnal slope and higher evening cortisol in ASD. |
| Putnam et al., 2015 | Salivary Cortisol | 16HFASD + 13LFASD + 14TD; 7–12 yrs | ASD with cognitive impairment (LFASD) significantly elevated cortisol at all time points relative to ASD with average or above average IQ (HFASD) and TD. |
| Tomarken et al., 2015 | Salivary Cortisol | 36ASD + 27TD; 7–16 yrs | Higher evening cortisol; Blunted diurnal slope in ~25% of ASD sample. |
| Tordjman et al., 2014 | Salivary and Urinary Cortisol | 55ASD + 32TD; mean 11 yrs; ASD IQ < 70 | ASD higher cortisol across time points; Flatter diurnal slopes; ASD severity associated with higher cortisol. |
| ANS | |||
| Althaus et al., 1999 | HRV, HR, respiration | 36PDD-NOS + 18TD; 7–12 yrs | Did not differ at rest. |
| Daluwatte et al., 2013 | HRV | 152ASD + 116TD + 36NDD; 5–19 yrs | ASD group higher resting HR compared to TD but lower than NDDs; No differences in HRV measures. |
| Edmiston et al., 2016 | RSA | 21ASD + 13TD; 12–18 yrs | Lower baseline RSA in ASD. |
| Edmiston et al., 2017 | PEP | 18ASD + 13TD; 12–17 yrs | Did not differ in baseline PEP. |
| Guy et al., 2014 | RSA | 14ASD + 22TD; mean 11.5–13 yrs | Lower baseline RSA in ASD; Decreased RSA and elevated anxiety, worsening social skills. |
| Hollocks et al., 2014 | HR, HF HRV, LF/HF HRV, Cortisol | 20ASD + 32ASDanx + 23TD; 10–16 yrs | ASD without anxiety elevated HR at rest; No differences at rest for HRV parameters or cortisol. |
| Kushki et al., 2014 | HR, RSA | 40ASD + 34TD; 8–18 yrs | ASD slightly elevated baseline HR; No differences in resting RSA. |
| Levine et al., 2012 | VT, EDA, Cortisol | 19ASD + 11TD; 8–12 yrs | No group differences; Resting PNS, SNS, and HPA function did not differ between groups. |
| Neuhaus et al., 2014 | RSA | 18ASD+ 18TD; mean 11 yrs | ASD lower resting RSA; Higher RSA and better socialization, fewer social problems, and fewer internalizing symptoms. |
| Neuhaus et al., 2016 | HR, PEP, RSA | 18ASD + 18TD; 8–11 yrs | Lower baseline RSA in ASD; No differences in baseline PEP. |
| Patriquin et al., 2013 | RSA | 23 ASD; 4–7 yrs | Low baseline RSA associated with language and cognitive delays. |
| Porges et al., 2013 | RSA | 78ASD + 68TD; 6–21 yrs | Lower baseline RSA in ASD. |
| Schaaf et al., 2015 | RSA, PEP | 59ASD + 30TD; 6–9 yrs | No differences in PEP or RSA at baseline. |
| Sheinkopf et al., 2013 | HR, RSA | 15ASD + 8TD; 2–6 yrs | No differences in HR or RSA at baseline. |
| Toichi et al., 2003 | R-R interval, SD of RRI | 20ASD + 20TD; mean 19–20 yrs | No differences in RRI or SD of RRI at rest. |
| Van Hecke et al., 2009 | RSA | 19ASD + 14TD; 8–12 yrs | Lower baseline RSA in ASD; Lower RSA associated with more social problems. |
| Watson et al., 2012 | RSA | 22ASD + 15LC + 14AC; 6 mos. – 3 yrs | No differences in RSA during nonsocial activity video activity. |
Note: HR, Heart Rate; HF HRV, High Frequency Heart Rate Variability; LF/HF, Low Frequency/High Frequency; PDD-NOS, Pervasive Development Disorder, Not Otherwise Specified; NDD, Neurodevelopmental Disorder; VT, Vagal Tone; EDA, Electrodermal Activity; AC, Age Comparison; LC, Language Comparison; ASDanx, ASD with Anxiety
Given the prevalence of physiological dysfunction in ASD, as well as the high rates of depressive disorders, clearly elucidating the relationship between physiology and internalizing symptoms in youth with ASD could serve to identify markers for risk of psychiatric comorbidities and to identify targets for intervention. While there is some previous evidence for associations between HPA and/or ANS dysfunction and anxiety in ASD (Corbett, Blain, Ioannou, & Balser, 2016; Hollocks, Howlin, Papadopoulos, Khondoker, & Simonoff, 2014; Hollocks, Pickles, Howlin, & Simonoff, 2016; Jansen, Gispen-de Wied, van der Gaag, & van Engeland, 2003; Kushki et al., 2013; Neuhaus, Bernier, & Beauchaine, 2014; Panju, Brian, Dupuis, Anagnostou, & Kushki, 2015), limited research exists regarding the correlation between physiological dysfunction and the high rates of depression in this population. One study of school-aged males with and without ASD found that across the full sample, lower parasympathetic regulation was associated with more internalizing symptoms and withdrawn/depressed symptoms (Neuhaus et al., 2014). Another study in females with ASD found that girls with a lower cortisol awakening response (opposite to the predicted response) were more likely to self-report symptoms of major depressive disorder (Sharpley, Bitsika, Andronicos, & Agnew, 2016). Yet others have cited no associations with internalizing symptoms and physiological response in ASD (Cai, Richdale, Dissanayake, & Uljarević, 2019; Kushki et al., 2014; Lanni, Schupp, Simon, & Corbett, 2012; Muscatello & Corbett, 2017; Tomarken et al., 2015).
The inconsistencies in previous research may be due in part to these relationships between physiological systems. Thereby, elucidating HPA axis and ANS interactions will convey greater information regarding internalizing disorder susceptibility compared to examining either system in isolation. To our knowledge, no study has investigated these interactions in ASD and their associations with internalizing symptoms. The current study argues that such an investigation carries substantial significance, as the systems are not entirely independent, and their interactions may better explain and predict differences in behavior and affect than a single system in isolation. Based upon previous findings in the ASD literature (e.g. Benevides & Lane, 2013), it was hypothesized that children with ASD would exhibit a hyperarousal profile, especially within the ANS, including lower PNS and greater SNS regulation at rest relative to typically developing (TD) controls. There were no a priori hypotheses regarding the within-diagnosis interactions and directionality of associations with depression given the exploratory nature of the study.
Methods
Participants
Initially, 100 children were enrolled in the study; however, thirteen participants were excluded due to incomplete physiological data from equipment malfunction or excessive artifact in the ECG or impedance signal (TD: n=4, ASD: n=9, χ2=2.21, p=0.14). Thus, a total of 87 children, ages 10–13 years, with ASD (N=41) or typical development (TD; N=46) were included in the analyses. The two groups had roughly equal ratios of males to females, with 14 females in the TD group and 11 in the ASD group. Age did not significantly differ between the two groups (Table 2). Participants were recruited from the community using university-wide research announcements, other autism-related studies, research registries, local pediatric and ASD diagnostic clinics, and social media. Participants were part of a larger, longitudinal study of pubertal development (Corbett, 2017). All children had to have an intelligence quotient (IQ) ≥ 70, based on the Wechsler Scale of Abbreviated Intelligence, 2nd edition (WASI-2, Wechsler, 1999) in order to complete self-report questionnaires as part of the larger study. Diagnosis of ASD was based on DSM-5 criteria (American Psychiatric Association, 2013), and established by (1) previous diagnosis by psychiatrist, psychologist, or clinician with autism expertise, (2) current clinical judgement, and (3) corroborated by the Autism Diagnostic Observation Schedule, 2nd edition (Lord et al., 2012), administered by research reliable personnel.
Table 2.
