Abstract
Background
Tocopherol isoforms may regulate child lung growth and spirometric measures.
Objective
To determine the extent to which plasma alpha (α-T) or gamma tocopherol (γ-T) isoform levels in early childhood or in-utero are associated with childhood lung function.
Methods
We included 622 participants in the Project Viva cohort, who had lung function at a mid-childhood visit (age 6–10 years old). Maternal and child tocopherol isoform levels were measured by HPLC at second trimester and 3 years old, respectively. Multivariable linear regression models (adjusted for mid-childhood BMI-z-scores, and maternal education, smoking in pregnancy, and prenatal PM2.5 particulate exposure), stratified by tertiles of child γ-T, were employed to assess the association of α-T levels with FEV1 and FVC percent predicted. Similarly, models stratified by child α-T tertile evaluated associations of γ-T levels with lung function. We performed similar analyses with maternal second trimester tocopherol isoform levels.
Results
The maternal second trimester α-T level was median;IQR: 63; 47–82 μM. The early childhood levels were median;IQR: 25; 20–33 μM. In the lowest tertile of early childhood γ-T, children with higher α-T levels (per 10 μM) had higher mid-childhood FEV1 %-predicted (β=3.09, 95%CI=0.58–5.59), and a higher FVC %-predicted (β=2.77, 95%CI=0.47,5.06). This protective association of α-T was lost at higher γ-T levels. We did not see any consistent associations of second trimester levels of either α-T or γ-T with mid-childhood FEV1 or FVC.
Conclusion
When γ-T levels were in the lowest tertile, higher early childhood α-T was associated with better lung function at mid-childhood. Second trimester maternal plasma α-T concentration was 3-fold higher than the adult female non-pregnant population.
Clinical Implication
α-Tocopherol and γ-tocopherol isoforms are potentially modifiable exposures that have differential associations with lung function in later childhood.
Keywords: tocopherol isoforms, lung function, child
Capsule summary
Early life α-tocopherol levels associated with better lung function if γ-tocopherol levels were low, suggesting the need for studies of α-tocopherol as a potential intervention in childhood asthma and lung function.
Introduction
Asthma is a heterogeneous disease resulting from complex interactions of environmental and genetic factors that result in reduced lung function (1). The World Health Organization reported that the prevalence of asthma from 1950 to the present has increased in countries regardless of whether they had high, intermediate or low rates of asthma (2–4). The marked rise in rates of asthma over a few decades and the differences in rates among countries and in migrating populations suggest an important role for environmental factors, such as diet, in development of asthma. In Project Viva, food frequency questionnaire measures of prenatal Vitamin E ingestion have been associated with increased measures of lung inflammation (FENO levels) in adolescence, but this is without information on tocopherol isoforms.(5) A better understanding of the tocopherol isoforms relevant for this association would be important, as reviewed by Camargo et al.(6) Indeed, an environmental change over the past 40 years that mirrors the rise in asthma prevalence has been an increase in d-γ-tocopherol (γ-T) in both maternal diet and in infant formulas. Infant formulas contain soybean oil which is rich in γ-T (7–10), whereas breast milk contains about 10 fold higher levels of α-T than γ-T.(11) A possible role of tocopherol isoforms on lung growth and inflammatory responses may be relevant to respiratory morbidity in this context.
γ-T is one of the two most abundant natural forms of tocopherols in diet and tissues; the other being d-α-tocopherol (α-T) (7–10). In preclinical studies in allergic asthma, these two isoforms have opposing effects on the allergic response, potentially via interactions with protein kinase Cα (PKCα) (12–14). In murine models, α-T blocks eosinophilic allergic responses whereas γ-T elevates eosinophilic allergic lung inflammation and airway responses (8, 13–16). Also, γ-T ablates the anti-inflammatory benefit of α-T, even when γ-T is 10 fold lower in tissue concentration than α-T, suggesting a potent effect of γ-T (13, 17). In humans, α-T and γ-T isoforms may have opposing effects on both childhood wheeze (18) and adult lung function and asthma. Specifically, in adults, α-T level associates with better lung function and high γ-T (>10 μM) associates with lower lung function by age 21 (8, 13, 15, 19), suggesting that early in life, α-T and γ-T may regulate lung function (19) Notably, high plasma γ-T (>10 μM) with low α-T (12–15 μM) also resulted in up to a 4 fold increase in odds ratio for asthma in an 8 year study of adults in China (20). However, prior studies have not assessed the role of tocopherol isoforms prenatally and in early childhood on later childhood lung function. This is important as tocopherol isoforms in the diet are impactable, and plateau lung function has effects on life course events including lung function trajectory, respiratory morbidity, and all cause mortality. (21, 22)
The hypothesis that tocopherol isoforms may be relevant for lung inflammation is supported by our preclinical work with pregnant murine models. We demonstrated that supplementation of allergic pregnant and nursing mice with α-T decreases (23) and γ-T increases (24) neonate allergic responsiveness. Moreover, when both of these tocopherol isoforms are administered together, murine studies indicate that γ-T ablates the benefit of α-T (13, 17). Similar regulation of allergic inflammation by α-T in utero and after birth were noted in cross-foster studies (23). This suggests that α-T and γ-T levels in combination with early life events may be relevant to lung function in children. Indeed, α-T and γ-T may regulate early life responses to lung events such as viral illnesses that effect both lung function and asthma (25–31). Previous reports have not determined associations for maternal and child plasma α-T and γ-T with child spirometric measures.
We hypothesized that higher early life or prenatal exposures to α-T would be associated with improved lung function. We secondarily hypothesized that these associations would be abrogated at higher levels of γ-T. To examine these hypotheses, we measured plasma tocopherol isoform levels in early childhood and in maternal second trimester plasma (a time which is relevant to the development of adaptive immunity in the fetus) and determined their associations with childhood lung function in the Project Viva cohort.
Methods
Cohort
Project Viva is a US cohort study of pregnant women and their offspring registered at clinicaltrials.gov as NCT02820402. The Viva cohort enrolled pregnant women in the first trimester from Atrius Harvard Vanguard Medical Associates in eastern Massachusetts between 1999 and 2002, with 2,128 live births and follow up of about 1600 mother-child dyads to the present time. Exclusion criteria included multiple gestations, inability to answer questions in English, gestational age ≥22 weeks at recruitment and plans to move away before delivery. Additionally, for this analysis, we excluded individuals who had gestational age <34 weeks (32). The institutional review board of Harvard Pilgrim Health Care approved this study protocol and mothers provided written informed consent at recruitment and follow-up.
For this analysis, we focused on a subset of subjects who had either second trimester maternal plasma or early childhood (age 3) plasma and had mid-childhood visit (age 6–10) with spirometry testing. Additional details are described elsewhere.(33) We analyzed plasma samples for tocopherol isoforms from 1117 participants of whom 622 also had data on mid-childhood spirometry.
