Abstract
To define the tolerability and outcome of allogeneic hematopoietic stem cell transplant (allo-HSCT) following CAR T cell therapy, we retrospectively reviewed pediatric/young adult patients with relapsed/refractory B-ALL who underwent this treatment. Fifteen patients (median age 13 years; range 1–20 years) with a median potential follow up of 39 months demonstrated 24-month cumulative incidence of relapse, cumulative incidence of TRM, and OS of 16% (95% CI: 0–37%), 20% (95% CI: 0–40%), and 80% (95% CI: 60–100%), respectively. Severe toxicity following CAR T cells did not impact OS (p=0.27) while greater time from CAR T cells to allo-HSCT (>80 days) was associated with a decrease in OS. In comparing CD34-selected T cell depleted (TCD; n=9) versus unmodified (n=6) allo-HSCT, the cumulative incidence of relapse, TRM, and OS at 24-months was 22% (95% CI: 0–49%) vs 0% (p=0.14), 0% vs. 50% [95% CI: 10–90%] (p = 0.02) and 100% vs. 50% [95% CI: 10–90%] (p=0.02). In this small cohort of patients, CAR T cells followed by a CD34-selected TCD allo-HSCT appears to result in less TRM and favorable OS when compared to unmodified allo-HSCT. There was no evidence that disease control was impacted by the type of consolidative allo-HSCT, which demonstrates the feasibility of this approach.
INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common malignancy occurring in children, and survival with risk-stratified therapy for B-cell ALL (B-ALL) approaches ≥90%.1–3 However, for pediatric/young adult patients with relapsed or refractory (R/R) disease the prognosis remains dismal.4–6 Traditionally patients with R/R disease have required allogeneic hematopoietic stem cell transplantation (allo-HSCT) for cure. The necessity of achieving minimal residual disease negative complete response (MRD-negative/CR) prior to allo-HSCT has precluded a large number of patients with R/R B-ALL from undergoing allo-HSCT.7–9 Several groups initiated investigation of CD19-specific CAR T cells as a potential therapeutic option for patients with R/R B-ALL.10–17 This led to the approval by the Food and Drug Administration of CD19-specific CAR T cells for R/R B-ALL in patients <26 years old.10–16 Despite this success, the incidence of relapse following treatment with CAR T cells in all patients with R/R B-ALL is unknown and has been reported to exceed 50% in some series, demonstrating a need to improve therapy.11–13, 16
The tolerability and outcome of allo-HSCT following CAR T cell therapy in pediatric/young adult patients is undefined and the use of allo-HSCT as consolidative therapy may reduce the incidence of disease relapse and improve overall survival (OS). Reported outcomes for adult B-ALL patients (age 26–74 years) who underwent allo-HSCT following CAR T cell therapy failed to demonstrate superior results as compared to patients who received CAR T cells alone.16 Additionally, in a cohort of adult patients with B-ALL, longer duration between CAR T cell therapy and allo-HSCT (≥80 days) was associated with higher risk of death and non-relapse mortality.18 Herein we report the results of a cohort of pediatric and young adult patients with B-ALL who received CAR T cells followed by allo-HSCT and describe that allo-HSCT as consolidative therapy following CAR T cells is well tolerated and CD34-selected T cell depleted (TCD) allo-HSCT has a low incidence of toxicity.
SUBJECTS AND METHODS
Study Design
We conducted a retrospective analysis of pediatric and young adult patients with B-ALL who received CAR T cells followed by an allo-HSCT. Patients received CAR T cells on an investigator-initiated protocol NCT01860937 or experimental CAR T cells at referring institutions. Allo-HSCT occurred at both our institution and five collaborating institutions. Only patients who achieved MRD-negative/CR proceeded to allo-HSCT based on treating physician preference and institutional guidelines. Timing, preperative regimen, and graft manipulation of allo-HSCT were determined by the treating physician. Data was captured as part of an IRB approved protocol (NCT01860937) or retrospective waiver for research. Informed consent was obtained from all subjects taking part in the IRB approved protocol (NCT01860937).
Outcomes of Interest
The main outcomes of interest were OS, transplant-related mortality (TRM), and relapse. Other outcomes of interest were neutrophil engraftment, platelet engraftment, occurrence of graft vs host disease (GVHD), occurrence of veno-occlusive disease (VOD), CD19-positive B cell recovery and donor chimerism. Relapse was defined as any (medullary or extramedullary) evidence of hematologic, cytogenetic, and/or molecular recurrence of primary disease. Late relapse was defined as relapse ≥12 months after allo-HSCT. Neutrophil engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count >0.5 ×109/L. Platelet engraftment was defined as a platelet count of >20 ×109/L for 7 days without transfusion. GVHD was diagnosed and scored based on the Center for International Blood and Marrow Transplant Research criteria for acute GVHD (aGVHD)19 and National Institute of Health consensus criteria for chronic GVHD (cGVHD).20 VOD was defined according to the modified Seattle criteria.21 Regimen-related toxicities were graded according to the standard National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. CD19-positive B cell recovery was defined as any detectable CD19-positive B cells (>0 cell/mcL) on a peripheral blood lymphocyte flow cytometry panel. Chimerism was obtained on bone marrow samples (bulk population short tandem repeat (STR) analysis) and peripheral blood (American Red Cross (ARC), lineage specific donor chimerism) and was considered all donor if ≥95% donor chimerism was reported in the bulk population (bone marrow sample) or 100% was reported in the myeloid population (peripheral blood sample).