Demographics and Dependent Variables
| ASD | TD | ||||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | Cohen’s d | |
| Age | 11.49 | 1.05 | 11.36 | 1.08 | −0.57 | 0.54 | 0.12 |
| IQ** | 101.68 | 17.43 | 118.85 | 12.95 | 5.16 | <0.001 | 1.12 |
| SCQ** | 17.29 | 7.81 | 2.48 | 2.23 | −11.73 | <0.001 | 2.58 |
| ADOS | 12.68 | 4.62 | -- | -- | -- | -- | -- |
| BMI Percentile* | 73.24 | 21.17 | 50.00 | 33.32 | −3.53 | 0.001 | 0.76 |
| CBCL Affective (Raw)** | 5.61 | 4.12 | 1.63 | 2.11 | −5.57 | <0.001 | 1.21 |
IQ, Intelligence Quotient; SCQ, Social Communication Questionnaire; ADOS, Autism Diagnostic Observation Schedule; BMI, Body Mass Index; CBCL, Child Behavior Checklist; ASD, Autism Spectrum Disorder; TD, Typically Developing
p<0.01
p<0.001
Inclusion and exclusion criteria were reviewed at phone screening, prior to enrollment. Typically developing participants were excluded if they had a biological sibling with ASD. Parents also completed the Social Communication Questionnaire-Lifetime (SCQ-L; Rutter, Bailey, & Lord, 2003) as a screening measure for the assessment of ASD symptoms. TD participants were required to have a total score <10 in order to be included in the study. In an effort to be more representative of the population of youths with ASD, the sample did not require medication-naïve participants. However, those on medications that may directly affect the HPA axis (e.g., corticosteroids; Granger, Hibel, Fortunato, & Kapelewski, 2009) or the ANS (e.g., stimulants; Shibao & Okamoto, 2012) were excluded from the study. Fourteen participants in the ASD group were on medications, including selective-serotonin reuptake inhibitors (n=5), melatonin (n=5), and antihistamines (n=10), or others (n=4). Some participants were on multiple medications. Three participants in the TD group were on antihistamines for daily allergy prevention. All regression analyses controlled for medication status.
The current study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Vanderbilt Institutional Review Board approved all study procedures. Informed written consent and verbal assent was obtained from a parent/guardian and the child, respectively, prior to inclusion in the study.
Procedures
The current study required two visits to a university laboratory. Visit 1 consisted of diagnostic and psychological testing to confirm eligibility, while parents completed the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001). During the first visit, participants also underwent a physical examination to measure height and weight. Body mass index (BMI) was calculated using the standard formula (lb./in2) x 703 then age- and sex-adjusted using the CDC growth charts for children and adolescents (2 through 19 years; https://www.cdc.gov/healthyweight/bmi/calculator.html). Detailed procedures for the physical exam are described elsewhere (Corbett, Muscatello, Tanguturi, McGinn, & Ioannou, 2019). At the second visit, participants underwent physiological data collection as part of a larger study of stress and social functioning. Visits were conducted in the same 10’ by 15’ room with highly trained research personnel.
Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001).
The CBCL is a parent-report questionnaire to assess problem behaviors. Scores are calculated as total raw scores, which are then converted to T-scores based on established national norms. The measure includes several DSM-oriented scales, with high scores suggesting diagnoses to be considered for further evaluation. The Affective Problems subscale includes items consistent with a diagnosis of dysthymia or major depressive disorder, such as feelings of apathy, worthlessness and guilt, presence of self-harm, and more, and it was a primary dependent variable in analyses. The measure has shown excellent sensitivity (0.93) and good specificity (0.74) for the Affective Problems subscale’s overall diagnostic accuracy in youth with ASD (Magyar & Pandolfi, 2017). Follow up exploratory analyses included the Anxiety subscale of the CBCL, which includes items consistent with generalized anxiety, separation anxiety, and phobias. Raw scores were used in analyses, as recommended in the CBCL manual (Achenbach & Rescorla, 2001). Internal consistency was good for Affective Problems (α=0.80) and Anxiety Problems (α=0.79).
Physiological Measures
HPA Axis: Salivary Cortisol
HPA axis regulation was measured through the collection of salivary cortisol. Collecting cortisol through saliva is noninvasive and was collected in the lab using well-established methods (Corbett et al., 2008). Samples were provided by passive drool approximately 40 minutes after arrival to the lab to allow for ample acclimation time. All visits began between 2pm – 3pm to control for the diurnal rhythm of cortisol. Participants were instructed not to eat and drink anything other than water starting one hour prior to their study visit.
Cortisol Assay
Prior to assay, samples were stored at −20°C. Salivary cortisol assay was performed using a Coat-A-Count® radioimmunoassay (RIA) kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA) modified to accommodate lower levels of cortisol in human saliva relative to plasma. Saliva samples were thawed and centrifuged at 3460 rpm for 15 min to separate the aqueous component from mucins and other suspended particles. All samples were duplicated. The coated tube from the kit was substituted with a glass tube into which 100ml of saliva, 100ml of cortisol antibody (courtesy of Wendell Nicholson, Vanderbilt University, Nashville, TN), and 100ml of 125I-cortisol were mixed. After incubation at 4°C for 24h, 100ml of normal rat serum in 0.1% PO4/ EDTA buffer (1:50) and precipitating reagent (PR81) were added. The mixture was centrifuged at 3460 rpm for 30 min, decanted, and counted. Serial dilution of samples indicated a linearity of 0.99. Interassay coefficient of variation was 1.03%.
ANS: Respiratory Sinus Arrhythmia (RSA) and Pre-ejection Period (PEP)
Cardiac autonomic measures were collected using MindWare Mobile Impedance Cardiograph units (MindWare Technologies LTD, Gahanna, OH) for synchronized electrocardiography (ECG) and respiration data using a seven-electrode configuration. A color cartoon was provided to all participants, illustrating where electrodes would be placed, and all children had the opportunity to place an electrode on their hand prior to placement in order to increase participant comfort with the protocol. If participants expressed significant discomfort from the electrode placement, the protocol was discontinued. All participants successfully completed the physiological collection protocol. Data collection began following a 35-minute acclimation period to the lab environment, immediately prior to the saliva sample collection. Baseline collection consisted of a five-minute rest period, with all participants sitting quietly and not engaging in any other tasks.
Parasympathetic regulation was indexed using respiratory sinus arrhythmia (RSA), a measure of high frequency heart rate variability, indicating variation in timing between successive heart beats in association with respiration. RSA was derived in accordance with the guidelines by the Society for Psychophysiological Research committee on heart rate variability (Berntson et al., 1997; Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996) and calculated on a minute-by-minute basis. ECG signal was sampled at 500 Hz and analyzed using the Heart Rate Variability Software Suite provided by MindWare Technologies (MindWare Technologies LTD, Gahanna, OH). RSA was quantified as the integral power within the respiratory frequency band (0.15 to 0.42 Hz). The respiration signal was processed by impedance cardiography and displayed to ensure that the values were within the designated respiratory frequency band. Of the total analyzed data, 0.4% were excluded due to excessive motion artifact or arrhythmia.
Sympathetic arousal was measured using impedance cardiography, a measurement of the mechanical activation of the heart, and can be quantified via pre-ejection period (PEP), which represents the interval from electrical stimulation to mechanical opening of the aorta. PEP was processed using MindWare Technologies Impedance Cardiography Analysis Software (MindWare Technologies, LTD, Gahanna, OH) and calculated as the distance (in ms) from the ECG Q-point of the QRS complex to the B point of the impedance waveform, which corresponds with the time from ventricular depolarization to aortic valve opening (Sherwood et al., 1990). PEP was ensemble-averaged for each one-minute epoch by the MindWare software, and B-point was calculated at 55% of the R-Z interval (time to dZ/dt peak) (Lozano et al., 2007). The QRS complex and dZ/dt signal was confirmed by visual inspection (RAM).
Statistical Analysis
Statistical analyses were performed using IBM SPSS Statistics 26 (IBM Corp, 2019). Independent sample t-tests were conducted to test for differences between ASD and TD groups on demographic and diagnostic variables, including age, IQ, BMI, SCQ, and the CBCL. If assumption of equal variance was not met, the Welch degree of freedom transformation was used. Effect sizes were reported as Cohen’s d. For internalizing symptoms, participants were characterized as reaching clinically significant depressive symptoms is CBCL T-scores ≥ 70. Differences in number of participants falling in clinically significant or non-significant categories were examined using chi-square.
Cortisol values were positively skewed and thus log-10 transformed to achieve normality; RSA and PEP were normally distributed. Between-group differences in physiological measures were tested using analysis of covariance, controlling for age and BMI percentile. Partial η2 was calculated to indicate effect size.