Tocopherol measurements
Concentrations of tocopherol isoforms were determined by high pressure liquid chromatography (HPLC) with an electrochemical detector in plasma from mothers in second trimester and child plasma. Briefly, 7 μl of 0.2 N HCl was added to each plasma sample (50 μl) on ice and vortexed. Then the following was added in consecutive order: the internal standard tocol (20 μl of 0.05 mg/ml tocol, Abcam), 2 μl of 10% ascorbic acid in water, 0.5 ml of 100% ethanol (HPLC grade), 0.5 ml water, and 1 ml hexane with 0.1% weight/volume butylated hydroxytoluene (BHT) to prevent oxidation and increase recovery of tocopherol. The samples were vortexed and then centrifuged for 3.5 minutes at 9,000 x g at 4oC and hexane layers collected. The samples were extracted a second time with addition of hexane/BHT. The hexane layers were combined, dried under nitrogen, and stored at −20oC. The samples were reconstituted in methanol and the tocopherols were separated using a reverse phase C18 HPLC column (catalog # WAT085711, Waters) and HPLC (Waters Co.) with 99% methanol-1% water as a mobile phase with detection with an electrochemical detector (potential 0.7V) (Waters Co.). Tocol standards which were equivalent to 100% of tocol added to the samples were measured after every 7 samples. Standard curves containing tocol, α-tocopherol, γ-tocopherol, β-tocopherol, and δ-tocopherol (25 μl injection volume of 15, 10, 5, 2.5, 1.25, 0.6, 0.3, and 0.15 μg standards/ml) were done every 3 days of the analysis, with a lower limit of detection of less than 0.003 μg tocopherol. β-tocopherol, and δ-tocopherol were near or below the detection limit in all the plasma samples. Concentrations were calculated using the area under the curve of the HPLC chromatograms, the standard curves and tocol percent recovery from extractions. All samples were analyzed within the linear standard curve and, additionally, maternal samples with high levels of α-tocopherol were periodically re-extracted and analyzed to confirm the accuracy of concentration in previous samples. Our co-efficient of variation for tocopherol analysis of human plasma is 6–8%, consistent with reports of less than 10% (19).
Pulmonary function measurements
Lung function measurements, including FEV1, FVC, and forced expiratory flow at 25–75% of FVC were obtained using the EasyOne Spirometer (NDD Medical Technologies, Andover, MA). Spirometry measurements met the American Thoracic Society criteria for acceptability and reproducibility: For inclusion, each child had to produce at least three acceptable spirograms, two of which must have been reproducible. (34) Mothers were instructed to hold any child inhaler medications on the day of spirometry testing. (35) Results were expressed as % predicted using the Global Lung Function initiative equations.(36)
Statistical Analysis
Descriptive statistics summarized all variables, including α-tocopherol and γ-tocopherol levels and potential confounders. Demographic and clinical variables were compared between the subgroup included in analyses and the excluded subgroup via chi-squared tests. All analyses were specified a priori based on mechanisms defined in preclinical studies (8, 13–17, 23, 24). Linear regression models, stratified by tertiles of child γ-tocopherol, were employed to assess the association of α-tocopherol levels (in 10 μM increments) with FEV1 and FVC percent predicted at 7 years of age. Similarly, models stratified by child α-tocopherol tertile, considered the association of γ-tocopherol levels (in 10 μM increments) with FEV1 and FVC %-predicted at the 7 year visit (mean age 7.9 years, range 6.6 to 10.6, SD 0.8 yrs). Analyses were replicated in a similar fashion for maternal 2nd trimester tocopherol levels.
Unadjusted linear regression models with the outcome FEV1 %-predicted were also used to evaluate potential confounders including gestational age, maternal age at enrollment, maternal education, household income, smoking during pregnancy, passive smoke exposure postnatally, child BMI z-scores at the mid childhood (7 year) visit, and pollution variables including distance to major roadways, traffic density, prenatal exposure to particulate matter (PM2.5) / and prenatal BC.) Sex, race, height and age of the child were not considered as percent predicted outcomes already adjust for these variables. There were a number of covariates which were retained in the model as potential confounders (associated with the outcome with an alpha level of p≤0.2) including maternal education level, smoking during pregnancy, and prenatal PM2.5 air pollutant exposure. Maternal education was dichotomized as less than 4 years of college and 4 or more years of college. Any self-report of maternal smoking on standardized questionnaires administered at study visits during pregnancy and prior to delivery was coded as “maternal pre-pregnancy smoking” with never or former smokers classified as not smoking during pregnancy. Prenatal PM2.5 air pollutant exposure were period averages from measures taken from each trimester based on temporally and spatially resolved, aerosolized optical depth data. (37) For consistency, these variables were also included in multivariable models with the FVC %-predicted outcome. All models with child three-year tocopherols were also adjusted for some hemolysis that occurred in 22% of the samples because lipids are released from cell membranes during hemolysis. Similar models were also fit within the subgroup of subjects or a history of wheezing at mid-childhood, additionally controlling for controller medication use as a confounder, to further explore the relationship between tocopherol levels and lung function. We classified subjects as being on a controller medication in the last year if they used any of the following: inhaled steroid, prednisone, nedocromil, montelukast, theophylline, and ICS/LABA.
Exploratory analyses considered t-tests or Wilcoxon rank sum tests, as appropriate, to compare FEV1 and FVC %-predicted between two subgroups of the cohort, defined by levels of α-T and γ-T at age 3. Specifically, the high alpha and low gamma subgroup was defined as subjects with alpha tocopherol > 40μM and gamma tocopherol ≤ 7μM. The high gamma and low alpha subgroup was defined as subjects with alpha tocopherol ≤ 40μM and gamma tocopherol > 7μM. These were selected based on the mean and 1.5 standard deviations within this cohort as in the CARDIA study (19). Analyses were replicated with subgroups defined by maternal tocopherol levels, with the high alpha and low gamma subgroup defined as subjects with alpha tocopherol > 100μM and gamma tocopherol ≤ 10μM; the low alpha and high gamma subgroup was defined as subjects with alpha tocopherol ≤ 100μM and gamma tocopherol > 10μM.
Additional exploratory analyses compared the distributions of alpha and gamma tocopherols by prenatal vitamin intake using Wilcoxon rank sum tests. Unless otherwise specified, analyses assumed a two-sided type one error rate of 0.05 and no adjustments were made for multiple tests.
Results
Sample characteristics
Of the 1117 with second trimester or early childhood exposure data, we included 622 mother-infant dyads who had had data for lung function in mid-childhood (age 6–10). Compared to those in the Project Viva cohort that were excluded from the analysis (1506), the sample of 622 dyads were not significantly different with respect to sex of infants (p=0.2), race/ethnicity (p=0.1), or proportion whose mothers had asthma or eczema as measures of maternal atopy (p=0.2). The mean difference in maternal age at enrollment was less than 1 year. The included sample was however, more likely to have higher education (≥ 4 year college) compared to those excluded.
