Abstract
In the last twenty years an impressive body of evidence in diverse inflammatory animal disease models and human tissues, has established polyunsaturated fatty acids (PUFA) derived specialized-pro-resolving mediators (SPM), as essential mediators for controlling acute inflammation, immune responses and wound healing and for resolving acute inflammation in many non-ocular tissues. SPM pathways and receptors are highly expressed in the ocular surface where they regulate wound healing, nerve regeneration, innate immunity and sex-specific regulation of auto-immune responses. Recent evidence indicates that in the eye these resident SPM networks are important for maintaining ocular surface health and immune homeostasis. Here, we will review and discuss evidence for SPMs and other PUFA-derived mediators as important endogenous regulators and biomarkers of ocular surface health and disease and their therapeutic potential.
1. PUFA-derived Mediators
Bioactive lipids derived from polyunsaturated fatty acids (PUFA) are potent signaling molecules that regulate the initiation, amplification and termination of inflammatory responses. They are autocrine and paracrine mediators that are produced rapidly and are one of the earliest cellular responses following cell activation or tissue injury. PUFAs are major constituents of mammalian membranes, and once cleaved from phospholipids are classified based on carbon chain length and the number and position of double bonds [1, 2]. Several ocular surface cell types have both the enzymatic machinery to generate and also respond to PUFA derived bioactive lipids that control downstream immune responses. Several standard of care ocular therapies such as corticosteroids, NSAIDS, and PGF2α-analogs either amplify the actions or inhibit endogenous production of these PUFA-derived mediators, demonstrating that selective therapeutic targeting of lipid mediator pathways are effective for treating ocular diseases. In human studies PUFA-derived lipid mediator profiles more recently have been evaluated as markers for cell-specific functional responses in homeostasis, disease, infection and tissue repair [3–9]. Hence, they have the potential as biomarkers for assessing DED symptoms, infection, ocular surface discomfort or perhaps monitoring the efficacy of other ocular therapies.
Essential PUFAs include arachidonic acid (ω−6 AA), docosahexaenoic acid (ω−3 DHA) eicosapentaenoic acid (ω−3 EPA) and docosapentaenoic acid (ω−3 DPA), and can be metabolized by cyclooxygenases (COX), lipoxygenases (LOX), and monooxygenases (CYP450) enzymes into a broad range of structurally distinct and short-lived oxygenated lipid mediators that regulate and fine-tune inflammatory and immune responses [10, 11]. The actions of these highly conserved oxygenated lipid networks on mammalian cells is mediated to a large extent by G-protein coupled receptors (GPCR) that drive cell and tissue specific responses [12, 13]. PUFA-derived mediators are intrinsic signals that initiate, amplify and regulate routine inflammatory cascades such as vasodilation, edema, platelet clotting, fibrosis, host defense, the migration and recruitment of innate and adaptive immune cells into tissues and their local functional responses [11, 12].
The same COX and LOX enzymes which generate bioactive lipid mediators that promote inflammatory responses, also generate counterregulatory lipids that keep routine inflammatory events and activation of adaptive immune responses controlled. AA, DHA, EPA and DPA are substrates for a large class of bioactive lipid molecules that collectively have been classified as specialized pro-resolving mediators (SPMs) as they limit cellular infiltration, control leukocyte and effector lymphocyte activation and promote the return to tissue homeostasis. SPMs have garnered considerable interest as therapeutic targets as they have immunomodulatory properties, resolve acute inflammation and promote healing in several pre-clinical animal models [14–18]. Furthermore, in a variety of diseases, diminished SPM levels in human patient samples correlate with elevated inflammatory markers and poor disease outcomes [19–22]. Research efforts have primarily focused on the formation of SPMs during sterile, allergic and microbial-induced inflammation and the efficacy of individual SPMs or stable mimetics to limit inflammation and enhance tissue regeneration. However, emerging data provides compelling evidence that several SPMs are also produced in healthy tissue in the ocular surface, where they regulate routine innate and adaptive immune responses, wound healing and support immune homeostasis. For example AA-derived Lipoxin A4 (LXA4), the first SPM discovered in 1984 [23] was identified as an intrinsic SPM in the ocular surface in 2005 that maintains corneal homeostasis [24]. We will discuss how LXA4 as well as other DHA and EPA-derived SPMs are vital for preserving ocular surface health, by providing counterregulatory and homeostatic signals.
The surface of the eye is remarkable at actively restraining and controlling inflammatory immune responses in order to maintain and preserve vision. This inherent and highly evolved immune privilege is reinforced by the compartmentalization of distinct cell types in the cornea, conjunctiva and the tear forming glands, that each have specialized roles in supporting ocular surface health. The dysregulation of normal cellular responses in these compartments can lead to prolonged ocular surface inflammation, adaptive immunity and ultimately vision loss. Here, we will review the evidence that endogenously produced bioactive lipid mediators play an essential role in maintaining the health of the ocular surface and discuss the therapeutic potential of harnessing SPMs networks for treating a range of ocular diseases.
2. Homeostatic SPM Networks in the Ocular Surface
To maintain transparency the cornea is devoid of lymphatic vessels or blood flow and therefore has evolved intrinsic protective mechanisms in the absence of immune cell surveillance. A large body of work has established that SPMs have key roles in maintaining ocular surface homeostasis and protecting the cornea. SPMs generation is dependent on LOX enzymes which catalyze the stereo-specific oxygenation of PUFA substrates [25]. There are three primary and conserved LOX enzymes (5-LOX, 15-LOX and 12-LOX) that generate hydroperoxides [26]. In most tissues except for blood, two enzymes 15-LOX and 5-LOX catalyze the sequential oxygenation of PUFA substrates to generate a large family of SPMs [10, 12]. Neutrophils, eosinophils and macrophages all highly express 5-LOX and their interactions with platelets, endothelial and epithelial cells that express 15-LOX or 12-LOX was considered the primary (transcellular) route of SPM formation that is activated specifically during the resolution of acute inflammation. It is now apparent that several cell types express 5-LOX such as retinal astrocytes, mesenchymal stromal cells, and several epithelial cell types such as tuft cells and corneal epithelial cells [24, 27–29]. More importantly, some of these cells such as corneal epithelial cells and retinal astrocytes express both 5-LOX and 15-LOX enzymes, thus have the capacity to produce SPM as part of their homeostatic function [24, 27]. Corneal epithelial cells are the main source of endogenous SPMs production in the healthy cornea and the AA-derived LXA4 and the DHA-derived SPM neuroprotectin D1 (NPD1) are abrogated following removal of the epithelium [24].
The receptor for LXA4 and the DHA-homolog RvD1, FPR2/ALX is expressed by many cell types in the healthy cornea, conjunctiva and retina as well as by lymphocytes, macrophages, neutrophils and dendritic cells [30–33] again underscoring that endogenous SPMs networks are essential for maintaining ocular surface homeostasis. Formation of the EPA-derived SPM, RvE1, have not been documented in the ocular surface or tears of animal or human so far but the receptor is expressed in corneal epithelial cells, stromal keratocytes and infiltrating leukocytes [30]. Additional GPCR for other DHA- and DPA-derived SPM have been identified in mice and humans [10, 13, 34] and it is likely that they are also expressed by cells in the healthy ocular surface and/or infiltrating lymphocytes and leukocytes.
Endogenous 5-LOX and 15-LOX products also play critical roles in regulating corneal angiogenesis and re-epithelization following injury. Corneal neovascularization can lead to compromised visual acuity and occurs in a wide variety of corneal pathologies. In a model of chronic ocular inflammation, genetic deletion of either 5-LOX or 15-LOX increased corneal neovascularization, demonstrating that endogenous generation of LXA4 in the cornea is important for regulating the amplitude of inflammatory angiogenesis [35]. Furthermore, treating LOX knockout mice with topical LXA4 rescued mice from neovascularization demonstrating the potential of amplifying endogenous LXA4 generation in the cornea as a therapeutic approach. Additionally the efficacy of LXA4 analogs and of SPMs (RvD1 and RvE1) to inhibit angiogenesis has been demonstrated in suture and micropellet corneal neovascularization mouse models [30, 35].
Corneal epithelial regeneration is another fundamental and inherent function for maintaining ocular surface homeostasis. Progenitor cells in the limbus region replace shedding corneal epithelial cells by differentiating and migrating centripetally into the center of the cornea [36]. If the epithelium is injured, neutrophils which are normally precluded from the cornea, infiltrate the stroma within a few hours and are necessary for proper wound healing. Abrogation of these innate immune cells in animal models of epithelial abrasion significantly delays re-epithelization and wound closure [24, 37]. Estrogen downregulates both neutrophil responses and re-epithelialization in the cornea by suppressing SPM generation causing sex-specific delayed wound healing in females and in estradiol treated male mice [38, 39]. Topical treatment with LXA4 promotes wound healing in females and restores normal wound healing in estradiol treated males [38]. These studies demonstrated that sex steroid-regulation of a homeostatic corneal SPM networks can drive sex-specific difference in acute corneal wound healing responses.
Tear production is a critical component in maintaining ocular health, and SPMs and their metabolic precursors are present in significant quantities in healthy human tears [8, 40]. Using metabolo-lipidomics, significant concentrations of AA-derived lipid mediators were identified in emotional tears from 12 healthy individuals, including LXA4 and 15-epi LXA4, HETEs, prostaglandins (PGs), as well as DHA-derived HDHAs resolvins RvD1, RvD2 and RvD5 [40]. Interestingly this study also observed sex-specific differences in the bioactive lipids detected at the ocular surface. DHA-derived resolvins were present at higher concentrations in male tears while AA-derived LXA4 and 15-epi LXA4 were more abundant in female tears [40]. As this study profiled human emotional tears rather than basal tears, it indicates that tear SPMs may have originated from the aqueous compartment. Tears are comprised of three layers, the inner mucin layer, the middle aqueous layer and the outer lipid layer [41]. The tear lipid layer is primarily comprised of wax esters and cholesteryl esters produced by the meibomian glands which are fundamental for preventing the evaporation of the aqueous layer and in spreading tears across the surface of the eye. These nonpolar esters belong to a structurally different class of lipids than PUFAs and their more polar fatty acid derivatives. Due to their amphipathic properties, PUFA-derived lipid mediators are likely to be found primarily in the aqueous tear layer which is mainly produced by the lacrimal gland. The lacrimal gland has been shown in murine models to produce significant amounts of LXA4 [42], but it is currently unknown if LXA4 and prostaglandins that are produced locally in the lacrimal gland are secreted in tears. More research is needed to determine tissue and cellular origins of SPMs in tears, which may include a combination of corneal epithelial cells, lacrimal glands, meibomian glands or the conjunctiva.