Hierarchal regression with bootstrapping for robust estimators (Efron & Tibshirani, 1993) and bias-corrected and accelerated confidence intervals (to account for the non-normal distribution of the outcome variable) assessed the contributions of cortisol, RSA, and PEP interactions in predicting parent-reported affective symptoms. Separate models were conducted for the ASD and TD groups. Medication use and BMI percentile were controlled in the first step of the model, and then the main effects of cortisol, RSA, and PEP were added in the second step. All possible two-way interaction terms between physiological variables were individually added to the base model in a third step to determine the extent to which each interaction contributed additional variance to the prediction of affective symptoms. Significant interactions were further examined using the PROCESS v3.4 macro (Hayes, 2013) to plot regression lines at high (+1 SD), mean, and low (−1 SD) values of the interacting variables (Aiken & West, 1991). All control and independent variables were mean centered prior to analyses (and prior to creating the interaction terms) to avoid multicollinearity effects.
Results
Demographic and diagnostic information between groups is presented in Table 2. There were no differences between the groups in age; however, the TD group did have higher average IQ than the ASD group. Nonetheless, the mean score for the ASD group fell well within the average range for IQ. Youth with ASD presented with higher average BMI (age- and gender-adjusted percentiles), as measured by physical examination, relative to the TD youth, thus all regression models controlled for BMI percentile. Within the ASD group, there were no significant relations between medication status (taking medication vs. not taking medication) and the primary outcome variables (i.e., cortisol, RSA, PEP, affective symptoms; p>0.05); nonetheless, medication status was included as a covariate in the initial step of all regression models.
Regarding depressive symptoms, relative to parents of TD children, parents of children with ASD reported that their sons/daughters had significantly more affective problems on the CBCL. Participants were further categorized according to whether or not they met clinical cutoffs for affective problems (T≥70). More youth with ASD met the clinically significant cutoff for affective problems relative to TD youth (39% ASD vs. 6.5% TD, p<0.001, Fisher’s exact test).
Results of between group comparisons for RSA, PEP, and cortisol are illustrated in Figure 1. After controlling for BMI percentile, youth with ASD and TD did not differ in baseline cortisol levels (F(1,83)=0.01, p=0.94, Partial η2<0.01, Figure1a), suggesting no significant differences in HPA axis regulation during a resting period. Similarly, the groups showed no statistically significant differences in baseline RSA (F(1,84)=2.55, p=0.11, Partial η2=0.03; Figure 1b) or PEP (F(1,84)=0.63, p=0.43, Partial η2=0.01; Figure 1c). Therefore, youth with ASD and TD had comparable parasympathetic and sympathetic regulation during a resting state.
Figure 1. Mean Baseline Physiological Levels for Children with ASD and TD.
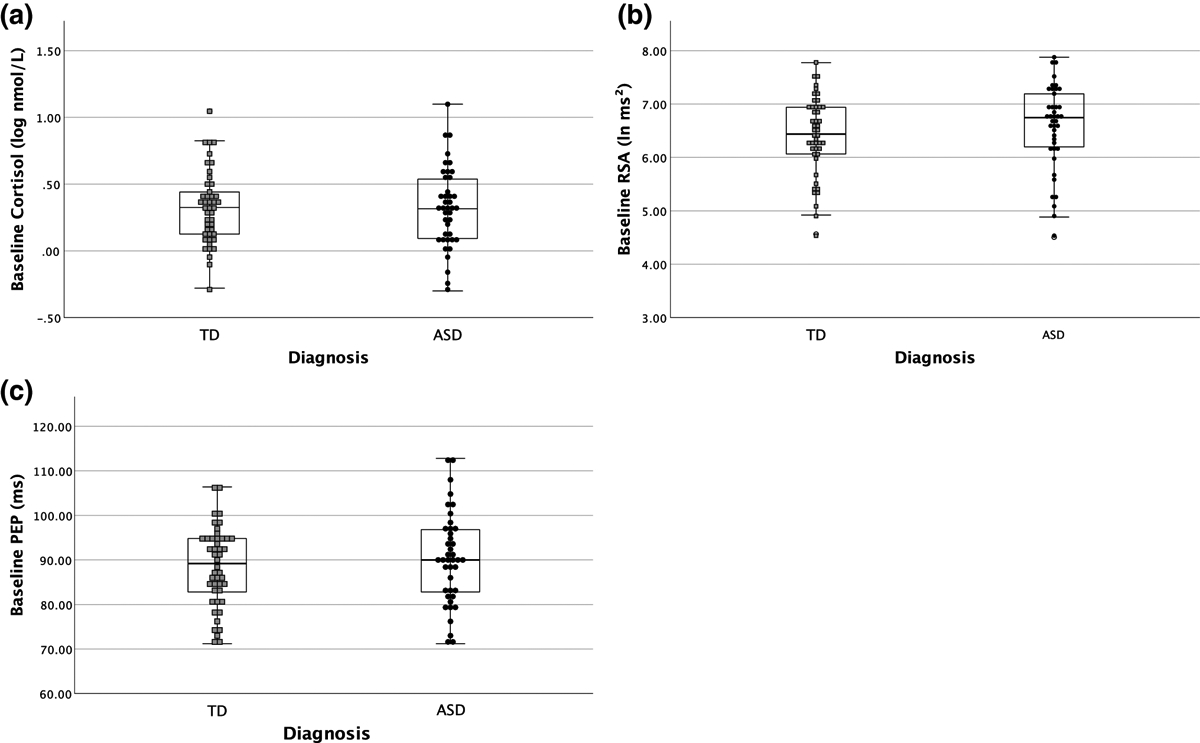
There were no significant differences between children with ASD and TD in (a) baseline log cortisol, (b) baseline respiratory sinus arrhythmia, or (c) baseline pre-ejection period.
When modeling predictors of depressive symptoms in the ASD group, after controlling for medication use and BMI percentile, addition of the main effects for RSA, PEP, and cortisol did not account for significant additional model variance. Separate models were run for each two-way interaction in order to test the individual contribution of each interaction (see Table 3). In the ASD group, the RSA*PEP interaction contributed an additional 11% of variance to the base model, while the cortisol*RSA and cortisol*PEP interactions did not contribute statistically significant additional variance (p>0.05).
Table 3.
Regression Models Predicting Scores on Affective Subscale of CBCL in ASD
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 3b | 3c | ||||||||
| Variable | b | pa | b | pa | b | pa | b | pa | b | pa |
| Medications | −0.82 | 0.25 | −0.67 | 0.76 | −0.80 | 0.29 | −0.83 | 0.22 | −0.64 | 0.41 |
| BMI | 0.02 | 0.51 | 0.01 | 0.59 | 0.01 | 0.76 | 0.02 | 0.40 | 0.01 | 0.62 |
| Cortisol | -- | -- | −2.18 | 0.24 | −0.36 | 0.86 | −2.05 | 0.26 | −2.20 | 0.28 |
| RSA | -- | -- | −1.18 | 0.17 | −0.71 | 0.42 | −0.90 | 0.23 | −1.25 | 0.16 |
| PEP | -- | -- | 0.01 | 0.88 | 0.02 | 0.76 | −0.02 | 0.70 | −0.01 | 0.86 |
| Cortisol*RSA | -- | -- | -- | -- | −6.67 | 0.13 | -- | -- | -- | -- |
| RSA*PEP | -- | -- | -- | -- | -- | -- | -0.15* | 0.01* | -- | -- |
| Cortisol*PEP | -- | -- | -- | -- | -- | -- | -- | -- | 0.22 | 0.47 |
| Model 1 | Model 2 | Model 3a | Model 3b | Model 3c | ||||||
| R2 | 0.06 | 0.13 | 0.45 | 0.49 | 0.37 | |||||
| ΔR2 | 0.06 | 0.06 | 0.07 | 0.11* | 0.01 | |||||
| ΔF(df) | 1.29(2,38) | 0.86(3,35) | 3.17(1,34) | 4.80(1,34)* | 0.46(1,34) | |||||
RSA, Respiratory Sinus Arrhythmia; PEP, Pre-ejection Period; BMI, Body Mass Index
p-value < 0.05
p-values based on bootstrapping with 1000 bootstrap samples
When further examining the RSA*PEP interaction using simple slopes, it was shown that only at high levels of PEP (+1 SD) was there a significant negative relationship between RSA and depressive symptoms (b=−2.60, p=0.02, 95% CI [−4.75, −0.44]; Figure 2). Further, Johnson-Neyman analysis (Hayes & Matthes, 2009; Johnson & Fay, 1950) confirmed the threshold of significance at a mean-centered PEP value of 3.13, and the effect for RSA and depression became stronger and more negative as PEP increased. Therefore, children with ASD and highest levels of PEP (less sympathetic arousal) had significantly fewer depressive symptoms with increasing parasympathetic regulation. At low and mean values of PEP, there was a nonsignificant relationship between RSA and CBCL affective symptoms (all p>0.05; see Figure 2).