In Table 1, we display the demographic characteristics of the analysis sample. The median (IQR) age of the mothers was 32.5 (29.3–35.9) years, with 51% of infants female. The median (IQR) FEV1 was 96.3 (86.7–104.3) percent predicted and FVC was 102.5 (95.1–110.6) percent predicted. The total IgE had a median level of 25.7 kU/L and an interquartile range of 9.7–73.4 kU/L. The proportion of children who were ever diagnosed with asthma or wheezing / reactive airways disease was 33%. We also classified subjects as having never wheezed at 48.5%, having transient wheeze (24.7% had wheezing at any time up to 6 years of age and not beyond that), persistent wheeze (19.5% had wheezing up to age 5 and continued wheeze at any of the visits at 7, 8 or 9 years of age), and late onset wheeze (7.3% had no wheezing up to age 5 years, but onset of wheezing at any of the visits at 6,7,8 or 9 years of age). There were no clinically meaningful differences in percent with asthma and wheeze by tertile of each tocopherol isoform (Table S1). We also present the levels of each tocopherol isoform between those subjects with asthma (or a history of wheezing) on controller medication versus those not on these medications (Table S2).
Table 1:
Demographics of included Project Viva subjects
| N (%) | Median(IQR) | |
|---|---|---|
| Maternal age at enrollment | 622 (100) | 32.5 (29.3, 35.9) |
| Gestational age (weeks) | 622 (100) | 39.7 (38.9, 40.6) |
| Total IgE kU/L(early childhood – age 3 years) | 400 (64.3) | 25.7 (9.7, 73.4) |
| Sex | ||
| Male | 307 (49.4) | -- |
| Female | 315 (50.6) | |
| Mid-childhood BMI Z-score | ||
| Male | 16.29 (15.30,17.77) | |
| Female | 16.58 (15.23, 18.33) | |
| Race | ||
| White | 410 (65.9) | -- |
| Black | 101 (16.2) | |
| Other | 111 (17.9) | |
| Maternal Education | ||
| < 4 year college | 176 (28.3) | -- |
| ≥ 4 year college + | 445 (71.5) | |
| Missing | 1 (0.2) | |
| Maternal Prenatal vitamin use (%) | 477 (96) | |
| Tocopherols | ||
| Alpha 3y child (μM) | 397 (63.8) | 25.5 (20.1, 33.3) |
| Gamma 3y child (μM) | 397 (63.8) | 2.6 (1.3, 4.0) |
| Alpha 2nd trimester (μM) | 549 (88.3) | 62.6 (46.7, 82.0) |
| Gamma 2nd trimester (μM) | 549 (88.3) | 4.5 (3.0, 6.5) |
| Children ever diagnosed with asthma or wheeze by questionnaire(%) | 207 (33.4) | -- |
| Frequency of controller medication in children with asthma or wheeze at age 7 y | ||
| Inhaled steroids (%) | 84 (41.2) | |
| Leukotriene antagonists (%) | 17 (8.3) | |
| Nedocromil (%) | 2 (1) | |
| inhaled corticosteroid / long acting beta agonist (%) | 2 (1) | |
| Wheezing phenotypes | ||
| Never wheezing children | 299 (48.5) | |
| Transient wheeze | 152 (24.7) | |
| Persistent wheeze | 120 (19.5) | |
| Late onset wheeze | 45 (7.3) | |
| Lung Function Outcomes mid-childhood (age6–10 years) | ||
| FEV1 % Predicted (age 6–10) | 622 (100) | 96.3 (86.7, 104.3) |
| FVC % Predicted | 622 (100) | 102.5 (95.1, 110.6) |
| FEV1/FVC Percent Predicted | 622 (100) | 93.7 (88.7, 97.8) |
Distribution of Tocopherol measures
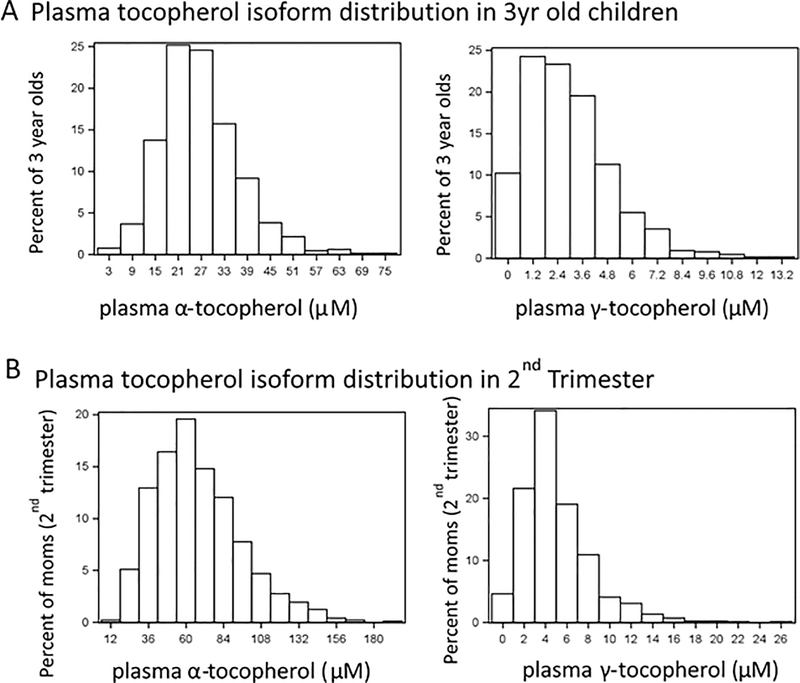
Figure 1A displays the distribution or tocopherol isoforms in the children at age 3 years from the entire study population with this information (n=656 with 3 year tocopherol levels and n=982 with 2nd trimester tocopherol levels). The median child plasma α-T was 25.48 μM (range 2.95–73.16 μM) and γ-T was 2.67 μM (range 0.00–13.52 μM). The α-T levels are higher than prior reference ranges in children (11.9–30 μM/L),(38) but are similar to the average adult α-T levels (19, 39). Figure 1B displays the distribution for tocopherol isoforms in the mothers measured in the second trimester, revealing markedly higher levels of α-T with a median of 63.13 μM (range 14.84–192.66 μM) compared to average non-pregnant adult α-T level (19) and compared to pregnant levels of γ-T with a median of 4.40 μM (range 0.00–26.39 μM). 89% of mothers took prenatal vitamins and 96% used prenatal vitamins or multivitamins, but there were no statistically significant differences in alpha-tocopherol levels between those reporting prenatal vitamin use and those who did not (Table S3). We elected to categorize these measurements in tertiles, to determine the associations of the opposing isoform of tocopherol in different strata of the variable as illustrated in Table 2.
Figure 1. Tocopherol Isoform Distributions in Children and in Mothers.
The x-axes for the maternal and child levels differ due to the dramatically greater maternal tocopherol levels.
