Abstract
Objectives:
The study aimed to evaluate the feasibility, acceptability, and preliminary clinical impact of BRIGHT (Building a Renewed ImaGe after Head & neck cancer Treatment), a novel telemedicine-based cognitive-behavioral intervention to manage body image disturbance (BID) in head and neck cancer (HNC) survivors.
Methods:
HNC survivors with BID were enrolled into a single-arm pilot trial. Participants completed study measures at baseline, 1- and 3-months post-BRIGHT to assess its acceptability and clinical impact. Participants completed semi-structured interviews to evaluate the feasibility and acceptability of BRIGHT and refine the intervention.
Results:
Ten HNC survivors with BID were enrolled into the trial of tablet-based BRIGHT. BRIGHT was feasible, as judged by low dropout (n=1), high session completion rates (100%; 45/45) and low rates of technical issues with the tablet-based delivery (11% minor; 0% major). Ninety percent of participants were highly likely to recommend BRIGHT, reflecting its acceptability. BRIGHT was associated with a 34.5% reduction in mean Body Image Scale scores at 1-month post-BRIGHT (mean difference from baseline = 4.56; 95% CI 1.55, 7.56), an effect that was durable at 3-months post-BRIGHT (mean decrease from baseline = 3.56; 95% CI 1.15 to 5.96). Program evaluation revealed high levels of satisfaction with BRIGHT, particularly the delivery platform. During the qualitative evaluation, participants highlighted that BRIGHT improved image-related coping behavior.
Conclusions:
BRIGHT is feasible, acceptable to HNC survivors, and has significant potential as a novel approach to manage BID in HNC survivors. Additional research is necessary to refine BRIGHT and evaluate its clinical efficacy and scalability.
Keywords: Body image, cancer, cognitive behavioral therapy, head and neck cancer, oncology, telemedicine
Introduction
Head and neck cancer (HNC), which arises in cosmetically and functionally critical areas, is diagnosed in 65,000 patients in the US annually1. Treatment for HNC includes various combinations and sequences of surgery, radiation, and chemotherapy2 which can result in substantial life-altering morbidity related to disfigurement, difficulty swallowing, impaired smiling, and challenges speaking3. These changes occur in highly visible, socially significant parts of the body that are integral to self-conception, communication, and interpersonal relationships4–6. Based on single-institution cross sectional and cohort studies of HNC patients, up to 75% express body image concerns when assessed using screening questionnaires or upon structured clinical interview7–9. When severe, these image concerns can result in high rates of body image disturbance (BID), a disorder characterized by a displeasing self-perceived change in appearance and/or function4,10,11. BID results in devastating psychosocial morbidity and is an important contributor to social isolation12, stigmatization4, depression5,13, decreased intimacy14, and worse quality of life (QOL)4,6 among HNC survivors.
Managing BID is emerging as an important component of HNC survivorship care3. Unfortunately, no effective interventions for BID in HNC survivors have been described6,11,15. As a result, treatment of BID in HNC survivors represents a significant unmet need16. It has been suggested that psychotherapeutic interventions may be effective to treat HNC-related BID8, although no such interventions have been developed or tested. In addition, HNC survivors encounter significant access-to-care barriers for face-to-face mental health care including travel burden, fatigue, and treatment toxicity. As a result, innovative approaches to deliver evidence-based psychosocial interventions to HNC survivors are needed
To address these gaps in clinical care, we developed BRIGHT (Building a Renewed ImaGe after Head & neck cancer Treatment), a novel one-on-one tele-cognitive behavioral therapy (CBT) intervention. BRIGHT was developed to target the cognitive, behavioral, and attitudinal components of HNC-related BID. This study aims to evaluate the feasibility and acceptability of BRIGHT and assess its preliminary clinical impact on BID among HNC survivors.