Figure 2. RSA and PEP Interact to Predict Depressive Symptoms in ASD.
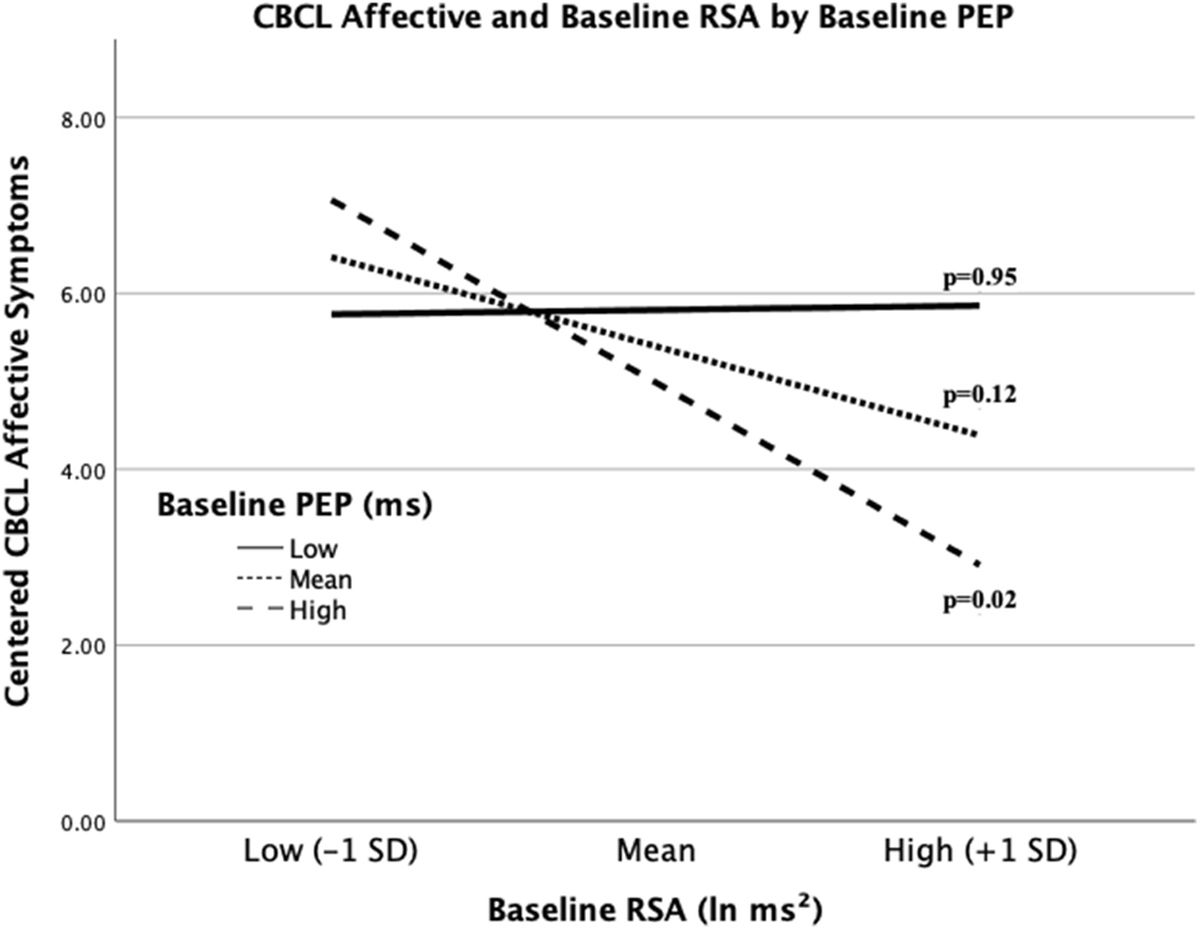
High PEP moderates the negative association between RSA and affective symptoms in youth with ASD. High and low values for PEP and RSA are equivalent to +/− 1 SD from the mean. P-values for each slope are provided.
Regression models in the TD group revealed neither the physiological main effects (RSA, cortisol, PEP) nor any of the interactions contributed significant additional variance (see Table 4). However, the main effect of PEP and the RSA*PEP interaction were significant bootstrapped model coefficients. Johnson-Neyman analysis found no points at which PEP moderated the relationship between RSA and affective symptoms (p>0.05), nor were the slopes statistically significant at any level of PEP or RSA (see Figure 3), suggesting a lack of association between PNS functioning and depression in TD youth at varying levels of SNS arousal or insufficient power to detect possible relationships within the current sample.
Table 4.
Regression Models Predicting Scores on Affective Subscale of CBCL in TD group.
| Model 1 | Model 2 | Model 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 3b | 3c | ||||||||
| Variable | b | pa | b | pa | b | pa | b | pa | b | pa |
| BMI | 0.004 | 0.66 | 0.004 | 0.63 | 0.004 | 0.66 | 0.005 | 0.48 | 0.003 | 0.74 |
| Cortisol | -- | -- | 2.11 | 0.08 | 2.04 | 0.09 | 2.20 | 0.09 | 2.15 | 0.09 |
| RSA | -- | -- | 0.12 | 0.76 | 0.09 | 0.83 | 0.05 | 0.86 | 0.11 | 0.76 |
| PEP | -- | -- | 0.07* | 0.04* | 0.07* | 0.04* | 0.08* | 0.04* | 0.07 | 0.07 |
| Cortisol*RSA | -- | -- | -- | -- | −0.45 | 0.79 | -- | -- | -- | -- |
| RSA*PEP | -- | -- | -- | -- | -- | -- | 0.09* | 0.05* | -- | -- |
| Cortisol*PEP | -- | -- | -- | -- | -- | -- | -- | -- | −0.08 | 0.56 |
| Model 1 | Model 2 | Model 3a | Model 3b | Model 3c | ||||||
| R2 | 0.003 | 0.15 | 0.15 | 0.20 | 0.16 | |||||
| ΔR2 | 0.003 | 0.15 | 0.001 | 0.05 | 0.01 | |||||
| ΔF(df) | 0.14(1,43) | 2.24(3,40) | 0.06(1,39) | 2.41(1,39) | 0.28(1,39) | |||||
RSA, Respiratory Sinus Arrhythmia; PEP, Pre-ejection Period; BMI, Body Mass Index
p-value < 0.05
p-values based on bootstrapping with 1000 bootstrap samples
Figure 3. Illustration of RSA and Depressive Symptom Associations by level of PEP in TD.