Table 2:
Plasma levels and Tertiles of Tocopherol isoforms in the second trimester in mothers and at 3 years of age in children
| Alpha-T 2nd Trimester (μM) |
Gamma-T 2nd Trimester (μM) |
Alpha-T 3 yrs (μM) |
Gamma-T 3 yrs (μM) |
|
|---|---|---|---|---|
| Level (median,IQR) | 63.13, 47.49–83.50 | 4.40, 2.93–6.38 | 25.48, 19.78–32.41 | 2.67, 1.43–4.02 |
| Tertiles | ||||
| 1st | ≤ 53.326 | ≤ 3.428 | ≤ 21.503 | ≤ 1.733 |
| 2nd | 53.329–75.945 | 3.429–5.471 | 21.504–29.520 | 1.734–3.538 |
| 3rd | ≥ 75.946 | ≥ 5.472+ | ≥ 29.521 | ≥ 3.539 |
Association of Tocopherol Isoform levels with Lung function in Children at the mid-childhood visit (age 6–10)
Given the opposing associations of γ-T and α-T on adult lung function (19) and the preclinical data which showed that effects of either isoform were only evident in subjects with low levels of the other (13, 23, 24), we analyzed associations of opposing effects of tocopherol isoforms. The association of each isoform was analyzed by strata based on tertiles of the opposing tocopherol.
In Table 3, we display the associations of both maternal 2nd trimester and child tocopherol at age 3 years with %-predicted FEV1 and FVC at the mid-childhood visit (age 6–10), adjusted for maternal education level, smoking in pregnancy, child BMI z-score in mid-childhood (7 yr visit), prenatal PM2.5, and presence of some hemolysis in some of the 3 year samples. In the lowest tertile of γ-T, children with higher α-T levels (per 10 μM) had higher expected FEV1 %-predicted (β=3.13, p=0.01), and a higher FVC %-predicted (β=2.85, p=0.01). We evaluated the same associations with maternal levels of tocopherol isoforms. Notably, with the very high levels of α-T in maternal plasma, we did not see any consistent associations for child year 7 FEV1 and FVC with second trimester levels of either α-T or γ-T. A finding for maternal tocopherols was that for each 10 μM increment in γ-T, individuals in the middle tertile of 2nd trimester α-T had a decrement in % predicted FVC only (β=−10.20, p=0.004).
Table 3:
Associations of Tocopherol isoform levels (in 10 μM increments) within tertile strata of the opposing Tocopherol isoform with lung function at the mid-childhood visit (age 6–10)
| Change in %predicted FEV1 per 10 μM increment | Change in %predicted FVC per 10 μM increment | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictor (per 10 uM increment) | Tertile of opposing isoform | N | β | 95% CI | P-value | β | 95% CI | P-value |
| Gamma-T 3Y | Alpha-T 3y (1) | 82 | 17.40 | −6.46–41.26 | 0.15 | 11.11 | −9.87–32.09 | 0.30 |
| Alpha-T 3y (2) | 86 | −1.90 | −14.2–10.41 | 0.76 | −6.41 | −17.80–4.94 | 0.26 | |
| Alpha-T 3y (3) | 103 | −5.71 | −16.90–5.50 | 0.31 | −5.13 | −15.60–5.37 | 0.33 | |
| Alpha-T 3Y | Gamma-T 3y (1) | 103 | 3.13 | 0.63–5.84 | 0.01 | 2.85 | 0.58–5.12 | 0.01 |
| Gamma-T 3y (2) | 80 | −0.19 | −3.58–2.3.20 | 0.91 | −1.39 | −4.47–1.69 | 0.37 | |
| Gamma-T 3y (3) | 88 | −0.89 | −4.71–2.93 | 0.64 | 0.59 | −3.24–4. 42 | 0.86 | |
| Gamma-T Tri 2 | Alpha-T Tri 2 (1) | 107 | 4.44 | −9.41–18.28 | 0.53 | 1.68 | −11.1–14.45 | 0.79 |
| Alpha-T Tri 2 (2) | 135 | −6.49 | −14.50–1.47 | 0.11 | −10.20 | −17.1–−3.30 | 0.004 | |
| Alpha-T Tri 2 (3) | 116 | −0.71 | −7.96–6.54 | 0.85 | −4.09 | −11.3–3.06 | 0.26 | |
| Alpha-T Tri 2 | Gamma-T Tri 2 (1) | 111 | 0.25 | −0.87–1.37 | 0.66 | 0.25 | −0.73–1.22 | 0.62 |
| Gamma-T Tri 2 (2) | 131 | 0.42 | −0.79–1.63 | 0.50 | 0.45 | −0.68–1.58 | 0.43 | |
| Gamma-T Tri 2 (3) | 116 | 0.79 | −0.04–1.62 | 0.06 | 0.54 | −0.32–1.40 | 0.22 | |
Adjusted for education level, smoking during pregnancy, and prenatal exposure to particulate matter (pm25) and child BMI z-scores based on CDC 2000 reference data at 7 years; 3 Year exposure models also adjusted for hemolysis
Abbreviations: Alpha-T, α-tocopherol; Gamma-T, γ-tocopherol; Tri 2, 2nd trimester; 3Y, 3 year old
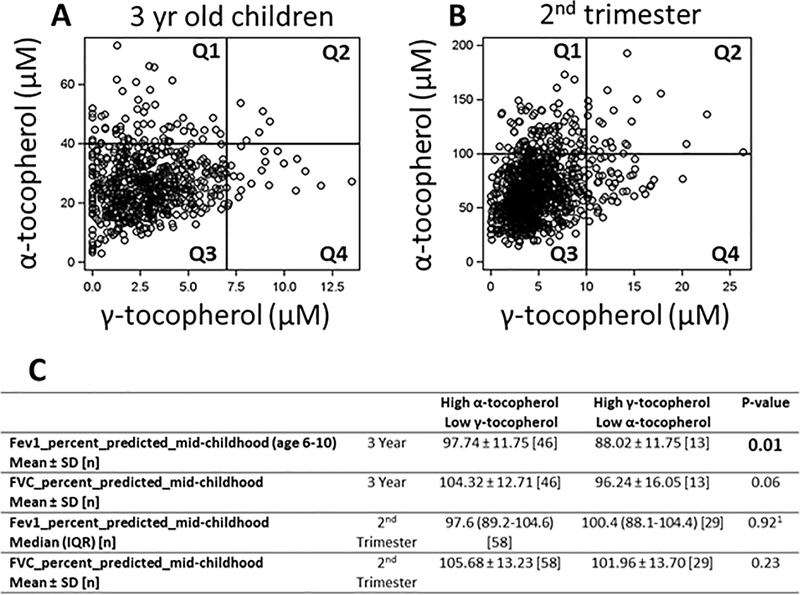
To further examine the association of γ-T with lung function in children with low α-T, in quadrants 1 and 4 of Figure 2, we carried out secondary analyses in children and mothers. In these unadjusted analyses shown in Figure 2C, we noted that those children with high α-T levels at age 3 years (> 40) and low γ-T (≤ 7) had greater FEV1 % predicted values (97.74±11.75%-predicted) at mid-childhood visit (age 6–10) compared to those who had high γ-T (> 7) and low α-T (≤ 40) (88.02±11.75%-predicted), consistent with opposing functions of tocopherol isoforms on lung spirometry in young children. This association remained present when limited to children with asthma even while controlling for use of a controller medication (for each 10 IU increase in alpha tocopherol, FEV1 increased by 4.43% (95% CI 0.39–8.46) and FVC increased by 4.36% (95% CI 0.26–8.47). When applying this approach to maternal 2nd trimester plasma levels which had very high levels of α-T, we did not find a significant difference in lung spirometry at the mid-childhood visit (age 6–10).
