Abstract
Background:
Rapid gastric emptying, increased food intake, and alterations in gastrointestinal hormones are associated with obesity. The effect of regular physical activity (PA) on food intake, gastric emptying (GE), gastric accommodation and gastrointestinal (GI) hormones in adults with obesity remains unclear. Our aim was to compare, at time of presentation, weight trends, eating behavior, GE and GI hormone levels among individuals with obesity who engage in regular PA compared to those who do not.
Methods:
In 270 participants with obesity, we performed validated measurements of GI phenotypes: GE of solids and liquids, gastric volume (GV) during fasting and after consumption of 200mL Ensure®, satiety by kcal intake (T-kcal) during a buffet meal, satiation [volume to fullness (VTF) and maximal tolerated volume (MTV)] of a liquid nutrient, and plasma levels of fasting and postprandial GLP-1, PYY, CCK, and ghrelin. Physical Activity Stages of Change Questionnaire was used to assess whether participants were regularly PA or not
Key Results:
PA was associated with lower BMI (Δ 2.01kg/m2, p=0.001) and body weight (Δ 4.42kg, p=0.0278). GE of solids (T-50% Δ 7.54min, p=0.021) and liquids (T-50% Δ 2.99min, p=0.029%) was significantly more rapid in physically active participants. PA was also associated with relatively higher postprandial ghrelin AUC (Δ 10.4pg/ml, p=0.015).There was no significant difference in postprandial satiation, satiety, GV, or other GI hormones (CCK, PYY, or GLP-1) between groups.
Conclusions & Inferences:
PA is associated with lower BMI, but faster GE and higher postprandial ghrelin levels, two factors that are also associated with obesity.
Keywords: Hunger, caloric intake, exercise, satiety, ghrelin
INTRODUCTION
Physical activity is known to play a role in the prevention of obesity and weight gain, but weight loss plans involving only exercise seem to have limited effectiveness1,2, possibly due to compensatory increase in food intake, and other gastric adaptations associated with regular physical activity3,4, although this effect is debatable.5 The effect of physical activity on the GI system and its relationship to obesity is unclear, as regular exercise has been demonstrated to accelerate gastric emptying time in a healthy population, and we recently showed in 509 participants that obesity is associated with an accelerated gastric emptying.6–8 These results are contrary to previously published results with smaller sample sizes showing no statistical difference in gastric emptying between obese and healthy participants.9,10 Gastrointestinal function and feelings of fullness appear to be linked, although the mechanism is not well understood.11
There is a mixed body of evidence on the effect of exercise on gastric emptying. Numerous investigations in normal weight individuals have demonstrated the effect of acute exercise on delaying gastric emptying in a dose response fashion.12–15 However, while multiple studies show this is true at high intensities, there is evidence showing the opposite at moderate intensity. It has also been shown that high intensity exercise does not influence gastric emptying immediately post exercise, demonstrating that this acute change is short lived.11 The chronic effect of exercise on the GI system has been investigated in healthy individuals and has shown adaptation to these acute changes, leading to accelerated gastric emptying in those who exercise regularly.7
Similar investigations also demonstrate a decrease in ghrelin and hunger, and an increase in satiation hormones following acute exercise.13,16,17 Aerobic exercise seems to have a greater hormonal effect than resistance exercise, but both lead to decreases in ghrelin.17
Obesity can be associated with altered satiation, altered gastric function including rapid gastric emptying, and lower levels of “satiation” hormones, potentially leading to overeating and gain of adipose tissue.6 However, these factors appear to be independently influenced by regular physical activity and by obesity, and little is known about the effects of physical activity on the brain-gut axis in obesity.
This study was designed to assess characteristics associated with obesity. Part of the initial study design included a basic questionnaire to assess physical activity behaviors. This questionnaire was later used to break the study participants into two groups. The purpose of this present study is to examine the difference in weight trends, eating behavior, gastric emptying and gastrointestinal hormone levels among overweight and obese individuals who engage in regular physical activity compared to those who do not.
MATERIALS AND METHODS
Participants
A total of 270 individuals consented to participation, signed an informed consent document, and were studied in an investigation approved by the Mayo Clinic Institutional Review Board, as previously published.6 These individuals were primarily Caucasian (91%) adults (37 ± 0.72 yrs) and were classified as overweight (N=89) or obese (N=181) based on World Health Organization classification. The main inclusion criteria were: men or women with a body mass index >25 kg/m2, age 18 years or older, and not on current treatment for other diseases other than hypothyroidism. Exclusion criteria were: a positive history of any systemic disease, concurrent treatment of gastrointestinal motility or psychological disorders (eating disorder, anxiety and depression) or weight loss medications. Permitted medications were stable doses (for at least 30 days prior to the studies) of birth control pills, estrogen, and L-thyroxine replacement. Women of childbearing potential had a negative pregnancy test within 48 hours of any test involving radioisotopes. Participants were informed of the risks involved in their participation, reviewed and signed an IRB approved informed consent form and were free to withdraw from the study at any time. Participants were recruited between 2010 and 2014 and all the studies were performed at the Mayo Clinic Clinical Research Unit.