Materials and Methods
Study Design and Procedures
Consistent with our feasibility, acceptability, and preliminary efficacy objectives, we designed a pilot single-arm clinical trial with a mixed methods approach for program evaluation. Patients were originally allowed to choose their method of BRIGHT delivery (tablet-based or face-to-face) upon accruing to the trial. Tablet-based BRIGHT was overwhelmingly preferred (100% of patients traveling >25 miles (8/8); 67% (2/3) of patients traveling ≤ 25 miles) because of travel considerations, convenience, and flexibility. After it became clear that telemedicine was the preferred strategy to deliver BRIGHT to HNC survivors with BID, we closed accrual to the face-to-face delivery arm. The results of the pilot trial of tablet-based BRIGHT are presented herein. Following provision of written informed consent, patients were enrolled in the trial. Patients completed study questionnaires at baseline, 1-, and 3-months after BRIGHT. Semi-structured interviews were performed with participants at 1-month post-BRIGHT to evaluate its acceptability, gather feedback about BRIGHT to refine the intervention, and assess its perceived behavioral mechanism of action. The semi-structured interviews were conducted with all participants in the trial as determined by the trial sample size estimate (i.e. no attempts were made to reach thematic saturation). The data from these interviews are intended to reflect the views of HNC patients with BID who would be interested in pursuing treatment. The trial is registered at ClinicalTrials.gov (NCT03518671). All procedures performed in studies involving human participants were in accordance with the ethical standards of the Medical University of South Carolina Institutional Review Board, which approved this study (Pro00072856).
Study Participants and Setting
Study eligibility criteria included: 1) ≥ 18 years of age, 2) history of squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx or a cutaneous malignancy of the head and neck, 3) definitive surgery with or without adjuvant therapy, 4) completion of treatment within the prior year, 5) no evidence of active disease, and 6) Body Image Scale (BIS) Score ≥ 5. Patients with cutaneous malignancies seen in the MUSC Head and Neck Tumor Center, similar to many academic HNC practices, generally have locoregionally advanced disease requiring extensive ablative surgery and free flap reconstructions with adjuvant therapy, predisposing them to similar image-related concerns as mucosal HNC patients. Patients were excluded due to inability to speak English, recurrence, or second primary malignancies. All HNC survivors returning for routine survivorship follow-up at a multidisciplinary HNC clinic at a single academic center were screened for the study; those meeting eligibility criteria were recruited by the project coordinator at the survivorship visit. We did not screen for comfort with technology nor avoid recruiting patients based on this perception. The CONSORT diagram is shown in Supplemental Figure 1.
Development of BRIGHT
BRIGHT was developed utilizing the cognitive model, the basis of CBT, and tailored to address key domains of HNC-related BID identified in our qualitative work11. We used an intervention mapping approach to optimize BRIGHT delivery17. A needs assessment was conducted within our prospective cohort study of BID in HNC survivors to inform the timing (immediately post-HNC treatment), setting (one-on-one psychotherapy), and delivery method (telemedicine or face-to-face) of BRIGHT18. We assessed the feasibility of delivering BRIGHT via patient-owned technology. Although a majority of our population owned a video-enabled device (smart phone=83%, tablet=36%, computer=64%, none=6%) and had home internet access (88%), we elected to provide each participant with the same video platform (tablet) and internet connection (cellular-enabled Wi-Fi) to standardize our approach.
BRIGHT Intervention
BRIGHT consists of 5 weekly 60-minute sessions delivered one-on-one via tablet. Session materials provided to patients included agendas, exercises which illustrate the session topic, and corresponding homework. BRIGHT focuses on adjustment to physical changes and changes in functioning, teaches coping and problem-solving skills, and aims to reduce avoidance behaviors among patients with BID. Session topics include: 1) psychoeducation regarding image concerns and appearance beliefs; 2) introduction to the cognitive model and cognitive restructuring; 3) avoidance behaviors and social support; 4) coping with role changes and finding self-worth; and 5) identifying personal values. BRIGHT is delivered by a licensed clinical psychologist (SM).
Upon enrollment, subjects received and were trained to use, a study-issued, Wi-Fi and 4G LTE cellular-enabled iPad. Each iPad was locked to prevent downloading of additional applications, pre-loaded with a SIM Card to enable cellular communication, and pre-loaded with Vidyo, a HIPAA-compliant, video teleconference platform. Vidyo allows face-to-face communication, but also includes a within-video text feature (useful for aphonic or severely dysarthric HNC patients). Following BRIGHT, subjects returned the iPads in pre-addressed, stamped, mailers. The additional costs of delivering BRIGHT using the tablet-based platform included: 1) cellular-enabled iPad with protective case ($384.99); 2) cellular service for the iPad ($37.99/month), and 3) mail-related expenses for the iPad ($13.40/patient for shipping).