Figure 2. Lung function between children with predominantly high α-tocopherol (and low γ-tocopherol) to children with high γ-tocopherol (and low α-tocopherol).
1 Distribution of α-tocopherol and γ-tocopherol within A) 3 year olds and B) 2nd trimester mothers .Q1, quadrant 1; Q2, quadrant 2; Q3, quadrant 3; Q4, quadrant 4. C) Comparison of lung function for subjects in quadrants Q1 versus Q4 of panels A and B. 1- Non-parametric Wilcoxon rank sum test for p-value, all other p values from t-test. The FEV1 data for FEV1 was not normally distributed in the sample with 2nd trimester tocopherol data.
Discussion
We sought to evaluate the association of early life plasma levels of tocopherol isoforms with mid-childhood lung function, especially since there are opposing associations of tocopherol isoforms with plateau lung function in adults by age 21.(19) This is the first population-based study that evaluated the association of plasma levels of tocopherol isoforms in young children with lung function. We found that higher α-T in 3 year olds was associated with better lung function at 7 years when γ-T levels were in the lowest tertile. This protective effect of αT was lost at higher γ-T levels. Interestingly, the mean second trimester mother α-T concentrations in plasma was 3-fold higher than that reported for non-pregnant female adults, whereas γ-T concentrations in the second trimester were similar to non-pregnant adult γ-T levels. (19, 39) Moreover, the 75th percentile of 2nd trimester α-T was about 5 times higher than the reported average non-pregnant α-T levels in the United States.(40) This high level of α-T in 2nd trimester plasma is similar to pregnancy levels in Australia in 2nd trimester, reporting high level of α-T (43 μM) and normal adult levels of γ-T (1.4 μM) for adults in Australia (41). Plasma tocopherol levels in pregnancy have been measured previously,(42–44) but their associations with childhood lung function have not been reported before. Of note, this elevation in second trimester α-T did not seem to differ by reported prenatal vitamin use. It is unclear if this is due to inaccurate reporting of prenatal vitamin use or if there may be other factors such as upregulation of α-T transfer protein and active transport which may mediate these high α-T levels. Also, changes in α-T metabolism may occur during pregnancy in humans.(45) In the presence of these high second trimester α-T plasma levels, the maternal tocopherol concentrations did not associate with 7 year old child lung function.
Our prior epidemiologic findings in adults suggest that α-T and γ-T tocopherol Isoforms may have opposing effects on lung function. In a normative aging study, γ-T associated with lower lung function (46). In a prospective 20 year cohort (CARDIA), we demonstrated that a 5-fold higher plasma α-T level associates with better lung spirometry and that a 5-fold higher plasma γ-T level associates with lower lung spirometry (10 to 17% decrease in FEV1) in adults by age 21 (8, 13, 15, 19), suggesting that early in life, α-T and γ-T may regulate lung development, potentially by mediating responses to environmental exposures.(19) This hypothesis, that tocopherol isoforms may be relevant for both lung growth and asthma development, is supported by our preclinical work with pregnant murine models. We have demonstrated that supplementation of allergic pregnant mice with α-T decreases (23) and γ-T increases (24) neonate allergic responsiveness. In our mechanistic mouse studies with cross-fostering of pups at birth, we demonstrated a contribution to regulation of allergic inflammation by α-T in utero and after birth (23). This suggests that α-T and γ-T levels in combination with early life events may be relevant to both asthma and lung development. Indeed, early life severe lower respiratory viral illnesses are associated with asthma and worse lung function with potential fixed obstruction in some subjects in later childhood.(47, 48) Furthermore, prior studies from the Viva cohort have suggested that vitamin E (tocopherol isoforms) may regulate early life responses and wheezing to lung events such as viral illness, which would impact both FVC and asthma.(25–31) The interaction of tocopherol levels with specific viral illness could not be addressed directly in the current study in the absence of virology. Furthermore, self-report of viral illness used in this study would be poorly sensitive with only 5% recalling a clinical diagnosis of bronchiolitis. With respect to rhinovirus, 50% of random sampling over a year on one cohort of children had detectable virus of which 72–99% of these were rhinovirus.(49) Similarly, 19% of children under 5 have had RSV, and this increases to 95% by 5 years of age.(50, 51) Further prospective studies with virology may help delineate any interaction of specific viral insults with tocopherol levels on later life lung function. While we recognize that FEV1 is typically more proportionally affected, pediatric asthma can have both lowered FEV1 and FVC (25–31). Along these lines, we also analyzed the outcome of childhood asthma (data not shown), but found no association with tocopherol isoforms. We assume that this is due to power considerations (insufficient numbers in subgroups with low levels of the opposing tocopherol) for this dichotomous and multifactorial outcome. An additional importance of our findings is that if tocopherols are important in early lung development, they may be modifiable risk factors for impaired lung function growth and early life risks for later lung disease in adulthood.(52)
The opposing effects of γ-T and α-T isoforms, the two most abundant natural forms of tocopherols in diet and tissues (7–10), on lung function is biologically plausible. There are a number of mechanisms previously postulated for the effects of tocopherols on the developing immune system: including decreased cord blood mononuclear cells responses to antigen stimulation(53), decreased IL-4 production in T cells,(54) and decreased sensitivity of DC’s to pro-inflammatory cytokines.(55) These all could have relevance to both response to viral insult, and the development of asthma. Indeed, in our preclinical mechanistic studies of signals for eosinophil recruitment in allergic asthma, we demonstrated that γ-T is an agonist and α-T is an antagonist of recombinant protein kinase Cα (PKCα) and that α-T and γ-T bind to a regulatory domain of PKCα (12–14), a molecule important in airway viral infection (56–58), T- cell responses (59), and eosinophil recruitment to tissues (8, 13–16). In vivo, we demonstrated that a 5-fold increase in α-T blocks eosinophilic allergic responses (65% decrease) in adult mice whereas a 5-fold increase in γ-T elevates eosinophilic allergic lung inflammation (175%) and airway responses in adult mice (8, 13–16). γ-T potently elevates allergic inflammation in preclinical models. Tissue levels of γ-T are 10-fold lower than α-T, because α-T is preferentially transferred to liver lipoproteins by α-tocopherol transfer protein (60–64). At 10-fold lower tissue levels, in adult mice, γ-T elevates allergic responses to OVA (13, 17) and house dust mite extract (HDM) (20) and potently ablates the anti-inflammatory benefit of α-T during allergic responses (13, 17). γ-T amplification of allergic lung inflammation in adult mice is partially reversible by switching to elevated α-T before allergen re-challenge(15). These findings of opposing effects of tocopherols in disease and lung development with effects of α-T blocked at high levels of γ-T may have relevance to prior conflicting results from vitamin E clinical trials on the modulation of established disease (39). Specifically, in the US population no effect of tocopherol was seen on asthma severity but the mean serum γ-tocopherol concentration in the US population was 2–6 fold higher than French and Italian populations where there were clear effects (65–68). Finally, due to sample size, we are not able to examine interactions between markers of Th2 predisposition such as eosinophilia and predominant gamma tocopherol levels (high levels of gamma tocopherol and low levels alpha tocopherol) on the outcome of lung function.