Endogenous SPMs production is also interconnected with the ocular surface cytoprotective enzymes heme-oxygenases (HO-1 and HO-2). HO-1 and HO-2 are critical in heme catabolism and the generation of antioxidants and carbon monoxide gas. These enzymes also play a fundamental role in corneal wound healing [43]. HO-2 is constitutively expressed in the healthy cornea, while HO-1 is upregulated following injury or chronic inflammatory responses [44, 45]. Both HO-1 and HO-2 have immunomodulatory functions and genetic deletion results in spontaneously induced, non-resolving sterile inflammation characterized by sustained leukocyte infiltration and inadequate wound healing [46, 47]. There is a well-recognized connection between protective SPM and HO endogenous pathways in the cornea. Chronic ocular inflammation following epithelial injury in HO-2 deficient mice correlates with a 50% reduction in endogenous LXA4 and impaired HO-1 induction [48]. Furthermore, in 15-LOX knockout mice, HO-1 expression is impaired and corneal wound healing delayed, a phenotype that can be rescued with LXA4 treatment [48]. In vitro studies have also verified the connection between HO and SPM endogenous pathways. In human corneal epithelial cells, treatment with either 15-LOX-derived SPMs, LXA4 and NPD1 or a metabolic precursor 17-HDHA, amplified HO-1 expression [48]. These studies suggest an interdependence of two resident endogenous protective pathways, which are both essential for maintaining an anti-inflammatory or counterregulatory tone at the ocular surface.
3. Corneal Wound Healing and SPMs
The cornea is continuously exposed to the environment and thus at high risk for chemical and physical insults. The incidence of corneal abrasion in the United states is estimated to be 400,000 annually [49]. Furthermore, corneal wound healing is a significant clinical problem due to the increasing frequency of diabetic neuropathy, refractive surgeries, and corneal transplants performed in the United States [50]. Therefore, a better understanding of the underlying molecular mechanisms involved in corneal healing as well as the development of therapeutics that accelerate and improve outcomes are needed.
In vitro models have demonstrated the benefit of SPM treatments on accelerating corneal wound healing. In a human corneal epithelial scratch model, treatment with the EPA-derived RvE1 enhanced wound closure, which was comparable to that of the well-known protective epidermal growth factor (EGF) [51]. In rabbit corneal epithelial cells, epidermal growth factor induced LXA4 production suggesting that EGF broad mitogenic activities may include amplification of protective SPM production [52]. LXA4 also enhanced epithelial cell migration in a monolayer scratch model, and reversed delayed re-epithelization obstructed by estradiol treatment [38]. These in vitro studies demonstrate that SPMs mediate direct actions on corneal epithelial cells and promote corneal epithelial wound healing. Furthermore, corneal epithelial express GPCRs (ALX/FPR2 and ChemR23) for LXA4, RvD1 and RvE1 [30], but additional studies are needed to define their cellular mechanisms and signal transduction pathways for regulating corneal epithelial cell migration and/or proliferation.
Multiple preclinical animal models have demonstrated the protective bioactions of SPM treatment during corneal wound healing (Figure 1). Neutrophils are early responder to danger signals, and well known for their ability to phagocytose debris, microorganisms and secrete a wide swath of potent inflammatory proteins [53]. However, neutrophils are also essential for proper wound healing, and their ability to abundantly produce SPMs is often overlooked as a key function for regulating subsequent immune responses [31, 53, 54]. Neutrophils have the capacity to amplify or initiate formation of multiple SPMs due their high expression of 5-LOX [55] making these innate cells vital in controlling downstream adaptive immune responses. At ocular homeostasis, neutrophils are excluded from the cornea; however, they do reside in the limbus, lacrimal gland, tears and lymph nodes that drain the ocular surface [42, 56, 57]. The unique role of neutrophils in the ocular surface is further evident by the large number of fully functional neutrophils that are present in human nocturnal tears every night without causing any tissue damage or clinical inflammation [56]. Following corneal injury, neutrophils are among the first effector cells to infiltrate the stroma. This innate neutrophilic response is vital for proper wound healing, but their sustained presence due to dysregulated immune responses in the cornea can lead to collateral tissue damage [58].
Figure 1: SPMs maintain ocular surface health and restore tissue homeostasis.
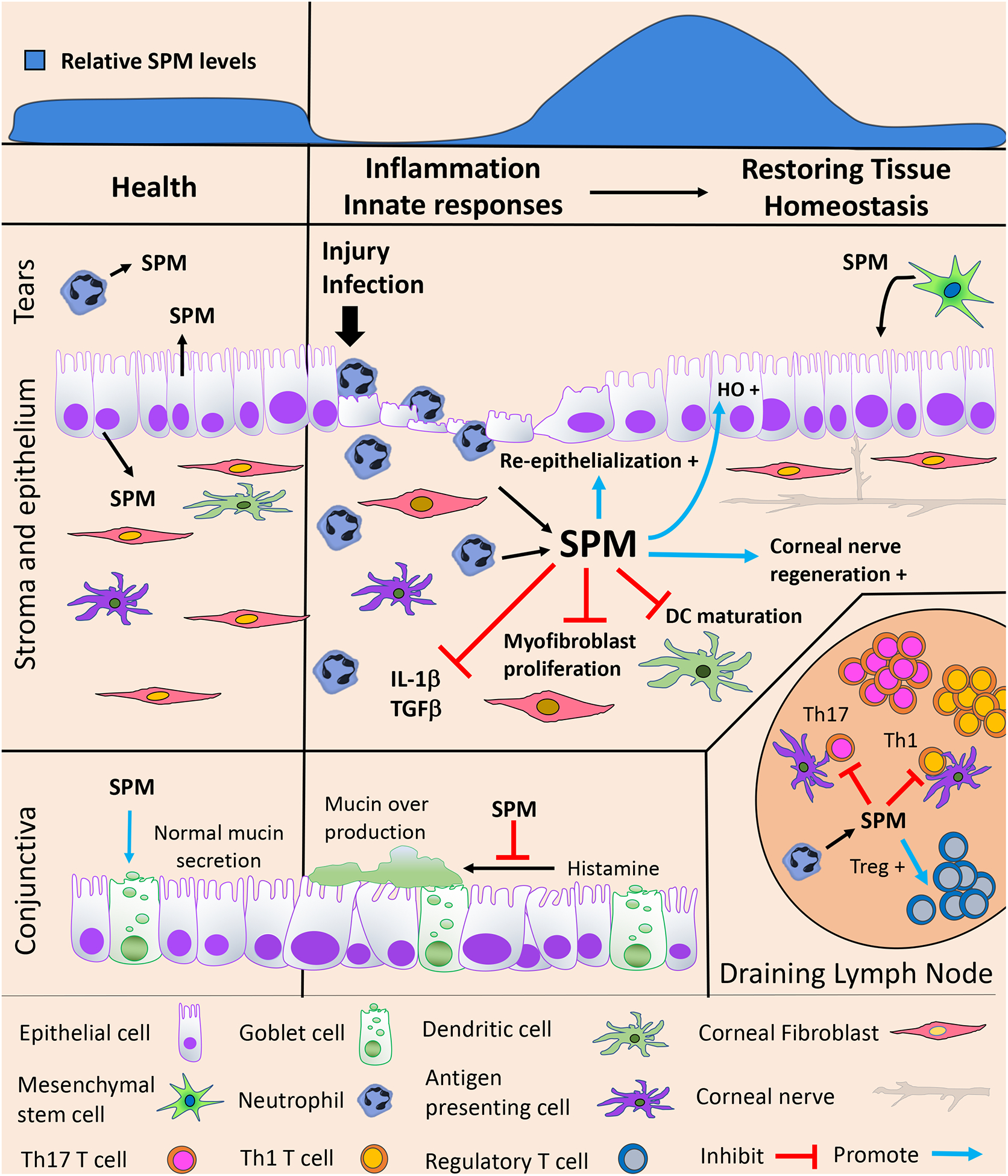
During ocular homeostasis tears, cornea, conjunctiva and lymph nodes contain a basal tone of SPMs. SPMs can be produced by corneal epithelial cells and potentially tear neutrophils, which express required biosynthetic enzymes 5-LOX and 15-LOX. Cognate SPM receptors (not shown) are expressed on corneal and conjunctival epithelium, DC, fibroblasts, neutrophil, APC and lymphocytes. Following corneal injury or infection, activated neutrophil are rapidly recruited to the ocular surface and amplify formation of SPMs, which can inhibit DC maturation, myofibroblast proliferation and IL-1β and TGFβ production. SPMs also promote corneal wound healing by enhancing re-epithelialization, heme-oxygenase expression and corneal nerve regeneration. Mesenchymal stem cells (MSC), which have immune-regulatory and wound healing properties can produce SPMs. In the healthy conjunctiva, SPMs promote normal mucin secretion by goblet cells, however, during allergic responses exogenous SPM treatment reduces histamine mediated production of mucins. In draining lymph nodes SPM control APC interaction with lymphocytes and directly act on effector T cells to inhibit Th1 and Th17 and enhance Treg responses.
A hallmark bioaction for most SPMs is the inhibition of neutrophil adhesion and migration during acute inflammation. However, in acute and self-resolving corneal abrasion injuries topical treatment with LXA4 or NPD1 significantly increases neutrophils and these beneficial neutrophils promote wound healing [24]. The source and exact phenotype of these wound healing neutrophils remains to be defined but it underscores that the cornea is a privileged tissue in terms of inflammation, wound healing and leukocyte functional responses.