Statistical Analysis
For all analyses, transplantation date was considered time 0, with a data cutoff of Nov 1, 2019. The median potential follow-up was calculated using the reverse Kaplan-Meier method. OS was defined as the time from transplant to death from all causes. Patients alive were censored at their last follow-up date. Time-to-relapse was defined as the time from the date of transplantation to the date of disease relapse. Patients alive without disease relapse were censored at their date of last follow-up and deaths without disease relapse were considered as competing events. Finally, TRM is defined as the time from transplant to death related to transplant. Kaplan-Meier analysis was used to estimate OS while an Aalen-Johansen estimator was used for the cumulative incidence of relapse and TRM. A comparative analysis was performed between CD34-selected TCD versus unmodified allo-HSCT on fifteen patients. Differences in survival endpoints between groups was tested using a log-rank test or Gray’s test for cumulative incidences.
RESULTS
Patient Characteristics
Between February 3, 2014 and June 8, 2018 fifteen pediatric/young adult patients with R/R B-ALL were treated with CAR T cells followed by an allo-HSCT. All fifteen patients received CD19-specific CAR T cells including fourteen who received 19–28z CAR T cell (NCT01860937) and one patient who received 19-BBz CAR T cells (NCT03792633). Baseline characteristics and toxicity following CAR T cell therapy are shown in Table 1.
Table 1:
Characteristics all Entire Cohort, CD34-Selected TCD Cohort, and Unmodified Cohort
| CAR + HSCT Entire Cohort N=15 | CAR + CD34-Selected HSCT Cohort N=9 | CAR + Unmodified HSCT Cohort N=6 | p-value* | ||
|---|---|---|---|---|---|
| Median Age (range, years) | 13 (1–20) | 13 (1–20) | 12.5 (4–20) | 0.81 | |
| Median time from CAR T cell therapy to allo-HSCT (range, days) | 57 (30–135) | 46 (30–135) | 65 (55–95) | 0.09 | |
|
Lymphodepleting regimen pre-CAR T cell therapy Cyclophosphamide/Fludarabine Cyclophosphamide |
6 9 |
4 5 |
2 4 |
>0.99 | |
|
CRS grade
0 1 2 3 4 |
2 6 5 1 1 |
2 4 2 0 1 |
0 2 3 1 0 |
>0.99 (0/1/2 vs 3/4) |
|
|
ICANS grade
0 1 2 3 4 |
2 4 5 3 1 |
2 2 3 1 1 |
0 2 2 2 0 |
>0.99 (0/1/2 vs 3/4) |
|
|
Disease Status
CR1 CR2 CR3 CR4 |
6 5 3 1 |
5 2 2 0 |
1 3 1 1 |
0.29 (CR1 vs ≥CR2) |
|
|
Conditioning Regimen TBI-based 1500 cGy 1200–1440 cGy unknown Chemotherapy only clo/mel/thio** flu/mel/thio*** |
12 8 3 1 3 2 1 |
7 7 0 0 2 2 0 |
5 1 3 1 1 0 1 |
>0.99 | |
|
HLA Matched Mismatched Related Unrelated |
10 5 8 7 |
6 3 4 5 |
4 2 4 2 |
>0.99 0.61 |
|
|
Graft Source BM PBSC |
7 8 |
1 8 |
6 0 |
n/a | |
|
GVHD prophylaxis
CD34+ selected CNI plus MTX CNI plus MMF Post HSCT Cyclophosphamide |
9 4 1 1 |
9 0 0 0 |
0 4 1 1 |
n/a |
CAR, chimeric antigen receptor; HSCT, hematopoietic stem cell transplantation; CRS, cytokine release syndrome; ICANS, immune effector cell-associated neurotoxicity syndrome; CR, complete remission; TBI, total body irradiation; BM, bone marrow; PBSC, peripheral blood stem cell; GVHD, graft-vs-host disease; CNI, calcineurin inhibitor; MTX, methotrexate; n/a, not applicable for statistical analysis because graft source and GVHD prophylaxis is dependent on the graft manipulation cohort.
p-values from Mann-Whitney-Wilcoxon rank test or Fisher’s exact test.
clofarabine (150 mg/m2), melphalan (140 mg/m2), and thiotepa (10mg/kg).
fludarabine (120 mg/m2), melphalan (140 mg/m2), and thiotepa (10 mg/kg).
All patients received lymphodepleting chemotherapy prior to CAR T cell therapy including: cyclophosphamide (3000 mg/m2) and fludarabine (75 mg/m2) (n=5), cyclophosphamide (500 mg/m2) and fludarabine (120 mg/m2) (n=1), cyclophosphamide (3000 mg/m2) alone (n=8), and cyclophosphamide (750 mg/m2) alone (n=1). Lymphodepleting chemotherapy regimens were determined based on CAR specific protocol guidelines and have been previously described.15 Cytokine release syndrome (CRS) and severe CRS were observed in 87% (13/15 patients) and 13% (2/15 patients) respectively. Immune effector cell-associated neurotoxicity syndrome (ICANS) and severe ICANS were observed in 87% (13/15 patients) and 27% (4/15 patients). Severe toxicity (grade 3/4 CRS or ICANS) following CAR T cell therapy occurred in 27% (4/15 patients). Eleven patients underwent allo-HSCT at MSKCC and four were transplanted in one of the four referring institutions after receiving CAR T cells.