The 9% difference in percent predicted FEV1 at the mid-childhood visit (age 6–10) comparing the children at the opposite spectrum of the tocopherol isoform distributions (high α-T with low γ-T compared to low α-T with high γ-T) at age 3 is clinically relevant. Even in subjects with who were diagnosed with asthma or wheezing at any point, this level of difference in FEV1 is larger than the amount of difference seen with allergen sensitization noted by a large longitudinal study.(69) The lower lung function in these Viva participants is similar to the 5–11% reduction in lung function reported for other environmental factors. It is reported that responders to cotton have a 5–8% decrease in FEV1 and that this decrease is associated with dyspnea, chest tightness, chronic bronchitis, and chronic cough.(70, 71) It is also reported that responders to particulate matter have a 2 to 6% decrease in FEV1,(72) responders to cold or exercise have a 5 to 11% decrease in FEV1 (73), and responders to house dust mite or dog/cat dander have a 2–8% decrease in FEV1.(74) These subsets represent the extremes of the spectrum, where there is minimal competition of the opposing tocopherol isoform. However, even while looking at the children with the lowest tertile of gamma tocopherol, the difference of α-T across the interquartile range for the population (13 μM) is associated with a 4% difference in %-predicted FEV1. To put this difference in perspective, this is the same level of difference in FEV1 %-predicted seen across the spectrum of pediatric asthma as reported by Bacharier et al (75).
Interestingly, we did not see associations of child lung function with in utero tocopherol isoform exposures. The levels of maternal α-T concentrations in plasma were at least 3-fold higher than that reported for non-pregnant female adults, whereas γ-T concentrations in the second trimester were similar to non-pregnant levels. This suggests that the high levels of second trimester α-T in these moms, which were above the average for non-pregnant adult, may be at maximum for child lung function outcomes. This is the first report which examines the association of childhood lung function with plasma levels of tocopherols in pregnancy. The high α-T during pregnancy likely reflects a combination of the overall increase in lipid transport during pregnancy, potential increases in the liver α-T transfer protein during pregnancy, potential changes in α-T metabolism, and increase in intake of α-T in prenatal vitamin supplements.
The study has potential limitations. Firstly, we do not have serial measurements of tocopherol levels in childhood between age 3 years and the mid-childhood visit (age 6–10). It would be important to determine if there was a critical period in which lung growth and development are more affected. Additionally, we made no formal adjustment for multiple testing because the analyses were selected based on preclinical mechanistic outcomes; however, lung function outcomes are correlated and do not represent independent outcomes. Furthermore, the associations tested were established a priori at the onset of the project with lung function as the primary analysis and all others were secondary. These novel findings in children are consistent with the suggestion that in adults, higher levels of α-T in the context of lower levels of γ-T is associated with higher plateau lung function (and presumably lung development) in our studies in the CARDIA study(19) and is consistent with differential effects of these tocopherol isoforms in animal models (8, 13–16, 23). While we do not see the same degree of effect of γ-T at age 3 that we saw in our CARDIA paper,(19) this data in the Project Viva cohort demonstrate the novel finding that in early childhood, children with high α-T and low γ-T isoforms have higher lung function than those with high γ-T and low α-T isoforms at mid-childhood. This finding is more direct evidence than cross sectional associations with plateau lung function. Additional studies in larger numbers of subjects are needed to further delineate any opposing effects of α-T and γ-T in lung development. Finally, further mechanistic ex vivo studies in humans will help to examine mechanism.
In summary, our findings and the previous preclinical studies suggest that early childhood consumption of tocopherol isoforms could influence childhood lung function. While we did not see similar associations with in-utero exposure to tocopherols and lung function, it is possible that this is due to a potential maximal effect of the levels of α-T in pregnancy in the Project Viva cohort. However, based on our findings, lung function in childhood may be modifiable by α-T and γ-T levels early in childhood regardless of the prenatal vitamins. Further studies in prospective trials are needed to determine if changes to tocopherol isoforms in formula and diet would improve lung function trajectory in childhood and into adult life.
Supplementary Material
Acknowledgements
Dr. Cook-Mills was supported by NIH R01AI127695. Dr. Kumar was supported by NIH R01AI127695, HHSN2752011300013C, U19AR06952, and UG1HL139125. Project Viva is supported by R01HD034568, R01AI102960, UH3OD023286.
Abbreviations
- α-T
alpha tocopherol
- γ-T
gamma tocopherol
- PKCα
protein kinase Cα
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
Footnotes
Disclosures: The authors declare that they have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez FD. Genes, environments, development and asthma: a reappraisal. Eur Respir J. 2007;29(1):179–84. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Bousquet PJ, Godard P, Daures JP. The public health implications of asthma. Bull World Health Organ. 2005;83(7):548–54. [PMC free article] [PubMed] [Google Scholar]
- 3.Vollmer WM, Osborne ML, Buist AS. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1079–84. [DOI] [PubMed] [Google Scholar]
- 4.Friebele E The attack of asthma. Environ Health Perspect. 1996;104(1):22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pike KC, Inskip HM, Robinson SM, Cooper C, Godfrey KM, Roberts G, et al. The relationship between maternal adiposity and infant weight gain, and childhood wheeze and atopy. Thorax. 2013;68(4):372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson PE, McDonald BW. Subcutaneous body fat in pregnant New Zealand women: association with wheeze in their infants at 18 months. Maternal and child health journal. 2013;17(5):959–67. [DOI] [PubMed] [Google Scholar]
- 7.Uauy R, Hoffman DR, Birch EE, Birch DG, Jameson DM, Tyson J. Safety and efficacy of omega-3 fatty acids in the nutrition of very low birth weight infants: soy oil and marine oil supplementation of formula. J Pediatr. 1994;124(4):612–20. [DOI] [PubMed] [Google Scholar]
- 8.Cook-Mills JM, McCary CA. Isoforms of Vitamin E Differentially Regulate Inflammation. Endocr Metab Immune Disord Drug Targets. 2010;10:348–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle FG, Yuhas RJ, Lien EL. Red blood cell and tissue phospholipid fatty acid profiles of weanling rats fed infant formula fat blends containing soy and/or corn oil. Ann Nutr Metab. 1996;40(4):234–42. [DOI] [PubMed] [Google Scholar]