Measurements
On different days, participants reported to the Mayo Clinic Clinical Research Unit at 7:00 a.m. after an 8-hour fasting period, and the following validated investigations were performed as in prior studies: satiety by ad-libitum buffet meal to measure total caloric intake and macronutrient distribution in the chosen food8 and selected plasma gastrointestinal hormones.8
Gastric emptying:
Gastric emptying of solids and liquids was assessed by scintigraphy8; the primary endpoint was gastric half-emptying time (GE T1/2). Participants ingested a solid and liquid caloric meal (total calories, 296 kcal, 32% protein, 35% fat, 33% carbohydrate) in which both phases of the meal were radiolabeled: 99mTc-sulfur colloid (1.0 mCi) was added to 2 raw eggs during the scrambling and cooking process for gastric emptying of solids. The scrambled eggs were served on one slice of buttered bread with 240 mL of 1% milk labeled with 111In-diethyl-enetriaminepentaacetate (0.1 mCi) for gastric emptying of liquids. Anterior and posterior gamma camera images were obtained immediately after radiolabeled meal ingestion, every 15 minutes for the first two hours and then every 30 minutes for the next two hours. Geometric means of decay-corrected counts in anterior and posterior gastric regions of interest were used to estimate the proportion of 99mTc or 111In-DTPA.6,8
Gastric volume:
Fasting and postprandial gastric volumes were measured by single photon emission computed tomography (SPECT) imaging of the stomach after intravenous injection of 99mTc-pertechnetate, which is taken up by the gastric mucosa. This method was developed and validated (including performance characteristics) in our laboratory and provides volume measurements during fasting and post-300mL Ensure®.18
GE and SPECT studies were performed at least 72 hours apart to avoid interference by the 111In (indium) from the meal ingested during the GE study with the measurement of GV by 99mTc-SPECT, because 111In has 2 peaks, 1 of which overlaps with the peak for 99mTc.
Satiation test by ingestion of Ensure® (1 kcal/mL, 11% fat, 73% carbohydrate, and 16% protein) ingested at a constant rate of 30 ml/minute was performed to measure volume to fullness (VTF) and maximum tolerated volume (MTV).19,20 Thirty minutes after reaching MTV, symptoms of fullness, nausea, bloating, and pain were measured using 100 mm horizontal visual analog scales (VAS), with the words “none” and “worst ever” anchored at each end.
Satiety:
Satiety test (a measure of appetite) by ad libitum buffet meal measured total caloric intake and macronutrient distribution in the chosen foods from standard foods of known nutrient composition19: vegetable lasagna (Stouffers, Nestle USA, Inc., Solon, OH, USA]; vanilla pudding (Hunts, Kraft Foods North America, Tarrytown, NY, USA); and skim milk. The total kilocalories of food consumed and macronutrients ingested at the ad libitum meal were analyzed by validated software (ProNutra 3.0; Viocare Technologies Inc., Princeton, NJ, USA).
Blood draws:
Blood was drawn after placement of an IV cannula at 15 minutes pre-prandial, and at 15, 45, and 90 minutes post-prandial. CCK, Ghrelin, GLP-1 and PYY were measured at each time point and the area under the curve was calculated for each hormone.
Physical activity:
Physical activity was assessed using the four-item Physical Activity Stages of Change Questionnaire, based on the “transtheoretical model” of behavior. This questionnaire is a well validated and used to explore the relationship between physical activity and many different conditions including obesity21. The questionnaire includes 4 Yes/No questions: 1) Are you currently physically active, 2) I intend to become physically active in the next 6 months; 3) I currently engage on regular physical activity; and 4) I have been regularly physically active for the past 6 months. . Participants’ response to question number 4 (Y/N) was used to determine regular physical activity. Regular physical activity was described using the following statement “For activity to be regular, it must add up to a total of 30 minutes or more per day, and be done at least 5 days per week. For example, you could take one 30 minute walk, or take three 10 minute walks each day.” Activity was described to participants with the following statement: “Physical activity or exercise includes activities such as walking briskly, jogging, bicycling, swimming or any other activity where the exertion is at least as hard as these activities. Your heart rate and breathing should increase.” Participants were not given any other instruction regarding physical activity.