The median age at time of allo-HSCT was 13 years (range 1–20 years). All patients achieved MRD-negative/CR confirmed by flow cytometry following CAR T cells and prior to allo-HSCT. The median time from last MRD testing prior to the start of conditioning was 14 days (range 6–53). The median time from CAR T cell infusion to allo-HSCT was 57 days (range 30 –135). Median time from CAR T cell infusion to allo-HSCT in patients with severe toxicity (n=4) vs without severe toxicity (n=11) was 56 days (range 39–65) vs 57 days (range 30–135) respectively (p=0.56). Two patients had prior allo-HSCT before CAR T cell therapy and subsequently underwent a second allo-HSCT at 24 (CD34-selected TCD cohort) and 39 months (unmodified cohort) from the first allo-HSCT. Conditioning regimens and allo-HSCT graft source/manipulation was determined by each transplant center. Conditioning regimens were myeloablative (total body irradiation (TBI)-based n=12 or chemotherapy only n=3). Individual characteristics for each patient are listed in Table 2.
Table 2:
Individual Characteristics of Patients Who Received allo-HSCT after CAR T cells
| Age at HSCT | Prior allo-HSCT | Disease status | Conditioning Regimen | Time from CAR T cells to allo-HSCT | Graft Source | Graft manipulation | Donor | GVHD ppx | Neutrophil engraftment | Platelet engraftment | Relapse | Cause of Death | Transplant Complications | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | no | CR2 | TBI/thio/cy | 39 | BM | CD34+ | Unrelated 9/10 | TCD | 14 | 215 | No | NA | aGVHD (grade 2) |
| 2 | 16 | yes | CR3 | TBI/thio/cy | 57 | PBSC | CD34+ | Related 10/10 | TCD | 11 | 16 | Yes | NA | None |
| 3 | 12 | no | CR2 | TBI/cy | 57 | BM | Unmodified | Related | Tacro/MMF | 16 | 26 | No | NA | aGVHD (grade 2), cGVHD (*) |
| 4 | 1 | no | CR1 | Clo/mel/thio | 30 | PBSC | CD34+ | Unrelated 9/10 | TCD | 12 | 34 | No | NA | None |
| 5 | 4 | no | CR1 | TBI/thio/cy | 46 | PBSC | CD34+ | Unrelated 9/10 | TCD | 15 | 217 | No | NA | cGVHD (score 3) |
| 6 | 11 | no | CR1 | TBI/thio/cy | 57 | PBSC | CD34+ | Unrelated 10/10 | TCD | 11 | 34 | No | NA | cGVHD (score 1) |
| 7 | 4 | no | CR2 | TBI/thio/cy | 55 | BM | Unmodified | Related 10/10 | Tacro/MTX | 13 | 32 | No | NA | None |
| 8 | 8 | no | CR2 | TBI/thio/cy | 66 | BM | Unmodified | Related 10/10 | Tacro/MTX | 13 | 30 | No | NA | None |
| 9 | 20 | no | CR3 | TBI/cy | 81 | BM | Unmodified | Unrelated 10/10 | CSA/MTX | 23 | 265 | No | GVHD | aGVHD (grade 3), cGVHD (score 3) |
| 10 | 5 | no | CR1 | TBI/thio/cy | 44 | PBSC | CD34+ | Unrelated 10/10 | TCD | 11 | 16 | Yes | NA | None |
| 11 | 13 | no | CR1 | TBI/flu/cy | 64 | BM | Unmodified | Related haplo | Post-cy Tacro/MMF |
29 | NA | No | VOD, MSOF | aGVHD (grade 4), VOD |
| 12 | 15 | no | CR2 | Clo/mel/thio | 45 | PBSC | CD34+ | Related 10/10 | TCD | 11 | 17 | No | NA | None |
| 13 | 20 | no | CR1 | TBI/thio/cy | 135 | PBSC | CD34+ | Related 10/10 | TCD | 9 | 17 | Yes | NA | aGVHD (grade 2) |
| 14 | 18 | yes | CR4 | Flu/mel/thio | 95 | BM | Unmodified | Unrelated 10/10 | Tacro/MTX | 22 | NA | No | VOD, MSOF | VOD |
| 15 | 15 | no | CR3 | TBI/thio/cy | 65 | PBSC | CD34+ | Related 10/10 | TCD | 10 | 26 | No | NA | None |
CAR, chimeric antigen receptor; allo-HSCT, allogeneic hematopoietic stem cell transplantation; CR, complete remission; TBI, total body irradiation; flu, fludarabine; cy, cyclophosphamide; mel, melphalan; thio, thiotepa; clo, clofarabine; BM, bone marrow; PBSC, peripheral blood stem cell; GVHD, graft-vs-host disease; CSA, cyclosporine; MTX, methotrexate; TCD, T cell depleted; tacro, tacrolimus; MMF, mycophenolate mofetil; post cy, post-transplant cyclophosphamide; VOD, veno-occlusive disease; MSOF, multi-system organ failure; aGVHD, acute graft-vs-host disease; cGVHD, chronic graft-vs-host disease.
information not available
The median potential follow-up after allo-HSCT was 39 months (range 1–48 months). The median CD34+ dose was 6.9 ×106/kg (range 1.98–11.9 × 106/kg; n=15) and the median CD3+ dose was 4.7 ×103/kg (range 0.36–37,590 ×103/kg; n=14). All fifteen patients achieved neutrophil engraftment and thirteen achieved platelet engraftment. The median time to neutrophil engraftment was 13 days (range 9–29). The median time to platelet engraftment was 30 days (range 16–265).