- 10.Nelson SE, Rogers RR, Frantz JA, Ziegler EE. Palm olein in infant formula: absorption of fat and minerals by normal infants. Am J Clin Nutr. 1996;64(3):291–6. [DOI] [PubMed] [Google Scholar]
- 11.Halonen M, Lohman IC, Stern DA, Ellis WL, Rothers J, Wright AL. Perinatal tumor necrosis factor-alpha production, influenced by maternal pregnancy weight gain, predicts childhood asthma. Am J Respir Crit Care Med. 2013;188(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchese ME, Berdnikovs S, Cook-Mills JM. Distinct Sites within the Vascular Cell Adhesion Molecule-1 (VCAM-1) Cytoplasmic Domain Regulate VCAM-1 Activation of Calcium Fluxes versus Rac1 during Leukocyte Transendothelial Migration. Biochemistry. 2012;51:8235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, et al. Isoforms of Vitamin E have Opposing Immunoregulatory Functions during Inflammation by Regulating Leukocyte Recruitment. J Immunol 2009;182:4395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Vitamin E isoforms differentially regulate intercellular adhesion molecule-1 activation of PKCalpha in human microvascular endothelial cells. PLoS ONE. 2012;7(7):e41054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of alpha-tocopherol and gamma-tocopherol’s effects. J Immunol. 2011;186(6):3674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCary CA, Yoon Y, Panagabko C, Cho W, Atkinson J, Cook-Mills JM. Vitamin E isoforms directly bind PKCalpha and differentially regulate activation of PKCalpha. Biochem J. 2012;441:189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook-Mills JM, Marchese M, Abdala-Valencia H. Vascular Cell Adhesion Molecule-1 Expression and Signaling during Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxid Redox Signal. 2011;15(6):1607–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2(10):748–59. [DOI] [PubMed] [Google Scholar]
- 19.Marchese ME, Kumar R, Colangelo LA, Avila PC, Jacobs DR Jr., Gross M, et al. The vitamin E isoforms alpha-tocopherol and gamma-tocopherol have opposite associations with spirometric parameters: the CARDIA study. Respir Res. 2014;15(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook-Mills J, Gebretsadik T, Abdala-Valencia H, Green J, Larkin EK, Dupont WD, et al. Interaction of vitamin E isoforms on asthma and allergic airway disease. Thorax. 2016;71(10):954–6. doi: 10.1136/thoraxjnl-2016-208494. Epub 2016 Jun 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zha K, Zuo C, Wang A, Zhang B, Zhang Y, Wang B, et al. LDL in patients with subclinical hypothyroidism shows increased lipid peroxidation. Lipids Health Dis. 2015;14:95.(doi): 10.1186/s12944-015-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundstrom SL, Yang J, Kallberg HJ, Thunberg S, Gafvelin G, Haeggstrom JZ, et al. Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS One. 2012;7(3):e33780. doi: 10.1371/journal.pone.0033780. Epub 2012 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdala-Valencia H, Berdnikovs S, Soveg F, Cook-Mills JM. alpha-Tocopherol Supplementation of Allergic Female Mice Inhibits Development of CD11c+CD11b+ Dendritic Cells in Utero and Allergic Inflammation in Neonates. Am J Physiol Lung Cell Mol Physiol. 2014;307(00132):L482–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdala-Valencia H, Soveg F, Cook-Mills JM. γ-Tocopherol Supplementation of Allergic Female Mice Augments Development of CD11c+CD11b+ Dendritic Cells in Utero and Allergic Inflammation in Neonates Am J Physiol Lung Cell Mol Physiol 2016;310(8):L759–L71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyvarinen MK, Kotaniemi-Syrjanen A, Reijonen TM, Korhonen K, Korppi MO. Lung function and bronchial hyper-responsiveness 11 years after hospitalization for bronchiolitis. Acta Paediatr. 2007;96(10):1464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135(4):607–16. doi: 10.1542/peds.2014-3060. Epub 2015 Mar 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luisi F, Pinto LA, Marostica L, Jones MH, Stein RT, Pitrez PM. Persistent pulmonary function impairment in children and adolescents with asthma. J Bras Pneumol. 2012;38(2):158–66. [DOI] [PubMed] [Google Scholar]
- 28.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163(1):37–42. [DOI] [PubMed] [Google Scholar]
- 29.Joseph-Bowen J, de Klerk NH, Firth MJ, Kendall GE, Holt PG, Sly PD. Lung function, bronchial responsiveness, and asthma in a community cohort of 6-year-old children. Am J Respir Crit Care Med. 2004;169(7):850–4. Epub 2004 Jan 23. [DOI] [PubMed] [Google Scholar]
- 30.Hollams EM, de Klerk NH, Holt PG, Sly PD. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med. 2014;189(4):401–7. doi: 10.1164/rccm.201302-0323OC. [DOI] [PubMed] [Google Scholar]
- 31.Chatkin MN, Menezes AM, Macedo SE, Fiss E. Asthma and lung function in a birth cohort at 6–7 years of age in southern Brazil. J Bras Pneumol. 2008;34(10):764–71. [DOI] [PubMed] [Google Scholar]
- 32.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA Jr., et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84(4):903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58(2):337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe A, Braback L, Ekeus C, Hjern A, Forsberg B. Maternal obesity during pregnancy as a risk for early-life asthma. The Journal of allergy and clinical immunology. 2011;128(5):1107–9. e1–2. [DOI] [PubMed] [Google Scholar]
- 35.Patel SP, Rodriguez A, Little MP, Elliott P, Pekkanen J, Hartikainen AL, et al. Associations between pre-pregnancy obesity and asthma symptoms in adolescents. Journal of epidemiology and community health. 2012;66(9):809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buschard K, Mansson JE, Roep BO, Nikolic T. Self-glycolipids modulate dendritic cells changing the cytokine profiles of committed autoreactive T cells. PLoS One. 2012;7(12):e52639. doi: 10.1371/journal.pone.0052639. Epub 2012 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedulov AV, Kobzik L. Allergy Risk is Mediated by Dendritic Cells with Congenital Epigenetic Changes. Am J Respir Cell Mol Biol. 2011;44:285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim RH, Kobzik L. Maternal transmission of asthma risk. Am J Reprod Immunol. 2009;61(1):1–10. [DOI] [PubMed] [Google Scholar]
- 40.Kumar R, Story RE, Pongracic JA, Hong X, Arguelles L, Wang G, et al. Maternal Pre-Pregnancy Obesity and Recurrent Wheezing in Early Childhood. Pediatric allergy, immunology, and pulmonology. 2010;23(3):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloise E, Ortiga-Carvalho TM, Reis FM, Lye SJ, Gibb W, Matthews SG. ATP-binding cassette transporters in reproduction: a new frontier. Hum Reprod Update. 2016;22(2):164–81. doi: 10.1093/humupd/dmv049. Epub 2015 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khare A, Chakraborty K, Raundhal M, Ray P, Ray A. Cutting Edge: Dual Function of PPARgamma in CD11c+ Cells Ensures Immune Tolerance in the Airways. J Immunol. 2015;195(2):431–5. doi: 10.4049/jimmunol.1500474. Epub 2015 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Legutko A, Marichal T, Fievez L, Bedoret D, Mayer A, de Vries H, et al. Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-gamma activity in dendritic cells. J Immunol. 2011;187(9):4517–29. doi: 10.049/jimmunol.1101493. Epub 2011 Sep 23. [DOI] [PubMed] [Google Scholar]