Waist measurement:
Waist and hip circumferences at the end of normal expiration were obtained by trained physicians following World Health Organization guidelines.22 Abnormal waist circumference was defined as >102 cm for men and >88 cm for women.
Statistical Analysis
Data are presented as means ± SEM. The participants were grouped based on whether they reported to be physically active or not in the last six months. We compared the effect of physical activity in the validated measurements of GI phenotypes using Student’s t-test. Variables distributions were tested for normality prior to performing the Student’s t-test. The primary end points in Table 1 are parameters that reflect unique physiological phenotypes, therefore, no adjustment of the P values was done for multiple tests among these end points, including ghrelin as the fasting sample function differs from the postprandial ghrelin function. Analyses were performed with JMP (PRO 13 from SAS, Cary, NC).
Table 1:
Participants demographics in physically active compared to non-physically active.
| Data (mean ± SEM) | |||
|---|---|---|---|
| Variables | Non-PA | PA | P-value |
| N | 154 | 113 | |
| Body weight, kg | 95.98 ± 1.2 | 91.56 ± 1.6 | 0.028* |
| Height , cm | 168.5 ± 0.7 | 169.2 ± 0.9 | 0.52 |
| BMI, kg/m2 | 33.83 ± 0.4 | 31.82 ± 0.5 | 0.001* |
| Waist circumference, cm | 102.08 ± 0.9 | 99.88 ± 1.2 | 0.14 |
| Hip circumference, cm | 117 ± 0.85 | 114 ± 0.9 | 0.01* |
| Usual satiation (VTF), ml | 688 ± 23 | 734 ± 30 | 0.22 |
| Maximal satiation (MTV), ml | 1255±34 | 1335± 39 | 0.13 |
| Ad libitum buffet meal intake, Kcal | 965 ± 24 | 980 ± 28.6 | 0.67 |
| Gastric emptying solids (T-50%), min | 102.78 ± 2.2 | 95.24 ± 2.2 | 0.02* |
| Gastric emptying liquids (T-50%), min | 20.31 ± 1.1 | 17.32 ± 0.6 | 0.03* |
| Fasting ghrelin, pg/ml | 69.45 ± 4.2 | 82.69 ± 6.2 | 0.06 |
| Postprandial ghrelin (AUC), pg/ml | 42.37 ± 1.7 | 52.77 ± 4.4 | 0.015* |
| Self Esteem Score (MBSRQ) | 26.75 ± 0.5 | 29.2 ± 0.5 | 0.001* |
Description: Anthropometric, satiation, satiety, ghrelin and self-esteem scores for PA and non-
RESULTS
All 270 participants responded the physical activity questionnaire; from those, 113 (42%) reported to be physically active. Participants who reported being regularly physically active for the past 6 months had a significantly lower BMI (31.82 ± 0.47 vs. 33.83 ± 0.39 kg/m2, p<0.001) and body weight (91.56 ± 1.52 vs. 95.98 ± 1.30 kg, p=0.028) compared to the non-physically active group (Table 1). There was no difference in waist circumference, hip circumference, or fasting blood glucose between the two groups (Table 1).
Gastrointestinal Phenotype
Participants who reported being regularly physically active for the past 6 months had a significantly accelerated gastric emptying T1/2 of solids (95.24 ± 2.48 vs. 102.78 ± 2.08 min, p=0.021) and liquids (17.32 ± 1.04 vs. 20.31 ± 0.88 min, p=0.029) compared to the non-physically active (Table 1, Figure 1a and 1b).