Treatment with SPM enhances the protective and limits pathogenic functions of other cell types such as keratocytes and counter-regulates proinflammatory signals in the cornea following ocular trauma [59]. Fibroblasts located in the stroma upregulate the secretion of extracellular matrix proteins during wound healing, providing a necessary scaffolding for rebuilding of the tissue [60]. In a model of corneal trauma, epithelial abrasion followed by treatment with the proinflammatory lipid platelet activating factor (PAF), caused delayed wound closure with reduced fibronectin deposition and elevated metalloproteases and inflammatory cytokines [59]. Co-administration of LXA4 in conjunction with PAF enhanced fibronectin production by fibroblast in the corneal stroma and significantly reduced inflammatory markers, resulting in less corneal injury [59]. The effect of LXA4 on fibroblasts have also been investigated using a rabbit corneal 3D in vitro model. Isolated corneal fibroblasts treated with LXA4 generated less IL-1β and metalloproteases, and their degradation of collagen was reduced [61]. SPM treatment is also beneficial for reducing chronic inflammation and neovascularization at the ocular surface. Subconjunctival treatment with DHA-derived RvD1, EPA-derived RvE1 or AA-derived 15-epi LXA4 reduced neovascularization and levels of IL-1α, IL-1β and TNFα [30]. Together these in vivo animal studies demonstrate that resident SPM networks in the cornea have multipronged protective bioactions for limiting inflammation and promoting wound healing to restore homeostasis.
It is important to note that multiple therapeutics traditionally used in the clinic specifically target bioactive lipid mediator pathways in the eye [62]. Surprisingly, despite the fact that topical NSAIDS and PGF2α analogs are effective and standard treatments for ocular surface diseases such as trauma and glaucoma, little is known about the endogenous formation, cell specific expression of receptors and role of prostanoids in the ocular surface. In vitro studies, human corneal epithelial cells (HCEs) can upregulate many of the enzymes needed to generate a wide variety of lipid mediators including leukotrienes, prostanoids and SPMs [63–65]. Following UVB induced oxidative stress, HCEs upregulate or induce mRNA expression of COX-2, prostaglandin and thromboxane synthases (mPGES-2, PGDS, PGFS and TXAS) and lipoxygenases (5-LOX, 15-LOX-2, 12-LOX) [63]. Receptors for AA-derived prostaglandin E2 (PGE2) are expressed in the cornea (EP1–4) but acute inflammation such as abrasion injury does not lead to robust formation of PGE2 in the mouse cornea nor does PGE2 effect corneal wound healing [64]. The corneal PGE2 circuit appears to be activated selectively during chronic inflammation where it promotes corneal neovascularization [64]. It is of interest that BLT2 a receptor for the COX pathway metabolite 12-HHT is expressed in corneal epithelial cells and the conjunctiva [65]. BLT2 KO mice have a phenotype of delayed wound healing, and as 12-HHT formation is inhibited by NSAIDS, this study provides a potential mechanism for the side effect of delayed wound healing that is associated with the use of topical NSAID treatment after ocular surgery [65].
BLT2 was originally identified as a low affinity receptor for the AA-derived lipid mediator, leukotriene B4 (LTB4). LTB4 is a potent neutrophil and lymphocyte chemoattractant, and a primary mediator of inflammation whose inflammatory actions are mediated primarily by the LTB4 receptor BLT1. In vitro studies have shown that corneal epithelial cells have the capacity to produce leukotriene LTB4 following LPS exposure [66] and several studies have demonstrated LTB4 in human tears of contact lens wearers [67]. However, LTB4 formation is not associated with the presence of large number of neutrophils in nocturnal tears or neutrophil stromal infiltration after an epithelial abrasion injury [24]. Hence, despite the expression of BLT1 in the ocular surface and by neutrophils and lymphocytes the role of LTB4 in ocular surface inflammation remains to be defined.
4. Harnessing SPM’s Protective Actions in Corneal Transplantation
Corneal transplantation is the most frequent type of transplantation worldwide and about 180,000 corneal transplants are performed each year [68]. Graft recipients frequently exhibit inflamed and vascularized corneas which increases graft failure rates despite maximal treatment with nonspecific immunosuppressive medications. There is evidence that SPM treatment of donor corneas may reduce graft rejection by enhancing the viability of the graft during preservation. Human corneas stored in 100nM of 15-epi LXA4 overnight before transferring to Optisol-GS had a 36–56% increased viability compared to control grafts [69]. The overnight pre-storage of corneal tissue in the 15-epi LXA4 solution increased the subsequent epithelial cell proliferation in the graft [69]. Consistent with immune-regulatory actions of ALX/FPR2 and its SPM ligands (LXA4 and RvD1), systemic treatment with RvD1 inhibits graft T cell infiltration and allosensitization, which enhances graft survival and suppresses inflammatory angiogenesis in mouse corneal transplant models [33]. While more research is needed, data with human corneas and mouse studies provide compelling evidence that SPM have the potential to improve graft viability and suppress initiation of alloimmunity, which are primary risk factors for graft rejection in the clinic.
5. SPM Treatment Promotes Corneal Nerve Regeneration
The cornea is the most innervated tissue in the body and corneal nerves are an essential component of maintaining ocular surface health. Several studies have demonstrated that the DHA-derived SPM NPD1 can promote corneal nerve regeneration. In animal models, treatment with NPD1 is neuroprotective in the brain and retina [70]. As a topical eye drop treatment NPD1 induces nerve regeneration in mice following corneal and stromal nerve injury [71] and in a rabbit lamellar keratectomy model [72]. Pigment epithelial-derived factor (PEDF) is a broad acting neuroprotective factor that strongly stimulates the synthesis of NPD1 by 15-LOX from its precursor DHA. A combination of PEDF and DHA applied topically following lamellar keratectomy, stimulated NPD1 generation and increased nerve density and corneal epithelial cell proliferation [72–74]. These studies demonstrated that therapeutic amplification of endogenous SPM networks improves nerve regeneration in the stroma and cornea. Recent identification of an SPM receptor (ALX/FPR2) in retinal ganglion cells and primary cortical neurons indicates that SPM can have direct action with neuronal cells [27]. The molecular mechanisms for how DHA-derived SPMs improve nerve regeneration and functional activity needs to be further investigated.
Diabetic eye diseases are a significant health problem, ocular problems include retinopathy, decreased corneal sensitivity, increasing risk for corneal infections and impaired wound healing [75]. The incidence of diabetes has risen significantly affecting approximately 9% of the US population and 28% of adult diabetics have diabetic retinopathy; therefore, new therapeutics are urgently needed [76]. Preclinical animal studies support the notion that DHA treatment amplifies local SPM networks in the cornea. In a mouse model of diabetes, the combination of PEDF and DHA was investigated for the treatment of nerve regeneration following corneal injury [77]. Co-administration of PEDF and DHA significantly increased corneal nerve regeneration, sensitivity and tear production in wounded corneas. PEDF and DHA treatment also accelerated corneal wound healing by selective recruitment of wound healing type 2 macrophages [77]. Treatment with the DHA-derived SPM (RvD1) alone and in a ALX/FPR2 dependent fashion, demonstrated efficacy in stimulating corneal nerve growth in a diabetic mouse model [78]. Hence, these studies provide proof of concept that amplification of DHA-derived SPM networks are potential topical therapies for treating corneal diabetic neuropathy and stimulating nerve regeneration.
6. PUFA-Derived Mediators and the Conjunctiva
The conjunctiva is a mucosal tissue that that contains innate immune cells and specialized goblet cells interspersed throughout stratified squamous cells [79]. Goblet cells are responsible for the synthesis and secretion of mucins into tears that help maintain eyelid lubrication, preventing desiccation and protecting the ocular surface from the external environment [79]. These cells are an important component of innate ocular immune responses and respond to inflammatory stimuli [80]. Dysregulated goblet cell function and mucin secretion in allergic responses is associated with increased ocular pathology [81].
Conjunctivitis is a common ocular surface disease caused by viral or bacterial infection, allergies or autoimmune diseases [82]. During allergic responses, the release of histamine by mast cells stimulates goblet cell intracellular Ca2+ release and enhances mucin secretion in the conjunctiva[80]. Leukotrienes (LTs) and prostaglandins (PGs) also stimulate rat goblet cell secretion in vitro [83]. High expression of epidermal lipoxygenase has been observed in conjunctiva of the eyelid [84] and in a viral induced conjunctivitis murine model, 5-LOX inhibitors reduced cysteinyl leukotrienes (CysLTs) and alleviated disease pathology [85]. Furthermore the CysLT receptors CysLT1 and CysLT2 are expressed in the rat conjunctiva and in human conjunctival goblet cells indicating leukotriene signaling is a component of mucosal physiology/pathophysiology in the ocular surface [83]. Prostaglandins also appear to play a role in the conjunctiva. The PGE2 receptor EP3 is constitutively expressed in mice conjunctival epithelium [86]. In a ragweed allergic challenge model EP3 knockout mice had significantly increased eosinophil infiltration in the conjunctiva compared with wild-type mice, which indicates that PGE2 signaling is necessary for controlling innate granulocytic responses in allergic conjunctivitis [86]. Together these studies provide strong evidence that eicosanoids production and signaling in the conjunctiva is a feature of ocular allergic immune responses.
In vitro experiments with primary rat and human conjunctival goblet cells have demonstrated that several SPM, including LXA4, RvD1, RvD2 and RvE1, can down regulate histamine triggered Ca2+ signaling and mucin secretion [87, 88]. Interestingly in the absence of an allergic agonists all SPM appear to also trigger Ca2+ signaling and mucin secretion in cultured conjunctival goblet cells similar to the bioaction of histamine and CysLTs [89, 90]. These conflicting findings may suggest that SPM have a homeostatic function for maintaining normal mucin secretion, in addition to counter-regulating histamine or leukotriene bioactions [91]. Multiple and complex signaling pathways that regulate mucin secretion have been identified for SPMs in cultured goblet cells, providing evidence for bioactions of SPM in the conjunctiva. Furthermore, receptors for LXA4/RvD1 (ALX/FPR2) and RvE1 (ChemR23) are expressed in conjunctival goblet cells [83, 90]. One study has investigated the actions of SPM treatment in vivo, and demonstrated that topical RvD1 treatment was effective in alleviating allergic conjunctivitis in a ovalbumin induced murine model [92]. Even though topical RvD1 alone did not alter systemic adaptive immunity induced by ovalbumin immunization, topical RvD1 treatment significantly improved symptoms of AED, including reducing mucin secretion, and the total number of conjunctival immune cells.