Measures
We assessed a comprehensive set of program delivery elements to examine the feasibility and acceptability of BRIGHT. Feasibility measure included study dropout, session completion, technical issues, and tablet return. Acceptability measures, scored from strongly disagree (0) to strongly agree (5), included timing, delivery method, content, number of sessions, and likelihood of recommending BRIGHT. The primary clinical endpoint was change in BIS scores from baseline to one-month post-BRIGHT. The BIS is a psychometrically valid10, 10-item patient-reported outcome measure (PROM) assessing the affective, cognitive, and emotional aspects of body image due to cancer or its treatment19 that has been used extensively in patients with HNC15. BIS scores range from 0–30, with higher scores indicating greater BID. Additional clinical endpoints included changes in depression (PROMIS SF v1.0-Depression-4a20), anxiety (PROMIS SF v1.0-Anxiety-4a20), social isolation (PROMIS SF v2.0-Social Isolation-4a21), shame and stigma (Shame and Stigma Scale22), and HN QOL (EORTC QLQ-H&N3523), at 1- and 3-months post-BRIGHT relative to baseline.
Statistical Analysis
Statistical analyses were completed using the R statistical software package (R version 3.6.0). Graphical displays were constructed to demonstrate patterns of individual continuous measurement and the difference between baseline, 1-month, and 3-months post-intervention and summary statistics reported. Pairwise test of means was performed using Wilcoxon signed rank test to evaluate the association of BRIGHT with a reduction in continuous measures (BIS, PROMIS SF, Shame and Stigma Scale, EORTC QLQ-H&N35) at 1- and 3-months post-BRIGHT relative to baseline. Response rate was computed as the proportion of patients whose continuous measures decreased from baseline with its 95% confidence interval. Sample size calculations performed using PASS 2008, version 08.0.13 revealed that 20 participants (n=10 tablet-based BRIGHT and n=10 face-to-face) were required to detect a difference of at least 2.5 points in the BIS from pre-treatment to 1-month post-BRIGHT with power = 0.80 and a critical α = .05. The effect size was chosen based on published interventions for BID among cancer survivors24–26.
Results
Ten HNC survivors with BID were enrolled into the single-arm pilot of tablet-based BRIGHT. Participants were predominantly female (n=7; 70%), had oral cavity cancer (n=4; 40%), underwent microvascular reconstruction (n=8; 80%), and had adjuvant therapy (n=7; 70%) (Supplemental Table 1).
Feasibility
One patient, the first in the trial, withdrew after completing one session of BRIGHT and had no further follow-up (Table 1). BRIGHT session completion was high; with the exception of the patient who dropped out after the first session, all of the remaining participants (n=9) completed all five BRIGHT sessions (n=45). The tablet-based telemedicine delivery platform showed low rates of technical issues, with 11% of sessions having minor technical issues (5/45) and no sessions having major technical issues requiring canceling/missing the session. All tablets (n=10) were returned at the conclusion of the study.
Table 1.
BRIGHT Feasibility
| N* (%) | |
|---|---|
| BRIGHT session length (median; IQR), minutes | 54; 5 |
| BRIGHT session completion | 45, 100 |
| Major technical issues during BRIGHT sessions | 0 (0) |
| Minor technical issues during BRIGHT sessions | 5 (11.1) |
| Tablet returned to study team | 10 (100) |
| Dropout | 1 (10) |
Abbreviations: IQR: Interquartile range
Data calculated for the n=9 participants who completed the study except for study dropout and tablet return (n=10 each), which reflect the patient who dropped out after the first BRIGHT session
Acceptability
Table 2 demonstrates the acceptability data. Eighty-nine percent of patients (8/9) moderately/strongly agreed that the timing of the program (starting 1 month after completion of cancer treatment) worked well. The delivery method was highly rated, as 89% of patients (8/9) strongly agreed that the telemedicine platform worked well. Overall satisfaction was high, as 89% of participants (8/9) reported that they were highly likely to recommend BRIGHT to other HNC survivors with BID.
Table 2.