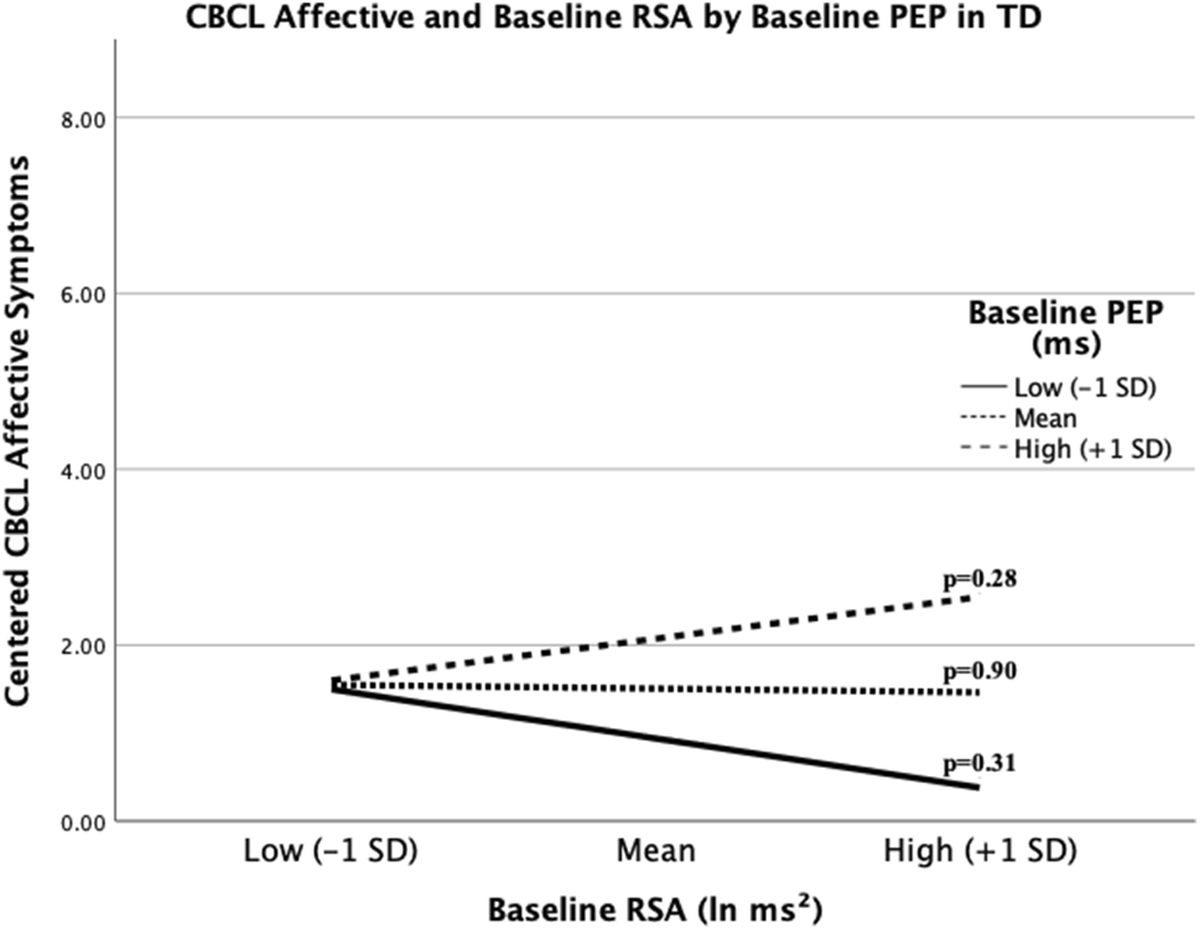
The relationship between RSA and affective symptoms in with TD is not significant at any value of PEP. High and low values for PEP and RSA are equivalent to +/− 1 SD from the mean. P-values for each slope are provided.
As depression and anxiety symptoms frequently co-occur (e.g. Kessler, Merikangas, and Wang, 2007), follow-up exploratory analyses were conducted within the ASD group in order to determine whether similar interaction effects contributed to anxiety symptoms in youth with ASD. When controlling for medications and BMI, main effects of cortisol, RSA, and PEP did not account for additional variance in Anxiety problems as reported on the CBCL (ΔR2=0.02, ΔF(3,35)=0.23, p=0.87). Contrary to depressive symptoms, there was no significant effect for the RSA*PEP interaction (ΔR2=0.07, ΔF(1,34)=2.62, p=0.12). Further, addition of the cortisol*RSA (ΔR2=0.03, ΔF(1,34)=1.19, p=0.28) and cortisol*PEP (ΔR2<0.001, ΔF(1,34)=0.02, p=0.90) interactions did not contribute significant additional variance. Results suggest the observed interactions may be specific to depressive symptomology in ASD.
Discussion
The current study aimed to examine HPA axis and ANS regulation in children with and without ASD, as well as the impact of physiological dysregulation on depressive symptoms in youth. The investigation uniquely sought to identify patterns of interaction between these physiological systems, which may especially contribute to elevated rates of depression in ASD. It was hypothesized that resting states characterized by greater sympathetic and HPA activation, as well as reduced parasympathetic regulation, would be more prevalent in ASD. Contrary to hypotheses, no differences were seen between the two groups on any of the physiological variables; however, as expected, children with ASD demonstrated significantly elevated depressive symptoms. Additionally, interactions between physiological systems uniquely predicted depressive symptoms, especially in children with ASD, which provided preliminary insight into the interrelated nature of these physiological systems and their potential role in effecting emotional states.
Prevalence of depression in youth with ASD has recently been reported to be as high 20.2% for adolescents with ASD (Greenlee et al., 2016). Our sample of 10–13-year-old youth similarly displayed elevated levels of depression compared to TD youth, as reported by their parents. When identifying those reaching clinically significant thresholds on the parent-report measures, children with ASD met the clinical threshold 39% of the time, while only 6.5% of TD children met the clinical significance threshold. This early age of onset for depression in youth with ASD is notable, as psychiatric symptoms may contribute to future maladaptive outcomes, including difficulties with employment and poor adaptive skills (e.g. Kraper, Kenworthy, Popal, Martin, & Wallace, 2017; Walsh, Lydon, & Healy, 2014). Moreover, depressive symptoms may exacerbate tendencies toward social withdrawal and social isolation (M. Ghaziuddin, Ghaziuddin, & Greden, 2002; Greenlee et al., 2016). Loneliness and social isolation are identified risk factors for suicidal behavior (Hedley, Uljarević, Foley, Richdale, & Trollor, 2018), with suicidality risk significantly higher in ASD relative to the general population (Cassidy et al., 2014). Clearly, early diagnosis and treatment is necessary to help avoid these negative consequences. Future studies with larger samples might further elucidate the associations between elevated depressive scores in children and adolescents with ASD and future maladaptive outcomes, as well as to highlight the impact of earlier diagnosis and intervention in this population.
One concern regarding the diagnosis of depression in ASD is that individuals with ASD may exhibit deficits in expressive affective states and challenges with language and social communication that impede their ability to effectively communicate symptoms (Losh & Capps, 2006). Physiological functioning, which has gained interest in its utility as a biological marker for ASD, may also be used in identifying risk or likelihood of being diagnosed with a depressive disorder in this population. However, as previously mentioned, the existing literature is unclear regarding the extent to which physiology and psychology are related in ASD, with several instances of conflicting results. Youth with ASD have been shown to demonstrate hyperarousal in the HPA axis (e.g. Muscatello & Corbett, 2017; Tomarken et al., 2015; Tordjman et al., 2014) and ANS (e.g. Kushki et al., 2014; Vaughan Van Hecke et al., 2009); however, others have cited no differences between youth with ASD and TD (Bitsika, Sharpley, Andronicos, & Agnew, 2015; Corbett et al., 2006; Watson et al., 2012). Similarly, some report associations between physiological arousal and internalizing symptoms (Neuhaus et al., 2014; Sharpley et al., 2016), while others cite no relationships (e.g. Tomarken et al., 2015). These contrasting findings suggest identifying patterns of interaction between the HPA axis and ANS may be more informative than examining individual systems when assessing associations with internalizing profiles.
In the current study, the main effects of each individual system revealed little information regarding the risk for internalizing disorders, as groups did not differ in baseline physiological response, nor systems singularly associated with depressive symptoms. However, upon looking at the interactions of the systems within-diagnosis, more distinct patterns of risk emerged. Autonomic balance, or the relationship between PNS and SNS regulation, was particularly associated with depressive symptom risk in children with ASD. Specifically, youth with a predominantly reciprocal parasympathetic response system (i.e. increased PNS with decreased SNS; Berntson et al., 2008) were noted to have the lowest levels of depressive symptoms. This tendency towards a lower baseline arousal pattern, with elevated inhibition from the PNS and removal of excitation of the SNS, may be suggestive of less chronic stress and/or be protective against the wear and tear (allostatic load; McEwen, 1998) associated with chronic stress exposure. Therefore, regions often implicated in depression (i.e. prefrontal cortex, amygdala, hippocampus; McEwen, 2003) would be less likely to experience neuronal damage associated with excitotoxicity. Caution should be taken in these interpretations, however, as these results are preliminary and only correlational, not causational. Future longitudinal studies with larger samples sizes may provide more information regarding the time course of these physiological and behavioral changes to elucidate the directionality of these relationships. Additionally, studies assessing stress reactivity may reveal different patterns of adaptive or maladaptive arousal, as previously symptoms of depression, such as loneliness, have been associated with a blunted (hypoarousal) cardiovascular and endocrinological stress response (e.g. Brown, Gallagher, & Creaven, 2018). Nonetheless, the current findings offer initial support for the added benefits of investigating interactions across the HPA axis and ANS, rather than their singular, isolated functions, when assessing their role in regulating behavior in ASD.