7. PUFA-Derived Mediators in Tears, Ocular Discomfort and DED in humans
The majority of studies examining lipid mediators in human tears have investigated their changes or presence in Dry Eye Disease (DED) or following contact lens wear. Contact lenses can mechanically modify the corneal surface, impact normal physiology and many individuals often report ocular discomfort [93]. There is evidence that presence of lipid mediators in tears of contact lens wearers may signify subclinical inflammation. Elevated LTB4 was observed in human tears from individuals experiencing red eye following lens wear and correlated with increased inflammatory cytokines, which may indicate contact lens induced neutrophil responses [67]. LTC4 was also detected in tears from symptomatic patients wearing contact lens compared to asymptomatic patients [94]. However, other studies that observed PGs and LTs in human tears, did not observe a difference in the levels of bioactive lipid concentrations between individuals who wore contact lenses compared to controls [95]. These conflicting results could be due to differences in sample processing and the primary reliance on antibody-based quantification of lipid mediators. Standardization of methods for processing tear samples and direct physical identification and quantification by LC/MS- based lipidomics has the potential to advance our understanding of tear lipid mediator levels in health, disease and how it is impacted by contact lens wear. Overall a small number of studies suggest that SPM and lipid mediators that drive leukocyte, allergy and pain response are present in human tears and thus can potentially be utilized as biomarkers for ocular inflammation, infection or discomfort which are potential risk factors associated with contact lens wear.
Dry Eye Disease (DED) is a multifactorial disease that causes debilitating morbidity in 20 million people worldwide and is the most common reasons for seeking eye care [96]. DED has a wide spectrum of etiologies including ocular discomfort, fatigue and visual disturbances resulting in significant morbidity for patients. Clinical studies in humans have observed significant differences between fatty acid lipid concentrations in the tears from DED patients compared to controls [8, 97]. One study, measured tear PG levels in DED patients and healthy volunteers whose ocular surface inflammation symptoms were assessed by questionnaire [97]. PGs are of particular interest as they often have opposing bioactions and their formation is inhibited by topical NSAIDS and corticosteroids. PGE2 levels in the tears of DED patients were significantly higher than in healthy subjects, whereas PGD2 concentration was significantly lower in DED patients compared to controls. The tear PGE2 to PGD2 ratio correlated strongly with scores in the questionnaire and indicated that high levels of PGE2 and low levels of PGD2 may be a marker of ocular surface inflammation in DED [97]. The physiological or pathophysiological roles and formation of PGE2 or PGD2 in the ocular surface remain to be explored. Tear lipid mediators and PUFA have been analyzed by LC/MS/MS-based lipidomics in a prospective 18 months clinical study of forty one individuals with DED [8]. The ratio of ω−6 (AA) to ω−3 (DHA+EPA) PUFA were quantified along with multiple measures of tear film dysfunction. PGE2 was detected in the majority of samples and correlated with low tear osmolarity, meibomian gland plugging, and corneal staining. Analysis also identified an increased ratio of omega-6 to omega-3 tear lipids that correlated with an increased tear film dysfunction and corneal staining in DED [8].
Supplementation with DHA and EPA has also been investigated in many DED clinical studies and there is epidemiological evidence that patients with ocular diseases improve when adding incorporating PUFAs into their treatment regimen [98, 99]. Increased dietary intake of ω−3 PUFA correlates with a decreased incidence of dry eye syndrome in a well-characterized population of women participating in the Women’s Health Study [98]. Furthermore, dietary DHA or topical administration appears to be a safe and effective treatment for DED [99]. However, a recent large clinical study indicates dietary supplementation with high doses of ω−3 PUFA as an adjunct treatment provides no additional clinical benefit for patients that are receiving standard treatment of care for active DED [100]. It is important to point out that compelling epidemiological data establishes dietary ω−3 PUFA as a protective factor in the prevalence, severity and development of DED. Hence, questions remain about long-term benefits and role of essential dietary ω−3 PUFA in the etiology of DED.
Although studies with human dietary ω−3 PUFA, which assessed their potential therapeutic in active DED versus their essential role in the etiology are contradictory, there is compelling evidence in preclinical models that treatment with protective SPMs derived from ω−3 and ω−6 PUFAs alleviate immune-driven DED. Animal studies further discussed below; have prompted EPA-derived RvE1 analogs (RX-10045) to be evaluated in human clinical trials. A phase 2 clinical trial assessed the safety and efficacy of RX-10045 applied as an ophthalmic solution reported positive outcomes on the signs and symptoms of dry eye (ClinicalTrials.gov, NCT01675570, 2012). RX-10045 has also been assessed for reducing anterior inflammation following cataract surgery (ClinicalTrials.gov NCT02329743 2018). 8 days following surgery, in both arms receiving RX-10045, 23% of patients had cleared anterior inflammation compared to 17% of patients in the placebo group. Furthermore, the patients treated with RX-10045 reported less ocular pain. These clinical trials support further development of RvE1 analogs and potentially other SPM analogs as topical treatments for DED and ocular surface inflammation.
8. SPMs Alleviate DED in Preclinical Animal Models
Preclinical animal models of immune-driven DED provide evidence that treatment with SPMs or amplification of endogenous SPM formation are effective monotherapies for reducing DED and can restore exocrine gland function in Sjögren’s Disease [31]. In the standard mouse model of DED, treatment with an analog of the EPA-derived RvE1 reduced corneal barrier permeability and loss of goblet cells [101]. A separate study demonstrated that topical treatment with RvE1 analogs increased tear flow, promoted healthy epithelial barrier integrity, decreased COX-2 expression and inhibited CD4+ T cell and macrophage infiltration [102]. In addition to ocular surface disease models, animal models in other organ systems have generated strong evidence for protective and immune regulatory actions of RvE1 and RvE1 analogs in preclinical animal models [10, 13, 15, 103], which let the first phase 1 and phase 2 clinical trials described in section 7.
Systemic treatment with DHA-derived RvD1 in murine DED model, restored saliva secretion in both male and female mice [104]. Topical application of the ω−3 PUFA α-linoleic acid (ALA), a metabolic precursor for EPA and DHA, in the mouse DED model demonstrated significant reduction in clinical and inflammatory markers of DED [105]. Even though ALA is an essential ω−3 PUFA precursor for the formation of EPA and subsequently DHA the conversion is not efficient in human (0–9%) [106]. A recent study investigated the effect of dietary DHA in DED. DHA dietary deficiency for 3 months markedly exacerbate clinical DED symptoms in the mouse DED model [57]. Acute DHA treatment rescued females on the DHA deficient diet from amplified DED by increasing tear volume, upregulating total numbers of Treg and reducing Th1 and Th17 effector cells in draining lymph nodes [57]. The mechanism of DHA’s protective effect, unexpectedly, included upregulation of 15-LOX expressing neutrophils in lymph nodes and corneal limbus which led to amplified LXA4 tissue levels in draining lymph nodes and cornea. The production of LXA4 by recently identified regulatory neutrophils in the lymph nodes are an endogenous pathway that regulates development and amplitude of adaptive T cell responses, which are triggered by ocular surface desiccating stress [42]. More importantly, reduced regulatory neutrophil numbers and suppressed LXA4 formation in draining lymph nodes has been identified as a sex-specific response that amplifies T effector cell functional responses and DED disease in female mice [42]. In summary, animal studies have established that 1) omega-3 PUFA and endogenous SPM networks in lymph nodes are protective in immune-driven DED, 2) sex-specific downregulation of the resident lymph node LXA4 circuit is linked to amplified DED in females and 3) therapeutic amplification of SPM is effective in limiting desiccating stress induced adaptive immune responses and DED in mice.
9. Protective Action of SPMs in Ocular Surface Infections
The actions of SPM in limiting infectious diseases is of considerable interest as they limit collateral damage of activated leukocytes, amplify phagocytosis of bacteria and apoptotic neutrophil, increase formation of bactericidal peptides and increase efficacy of antibiotics [10, 107, 108]. Current standard treatment of care for various ocular infections currently rely on antibiotics and or immunosuppressive drugs for treatment of chronic inflammatory conditions. However, these therapies do not aid in healing damaged ocular tissue. SPM networks as a treatment of ocular infections may be a new approach for reducing destructive infectious inflammation, preventing corneal opacity and scarring, increase topical antibiotic efficacy and enhance innate ocular surface host defense.
SPM treatment has been shown to be effective at reducing ocular surface damage following viral infection. Herpes simplex virus type-1 (HSV-1) infection leads to impaired corneal sensation and in severe cases corneal ulceration, melting and perforation. Topical administration of an RvE1 analog reduced corneal neovascularization and stromal keratitis lesions following HSV-1 infection and significantly reduced T cells and neutrophil numbers in the cornea [109]. In another study PEDF and DHA administered in combination following HSV-1 infection resulted in fewer dendritic corneal lesions, opacity and neovascularization [110]. These studies highlight that treatment with EPA- and DHA-derived SPMs can ameliorate corneal pathology following viral infection.
Pseudomonas aeruginosa is a clinically important, opportunistic bacterial pathogen and one of the leading causes of microbial keratitis in the US, especially in contact lens wearers [111]. Ocular P. aeruginosa infection induces robust corneal epithelial inflammatory responses and the release of PGE2 [112], which can result in permanent damage to the ocular surface. Topical treatment with RvE1 in a murine model following administration of bacterial LPS or heat kill whole P. aeruginosa, reduced stromal thickness, corneal apoptosis, cellular infiltrates and production of CXCL1, TNFα and IL-1β [113]. In a model of wound healing and bacterial keratitis, topical treatment of corneal abrasion wounds with LPS significantly amplified inflammation and reduced re-epithelialization [48]. Topical treatment with LXA4 reduced exacerbated inflammation by reducing corneal CXCL1 levels and amplified inflammatory neutrophil infiltration while increasing re-epithelialization by 83% [48]. In rat model of endotoxin induced anterior uveitis treatment with LXA4 or LXA4 analogues reduced clinical inflammation score and cellular infiltrates in the aqueous humor [114]. Although most of these studies did not use live bacteria, they provide strong evidence that SPMs as adjuvant therapy could potentially inhibit pathogenic inflammation in ocular microbial infections without impairing innate host defense while promoting tissue regeneration.
15-LOX expression in the corneal epithelium is important for host defense and protecting the cornea during Pseudomonas aeruginosa infection [24]. It is well known that C57BL/6 mice are highly susceptibility to Pseudomonas aeruginosa infection and develop severe keratitis whereas BALB/c mice are refractory to symptoms following bacterial infection [115]. Lipidomics analysis from these two mouse lines revealed divergent lipid profiles of the corneal epithelium following P. aeruginosa inoculation [116]. Five days post infection C57BL/6 mice had elevated bacterial loads and COX2 products including PGE2 and PGD2 and perforated corneas. Conversely, BALB/c mice had lower bacterial loads and ocular damage that correlated with increased 15-LOX expression and levels of the SPM pathway markers 14-HDHA and 17-HDHA in the cornea [116]. When 15-LOX was genetically deleted, the bacterial burden dramatically increased and lead to corneal perforation [116]. This illustrates that an imbalance of 15-LOX enzymatic pathway at the ocular surface can contribute to dysregulated immune responses resulting in elevated susceptibility to bacterial infection.