Quantitative and Qualitative Evaluation of BRIGHT Acceptability
| Measure | Mean (SD)* | Illustrative Quotations from Semi-Structured Interviews |
|---|---|---|
| How well did the timing of the program work for you? | 4.44 (0.73) | “Better to have it soon after treatment so you don’t have to deal with these issues all by yourself and so you have some tools to help along the way.” (Subject 1) |
| How well did the method of program delivery work? | 4.67 (0.5) | “The iPad allowed me to do the study, otherwise I wouldn’t have been able to participate.” (Subject 3) “The iPad was really convenient. Nice to be able to do the sessions from the comfort of home- in comfortable clothes, without having to drive.” (Subject 6) “Telemedicine was a better option since I live far away. Even for patients who live in/near Charleston…the iPad option may be better.” (Subject 8) |
| How well did the number of sessions work for you? | 4.56 (0.53) | “After 5 sessions I wanted to keep going.” (Subject 9) “It took a while for me to open up fully and feel comfortable.” (Subject 10) |
| How relevant was the content of each session? | 4.56 (0.30) | “BRIGHT dealt effectively with what I was experiencing.” (Subject 7) |
| Session 1 (psychoeducation regarding BID) | 4.11 (1.27) | “The whole idea of talking about body image and how you should feel is a good idea, because people don’t know it’s something they should think about.” (Subject 9) |
| Session 2 (cognitive restructuring) | 4.44 (0.73) | |
| Session 3 (avoidance behaviors) | 4.56 (0.53) | “Before: you go out in public and you’re uncomfortable and people do a double-take…never got used to it. Now: go out into public more…I understand the thought processes of other people. Recently had an event at Red Lobster with friends and they treated me like normal and that was a big event for me.” (Subject 7) |
| Session 4 (coping strategies) | 4.89 (0.33) | |
| Session 5 (identifying personal value) | 4.78 (0.44) | |
| How likely are you to recommend BRIGHT? | 4.89 (0.33) |
Scale 0–5; higher scores indicate greater satisfaction.
At 1- and 3-months post-BRIGHT, eight of nine patients (89%; 95% CI 51%, 99%) and all nine patients (100%; 95% CI 63%, 100%) experienced a reduction in the severity of their BID, respectively (Figure 1). BRIGHT was associated with a 34.5% relative reduction in mean BIS scores at 1-month post BRIGHT relative to baseline (mean decrease of BIS scores from baseline to 1-month post-BRIGHT = 4.56; 95% CI 1.55 to 7.56). This clinical effect persisted at 3 months post-BRIGHT relative to baseline (mean decrease in BIS scores from