TRM and Overall Survival
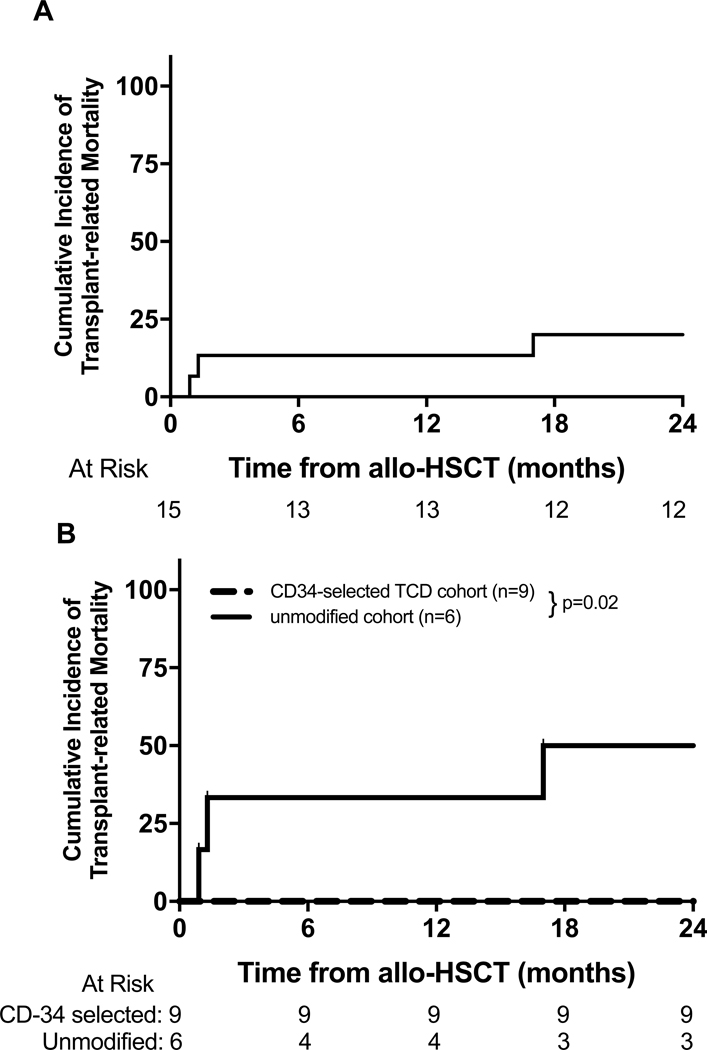
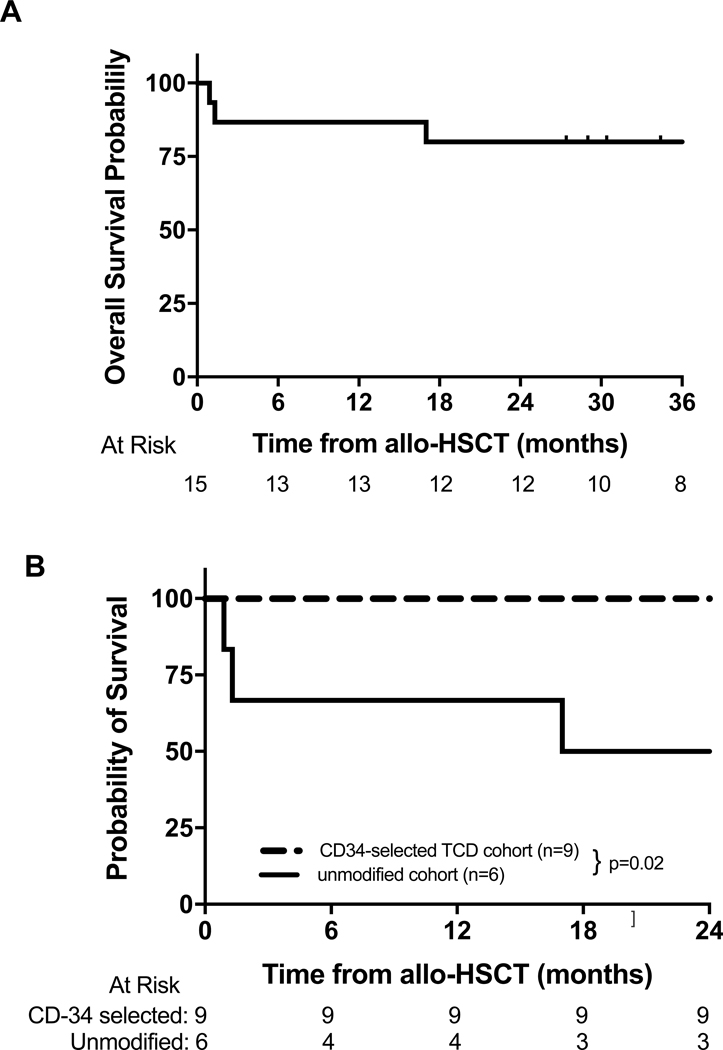
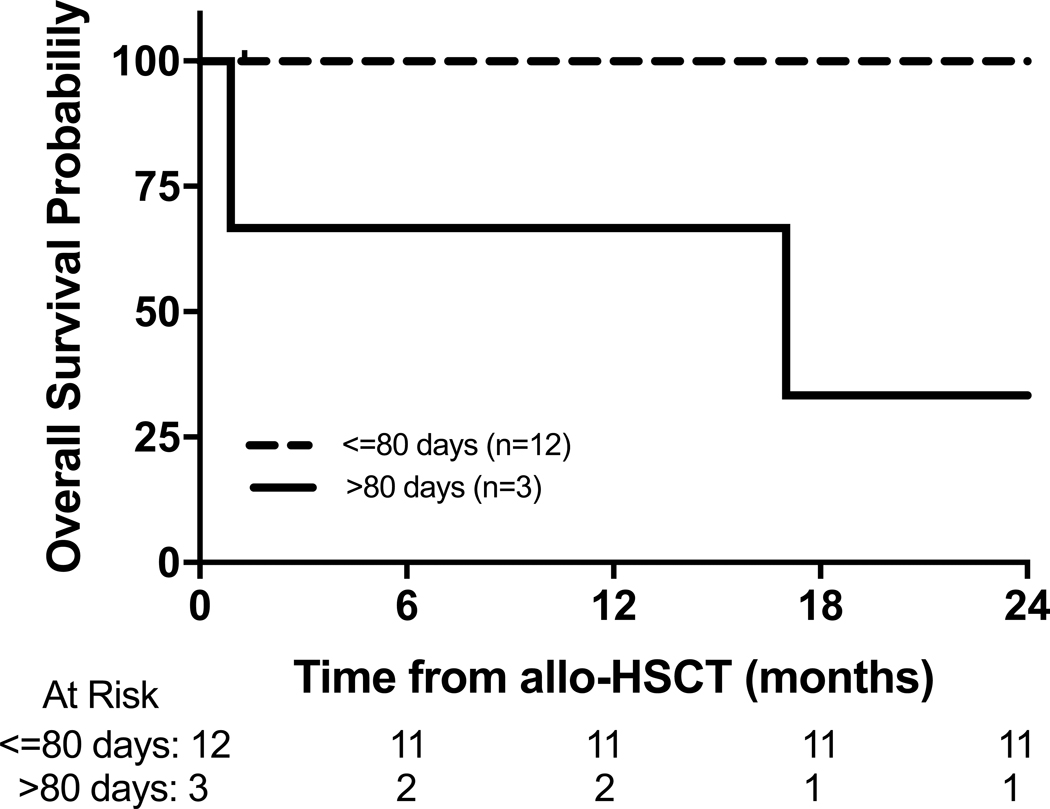
Post transplantation outcomes for all patients are shown in Table 3. The cumulative incidence of TRM for the entire cohort at 12 months and 24 months was 13% (95% CI: 0–31%) and 20% (95% CI: 0–40%) respectively (Figure 1A). Three patients in the unmodified cohort died due to TRM, including veno-occlusive disease (multi-organ failure/infection) (n=2) and GVHD (n=1). The cumulative incidence of TRM for the unmodified cohort (n=6) at 12-months and 24-months was 33% (95% CI: 0–71%) and 50% (95% CI: 10–90%) respectively, while TRM was absent in the CD34-selected TCD cohort (n=9) (p=0.02) (Figure 1B). OS at 12-months and 24-months for the entire cohort was 87% (95% CI: 69–100%) and 80% (95% CI: 60–100%) respectively (Figure 2A), with a median potential follow-up of 39 months. Three patients (unmodified cohort only) died during follow up and the 12-month and 24-month OS probabilities for the unmodified and CD34-selected TCD allo-HSCT cohorts were 67% (95% CI: 29–100%) and 50% (95% CI: 10–90%) vs 100% respectively (p=0.02, Figure 2B). The 12-month and 24-month OS in patients who underwent allo-HSCT >80 days vs ≤80 days after receiving CAR T cell therapy was 67% (95% CI: 13–100%) and 33% (95% CI: 0–87%) vs 92% (95% CI: 76–100%) and 92% (95% CI: 76–100%) respectively (Figure 3). Peripheral blood counts prior to allo-HSCT between these two groups are noted in supplementary table 1. The 12-month and 24-month OS in patients with severe toxicity following CAR T cells (grade 3 or 4 CRS or ICANS) vs without severe toxicity was 100%vs 82% (95% CI: 59–100%) and 73% (95% CI: 46–99%) respectively (p=0.27).
Table 3:
Post Transplantation Outcomes of Entire Cohort, CD34-selected Cohort, and Unmodified Cohort