Several studies have also established that SPMs stimulate antimicrobial activities at epithelial surfaces. Bactericidal permeability increasing protein (BPI) production in intestinal epithelium was enhanced following treatment with an LXA4 analogue and was shown to decrease S. typhimurium bacterial burden [108]. Additionally, RvE1 treatment of gut epithelial cells induces intestinal alkaline phosphatase, which detoxifies bacterial LPS in the gut [117]. These studies show that SPM treatment can enhance natural host defense against bacterial pathogens.
The administration of SPMs has been explored as a treatment for both viral and bacterial infections. Treatment with an isomer of NPD1 reduced influenza infection in mice and improve survival even when administered as late as 48 hours after infection [118]. LXA4 analogues also reduce bacterial burden and pathogen associated inflammation. Periodontitis is caused by an overgrowth of resident Gram-negative bacteria in the oral cavity and mucosal inflammation [119]. In Porphyromanas gingivalis infection models the introduction of stable analogues of LXA4 result in a reduction of neutrophil recruitment to the site of infection [120]. Administration of an LXA4 analog in mice challenged intratracheally with P. aeruginosa reduced neutrophil infiltration, weight loss, and bacterial burden [121]. These studies indicate that SPM treatment could be helpful in targeting bacterial pathogens and lessening of disease severity. Furthermore, supplementing antimicrobial therapies with SPMs might be a synergistic approach for combating ocular infections. For example, RvD1 treatment in combination with antibiotics, was shown to protect mice in Escherichia coli infection model by enhancing host antimicrobial responses and accelerating the resolution of inflammation compared to antibiotics alone [107]. Together this body of work suggests that SPM treatment could be beneficial in alleviating tissue damage and disease pathology associated with ocular infections.
However, it is important to note SPM treatment may not always be advantageous for combating all infections. For example the pathogens Mycobacterium tuberculosis and Toxoplasma gondii favor the generation of LXA4 and P. aeruginosa express a 15-LOX enzyme, most likely to promote immune evasion [122, 123]. The fungal pathogen Candida albicans can biosynthesize its own RvE1 that limits IL-8 mediated neutrophil infiltration in the host enabling colonization [124]. These examples illustrate the complicated relationship between the host response and microbial adaptation and survival in an inflammatory environment. Further work is needed to understand how either exogenous administration or host SPM production controls immune responses to pathogens at the ocular surface.
10. New Cellular Players in SPM Signaling in the Ocular Surface
Mast cells are sentinel cells that reside in the ocular tissue in the peripheral cornea, limbus and conjunctiva, and their central role in the initiation of ocular surface inflammatory immune responses has recently been discovered [125, 126]. Following corneal transplantation in mice, mast cells are recruited to the ocular surface and promote Th1 and APC responses that can lead to graft rejection [127]. Ablation of mast cells in the mouse transplant model resulted in reduced graft rejection, indicating that these cells play a role in regulating adaptive immune response at the ocular surface [127]. Furthermore, inhibition of mast cells in a corneal wound model, reduced CXCL2, IL1β and TNFα expression and prevented early neutrophil recruitment and ocular inflammation [128]. It appears that mast cells are early effector cells that regulate inflammation and neutrophil recruitment to injured corneas.
Investigations modeling disease in other tissues, have demonstrated that mast cells respond to SPM treatment. Treatment of Human lung mast cells in vitro with RvD1, RvD2 or LXA4 attenuated histamine release, with RvD1 having the most potent bioactions [129]. In an allergic murine model, LXB4 treatment resulted in less mast cell IgE mediated degranulation and reduced airway inflammation [130]. Bone marrow-derived mast cells have been reported to express FPR2 [131]; however, additional studies are needed to confirm this finding in tissue resident cells. Hence, multiple lines of evidence have established that mast cell function can be regulated by SPM in other tissues. Mast cell are a prominent source of several lipid mediators such CysLTs and PGD2 [132]. However, if mast cells can themselves produce protective SPMs is still unknown. Future studies are required to explore the endogenous production of lipid mediators by mast cells and the potential regulation of functional responses of mast cells in the ocular surface by SPM.
Mesenchymal stromal cells (MSCs) are multipotent stem cells that can migrate to damaged tissue sites, and participate in regeneration and repair [133]. MSCs have substantial immunoregulatory functional activity and have been reported to modulate both innate and adaptive immune responses [133]. Generating MSCs for therapeutic use in ocular disorders and tissue repair is currently an area of active investigation [134]. In a murine corneal transplant model, animals receiving MSCs treatment had enhanced graft survival and reduced leukocyte infiltration and APC maturation [135]. IV administration of MSCs following corneal abrasion in mice reduced Ly6G+ CD11b+ neutrophil infiltration and MPO concentrations at the ocular surface [135]. Topical application of cornea-derived MSCs to injured murine corneas promoted macrophage CD206 expression and enhanced their wound healing activity [136]. Immunoregulatory MSCs properties are mainly attributed to the secretion of immunomodulatory factors [68] and many studies have demonstrated that MSC-derived exosomes also display regenerative functions [137]. Exosome-derived from MSCs contains an abundance of miRNAs that regulate inflammatory processes and investigations have shown these vesicles are effective in driving corneal cell wound healing [68, 138].
The precise bioactions of how MSCs mediate tissue repair are not well defined and less is known about the endogenous lipid profile produced by these cells. One investigation demonstrated that human MSCs express 15-LOX and have the capacity to produce LXA4 [139]. This observation indicates that MSCs immunoregulatory properties may be mediated in part by the secretion of SPMs. In the same study, LXA4 production by human MSCs was enhanced when cultured in the presence of three proinflammatory cytokines IL-1β, TNFα and IFNγ [139]. These findings support other studies that demonstrated MSC immunoregulatory functions are not constitutively active, but induced or licensed by inflammatory cytokines, such as those in the inflammatory microenvironment [140]. Similarly, unpublished data from the Gronert laboratory identified increased release of LXA4 in cytokine activated human MSCs by targeted LC/MS/MS-based lipidomics. Moreover, unexpectedly, we identified 350-times increase in a second lipoxin, LXB4, indicating this SPM is a primary product of activated MSCs in vitro (Fig 2). LXB4 is of particular interest as it recently was identified as a novel protective signal that is released by homeostatic retinal astrocytes [27]. LXB4 displayed neuroprotective activity by directly acting on retinal ganglion cells and primary cortical neurons and in vivo increased retinal ganglion cells survival and function in murine models of glaucoma and neurotoxic stress [27]. Formation and actions of LXB4 in the ocular surface have not been investigated but there is a body of evidence demonstrating that this SPM is protective in several mouse models of inflammatory diseases [130, 141]. Future studies are needed that investigate if MSCs release SPM in vivo and if these lipid signals are part of MSC’s protective mechanisms at the ocular surface.
Figure 2: Cytokine activation induces MSC secretion of SPMs.
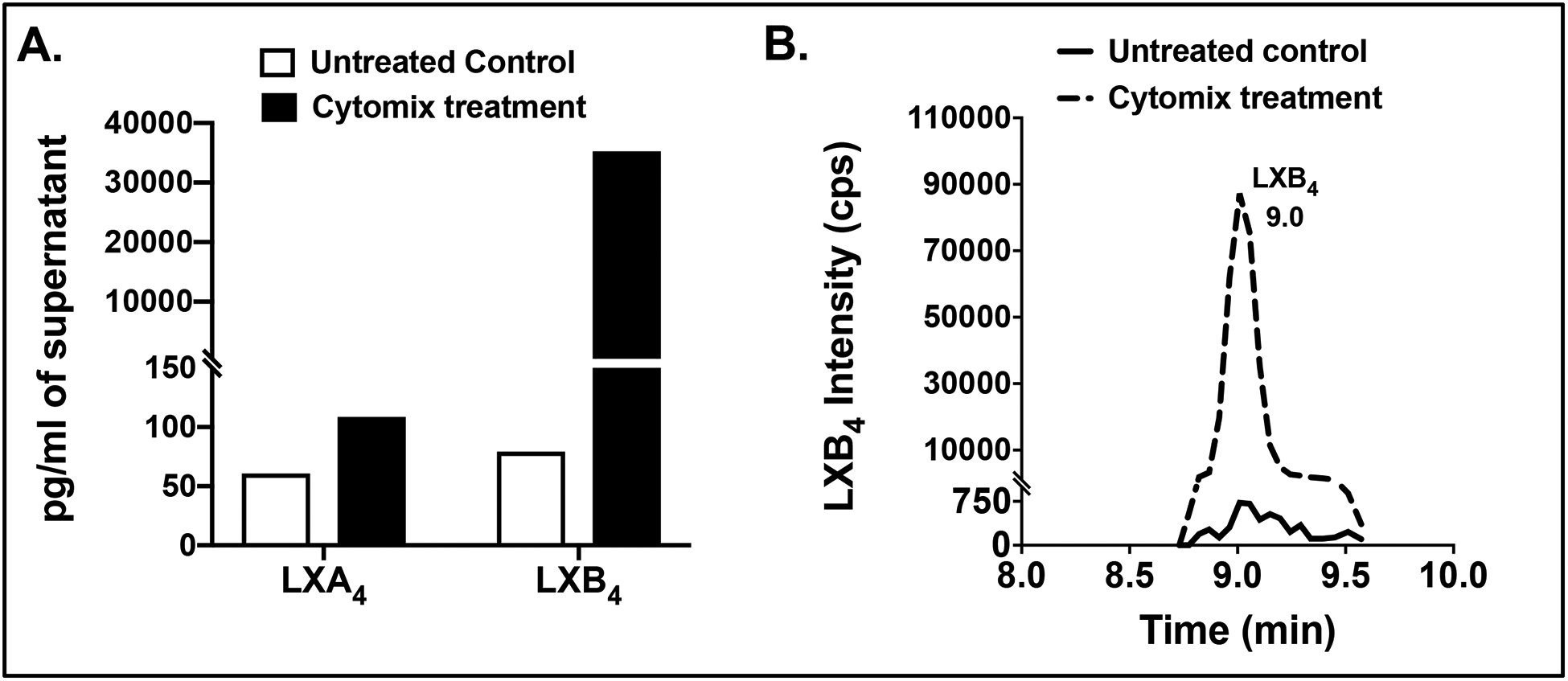
(A) LXA4 and LXB4 were identified and measured by LC/MS/MS lipidomics [27] in supernatants from human MSCs (3.5×105). MSC were activated with cytomix (IL-1β, TNFα and IFNγ 50ng/ml) for 24 hours prior to collection of supernatents. (B) Representative multiple reaction monitoring LC/MS/MS chromatograms for LXB4 (m/z 351.2 > 163) from untreated and cytomix activated MSCs.