- 44.Uderhardt S, Kronke G. 12/15-lipoxygenase during the regulation of inflammation, immunity, and self-tolerance. J Mol Med (Berl). 2012;90(11):1247–56. doi: 10.007/s00109-012-0954-4. Epub 2012 Sep 15. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Zhu Z, Zhu B, Ma Z. Peroxisome proliferator-activated receptor-gamma agonist induces regulatory T cells in a murine model of allergic rhinitis. Otolaryngol Head Neck Surg. 2011;144(4):506–13. doi: 10.1177/0194599810396133. Epub 2011 Feb 24. [DOI] [PubMed] [Google Scholar]
- 46.Leme AS, Hubeau C, Xiang Y, Goldman A, Hamada K, Suzaki Y, et al. Role of breast milk in a mouse model of maternal transmission of asthma susceptibility. J Immunol. 2006;176(2):762–9. [DOI] [PubMed] [Google Scholar]
- 47.Backman K, Ollikainen H, Piippo-Savolainen E, Nuolivirta K, Korppi M. Asthma and lung function in adulthood after a viral wheezing episode in early childhood. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2018;48(2):138–46. [DOI] [PubMed] [Google Scholar]
- 48.Guilbert TW, Singh AM, Danov Z, Evans MD, Jackson DJ, Burton R, et al. Decreased lung function after preschool wheezing rhinovirus illnesses in children at risk to develop asthma. The Journal of allergy and clinical immunology. 2011;128(3):532–8 e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arlt O, Schwiebs A, Japtok L, Ruger K, Katzy E, Kleuser B, et al. Sphingosine-1-phosphate modulates dendritic cell function: focus on non-migratory effects in vitro and in vivo. Cell Physiol Biochem. 2014;34(1):27–44. doi: 10.1159/000362982. Epub 2014 Jun 16. [DOI] [PubMed] [Google Scholar]
- 50.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12(9):688–702. doi: 10.1038/nrd4099. Epub 2013 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bu S, Kapanadze B, Hsu T, Trojanowska M. Opposite effects of dihydrosphingosine 1-phosphate and sphingosine 1-phosphate on transforming growth factor-beta/Smad signaling are mediated through the PTEN/PPM1A-dependent pathway. J Biol Chem. 2008;283(28):19593–602. doi: 10.1074/jbc.M802417200. Epub 2008 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G, et al. 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J Immunol. 2009;183(5):3383–9. doi: 10.4049/jimmunol.0900327. Epub 2009 Aug 12. [DOI] [PubMed] [Google Scholar]
- 53.Ilan Y Alpha versus beta: are we on the way to resolve the mystery as to which is the endogenous ligand for natural killer T cells? Clin Exp Immunol. 2009;158(3):300–7. doi: 10.1111/j.365-2249.009.04030.x. Epub 2009 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barcelo-Coblijn G, Murphy EJ, Mills K, Winchester B, Jakobs C, Snead OC 3rd, et al. Lipid abnormalities in succinate semialdehyde dehydrogenase (Aldh5a1−/−) deficient mouse brain provide additional evidence for myelin alterations. Biochim Biophys Acta. 2007;1772(5):556–62. Epub 2007 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatterjee S, Bell WR, Kwiterovich PO Jr. Distribution of antithrombin III and glucosylceramide in human plasma lipoproteins and lipoprotein deficient plasma. Lipids. 1984;19(5):363–6. [DOI] [PubMed] [Google Scholar]
- 56.Chu SY, Kim SY, Bish CL. Prepregnancy obesity prevalence in the United States, 2004–2005. Maternal and child health journal. 2009;13(5):614–20. [DOI] [PubMed] [Google Scholar]
- 57.Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Preventive medicine. 2013;56(6):372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ezzati M, Martin H, Skjold S, Vander Hoorn S, Murray CJ. Trends in national and state-level obesity in the USA after correction for self-report bias: analysis of health surveys. Journal of the Royal Society of Medicine. 2006;99(5):250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harpsoe MC, Basit S, Bager P, Wohlfahrt J, Benn CS, Nohr EA, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. The Journal of allergy and clinical immunology. 2013;131(4):1033–40. [DOI] [PubMed] [Google Scholar]
- 60.Wolf G How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr Rev. 2006;64(6):295–9. [DOI] [PubMed] [Google Scholar]
- 61.Leonard SW, Terasawa Y, Farese RV Jr., Traber MG. Incorporation of deuterated RRR- or all-rac-alpha-tocopherol in plasma and tissues of alpha-tocopherol transfer protein--null mice. Am J Clin Nutr. 2002;75(3):555–60. [DOI] [PubMed] [Google Scholar]
- 62.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. The FASEB journal. 1999;13:1145–55. [PubMed] [Google Scholar]
- 63.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. Journal of Lipid Research. 1996;37:893–901. [PubMed] [Google Scholar]
- 64.Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. American Journal of Clinical Nutrition. 1989;49:517–26. [DOI] [PubMed] [Google Scholar]
- 65.van Schayck CP, Smit HA. The prevalence of asthma in children: a reversing trend. Eur Respir J. 2005;26(4):647–50. [DOI] [PubMed] [Google Scholar]
- 66.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS data brief. 2012(94):1–8. [PubMed] [Google Scholar]
- 67.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, et al. National surveillance for asthma--United States, 1980–2004. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2007;56(8):1–54. [PubMed] [Google Scholar]
- 68.Otto C, Barthel D, Klasen F, Nolte S, Rose M, Meyrose AK, et al. Predictors of self-reported health-related quality of life according to the EQ-5D-Y in chronically ill children and adolescents with asthma, diabetes, and juvenile arthritis: longitudinal results. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2017. [DOI] [PubMed] [Google Scholar]
- 69.Clarke JT. The glycosphingolipids of human plasma lipoproteins. Can J Biochem. 1981;59(6):412–7. [DOI] [PubMed] [Google Scholar]
- 70.Rapp KI, Jack L Jr., Wilson C, Hayes SC, Post R, McKnight E, et al. Improving Asthma-Related Outcomes Among Children Participating in the Head-Off Environmental Asthma in Louisiana (HEAL), Phase II Study. Health promotion practice. 2017:1524839917740126. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan PW, Ghushchyan V, Navaratnam P, Friedman HS, Kavati A, Ortiz B, et al. The national cost of asthma among school-aged children in the United States. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017;119(3):246–52 e1. [DOI] [PubMed] [Google Scholar]
- 72.Oskeritzian CA, Milstien S, Spiegel S. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol Ther. 2007;115(3):390–9. Epub 2007 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Argraves KM, Sethi AA, Gazzolo PJ, Wilkerson BA, Remaley AT, Tybjaerg-Hansen A, et al. S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 2011;10:70.(doi): 10.1186/476-511X-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Levkau B HDL-S1P: cardiovascular functions, disease-associated alterations, and therapeutic applications. Front Pharmacol. 2015;6:243.(doi): 10.3389/fphar.2015.00243. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reichman NE, Nepomnyaschy L. Maternal pre-pregnancy obesity and diagnosis of asthma in offspring at age 3 years. Maternal and child health journal. 2008;12(6):725–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.