| CAR + HSCT Entire Cohort N=15 | CAR + CD34-Selected HSCT Cohort N=9 | CAR + Unmodified HSCT Cohort N=6 | |
|---|---|---|---|
| Relapse | 3 | 3 | 0 |
| TRM | 3 | 0 | 3 |
|
GVHD Acute Chronic |
5 4 |
2 2 |
3 2 |
|
Viral Infections Adenovirus CMV BK EBV HHV-6 Toxoplasmosis |
4 4 4 1 2 1 |
3 3 2 1 0 1 |
1 1 2 0 2 0 |
|
Bacterial Infections CONS Klebsiella C. Difficile |
1 2 4 |
1 2 4 |
0 0 0 |
|
Death VOD/MSOF GVHD |
2 1 |
0 0 |
2 1 |
CAR, chimeric antigen receptor; HSCT, hematopoietic stem cell transplantation; TRM, transplant-related mortality; GVHD, graft-vs-host disease; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV-6, human herpesvirus 6; CONS, coagulase negative staphylococci; VOD, veno-occlusive disease; MSOF, multi-system organ failure.
Figure 1.
(A) Cumulative incidence of treatment-related mortality in entire cohort and (B) cumulative incidence of treatment-related mortality in CD34-selected TCD allo-HSCT cohort and unmodified cohort (p=0.02).
Figure 2.
(A) Overall survival of entire cohort and (B) overall survival of CD34-selected TCD allo-HSCT cohort and unmodified cohort (p=0.02).
Figure 3.
Overall survival of patients who went to allo-HSCT less than 80 days from CAR T cell therapy compared to patients who went to allo-HSCT >80 days from CAR T cell therapy.
Relapse
A total of three patients relapsed following consolidative allo-HSCT, all in the CD34-selected cohort. One patient experienced an antigen negative relapse (CD19-negative n=1) following targeting of that antigen by CAR T cells. The 12-month and 24-month cumulative incidence of relapse for the entire cohort was 0% and 16% (95% CI: 0–37%) respectively. In the comparative analysis, 24-month cumulative incidence of relapse was 22% (95% CI: 0–49%) in the CD34-selected TCD allo-HSCT cohort (n=9), versus 0% in the unmodified allo-HSCT (n=6; p=0.14) respectively.