baseline to 3-months post-BRIGHT = 3.56; 95% CI 1.15 to 5.96). BRIGHT was associated with improvements in the trouble with social eating and trouble with social contact subdomains of the EORTC-QLQHN35 (median trouble with social eating scores = 66.67, 45.83, and 25, at baseline, 1-, and 3- months post-BRIGHT respectively; median trouble with social contact scores = 40, 26.67, and 16.67 at baseline, 1-, and 3- months post-BRIGHT respectively; Supplemental Figure 2). BRIGHT was not associated with improvements in depression, anxiety, social isolation or shame and stigma post-treatment relative to baseline.
Figure 1:
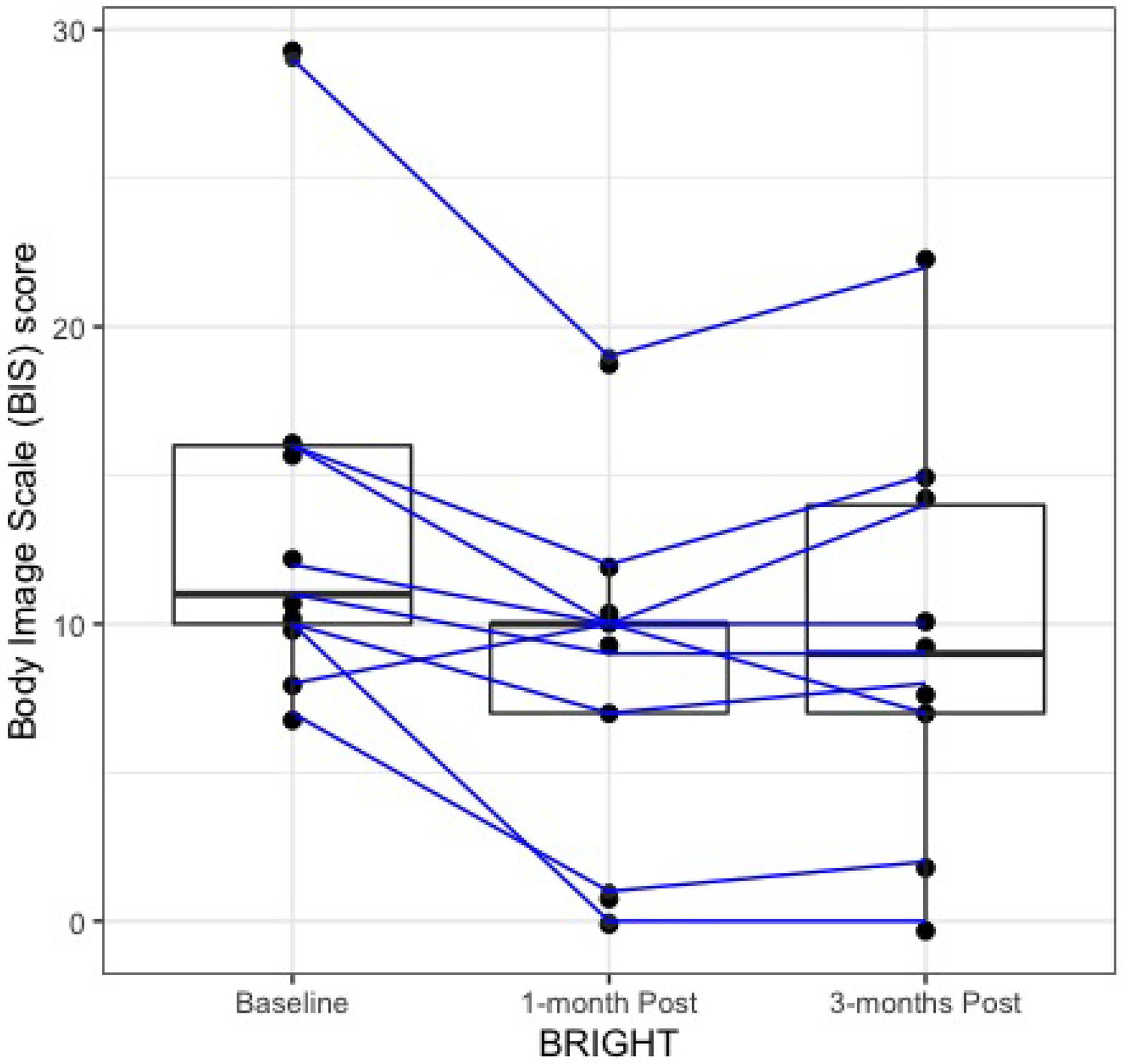
Decrease in the severity of body image disturbance (as determined by Body Image Scale scores) at 1-month and 3-months post-BRIGHT relative to baseline. The mean BIS scores at baseline, 1-month post, and 3-month post are 13.22, 8.67, and 9.76, respectively.
Semi-structured interviews with participants provided preliminary data suggesting that BRIGHT improved image coping behaviors (Table 3). As subject 4 reported, “BRIGHT gave me new tools to help cope with things that I didn’t know how to think about…being grounded with image issues when I have to go out in public to have the courage to do it and know how to cope with it.”
Table 3.
BRIGHT Improves Image-Related Coping Strategies Among HNC Survivors – Representative Quotations
| “I used the tools I learned during BRIGHT to get the courage to take a trip to visit my son….and held it together when others stared at me” (Subject 2) “BRIGHT brought attention to thinking about myself rather than just feeling sorry for myself” (Subject 3) “BRIGHT helped me find ways to handle changes in my appearance instead of just having a ‘pity party’” (Subject 6) “BRIGHT helped me handle uncomfortable situations to get back to activities.” (Subject 8) “BRIGHT gave me a lot of smart ideas/thoughts/techniques for how to deal with things and what to think about my body and the surgery” (Subject 9) |
Discussion
Even though BID is a critical issue for HNC survivors due to its prevalence, association with psychosocial morbidity, and negative impact on QOL4,6, effective treatments are lacking6,11,15. To our knowledge, BRIGHT is the first psychosocial intervention specifically developed to target BID in HNC survivors. Herein, we demonstrate that tablet-based BRIGHT is feasible to deliver and acceptable to HNC survivors with BID and provide preliminary data suggesting that BRIGHT has potential as a novel paradigm for treating BID in HNC survivors that may increase access to psychosocial care.