Other factors are important to consider when interpreting these findings. The study focused on a narrow age range of 10–13-year old children, yet incidence of depression is known to increase with age, with a particular risk during the adolescent period of development (Gotham, Brunwasser, & Lord, 2015). Yet the current sample already included five children being actively treated with SSRIs and up to 39% reporting clinically significant depressive symptoms on behavioral report. Future longitudinal studies (Corbett, 2017) following youth from childhood through the adolescent period may reveal important changes in symptom profile through development and the ways in which physiological systems interact and relate with mental health status. Moreover, while results suggested a possible interaction with RSA and PEP in the TD group, no significant relationships were seen between physiology and affect within these youth, despite previous research finding inter-relations across systems and with internalizing symptoms (e.g. El-Sheikh et al., 2008; 2011). Importantly, the current TD sample was smaller than previous studies and without psychiatric incidence, with only 6.5% of the sample falling in the clinically significant range for symptoms on the affective subscale on the CBCL. Therefore, future research with larger samples comparing across ASD and TD should also consider a more variable TD group, while also including individuals with a diagnosed depressive disorder as a third comparison group.
The investigation is strengthened by the novel examination of the interrelated actions of the HPA axis and ANS in predicting depressive symptoms in children with ASD, thereby helping to fill an important gap in our understanding of the connection between physiological arousal and affect. The study, however, is not without limitations. First, the sample was relatively limited by the narrow age range and use of a parent-report measure indexing a number of problem behaviors, not just depression. Future investigations will benefit from the use of multiple robust measures specific to depression, including clinical assessment of symptoms. Second, inclusion of only individuals without cognitive impairment limits interpretations of findings to only a subset of the ASD population. Similarly, it is important to acknowledge the heterogeneity within ASD and variability in physiological and psychological responses; therefore, current findings and interpretations may not apply to every child. Third, the sample size is comparable to many empirical studies in ASD, but a larger sample with greater power to handle the interaction analyses may have resulted in stronger findings. This is especially emphasized as there were likely three-way interactions between HPA axis, ANS, and diagnostic group, as seen by the differential findings within the ASD vs. TD group; however, we did not have sufficient power to statistically evaluate these higher-order interactions. Fourth, the current study utilized methods collected in the lab on a single day in an effort to have all three measures reflect arousal at approximately the same point in time, which is not uncommon amongst physiological studies (e.g., El-Sheikh et al., 2008; 2011). Nevertheless, more comprehensive methods, such as at-home collection of diurnal cortisol rhythm and 24-hour ambulatory monitoring of heart rate variability, as done in recent studies (Rotenberg & McGrath, 2016), may yield different results with a more complete profile of regulation. Fifth, the lab environment and other daily events may include additional unforeseen stress which we are unable to control. Lastly, the current study focused on baseline, resting physiological functioning. Future studies will want to investigate these interactions and associations in response to a stressor, as previous research suggests critical differences in physiological reactivity in youth with ASD, such as in response to social stress (see Benevides & Lane, 2013 for review).
Conclusions
The current study aimed to examine the interactions between the HPA axis and ANS and their associations with depression. The results extend previous findings on physiological dysregulation in children with ASD, identifying preliminary evidence for unique multi-system interactions, which may predict parent-reported symptoms of depression. While children with ASD displayed significantly more depressive symptoms, physiological arousal did not differ between diagnostic groups; however, distinct interactions, especially within the opposing branches of the ANS, were evident. As depressive disorders continue to be a prevalent concern for many youth and young adults with ASD, accurate diagnosis and early intervention are critical to effective management and improved quality of life. Our findings underscore the importance and benefits of examining arousal across multiple systems to more accurately identify these physiological profiles associated with psychological risk and behavioral outcomes in ASD, which may eventually aid in earlier diagnosis of comorbid symptom profiles and inform treatment to facilitate more favorable outcomes for those with these symptoms.
Acknowledgments
This study was funded by an Autism Speaks Weatherstone Predoctoral Fellowship (#10616) awarded to Rachael A. Muscatello, and a National Institute of Mental Health R01 (MH111599) awarded to Blythe A. Corbett, Ph.D. Additional core support was provided by the National Center for Advancing Translation Sciences (CTSA UL1 TR000445) and the National Institute of Child Health and Human Development (U54 HD083211, PI: Neul). Cortisol assays were completed by the VUMC Hormone Assay and Analytical Services Core, supported by NIH grants DK059637 and DK020593. We are grateful to all the children and families who continue to support our research. These data were completed as part of Rachael Muscatello’s doctoral dissertation.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical stands of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed written consent and assent was obtained from all parents and study participants, respectively, prior to inclusion in the study.
References
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT. [Google Scholar]
- Aiken LS, & West SG (1991). Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: SAGE Publications, Inc. [Google Scholar]
- Althaus M, Mulder LJ, Mulder G, Aarnoudse CC, & Minderaa RB (1999). Cardiac adaptivity to attention-demanding tasks in children with a pervasive developmental disorder not otherwise specified (PDD-NOS). Biological Psychiatry, 46(6), 799–809. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association. [Google Scholar]
- Badanes LS, Watamura SE, & Hankin BL (2011). Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Development and Psychopathology, 23(3), 881–896. 10.1017/S095457941100037X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal E, Harden E, Lamb D, Van Hecke AV, Denver JW, & Porges SW (2010). Emotion recognition in children with autism spectrum disorders: relations to eye gaze and autonomic state. Journal of Autism and Developmental Disorders, 40(3), 358–370. 10.1007/s10803-009-0884-3 [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, & Boyce WT (2002). Associations between physiological reactivity and children’s behavior: advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics:JDBP, 23(2), 102–113. [DOI] [PubMed] [Google Scholar]
- Benarroch EE (1993). The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clinic Proceedings, 68(10), 988–1001. [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2004). Central Autonomic Control In Robertson D, Biaggioni I, Burnstock G, & Low PA (Eds.), Primer on the Autonomic Nervous System (2nd ed., pp. 17–19). Elsevier Inc. [Google Scholar]
- Benevides TW, & Lane SJ (2013). A Review of Cardiac Autonomic Measures: Considerations for Examination of Physiological Response in Children with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 45(2), 560–575. 10.1007/s10803-013-1971-z [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Binkley PF, Uchino BN, Quigley KS, & Fieldstone A (1994). Autonomic cardiac control. III. Psychological stress and cardiac response in autonomic space as revealed by pharmacological blockades. Psychophysiology, 31(6), 599–608. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, & Cacioppo JT (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. 10.1111/j.1469-8986.2008.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsika V, Sharpley CF, Andronicos NM, & Agnew LL (2015). Hypothalamus-pituitary-adrenal axis daily fluctuation, anxiety and age interact to predict cortisol concentrations in boys with an autism spectrum disorder. Physiology & Behavior, 138, 200–207. 10.1016/j.physbeh.2014.11.010 [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Deeg DJH, Beekman ATF, Penninx BWJH, Lips P, & Hoogendijk WJG (2007). Major depression in late life is associated with both hypo- and hypercortisolemia. Biological Psychiatry, 62(5), 479–486. 10.1016/j.biopsych.2006.11.033 [DOI] [PubMed] [Google Scholar]