All three of the patients in the CD34-selected TCD cohort who relapsed experienced late relapse (range 16.1–24.4 months). All three patients with relapsed disease were successfully re-treated with subsequent CAR T cell therapy (CD19-specific n=2; CD22-specific n=1) (Supplementary Table 2). The time from allo-HSCT to leukapheresis ranged from 16.4 to 41.5 months and the time from relapse to infusion of second CAR T cell product ranged from 2.4 to 3.4 months. All three patients achieved MRD-negative/CR following CAR T cell infusion and remain alive without disease. Follow up since second CAR T cell infusion ranges between 1.3 and 16.6 months.
GVHD and Organ Toxicity
aGVHD occurred in 33% (5/15) of patients after allo-HSCT including two patients in the CD34-selected TCD cohort and 3 patients in the unmodified cohort (Table 2). cGVHD was seen in 27% (4/15) of patients after allo-HSCT including two patients in the CD34-selected TCD cohort and two patients in the unmodified graft cohorts (Table 2). Donor lymphocyte infusion for persistent adenovirus infection contributed to cGVHD for one patient (CD34-selected TCD cohort). Death from cGVHD (TRM) was seen in one patient with skin cGVHD and a late flair of GI cGVHD (unmodified cohort).
VOD did not occur in the CD34-selected cohort, while two patients developed VOD in the unmodified cohort. These patients with VOD ultimately died secondary to VOD and related complications, including one patient with a history of prior allo-HSCT. No post-transplant lymphoproliferative disease was seen in either cohort. No death secondary to infections occurred and pattern of infection was typical for this patient population (Table 3).
B cell Reconstitution and Donor Chimerism
Lymphocyte subset flow cytometry panel data for CD19-positive B cell recovery and chimerism data was available for eleven patients, nine in the CD34-selected cohort and two in the unmodified cohort. All measured patients had evidence of CD19-positive B cell recovery with a median time of 56 days (range 36–117 days). IVIG replacement was not standardized across the cohort, however twelve patients, nine in the CD34-selected cohort and three in the unmodified cohort, received IVIG replacement following allo-HSCT.
Chimerism data were available for eleven patients (CD34-selected cohort, n=9; unmodified cohort n=2). Bone marrow donor chimerism (median sample number 4, range 2–17)) demonstrated complete donor chimerism in 82% (9/11) of patients with median time of 51.5 days (19–158 days). Peripheral blood lineage specific donor chimerism (median number of samples 5, range 1–11) was obtained for the same eleven patients. Complete myeloid chimerism was achieved in 91% (10/11) of patients with a median time of 109.5 days (range 44–221 days). One patient (CD34-selected TCD cohort) who received a chemotherapy only conditioning regimen failed to achieve full donor chimerism (both BM/PB samples) and another patient (CD34-selected TCD cohort) is doing well with mostly host chimerism (both BM/PB samples) after losing his myeloid chimerism.
DISCUSSION
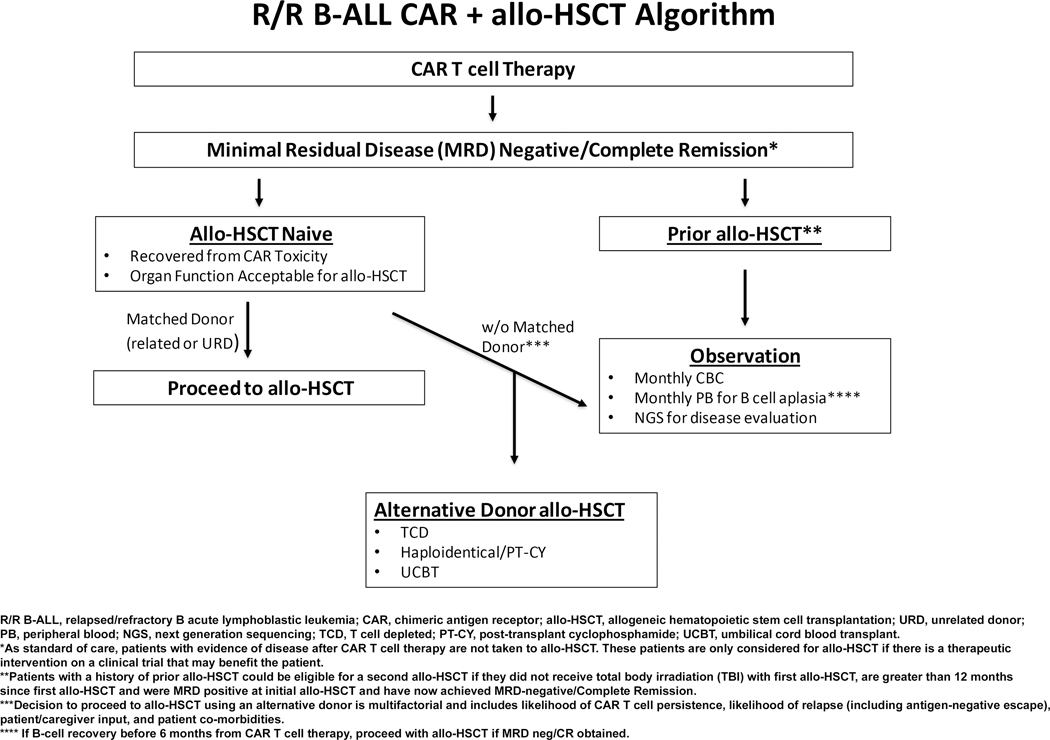
Allo-HSCT is the standard of care for patients with R/R B-ALL who achieve MRD-negative remission.22–26 The use of CAR T cells in R/R B-ALL has enabled chemotherapy refractory patients to achieve MRD-neg/CR however, the durability of this response is untested. Management of pediatric/young adult patients post CAR T cell therapy and the role of allo-HSCT as consolidative therapy is yet to be defined. This is in part due to the paucity of data on the tolerability and outcomes of patients who undergo allo-HSCT following CAR T cells. Our institution commonly offers allo-HSCT with particular focus on donor options and disease response after CAR T cell therapy (Figure 4). This report presents the outcomes of a cohort of patients who received CAR T cells followed by allo-HSCT and demonstrates the feasibility of this approach. In this small cohort of patients it appears that CD34-selected T cell depleted (TCD) allo-HSCT as compared to unmodified allo-HSCT is well tolerated with a low incidence of toxicity and favorable OS.