Other cell types at the ocular surface such as fibroblasts and dendritic cells have been shown in other tissues to be regulated by SPMs, enhancing their pro-resolving functions. However, if these cell types respond to SPMs at the ocular surface has yet to be determined. Following corneal injury, differentiated myofibroblasts generate collagens and matrix proteins necessary for proper cellular tissue rebuilding [60]. However, robust proliferation or dysregulation of myofibroblast functions lead to disorganized extracellular matrix scaffolding deposition resulting in corneal haze and fibrosis [142]. TGFβ production by corneal keratinocytes promotes myofibroblast differentiation and investigators have focused on reducing the production of this cytokine to treat corneal fibrosis [142]. Emerging evidence in other tissue models indicates SPMs may be valuable for use as anti-fibrotic therapeutics by inhibiting myofibroblast production of matrix proteins [116]. An in vitro airway cellular model, fibroblasts cultured in the presence of TGFβ upregulate FPR2/ALX and respond to LXA4 [116]. Exogenous addition of LXA4 also slowed myofibroblast proliferation in a scratch model and enhanced wound closure [143, 144]. In an airway model of inflammation, systemic administration of 15-epi LXA4 reduced collagen deposition and inhibited lung production of TGFβ, IL-1β, IL-17 and TNFα [145]. These studies establish that SPM alter fibroblast function in several tissues, however, whether endogenous SPM or treatment with SPM limits corneal fibrosis or scaring remains to be investigated.
Dendritic cells (DCs) which are located in the stroma and conjunctiva and are professional antigen presenting cells (APC) in the cornea that mediate T cell responses and the induction of immune tolerance [146]. DC responses at the ocular surface are vital for maintaining homeostasis and in the initiation of downstream immune responses after acute injury [147]. However, in many ocular surface disorders such as herpes keratitis, DED, diabetes and corneal graft rejection, prolonged activation or maturation of DCs can lead to permanent damage and vision loss [148, 149]. SPMs have been shown to have direct actions on DC functions. LXA4 treatment in a Toxoplasma gondii infection model reduces DC migration and IL-12 production [150]. RvE1 also reduces DC migration in a contact dermatitis [151] model and has been shown to suppress production of IL-6 and IL-23, two cytokines important for driving Th17 responses [103]. Therefore, endogenous SPM in the cornea may potentially have significant roles in controlling DC activation and maturation. Hence, therapeutic amplification of SPM networks at the ocular surface may reduce pathogenic DC activity that can drive chronic inflammation and adaptive immune responses.
Dysregulated execution of routine and frequent functional responses of mast cells, dendritic cells and fibroblasts can drive chronic inflammation and fibrosis leading to scarring and disease pathology at the ocular surface [149]. A body of work has established that SPM treatment in several non-ocular disease models counter-regulates and control aberrant activation of mast cells, dendritic cells and fibroblasts, yet, it remains to be determined if these cell-specific SPM actions are conserved in the ocular surface.
Summary
Lipid mediators are potent immune rheostats for inflammation, wound healing and tissue homeostasis. PUFA-derived mediators are central in both orchestrating rapid cellular infiltration to sites of injury and limiting the initiation and amplitude of adaptive immune responses. In the last twenty years an impressive body of evidence in diverse inflammatory animal disease models and human tissues, has established the PUFA-derived lipid mediators, SPMs, as essential mediators for controlling acute inflammation, immune responses and wound healing and for restoring and maintaining tissue homeostasis. Studies in the eye have expanded the roles of SPMs not only in wound healing, nerve regeneration, innate immunity and sex-specific regulation of auto-immune responses but also demonstrated that resident SPM networks in the ocular surface are important for maintaining ocular surface health and immune homeostasis. Several research groups have established that enzymes for SPM generation are highly expressed in corneal epithelial cells and leukocytes in the ocular surface. More importantly, receptors for the SPM’s LXA4, RvD1 and RvE1 are functionally expressed in resident or infiltrating ocular surface cell types including epithelial cells, goblet cells, neutrophils, macrophages and effector T cells, underscoring the relevance of SPM networks in ocular health and diseases. Pre-clinical animal studies have established proof of concept that SPM treatments are effective in limiting pathogenesis of inflammation, microbial keratitis, allergic conjunctivitis and autoimmune responses while promoting wound healing and nerve regeneration. Several key cell types in the ocular surface such as mast cells, fibroblasts and dendritic cells are established targets for SPM treatment in other tissues where they abate their activation and overexuberant inflammatory functions. Lastly, MSCs which promote wound healing at the ocular surface, have the capacity to generate SPMs, offering new insight to this promising new avenue for treating ocular wound healing and inflammatory disease.
SPMs like most eicosanoid are autocrine and paracrine acting cell signals that are rapidly inactivated by cells in vitro. Structural analogs have been designed for several SPMs to resist metabolic inactivation and to increase their bioavailability. An impressive body of work has established the efficacy of SPMs and SPM analogs at very low doses in preclinical animal models [10, 12, 13, 15, 107, 141, 152]. However, there are always challenges for meeting industry level drug production and stability standards, which may have delayed moving more SPMs or their structural analogs from bench to bedside. Identification of several GPCR that mediate the protective actions of SPMs should also enabled a targeted and standard industry approach for identifying and optimizing small molecules as SPM mimetics. Several drug companies have invested in developing small molecule FPR2/ALX agonists for treating immune/inflammatory diseases [153].
Given that SPM intrinsic pathways are highly expressed in the ocular surface and in view of compelling data that have established their protective actions and homeostatic functions in the eye, SPMs are therapeutic targets and potentially useful biomarkers for ocular health and disease.
Acknowledgments
Research from the authors that was cited in this review was supported in part by the following grants from the National Institutes of Health: EY026082, EY022208, and EY016136 to KG and NHLBI HL134828 to MAM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts to report: None
References
- [1].de Carvalho C, Caramujo MJ. The Various Roles of Fatty Acids. Molecules. 2018;23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Radzikowska U, Rinaldi AO, Celebi Sozener Z, Karaguzel D, Wojcik M, Cypryk K, et al. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Becares N, Harmala S, China L, Colas RA, Maini AA, Bennet K, et al. Immune Regulatory Mediators in Plasma from Patients with Acute Decompensation are Associated With 3-month Mortality. Clin Gastroenterol Hepatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fosshaug LE, Colas RA, Anstensrud AK, Gregersen I, Nymo S, Sagen EL, et al. Early increase of specialized pro-resolving lipid mediators in patients with ST-elevation myocardial infarction. EBioMedicine. 2019;46:264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shivakoti R, Dalli J, Kadam D, Gaikwad S, Barthwal M, Colas RA, et al. Lipid mediators of inflammation and Resolution in individuals with tuberculosis and tuberculosis-Diabetes. Prostaglandins Other Lipid Mediat. 2020;147:106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Souza PR, Marques RM, Gomez EA, Colas RA, De Matteis R, Zak A, et al. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ Res. 2020;126:75–90. [DOI] [PubMed] [Google Scholar]
- [7].Kalish BT, Le HD, Fitzgerald JM, Wang S, Seamon K, Gura KM, et al. Intravenous fish oil lipid emulsion promotes a shift toward anti-inflammatory proresolving lipid mediators. Am J Physiol Gastrointest Liver Physiol. 2013;305:G818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A. omega-3 Tear Film Lipids Correlate With Clinical Measures of Dry Eye. Investigative ophthalmology & visual science. 2016;57:2472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Norris PC, Skulas-Ray AC, Riley I, Richter CK, Kris-Etherton PM, Jensen GL, et al. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Scientific reports. 2018;8:18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. 2017;58:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krishnamoorthy N, Abdulnour RE, Walker KH, Engstrom BD, Levy BD. Specialized Proresolving Mediators in Innate and Adaptive Immune Responses in Airway Diseases. Physiol Rev. 2018;98:1335–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med. 2017;58:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Borgeson E, Johnson AM, Lee YS, Till A, Syed GH, Ali-Shah ST, et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab. 2015;22:125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat Med. 2002;8:1018–23. [DOI] [PubMed] [Google Scholar]
- [17].Krashia P, Cordella A, Nobili A, La Barbera L, Federici M, Leuti A, et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat Commun. 2019;10:3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bannenberg G, Moussignac RL, Gronert K, Devchand PR, Schmidt BA, Guilford WJ, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Flitter BA, Hvorecny KL, Ono E, Eddens T, Yang J, Kwak DH, et al. Pseudomonas aeruginosa sabotages the generation of host proresolving lipid mediators. Proc Natl Acad Sci U S A. 2017;114:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ho KJ, Spite M, Owens CD, Lancero H, Kroemer AH, Pande R, et al. Aspirin-triggered lipoxin and resolvin E1 modulate vascular smooth muscle phenotype and correlate with peripheral atherosclerosis. Am J Pathol. 2010;177:2116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Colas RA, Nhat LTH, Thuong NTT, Gomez EA, Ly L, Thanh HH, et al. Proresolving mediator profiles in cerebrospinal fluid are linked with disease severity and outcome in adults with tuberculous meningitis. FASEB J. 2019;33:13028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984;81:5335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. The Journal of biological chemistry. 2005;280:15267–78. [DOI] [PubMed] [Google Scholar]