An important study finding is that delivery of BRIGHT to HNC survivors with BID using a tablet-based telemedicine platform was highly feasible and strongly preferred relative to face-to-face delivery. The pilot trial originally allowed patients to choose the method of BRIGHT delivery. The telemedicine delivery platform was vastly preferred over face-to-face delivery of BRIGHT (100% of patients traveling >25 miles (8/8); 67% (2/3) of patients traveling ≤ 25 miles) because of travel considerations, convenience, and flexibility. Numerous participants explained that participation in BRIGHT would have been impossible without the telemedicine platform due to competing time commitments, travel burden, treatment-related fatigue, and treatment toxicity. Our study expands upon prior studies showing that telemedicine decreases travel burden27, increases access to care28, and provides effective evidence-based interventions29 (including CBT30,31), and is thus an appealing strategy to improve care delivery for HNC survivors. Our tablet-based telemedicine platform has significant potential as a disseminatable and scalable method of delivering interventions to HNC survivors with broad significance and therapeutic implications beyond BID.
The feasibility of BRIGHT was supported by high rates of intervention completion. Although one patient dropped out due to lack of perceived relevance of BRIGHT, the nine participants who remained in the study completed 100% of their BRIGHT sessions and 100% of study tablets were returned following BRIGHT. Although we did experience some technical issues (e.g. problems with audio or video connectivity), they were all minor in nature and solved efficiently with a telephone call to the patient by the psychologist or program coordinator such that the BRIGHT session was not derailed. We attribute the low rate of observed technical problems to the simple tablet setup (Wi-Fi enabled; cleared of all other apps/content except Vidyo), ease of connecting to the video teleconference room (no user names, logins, or URLs necessary), hands-on tutorial about how to use the telemedicine application upon study enrollment, and supplemental pictorial instructional booklet for home reference.
These data also support the high levels of acceptability of BRIGHT to HNC survivors with BID. Although structured program evaluation with participants revealed favorable ratings for BRIGHT in terms of its timing (immediately post-treatment), others have proposed managing BID at different timepoints along the care continuum (e.g. prior to treatment7). Interventions to manage BID in breast cancer survivors have primarily focused on survivors many years post-treatment24,25. Although survivors who were within one-year post-treatment were eligible for our study, 100% of the patients who enrolled were within 5 months of completing therapy. Future studies may consider evaluating the feasibility and impact of BRIGHT at different timepoints along the care continuum to determine optimal timing.
Clinical Implications
These pilot data reflect the potential of BRIGHT as a novel approach to managing BID in HNC survivors. To our knowledge, effective interventions to manage BID in HNC survivors are lacking6,11,15. Cosmetic rehabilitation32 and skin camouflaging26 interventions for HNC-related BID, both of which focus on concealing disfigurement, are ineffective. Instead of attempting to manage HNC-related BID by concealing external disfigurement, BRIGHT proposes a paradigm shift by focusing on the key behavioral and cognitive challenges faced by HNC survivors with BID11. CBT produces durable reductions in the severity of BID in non-disfigured, non-oncologic patients (e.g. anorexia nervosa33), in part by addressing negative or distorted views about body image34. However, because HNC survivors have highly visible and socially significant functional impairments and disfigurement11, they face a different set of body image concerns. Our preliminary data, with high response rates and a large effect size suggest that psychosocial interventions (e.g. CBT) to treat BID in HNC patients merit further study. A recent prospective cohort study aiming to characterize the natural history of BID demonstrated that HNC survivors with body image concerns (i.e. a historical control for BRIGHT) show no improvement in BID (as measured by BIS scores) in the first 12-months post-treatment9. The large effect size of BRIGHT on BID observed in this study thus appears to be substantial and differs from the temporal trajectory of untreated BID in the target population.
Unfortunately limited psychometric data prevent us from making more definitive claims about the clinical significance of changes in BIS scores over time observed in this trial. In addition, although the BIS, is the most widely used PROM to study BID among HNC patients15, is not specifically validated in this population and may lack content validity. To enable further clinically-meaningful research on this topic, there is a critically important need to 1) develop and validate psychometrically robust PROMs of HNC-related BID (to ensure that clinicians and researchers are accurately measuring the disorder of interest); 2) develop rigorous quantitative definitions of BID among HNC survivors (to ensure precise definition of the population of interest for clinical trial eligibility); and 3) determine the minimal clinically important difference for a changes in the scores of the PROM over time at the individual and/or group level (to ensure that novel therapeutic approaches result in clinically-meaningful improvements).