Figure 4.
MSKCC institutional post-CAR T cell therapy treatment algorithm
The toxicities following allo-HSCT (VOD, organ dysfunction, GVHD) and CAR T cell therapy (CRS, ICANS, HLH) have been well described.11, 13, 15, 16, 19, 20, 27, 28 Severe toxicity following CAR T cell toxicity did not impact the timing of allo-HSCT in this cohort. Timing from CAR T cell therapy to allo-HSCT does appear to impact OS in this cohort (Figure 3), however the number of patients who received an allo-HSCT >80 days (n=3) is too small to discern statistical significance. This finding is consistent with a previous findings in a cohort of adult patients with worse outcomes if allo-HSCT was >80 days from CAR T cell therapy.18 The mechanism as to why a delay in allo-HSCT impacts OS remains speculative, however any delay was not related to the severity of CAR T cell toxicity in this cohort. Of note, the toxicity profile for the majority of patients included in this cohort has been previously reported and while not impacting the timing or outcome of allo-HSCT, the incidence of ICANS is different from the commercially approved CAR T cell product (Tisagenlecleucel).15 Prospective studies are needed to define the optimal the time between allo-HSCT and CAR T cell therapy but in cases where allo-HSCT is certain there should be minimal delay.
Overall survival for the entire cohort and particularly the CD34-selected TCD cohort was favorable. This included three patients in the CD34-TCD cohort that experienced a late relapse and achieved MRD-negative CR following a second CAR T cell infusion. The ability to salvage patients with re-collection (sufficient circulating lymphocytes) and use of a different CAR T cell product demonstrates that re-treatment with immunotherapies can provide benefit for select patients. TRM was notably absent in the CD34-seleted TCD cohort following CAR T cells whereas TRM complications (VOD and GVHD) impacted survival in the unmodified cohort. Improved tolerability in the CD34-selected cohort would be futile if disease control was not equivalent.
To this point, this limited cohort demonstrates no increased risk of relapse using a TCD graft compared to an unmodified graft despite the concern of the elimination of graft vs leukemia in a TCD graft.29–32 Additionally, Shalabi et al. have previously reported CAR T cell therapy as an effective bridge to allo-HSCT with a low incidence of relapse.33 Not included in this analysis are the consequences of any financial toxicity novel immunotherapies will have within our healthcare system.34 Regardless, a significant proportion of patients will experience disease relapse following CAR T cells therapy which requires further investigation to improve consolidative therapy and durability of response 11–13, 16.
In summary, CAR T cells, when followed by allo-HSCT, appears to be a tolerable option with favorable OS for patients. This retrospective study represents a heterogeneous group of patients with diverse transplant conditioning regimens and graft source/manipulation. This data suggests further research incorporating CAR T cell persistence, timing of allo-HSCT, ongoing post CAR T cell toxicity, likelihood of antigen negative disease escape, and donor transplant specific factors including graft manipulation is required to determine the optimal strategy for these patients. Once these questions are answered, a prospective study is needed to determine if consolidation with allo-HSCT should be the standard of care after CAR T cell therapy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the patients and families who participated in this trial; Georgia Flynn, and the Cellular Therapeutic Center at MSKCC for help with this study; Joseph Olechnowicz (Editor, Department of Pediatrics, Memorial Sloan-Kettering Cancer Center), for editorial assistance; and Audrey Mauguen (PhD) for biostatistical support.
This work was supported by the William Lawrence and Blanche Hughes Foundation (K.J.C.), St. Baldrick’s Foundation provided an A.V.M. Traders Scholar Award (K.J.C.), and National Institutes of Health (NIH), National Cancer Institute (NCI) Cancer Center Support Grant (P30CA008748).