- [25].Newcomer ME, Brash AR. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015;24:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mashima R, Okuyama T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015;6:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Livne-Bar I, Wei J, Liu HH, Alqawlaq S, Won GJ, Tuccitto A, et al. Astrocyte-derived lipoxins A4 and B4 promote neuroprotection from acute and chronic injury. The Journal of clinical investigation. 2017;127:4403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alaseem AM, Madiraju P, Aldebeyan SA, Noorwali H, Antoniou J, Mwale F. Naproxen induces type X collagen expression in human bone-marrow-derived mesenchymal stem cells through the upregulation of 5-lipoxygenase. Tissue Eng Part A. 2015;21:234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, et al. Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018;49:33–41 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Investigative ophthalmology & visual science. 2009;50:4743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wei J, Gronert K. The role of pro-resolving lipid mediators in ocular diseases. Mol Aspects Med. 2017;58:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wei J, Mattapallil MJ, Horai R, Jittayasothorn Y, Modi AP, Sen HN, et al. A novel role for lipoxin A4 in driving a lymph node-eye axis that controls autoimmunity to the neuroretina. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hua J, Jin Y, Chen Y, Inomata T, Lee H, Chauhan SK, et al. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Investigative ophthalmology & visual science. 2014;55:5944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Flak MB, Koenis DS, Sobrino A, Smith J, Pistorius K, Palmas F, et al. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. The Journal of clinical investigation. 2020;130:359–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Leedom AJ, Sullivan AB, Dong B, Lau D, Gronert K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010;176:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yazdanpanah G, Jabbehdari S, Djalilian AR. Limbal and corneal epithelial homeostasis. Curr Opin Ophthalmol. 2017;28:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Li Z, Burns AR, Smith CW. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Investigative ophthalmology & visual science. 2006;47:1947–55. [DOI] [PubMed] [Google Scholar]
- [38].Wang SB, Hu KM, Seamon KJ, Mani V, Chen Y, Gronert K. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012;26:1506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Krishnan AV, Swami S, Feldman D. Estradiol inhibits glucocorticoid receptor expression and induces glucocorticoid resistance in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol. 2001;77:29–37. [DOI] [PubMed] [Google Scholar]
- [40].English JT, Norris PC, Hodges RR, Dartt DA, Serhan CN. Identification and Profiling of Specialized Pro-Resolving Mediators in Human Tears by Lipid Mediator Metabolomics. Prostaglandins, leukotrienes, and essential fatty acids. 2017;117:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Experimental Eye Research. 2004;78:347–60. [DOI] [PubMed] [Google Scholar]
- [42].Gao Y, Min K, Zhang Y, Su J, Greenwood M, Gronert K. Female-Specific Downregulation of Tissue Polymorphonuclear Neutrophils Drives Impaired Regulatory T Cell and Amplified Effector T Cell Responses in Autoimmune Dry Eye Disease. J Immunol. 2015;195:3086–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gronert K. Resolution, the grail for healthy ocular inflammation. Exp Eye Res. 2010;91:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Halilovic A, Patil KA, Bellner L, Marrazzo G, Castellano K, Cullaro G, et al. Knockdown of heme oxygenase-2 impairs corneal epithelial cell wound healing. J Cell Physiol. 2011;226:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Patil K, Bellner L, Cullaro G, Gotlinger KH, Dunn MW, Schwartzman ML. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Investigative ophthalmology & visual science. 2008;49:3379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Seta F, Bellner L, Rezzani R, Regan RF, Dunn MW, Abraham NG, et al. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169:1612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Biteman B, Hassan IR, Walker E, Leedom AJ, Dunn M, Seta F, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. Faseb j. 2007;21:2257–66. [DOI] [PubMed] [Google Scholar]
- [49].Ramirez DA, Porco TC, Lietman TM, Keenan JD. Ocular Injury in United States Emergency Departments: Seasonality and Annual Trends Estimated from a Nationally Representative Dataset. Am J Ophthalmol. 2018;191:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40:136–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang F, Yang H, Pan Z, Wang Z, Wolosin JM, Gjorstrup P, et al. Dependence of resolvin-induced increases in corneal epithelial cell migration on EGF receptor transactivation. Investigative ophthalmology & visual science. 2010;51:5601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kenchegowda S, Bazan NG, Bazan HE. EGF stimulates lipoxin A4 synthesis and modulates repair in corneal epithelial cells through ERK and p38 activation. Investigative ophthalmology & visual science. 2011;52:2240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang J, Hossain M, Thanabalasuriar A, Gunzer M, Meininger C, Kubes P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science. 2017;358:111–6. [DOI] [PubMed] [Google Scholar]
- [54].Wei J, Gronert K. Eicosanoid and Specialized Proresolving Mediator Regulation of Lymphoid Cells. Trends in biochemical sciences. 2019;44:214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Radmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends in biochemical sciences. 2007;32:332–41. [DOI] [PubMed] [Google Scholar]
- [56].Gorbet M, Postnikoff C, Williams S. The Noninflammatory Phenotype of Neutrophils From the Closed-Eye Environment: A Flow Cytometry Analysis of Receptor Expression. Investigative ophthalmology & visual science. 2015;56:4582–91. [DOI] [PubMed] [Google Scholar]
- [57].Gao Y, Su J, Zhang Y, Chan A, Sin JH, Wu D, et al. Dietary DHA amplifies LXA4 circuits in tissues and lymph node PMN and is protective in immune-driven dry eye disease. Mucosal immunology. 2018;11:1674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Arafat SN, Robert MC, Abud T, Spurr-Michaud S, Amparo F, Dohlman CH, et al. Elevated Neutrophil Elastase in Tears of Ocular Graft-Versus-Host Disease Patients. Am J Ophthalmol. 2017;176:46–52. [DOI] [PubMed] [Google Scholar]
- [59].Kakazu A, He J, Kenchegowda S, Bazan HE. Lipoxin A(4) inhibits platelet-activating factor inflammatory response and stimulates corneal wound healing of injuries that compromise the stroma. Exp Eye Res. 2012;103:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Petroll WM, Miron-Mendoza M. Mechanical interactions and crosstalk between corneal keratocytes and the extracellular matrix. Exp Eye Res. 2015;133:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhou HY, Hao JL, Bi MM, Wang S, Zhang H, Zhang WS. Molecular mechanism of the inhibition effect of Lipoxin A4 on corneal dissolving pathology process. International journal of ophthalmology. 2013;6:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Black AT, Gordon MK, Heck DE, Gallo MA, Laskin DL, Laskin JD. UVB light regulates expression of antioxidants and inflammatory mediators in human corneal epithelial cells. Biochemical pharmacology. 2011;81:873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Liclican EL, Nguyen V, Sullivan AB, Gronert K. Selective activation of the prostaglandin E2 circuit in chronic injury-induced pathologic angiogenesis. Investigative ophthalmology & visual science. 2010;51:6311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Iwamoto S, Koga T, Ohba M, Okuno T, Koike M, Murakami A, et al. Non-steroidal anti-inflammatory drug delays corneal wound healing by reducing production of 12-hydroxyheptadecatrienoic acid, a ligand for leukotriene B4 receptor 2. Scientific reports. 2017;7:13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Sahin A, Kam WR, Darabad RR, Topilow K, Sullivan DA. Regulation of leukotriene B4 secretion by human corneal, conjunctival, and meibomian gland epithelial cells. Archives of ophthalmology (Chicago, Ill : 1960). 2012;130:1013–8. [DOI] [PubMed] [Google Scholar]
- [67].Thakur A, Willcox MD. Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases. Exp Eye Res. 1998;67:9–19. [DOI] [PubMed] [Google Scholar]
- [68].Mansoor H, Ong HS, Riau AK, Stanzel TP, Mehta JS, Yam GH. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].He J, Kakazu AH, Bazan NG, Bazan HE. Aspirin-triggered lipoxin A4 (15-epi-LXA4) increases the endothelial viability of human corneas storage in Optisol-GS. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2011;27:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Asatryan A, Bazan NG. Molecular mechanisms of signaling via the docosanoid neuroprotectin D1 for cellular homeostasis and neuroprotection. The Journal of biological chemistry. 2017;292:12390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kenchegowda S, He J, Bazan HE. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cortina MS, He J, Russ T, Bazan NG, Bazan HE. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Investigative ophthalmology & visual science. 2013;54:4109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Cortina MS, He J, Li N, Bazan NG, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Investigative ophthalmology & visual science. 2010;51:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].He J, Cortina MS, Kakazu A, Bazan HE. The PEDF Neuroprotective Domain Plus DHA Induces Corneal Nerve Regeneration After Experimental Surgery. Investigative ophthalmology & visual science. 2015;56:3505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular Complications of Diabetes and Therapeutic Approaches. Biomed Res Int. 2016;2016:3801570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fitch K, Weisman T, Engel T, Turpcu A, Blumen H, Rajput Y, et al. Longitudinal Commercial Claims-Based Cost Analysis of Diabetic Retinopathy Screening Patterns. Am Health Drug Benefits. 2015;8:300–8. [PMC free article] [PubMed] [Google Scholar]
- [77].He J, Pham TL, Kakazu A, Bazan HEP. Recovery of Corneal Sensitivity and Increase in Nerve Density and Wound Healing in Diabetic Mice After PEDF Plus DHA Treatment. Diabetes. 2017;66:2511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhang Z, Hu X, Qi X, Di G, Zhang Y, Wang Q, et al. Resolvin D1 promotes corneal epithelial wound healing and restoration of mechanical sensation in diabetic mice. Molecular vision. 2018;24:274–85. [PMC free article] [PubMed] [Google Scholar]
- [79].Gipson IK. Goblet cells of the conjunctiva: A review of recent findings. Prog Retin Eye Res. 2016;54:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Dartt DA, Masli S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr Opin Allergy Clin Immunol. 2014;14:464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Saban DR, Calder V, Kuo CH, Reyes NJ, Dartt DA, Ono SJ, et al. New twists to an old story: novel concepts in the pathogenesis of allergic eye disease. Curr Eye Res. 2013;38:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Azari AA, Barney NP. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310:1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011;186:4455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Funk CD, Keeney DS, Oliw EH, Boeglin WE, Brash AR. Functional expression and cellular localization of a mouse epidermal lipoxygenase. The Journal of biological chemistry. 1996;271:23338–44. [DOI] [PubMed] [Google Scholar]
- [85].Musiyenko A, Correa L, Stock N, Hutchinson JH, Lorrain DS, Bain G, et al. A novel 5-lipoxygenase-activating protein inhibitor, AM679, reduces inflammation in the respiratory syncytial virus-infected mouse eye. Clin Vaccine Immunol. 2009;16:1654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ueta M. Regulation of ocular surface inflammation by prostaglandin E receptor subtype EP3. Cornea. 2010;29 Suppl 1:S57–61. [DOI] [PubMed] [Google Scholar]