Study Limitations
There are numerous limitations to this study. Although BID is thought to be common among HNC survivors7, the true prevalence is not known due to limitations of study design and validated PROMs with clinically relevant cutoff values in this population15. Consistent with its pilot nature, we had a small sample size, single-site, single-arm design. Significant additional research is necessary to evaluate BRIGHT in a larger sample, compare BRIGHT to a control group, and assess the feasibility, acceptability, and clinical impact of BRIGHT in diverse clinical and patient populations. Female HNC survivors were disproportionately represented in our pilot study. Whether this is due to chance alone in a small sample, the willingness of female HNC survivors to accept psychotherapy, or the underlying prevalence of BID in female HNC survivors is unclear and should be explored in future work. We also lack demographic and oncologic data about HNC survivors with BID who were eligible for the trial but declined. We therefore do not know whether they systematically differed from HNC survivors with BID who did accrue to the study, and if so, how.
Conclusions
BRIGHT is a novel tailored psychotherapeutic intervention that targets the cognitive, behavioral, and attitudinal components of HNC-related BID and is delivered using a tablet-based telemedicine platform to HNC survivors. BRIGHT is feasible and acceptable to HNC survivors with BID and has the potential to increase access to care and decrease psychosocial morbidity in this patient population. Additional research is necessary to evaluate fully the clinical efficacy of BRIGHT and identify accessibility and scalability solutions.
Supplementary Material
Acknowledgements:
This work was supported by the American Cancer Society (IRG-16-185-17), National Institutes of Health (P30CA138313, UL1TR000062), and HRSA of the U.S. Department of HHS (U66 RH31458-01-00).
Footnotes
Conflict of Interest: None.
Data Sharing: The study data are available from the corresponding author upon reasonable request.
References
- 1.American Cancer Society. Cancer Facts & Figures 2019. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2019.pdf. Accessed February 26, 2019.
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Head and Neck Cancers. Fort Washington, PA: National Comprehensive Cancer Network, 2019. [Google Scholar]
- 3.Cohen EE, LaMonte SJ, Erb NL et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin 2016; 66:203–239. [DOI] [PubMed] [Google Scholar]
- 4.Rhoten BA, Murphy B, Ridner SH. Body image in patients with head and neck cancer: a review of the literature. Oral Oncol 2013; 49:753–760. [DOI] [PubMed] [Google Scholar]
- 5.Katz MR, Irish JC, Devins GM, Rodin GM, Gullane PJ. Psychosocial adjustment in head and neck cancer: the impact of disfigurement, gender and social support. Head Neck 2003; 25:103–112. [DOI] [PubMed] [Google Scholar]
- 6.Fingeret MC, Teo I, Goettsch K. Body image: a critical psychosocial issue for patients with head and neck cancer. Curr Oncol Rep 2015; 17:422. [DOI] [PubMed] [Google Scholar]
- 7.Fingeret MC, Yuan Y, Urbauer D, Weston J, Nipomnick S, Weber R. The nature and extent of body image concerns among surgically treated patients with head and neck cancer. Psychooncology 2012; 21:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fingeret MC, Vidrine DJ, Reece GP, Gillenwater AM, Gritz ER. Multidimensional analysis of body image concerns among newly diagnosed patients with oral cavity cancer. Head Neck 2010; 32:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graboyes EM, Hill EG, Marsh CH et al. Temporal Trajectory of Body Image Disturbance in Patients with Surgically Treated Head and Neck Cancer. Otolaryngol Head Neck Surg 2020; 162:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teo I, Fronczyk KM, Guindani M et al. Salient body image concerns of patients with cancer undergoing head and neck reconstruction. Head Neck 2016; 38:1035–1042. [DOI] [PubMed] [Google Scholar]
- 11.Ellis MA, Sterba KR, Day TA et al. Body Image Disturbance in Surgically Treated Head and Neck Cancer Patients: A Patient-Centered Approach. Otolaryngol Head Neck Surg 2019; 161:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millsopp L, Brandom L, Humphris G, Lowe D, Stat C, Rogers S. Facial appearance after operations for oral and oropharyngeal cancer: a comparison of casenotes and patient-completed questionnaire. Br J Oral Maxillofac Surg 2006; 44:358–363. [DOI] [PubMed] [Google Scholar]