Conflict of interest statement: KJC has received research support from Juno Therapeutics and Novartis; has consulted, participated in advisory boards, or participated in educational seminars for Juno Therapeutics, Novartis, and Mesoblast; SM has participated in advisory boards for Novartis; RJB is a co-founder and received royalties for Juno Therapeutics. The remaining authors declare no competing financial interests.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67(1): 7–30. e-pub ahead of print 2017/01/05; doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012; 30(14): 1663–1669. doi: 10.1200/JCO.2011.37.8018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. The New England journal of medicine 2015; 373(16): 1541–1552. doi: 10.1056/NEJMra1400972 [DOI] [PubMed] [Google Scholar]
- 4.Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia 2008; 22(12): 2142–2150. doi: 10.1038/leu.2008.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol 2005; 131(5): 579–587. doi: 10.1111/j.1365-2141.2005.05773.x [DOI] [PubMed] [Google Scholar]
- 6.von Stackelberg A, Volzke E, Kuhl JS, Seeger K, Schrauder A, Escherich G et al. Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM Study Group. European journal of cancer 2011; 47(1): 90–97. doi: 10.1016/j.ejca.2010.09.020 [DOI] [PubMed] [Google Scholar]
- 7.Campana D, Leung W. Clinical significance of minimal residual disease in patients with acute leukaemia undergoing haematopoietic stem cell transplantation. Br J Haematol 2013; 162(2): 147–161. e-pub ahead of print 2013/05/10; doi: 10.1111/bjh.12358 [DOI] [PubMed] [Google Scholar]
- 8.Shen Z, Gu X, Mao W, Yin L, Yang L, Zhang Z et al. Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC Cancer 2018; 18(1): 755. e-pub ahead of print 2018/07/25; doi: 10.1186/s12885-018-4670-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant 2013; 48(5): 630–641. e-pub ahead of print 2012/07/25; doi: 10.1038/bmt.2012.139 [DOI] [PubMed] [Google Scholar]
- 10.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6(224): 224ra225. doi: 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine 2014; 371(16): 1507–1517. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385(9967): 517–528. e-pub ahead of print 2014/10/13; doi: 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude SL. Tisagenlecleucel in pediatric patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol 2018; 16(10): 664–666. [PubMed] [Google Scholar]
- 14.Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017; 129(25): 3322–3331. e-pub ahead of print 2017/04/13; doi: 10.1182/blood-2017-02-769208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran KJ, Margossian S, Kernan NA, Silverman LB, Williams DA, Shukla NN et al. Toxicity and Response following CD19-specific CAR T cells in pediatric/young adult relapsed/refractory B-ALL. Blood 2019. e-pub ahead of print 2019/10/28; doi: 10.1182/blood.2019001641 [DOI] [Google Scholar]
- 16.Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. The New England journal of medicine 2018; 378(5): 449–459. e-pub ahead of print 2018/02/01; doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol 2019: JCO1901892. e-pub ahead of print 2019/12/10; doi: 10.1200/JCO.19.01892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shadman M, Gauthier J, Hay KA, Voutsinas JM, Milano F, Li A et al. Safety of allogeneic hematopoietic cell transplant in adults after CD19-targeted CAR T-cell therapy. Blood Adv 2019; 3(20): 3062–3069. e-pub ahead of print 2019/10/28; doi: 10.1182/bloodadvances.2019000593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15(6): 825–828. [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11(12): 945–956. doi: 10.1016/j.bbmt.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 21.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118(4): 255–267. doi: 10.7326/0003-4819-118-4-199302150-00003 [DOI] [PubMed] [Google Scholar]
- 22.Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood 2006; 107(12): 4961–4967. e-pub ahead of print 2006/02/21; doi: 10.1182/blood-2005-12-4942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balduzzi A, De Lorenzo P, Schrauder A, Conter V, Uderzo C, Peters C et al. Eligibility for allogeneic transplantation in very high risk childhood acute lymphoblastic leukemia: the impact of the waiting time. Haematologica 2008; 93(6): 925–929. e-pub ahead of print 2008/04/15; doi: 10.3324/haematol.12291 [DOI] [PubMed] [Google Scholar]
- 24.Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant 2012; 18(4): 505–522. e-pub ahead of print 2011/12/29; doi: 10.1016/j.bbmt.2011.12.585 [DOI] [PubMed] [Google Scholar]
- 25.Wood WA, Lee SJ, Brazauskas R, Wang Z, Aljurf MD, Ballen KK et al. Survival improvements in adolescents and young adults after myeloablative allogeneic transplantation for acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2014; 20(6): 829–836. e-pub ahead of print 2014/03/07; doi: 10.1016/j.bbmt.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters C, Schrappe M, von Stackelberg A, Schrauder A, Bader P, Ebell W et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol 2015; 33(11): 1265–1274. e-pub ahead of print 2015/03/09; doi: 10.1200/JCO.2014.58.9747 [DOI] [PubMed] [Google Scholar]
- 27.Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant 2010; 16(2): 157–168. e-pub ahead of print 2009/09/18; doi: 10.1016/j.bbmt.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant 2019; 25(4): 625–638. e-pub ahead of print 2018/12/25; doi: 10.1016/j.bbmt.2018.12.758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood 2007; 110(13): 4552–4559. e-pub ahead of print 2007/08/23; doi: 10.1182/blood-2007-06-093880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakubowski AA, Small TN, Kernan NA, Castro-Malaspina H, Collins N, Koehne G et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant 2011; 17(9): 1335–1342. e-pub ahead of print 2011/01/11; doi: 10.1016/j.bbmt.2011.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldberg JD, Linker A, Kuk D, Ratan R, Jurcic J, Barker JN et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant 2013; 19(2): 208–213. e-pub ahead of print 2012/09/13; doi: 10.1016/j.bbmt.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hobbs GS, Hamdi A, Hilden PD, Goldberg JD, Poon ML, Ledesma C et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant 2015; 50(4): 493–498. e-pub ahead of print 2015/01/26; doi: 10.1038/bmt.2014.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shalabi H, Delbrook C, Stetler-Stevenson M, Yuan C, Steinberg S, Yates B, Fry T, Lee D, Shah N. Chimeric Antigen Receptor T-Cell (CAR-T) Therapy Can Render Patients with ALL Into PCR-Negative Remission and Can be an Effective Bridge to Transplant (HCT). Biol Blood Marrow Transplant 2018; 24(3). doi: 10.1016/j.bbmt.2017.12.018 [DOI] [Google Scholar]
- 34.Perica K, Curran KJ, Brentjens RJ, Giralt SA. Building a CAR Garage: Preparing for the Delivery of Commercial CAR T Cell Products at Memorial Sloan Kettering Cancer Center. Biol Blood Marrow Transplant 2018; 24(6): 1135–1141. e-pub ahead of print 2018/03/03; doi: 10.1016/j.bbmt.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.