- Brown EG, Gallagher S, & Creaven A-M (2018). Loneliness and acute stress reactivity: A systematic review of psychophysiological studies. Psychophysiology, 55(5), e13031 10.1111/psyp.13031 [DOI] [PubMed] [Google Scholar]
- Byrne ML, Sheeber L, Simmons JG, Davis B, Shortt JW, Katz LF, & Allen NB (2010). Autonomic cardiac control in depressed adolescents. Depression and Anxiety, 27(11), 1050–1056. 10.1002/da.20717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai RY, Richdale AL, Dissanayake C, & Uljarević M (2019). Resting heart rate variability, emotion regulation, psychological wellbeing and autism symptomatology in adults with and without autism. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 137, 54–62. 10.1016/j.ijpsycho.2018.12.010 [DOI] [PubMed] [Google Scholar]
- Cassidy S, Bradley P, Robinson J, Allison C, McHugh M, & Baron-Cohen S (2014). Suicidal ideation and suicide plans or attempts in adults with Asperger’s syndrome attending a specialist diagnostic clinic: a clinical cohort study. The Lancet. Psychiatry, 1(2), 142–147. 10.1016/S2215-0366(14)70248-2 [DOI] [PubMed] [Google Scholar]
- Corbett BA (2017). Examining stress and arousal across pubertal development in ASD. NationalInstitute of Mental Health. [Google Scholar]
- Corbett BA, Blain SD, Ioannou S, & Balser M (2016). Changes in anxiety following a randomized control trial of a theatre-based intervention for youth with autism spectrum disorder. Autism: the International Journal of Research and Practice, 1362361316643623 10.1177/1362361316643623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, & Levine S (2006). Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology, 31(1), 59–68. 10.1016/j.psyneuen.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, Carmean V, & Levine S (2008). Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of Psychiatry & Neuroscience, 33(3), 227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Muscatello RA, Tanguturi Y, McGinn E, & Ioannou S (2019). Pubertal Development Measurement in Children With and Without Autism Spectrum Disorder: A Comparison Between Physical Exam, Parent- and Self-Report. Journal of Autism and Developmental Disorders, 75(4), 594–13. 10.1007/s10803-019-04192-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Levine S, & Mendoza S (2009). Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Research, 2(1), 39–49. 10.1002/aur.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, & Perel J (1991). 24-hour cortisol measures in adolescents with major depression: a controlled study. Biological Psychiatry, 30(1), 25–36. [DOI] [PubMed] [Google Scholar]
- Daluwatte C, Miles JH, Christ SE, Beversdorf DQ, Takahashi TN, & Yao G (2013). Atypical pupillary light reflex and heart rate variability in children with autism spectrum disorder. Journal of autism and developmental disorders, 43(8), 1910–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, & Putnam K (2002). Depression: perspectives from affective neuroscience. Annual Review of Psychology, 53(1), 545–574. 10.1146/annurev.psych.53.100901.135148 [DOI] [PubMed] [Google Scholar]
- Edmiston EK, Jones RM, & Corbett BA (2016). Physiological Response to Social Evaluative Threat in Adolescents with Autism Spectrum Disorder. Journal of Autism and Developmental Disorders, 1–14. 10.1007/s10803-016-2842-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston EK, Muscatello RA, & Corbett BA (2017). Altered pre-ejection period response to social evaluative threat in adolescents with autism spectrum disorder. Research in Autism Spectrum Disorders, 36, 57–65. 10.1016/j.rasd.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, & Tibshirani RJ (1993). An Introduction to the Bootstrap. Boca Raton: Chapman & Hall/CRC. [Google Scholar]
- El-Sheikh M, Arsiwalla DD, Hinnant JB, & Erath SA (2011). Children’s internalizing symptoms: the role of interactions between cortisol and respiratory sinus arrhythmia. Physiology & Behavior, 103(2), 225–232. 10.1016/j.physbeh.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, & Mize J (2008). Cortisol and Children’s Adjustment: The Moderating Role of Sympathetic Nervous System Activity. Journal of Abnormal Child Psychology, 36(4), 601–611. 10.1007/s10802-007-9204-6 [DOI] [PubMed] [Google Scholar]
- Friedman BH (2007). An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology, 74(2), 185–199. 10.1016/j.biopsycho.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Ghaziuddin M, Ghaziuddin N, & Greden J (2002). Depression in persons with autism: implications for research and clinical care. Journal of Autism and Developmental Disorders, 32(4), 299–306. [DOI] [PubMed] [Google Scholar]
- Gotham K, Brunwasser SM, & Lord C (2015). Depressive and anxiety symptom trajectories from school age through young adulthood in samples with autism spectrum disorder and developmental delay. Journal of the American Academy of Child and Adolescent Psychiatry, 54(5), 369–76.e3. 10.1016/j.jaac.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, & Kapelewski CH (2009). Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrinology, 34(10), 1437–1448. 10.1016/j.psyneuen.2009.06.017 [DOI] [PubMed] [Google Scholar]
- Greenlee JL, Mosley AS, Shui AM, Veenstra-VanderWeele J, & Gotham KO (2016). Medical and Behavioral Correlates of Depression History in Children and Adolescents With Autism Spectrum Disorder. Pediatrics, 137 Suppl 2(Supplement), S105–14. 10.1542/peds.2015-2851I [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy L, Souders M, Bradstreet L, DeLussey C, & Herrington JD (2014). Brief report: Emotion regulation and respiratory sinus arrhythmia in autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(10), 2614–2620. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis. New York, NY: Guilford Press. [Google Scholar]
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996, March). Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European Heart Journal. [PubMed] [Google Scholar]
- Hedley D, Uljarević M, Foley K-R, Richdale A, & Trollor J (2018). Risk and protective factors underlying depression and suicidal ideation in Autism Spectrum Disorder. Depression and Anxiety, 35(7), 648–657. 10.1002/da.22759 [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, & Hellhammer DH (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25(1), 1–35. [DOI] [PubMed] [Google Scholar]
- Herman JP, & Cullinan WE (1997). Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences, 20(2), 78–84. [DOI] [PubMed] [Google Scholar]
- Hollocks MJ, Howlin P, Papadopoulos AS, Khondoker M, & Simonoff E (2014). Differences in HPA-axis and heart rate responsiveness to psychosocial stress in children with autism spectrum disorders with and without co-morbid anxiety. Psychoneuroendocrinology, 46, 32–45. 10.1016/j.psyneuen.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Hollocks MJ, Pickles A, Howlin P, & Simonoff E (2016). Dual Cognitive and Biological Correlates of Anxiety in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders, 46(10), 3295–3307. 10.1007/s10803-016-2878-2 [DOI] [PubMed] [Google Scholar]
- Hudson CC, Hall L, & Harkness KL (2019). Prevalence of Depressive Disorders in Individuals with Autism Spectrum Disorder: a Meta-Analysis. Journal of Abnormal Child Psychology, 47(1), 165–175. 10.1007/s10802-018-0402-1 [DOI] [PubMed] [Google Scholar]
- IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp. [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, van der Gaag R-J, & van Engeland H (2003). Differentiation between autism and multiple complex developmental disorder in response to psychosocial stress. Neuropsychopharmacology:Official Publication of the American College of Neuropsychopharmacology, 28(3), 582–590. 10.1038/sj.npp.1300046 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Merikangas KR, & Wang PS (2007). Prevalence, comorbidity, and service utilization for mood disorders in the United States at the beginning of the twenty-first century. Annu. Rev. Clin. Psychol, 3, 137–158. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, & Hellhammer DH (1989). Salivary cortisol in psychobiological research: an overview. Neuropsychobiology, 22(3), 150–169. [DOI] [PubMed] [Google Scholar]
- Koenigs M, & Grafman J (2009). The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research, 201(2), 239–243. 10.1016/j.bbr.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraper CK, Kenworthy L, Popal H, Martin A, & Wallace GL (2017). The Gap Between Adaptive Behavior and Intelligence in Autism Persists into Young Adulthood and is Linked to Psychiatric Co-morbidities. Journal of Autism and Developmental Disorders, 47(10), 3007–3017. 10.1007/s10803-017-3213-2 [DOI] [PubMed] [Google Scholar]