- 13.Rhoten BA, Deng J, Dietrich MS, Murphy B, Ridner SH. Body image and depressive symptoms in patients with head and neck cancer: an important relationship. Support Care Cancer 2014; 22:3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manne S, Badr H. Intimacy and relationship processes in couples’ psychosocial adaptation to cancer. Cancer 2008; 112:2541–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis MA, Sterba KR, Brennan EA et al. A Systematic Review of Patient-Reported Outcome Measures Assessing Body Image Disturbance in Patients with Head and Neck Cancer. Otolaryngol Head Neck Surg 2019; 160:941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliani M, McQuestion M, Jones J et al. Prevalence and nature of survivorship needs in patients with head and neck cancer. Head Neck 2016; 38:1097–1103. [DOI] [PubMed] [Google Scholar]
- 17.Eldredge L, Markham C, Ruiter R, Fernandez ME, Kok G, Parcel G. Planning Health Promotion Interventions: An Intervention Mapping Approach. San Francisco, CA: Jossey-Bass, 2016. [Google Scholar]
- 18.Graboyes EM, Hill EG, Marsh CH, Maurer S, Day TA, Sterba KR. Body Image Disturbance in Surgically Treated Head and Neck Cancer Patients: A Prospective Cohort Pilot Study. Otolaryngol Head Neck Surg 2019; 161:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer 2001; 37:189–197. [DOI] [PubMed] [Google Scholar]
- 20.Pilkonis PA, Choi SW, Reise SP et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment 2011; 18:263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn EA, DeWalt DA, Bode RK et al. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol 2014; 33:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kissane DW, Patel SG, Baser RE et al. Preliminary evaluation of the reliability and validity of the Shame and Stigma Scale in head and neck cancer. Head Neck 2013; 35:172–183. [DOI] [PubMed] [Google Scholar]
- 23.Bjordal K, de Graeff A, Fayers PM et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur J Cancer 2000; 36:1796–1807. [DOI] [PubMed] [Google Scholar]
- 24.Sherman KA, Przezdziecki A, Alcorso J et al. Reducing Body Image-Related Distress in Women With Breast Cancer Using a Structured Online Writing Exercise: Results From the My Changed Body Randomized Controlled Trial. J Clin Oncol 2018; 36:1930–1940. [DOI] [PubMed] [Google Scholar]
- 25.Esplen MJ, Wong J, Warner E, Toner B. Restoring Body Image After Cancer (ReBIC): Results of a Randomized Controlled Trial. J Clin Oncol 2018; 36:749–756. [DOI] [PubMed] [Google Scholar]
- 26.Chen SC, Huang BS, Lin CY et al. Psychosocial effects of a skin camouflage program in female survivors with head and neck cancer: A randomized controlled trial. Psychooncology 2017; 26:1376–1383. [DOI] [PubMed] [Google Scholar]
- 27.Wootton R, Bahaadinbeigy K, Hailey D. Estimating travel reduction associated with the use of telemedicine by patients and healthcare professionals: proposal for quantitative synthesis in a systematic review. BMC Health Serv Res 2011; 11:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doolittle GC, Spaulding AO. Providing Access to Oncology Care for Rural Patients via Telemedicine. J Oncol Pract 2006; 2:228–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agnisarman SO, Chalil Madathil K, Smith K, Ashok A, Welch B, McElligott JT. Lessons learned from the usability assessment of home-based telemedicine systems. Appl Ergon 2017; 58:424–434. [DOI] [PubMed] [Google Scholar]
- 30.van Beugen S, Ferwerda M, Hoeve D et al. Internet-based cognitive behavioral therapy for patients with chronic somatic conditions: a meta-analytic review. J Med Internet Res 2014; 16:e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson G, Cuijpers P, Carlbring P, Riper H, Hedman E. Guided Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: a systematic review and meta-analysis. World Psychiatry 2014; 13:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S, Liu HE. Effectiveness of cosmetic rehabilitation on the body image of oral cancer patients in Taiwan. Support Care Cancer 2008; 16:981–986. [DOI] [PubMed] [Google Scholar]
- 33.Jarry JL, Ip K. The effectiveness of stand-alone cognitive-behavioural therapy for body image: a meta-analysis. Body Image 2005; 2:317–331. [DOI] [PubMed] [Google Scholar]
- 34.Przezdziecki A, Sherman KA, Baillie A, Taylor A, Foley E, Stalgis-Bilinski K. My changed body: breast cancer, body image, distress and self-compassion. Psychooncology 2013; 22:1872–1879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