- [87].Li D, Hodges RR, Jiao J, Carozza RB, Shatos MA, Chiang N, et al. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal immunology. 2013;6:1119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Lippestad M, Hodges RR, Utheim TP, Serhan CN, Dartt DA. Resolvin D1 Increases Mucin Secretion in Cultured Rat Conjunctival Goblet Cells via Multiple Signaling Pathways. Investigative ophthalmology & visual science. 2017;58:4530–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hodges RR, Li D, Shatos MA, Serhan CN, Dartt DA. Lipoxin A4 Counter-regulates Histamine-stimulated Glycoconjugate Secretion in Conjunctival Goblet Cells. Scientific reports. 2016;6:36124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hodges RR, Li D, Shatos MA, Bair JA, Lippestad M, Serhan CN, et al. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal immunology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Dartt DA, Hodges RR, Serhan CN. Immunoresolvent Resolvin D1 Maintains the Health of the Ocular Surface. Adv Exp Med Biol. 2019;1161:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Saban DR, Hodges RR, Mathew R, Reyes NJ, Yu C, Kaye R, et al. Resolvin D1 treatment on goblet cell mucin and immune responses in the chronic allergic eye disease (AED) model. Mucosal immunology. 2019;12:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Willcox MD. Is There a Role for Inflammation in Contact Lens Discomfort? Eye Contact Lens. 2017;43:5–16. [DOI] [PubMed] [Google Scholar]
- [94].Sengor T, Irkec M, Gulen Y, Taseli M, Erker H. Tear LTC4 levels in patients with subclinical contact lens related giant papillary conjunctivitis. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 1995;21:159–62. [PubMed] [Google Scholar]
- [95].Masoudi S, Zhao Z, Stapleton F, Willcox M. Contact Lens-Induced Discomfort and Inflammatory Mediator Changes in Tears. Eye Contact Lens. 2017;43:40–5. [DOI] [PubMed] [Google Scholar]
- [96].Sullivan DA, Hammitt KM, Schaumberg DA, Sullivan BD, Begley CG, Gjorstrup P, et al. Report of the TFOS/ARVO Symposium on global treatments for dry eye disease: an unmet need. The ocular surface. 2012;10:108–16. [DOI] [PubMed] [Google Scholar]
- [97].Shim J, Park C, Lee HS, Park MS, Lim HT, Chauhan S, et al. Change in prostaglandin expression levels and synthesizing activities in dry eye disease. Ophthalmology. 2012;119:2211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr. 2005;82:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cortina MS, Bazan HE. Docosahexaenoic acid, protectins and dry eye. Current opinion in clinical nutrition and metabolic care. 2011;14:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dry Eye A, Management Study Research G, Asbell PA, Maguire MG, Pistilli M, Ying GS, et al. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N Engl J Med. 2018;378:1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].de Paiva CS, Schwartz CE, Gjorstrup P, Pflugfelder SC. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31:1299–303. [DOI] [PubMed] [Google Scholar]
- [102].Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26:431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Dean S, Wang CS, Nam K, Maruyama CL, Trump BG, Baker OJ. Aspirin Triggered Resolvin D1 reduces inflammation and restores saliva secretion in a Sjogren’s syndrome mouse model. Rheumatology (Oxford). 2019;58:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Archives of ophthalmology (Chicago, Ill : 1960). 2008;126:219–25. [DOI] [PubMed] [Google Scholar]
- [106].Stark AH, Crawford MA, Reifen R. Update on alpha-linolenic acid. Nutr Rev. 2008;66:326–32. [DOI] [PubMed] [Google Scholar]
- [107].Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, et al. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, et al. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci U S A. 2002;99:3902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rajasagi NK, Reddy PB, Suryawanshi A, Mulik S, Gjorstrup P, Rouse BT. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186:1735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].He J, Neumann D, Kakazu A, Pham TL, Musarrat F, Cortina MS, et al. PEDF plus DHA modulate inflammation and stimulate nerve regeneration after HSV-1 infection. Exp Eye Res. 2017;161:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Lakhundi S, Siddiqui R, Khan NA. Pathogenesis of microbial keratitis. Microb Pathog. 2017;104:97–109. [DOI] [PubMed] [Google Scholar]
- [112].Kernacki KA, Berk RS. Characterization of arachidonic acid metabolism and the polymorphonuclear leukocyte response in mice infected intracorneally with Pseudomonas aeruginosa. Investigative ophthalmology & visual science. 1995;36:16–23. [PubMed] [Google Scholar]
- [113].Lee JE, Sun Y, Gjorstrup P, Pearlman E. Inhibition of Corneal Inflammation by the Resolvin E1. Investigative ophthalmology & visual science. 2015;56:2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Karim MJ, Bhattacherjee P, Biswas S, Paterson CA. Anti-inflammatory effects of lipoxins on lipopolysaccharide-induced uveitis in rats. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2009;25:483–6. [DOI] [PubMed] [Google Scholar]
- [115].Hazlett LD, McClellan S, Kwon B, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Investigative ophthalmology & visual science. 2000;41:805–10. [PubMed] [Google Scholar]
- [116].Carion TW, Greenwood M, Ebrahim AS, Jerome A, Suvas S, Gronert K, et al. Immunoregulatory role of 15-lipoxygenase in the pathogenesis of bacterial keratitis. FASEB J. 2018;32:5026–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107:14298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–25. [DOI] [PubMed] [Google Scholar]
- [119].Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–52, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A(4) analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–8. [DOI] [PubMed] [Google Scholar]
- [121].Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–92. [DOI] [PubMed] [Google Scholar]
- [122].Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002;196:1253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. The Journal of clinical investigation. 2005;115:1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Haas-Stapleton EJ, Lu Y, Hong S, Arita M, Favoreto S, Nigam S, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One. 2007;2:e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Royer DJ, Zheng M, Conrady CD, Carr DJ. Granulocytes in Ocular HSV-1 Infection: Opposing Roles of Mast Cells and Neutrophils. Investigative ophthalmology & visual science. 2015;56:3763–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Liu J, Fu T, Song F, Xue Y, Xia C, Liu P, et al. Mast Cells Participate in Corneal Development in Mice. Scientific reports. 2015;5:17569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li M, Mittal SK, Foulsham W, Amouzegar A, Sahu SK, Chauhan SK. Mast cells contribute to the induction of ocular mucosal alloimmunity. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2019;19:662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Sahu SK, Mittal SK, Foulsham W, Li M, Sangwan VS, Chauhan SK. Mast Cells Initiate the Recruitment of Neutrophils Following Ocular Surface Injury. Investigative ophthalmology & visual science. 2018;59:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Martin N, Ruddick A, Arthur GK, Wan H, Woodman L, Brightling CE, et al. Primary human airway epithelial cell-dependent inhibition of human lung mast cell degranulation. PLoS One. 2012;7:e43545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Karra L, Haworth O, Priluck R, Levy BD, Levi-Schaffer F. Lipoxin B(4) promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal immunology. 2015;8:852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Sinniah A, Yazid S, Perretti M, Solito E, Flower RJ. The role of the Annexin-A1/FPR2 system in the regulation of mast cell degranulation provoked by compound 48/80 and in the inhibitory action of nedocromil. Int Immunopharmacol. 2016;32:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kulinski JM, Munoz-Cano R, Olivera A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur J Pharmacol. 2016;778:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–16. [DOI] [PubMed] [Google Scholar]
- [134].Sahu A, Foulsham W, Amouzegar A, Mittal SK, Chauhan SK. The therapeutic application of mesenchymal stem cells at the ocular surface. The ocular surface. 2019;17:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, et al. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports. 2016;7:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Eslani M, Putra I, Shen X, Hamouie J, Tadepalli A, Anwar KN, et al. Cornea-Derived Mesenchymal Stromal Cells Therapeutically Modulate Macrophage Immunophenotype and Angiogenic Function. Stem Cells. 2018;36:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, et al. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investigative ophthalmology & visual science. 2018;59:5194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, et al. Mesenchymal Stem Cells Reduce Corneal Fibrosis and Inflammation Via Extracellular Vesicle-Mediated Delivery of miRNA. Stem Cells Transl Med. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, et al. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J Immunol. 2015;195:875–81. [DOI] [PubMed] [Google Scholar]
- [140].Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50. [DOI] [PubMed] [Google Scholar]
- [141].McMahon B, Mitchell S, Brady HR, Godson C. Lipoxins: revelations on resolution. Trends Pharmacol Sci. 2001;22:391–5. [DOI] [PubMed] [Google Scholar]
- [142].Jeon KI, Kulkarni A, Woeller CF, Phipps RP, Sime PJ, Hindman HB, et al. Inhibitory effects of PPARgamma ligands on TGF-beta1-induced corneal myofibroblast transformation. Am J Pathol. 2014;184:1429–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zheng S, D’Souza VK, Bartis D, Dancer RC, Parekh D, Naidu B, et al. Lipoxin A4 promotes lung epithelial repair whilst inhibiting fibroblast proliferation. ERJ Open Res. 2016;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Herrera BS, Kantarci A, Zarrough A, Hasturk H, Leung KP, Van Dyke TE. LXA4 actions direct fibroblast function and wound closure. Biochem Biophys Res Commun. 2015;464:1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Guilherme RF, Xisto DG, Kunkel SL, Freire-de-Lima CG, Rocco PR, Neves JS, et al. Pulmonary antifibrotic mechanisms aspirin-triggered lipoxin A(4) synthetic analog. Am J Respir Cell Mol Biol. 2013;49:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–8. [DOI] [PubMed] [Google Scholar]
- [147].Foulsham W, Coco G, Amouzegar A, Chauhan SK, Dana R. When Clarity Is Crucial: Regulating Ocular Surface Immunity. Trends Immunol. 2018;39:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lagali NS, Badian RA, Liu X, Feldreich TR, Arnlov J, Utheim TP, et al. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Scientific reports. 2018;8:14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Forrester JV, Xu H, Kuffova L, Dick AD, McMenamin PG. Dendritic cell physiology and function in the eye. Immunol Rev. 2010;234:282–304. [DOI] [PubMed] [Google Scholar]
- [150].Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3:76–82. [DOI] [PubMed] [Google Scholar]
- [151].Sawada Y, Honda T, Hanakawa S, Nakamizo S, Murata T, Ueharaguchi-Tanada Y, et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J Exp Med. 2015;212:1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91–106. [DOI] [PubMed] [Google Scholar]
- [153].Corminboeuf O, Leroy X. FPR2/ALXR agonists and the resolution of inflammation. J Med Chem. 2015;58:537–59. [DOI] [PubMed] [Google Scholar]