- Kushki A, Brian J, Dupuis A, & Anagnostou E (2014). Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism, 5(1), 39 10.1186/2040-2392-5-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Pla Mobarak M, Tanel N, Dupuis A, Chau T, & Anagnostou E (2013). Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PloS One, 8(4), e59730 10.1371/journal.pone.0059730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni KE, Schupp CW, Simon D, & Corbett BA (2012). Verbal ability, social stress, and anxiety in children with autistic disorder. Autism : the International Journal of Research and Practice, 16(2), 123–138. 10.1177/1362361311425916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine TP, Sheinkopf SJ, Pescosolido M, Rodino A, Elia G, & Lester B (2012). Physiologic Arousal to Social Stress in Children with Autism Spectrum Disorders: A Pilot Study. Research in Autism Spectrum Disorders, 6(1), 177–183. 10.1016/j.rasd.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop SL (2012). Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Torrance, CA: Western Psychological Services. [Google Scholar]
- Losh M, & Capps L (2006). Understanding of emotional experience in autism: insights from the personal accounts of high-functioning children with autism. Developmental Psychology, 42(5), 809–818. 10.1037/0012-1649.42.5.809 [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, & Berntson GG (2007). Where to B in dZ/dt. Psychophysiology, 44(1), 113–119. 10.1111/j.1469-8986.2006.00468.x [DOI] [PubMed] [Google Scholar]
- Magyar CI, & Pandolfi V (2017). Utility of the CBCL DSM-Oriented Scales in Assessing Emotional Disorders in Youth with Autism. Research in Autism Spectrum Disorders, 37, 11–20. 10.1016/j.rasd.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL, Murray MJ, Ahuja M, & Smith LA (2011). Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Research in Autism Spectrum Disorders, 5(1), 474–485. 10.1016/j.rasd.2010.06.012 [DOI] [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. The New England Journal of Medicine, 338(3), 171–179. 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2003). Mood disorders and allostatic load. Biological Psychiatry, 54(3), 200–207. [DOI] [PubMed] [Google Scholar]
- Ming X, Julu POO, Brimacombe M, Connor S, & Daniels ML (2005). Reduced cardiac parasympathetic activity in children with autism. Brain & Development, 27(7), 509–516. 10.1016/j.braindev.2005.01.003 [DOI] [PubMed] [Google Scholar]
- Muscatello RA, & Corbett BA (2017). Comparing the effects of age, pubertal development, and symptom profile on cortisol rhythm in children and adolescents with autism spectrum disorder. Autism Research, 56, 1 10.1002/aur.1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E, Bernier RA, & Beauchaine TP (2016). Children with Autism Show Altered Autonomic Adaptation to Novel and Familiar Social Partners. Autism Research, 9(5), 579–591. 10.1002/aur.1543 [DOI] [PubMed] [Google Scholar]
- Neuhaus E, Bernier R, & Beauchaine TP (2014). Brief report: social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders, 44(3), 730–737. 10.1007/s10803-013-1923-7 [DOI] [PubMed] [Google Scholar]
- Panju S, Brian J, Dupuis A, Anagnostou E, & Kushki A (2015). Atypical sympathetic arousal in children with autism spectrum disorder and its association with anxiety symptomatology. Molecular Autism, 6(1), 64 10.1186/s13229-015-0057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin MA, Scarpa A, Friedman BH, & Porges SW (2013). Respiratory sinus arrhythmia: a marker for positive social functioning and receptive language skills in children with autism spectrum disorders. Developmental Psychobiology, 55(2), 101–112. 10.1002/dev.21002 [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Macellaio M, Stanfill SD, McCue K, Lewis GF, Harden ER, … & Heilman KJ (2013). Respiratory sinus arrhythmia and auditory processing in autism: Modifiable deficits of an integrated social engagement system?. International Journal of Psychophysiology, 88(3), 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S, & McGrath JJ (2016). Inter-relation between autonomic and HPA axis activity in children and adolescents. Biological Psychology, 117, 16–25. 10.1016/j.biopsycho.2016.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Clift A, Bolden S, & Salomon K (2007). RSA fluctuation in major depressive disorder. Psychophysiology, 44(3), 450–458. 10.1111/j.1469-8986.2007.00509.x [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). The Social Communication Questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Sapolsky RM, Krey LC, & McEwen BS (1986). The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocrine Reviews, 7(3), 284–301. 10.1210/edrv-7-3-284 [DOI] [PubMed] [Google Scholar]
- Schaaf RC, Benevides TW, Leiby BE, & Sendecki JA (2015). Autonomic dysregulation during sensory stimulation in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(2), 461–472. [DOI] [PubMed] [Google Scholar]
- Schumann A, Andrack C, & Bär K-J (2017). Differences of sympathetic and parasympathetic modulation in major depression. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 79(Pt B), 324–331. 10.1016/j.pnpbp.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Sharpley CF, Bitsika V, Andronicos NM, & Agnew LL (2016). Further evidence of HPA-axis dysregulation and its correlation with depression in Autism Spectrum Disorders: Data from girls. Physiology & Behavior, 167, 110–117. 10.1016/j.physbeh.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Sheinkopf SJ, Neal-Beevers AR, Levine TP, Miller-Loncar C, & Lester B (2013). Parasympathetic response profiles related to social functioning in young children with autistic disorder. Autism research and treatment, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, & van Doornen LJ (1990). Methodological guidelines for impedance cardiography. Psychophysiology, 27(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Shibao C, & Okamoto L (2012). Agents Potentiating Sympathetic Tone In Robertson D, Biaggioni I, Burnstock G, Low PA, & Paton JFR (Eds.), Primer on the Autonomic Nervous System. Academic Press. [Google Scholar]
- Stetler C, & Miller GE (2011). Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosomatic Medicine, 73(2), 114–126. 10.1097/PSY.0b013e31820ad12b [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality,Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Taylor JL, & Corbett BA (2014). A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology, 49, 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, & Brosschot JF (2005). Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology, 30(10), 1050–1058. 10.1016/j.psyneuen.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81–88. 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Smith M, Rossy LA, Sollers JJ, & Friedman BH (1998). Heart period variability and depressive symptoms: gender differences. Biological Psychiatry, 44(4), 304–306. [DOI] [PubMed] [Google Scholar]
- Toichi M, & Kamio Y (2003). Paradoxical autonomic response to mental tasks in autism. Journal of Autism and Developmental Disorders, 33(4), 417–426. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Han GT, & Corbett BA (2015). Temporal patterns, heterogeneity, and stability of diurnal cortisol rhythms in children with autism spectrum disorder. Psychoneuroendocrinology, 62, 217–226. 10.1016/j.psyneuen.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Anderson GM, Kermarrec S, Bonnot O, Geoffray M-M, Brailly-Tabard S, et al. (2014). Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology, 50, 227–245. 10.1016/j.psyneuen.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, & Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, & Van Calster B (2009). Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children’s Depression Inventory. Psychoneuroendocrinology, 34(5), 791–794. 10.1016/j.psyneuen.2008.12.008 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Van Calster B, Pinna Puissant S, & Van Huffel S (2008). Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Hormones and Behavior, 54(2), 253–257. 10.1016/j.yhbeh.2008.03.015 [DOI] [PubMed] [Google Scholar]
- Vaughan Van Hecke A, Lebow J, Bal E, Lamb D, Harden E, Kramer A, et al. (2009). Electroencephalogram and heart rate regulation to familiar and unfamiliar people in children with autism spectrum disorders. Child Development, 80(4), 1118–1133. 10.1111/j.1467-8624.2009.01320.x [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJG, van Pelt J, Derijk RH, Verhagen JCM, van Dyck R, et al. (2009). Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Archives of General Psychiatry, 66(6), 617–626. 10.1001/archgenpsychiatry.2009.50 [DOI] [PubMed] [Google Scholar]
- Walsh L, Lydon S, & Healy O (2014). Employment and Vocational Skills Among Individuals with Autism Spectrum Disorder: Predictors, Impact, and Interventions. Review Journal of Autism and Developmental Disorders, 1(4), 266–275. 10.1007/s40489-014-0024-7 [DOI] [Google Scholar]
- Watson LR, Roberts JE, Baranek GT, Mandulak KC, & Dalton JC (2012). Behavioral and physiological responses to child-directed speech of children with autism spectrum disorders or typical development. Journal of Autism and Developmental Disorders, 42(8), 1616–1629. 10.1007/s10803-011-1401-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Yaptangco M, Crowell SE, Baucom BR, Bride DL, & Hansen EJ (2015). Examining the relation between respiratory sinus arrhythmia and depressive symptoms in emerging adults: A longitudinal study. Biological Psychology, 110, 34–41. 10.1016/j.biopsycho.2015.06.004 [DOI] [PubMed] [Google Scholar]