Figure 1:
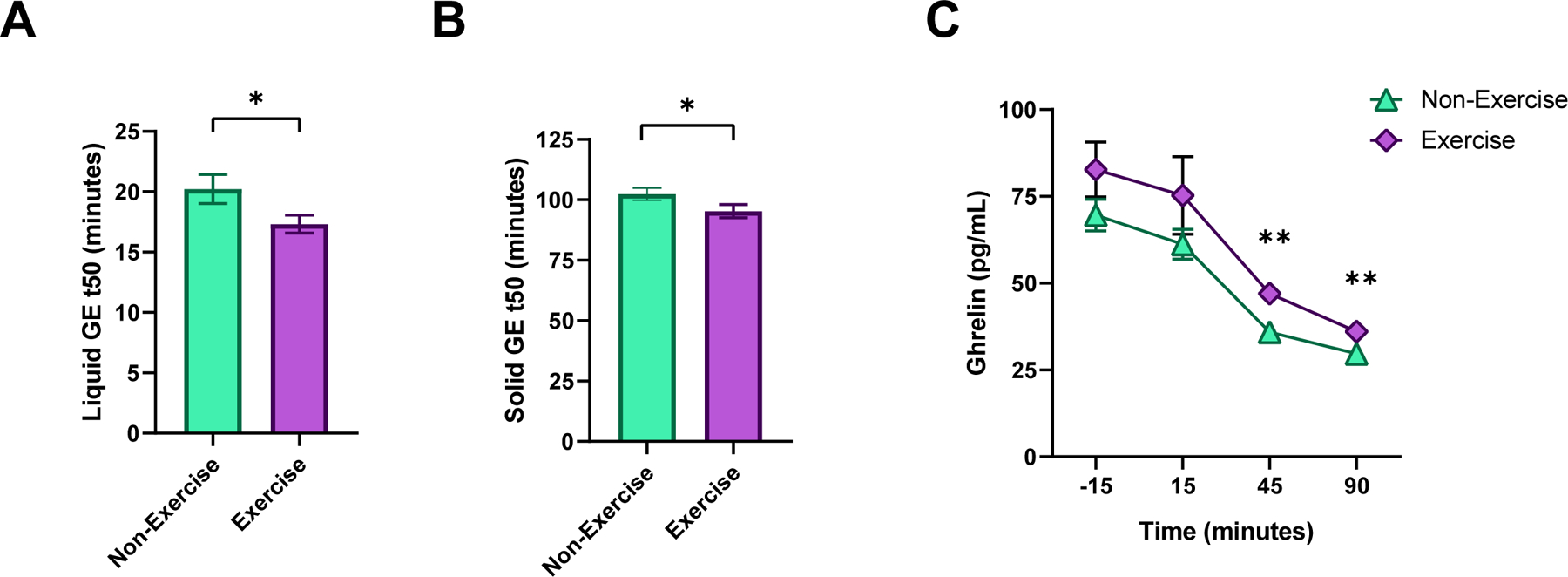
Gastric emptying and ghrelin in physically active participants compared to sedentary participants. Figure 1a: Gastric Emptying of Liquids (T50: time to emptied 50% of consumed meal, minutes). Figure 1b: Gastric Emptying of Solids (T50, minutes). Figure 1c: Total ghrelin at fasting and postprandial 15, 45 and 90 minutes. P values: *p<0.05, **p<0.01 Student t-test.
Participants who reported being regularly physically active had a significantly higher level of postprandial ghrelin (52.77 ± 3.26 vs 42.37 ± 2.72, p=0.015), and borderline high fasting ghrelin (82.69 ± 5.50 vs 69.45 ± 4.59, p=0.066) compared to the non-physically active (Figure 1c). There was no association with satiation tested by nutrient drink test or ad libitum buffet meal, satiety hormones (PYY or GLP-1) (Table 1).
Per gender Analysis
Women who reported being physically active had significantly lower body weight (87.56 ± 1.98 kg vs 92.67 ± 1.31 kg, p=0.038), BMI (32.44 ± 0.64 kg/m2 vs 34.15 ± 0.47 kg/m2, p=0.0324) and hip circumference (115.56 ± 1.28 cm vs 118.66 ± 1.02 cm, p=0.0459), and significantly higher fasting ghrelin (90.80 ± 9.22 pg/ml vs 69.36 ± 3.33 pg/ml , p=0.0319) and postprandial ghrelin AUC (57.54 ± 7.07 pg/ml vs 42.11 ± 1.74 pg/ml, p=0.0376) than those who reported not being physically active. There was no difference in VTF, MTV, energy intake, gastric emptying of liquids or solids between groups (Table 2).
Table 2:
Participants demographics based on sex and activity level.
| Females (mean ± SEM) | |||
|---|---|---|---|
| Variables | Non-PA | PA | P value |
| N | 116 | 68 | |
| Body weight, kg | 92.7 ± 1.4 | 87.6 ± 1.8 | 0.04* |
| Height , cm | 164.8 ± 0.6 | 163.9 ± 0.8 | 0.34 |
| BMI, kg/m2 | 34.2 ± 0.5 | 32.4 ± 0.62 | 0.03* |
| Waist circumference, cm | 100.17 ± 1.1 | 97.92 ± 1.4 | 0.22 |
| Hip circumference, cm | 118.66 ± 1 | 115.56 ± 1.3 | 0.046* |
| Usual satiation (VTF), ml | 634 ± 24 | 638 ± 32.4 | 0.92 |
| Maximal satiation (MTV), ml | 1155±33 | 1182±44 | 0.63 |
| Ad libitum buffet meal intake, Kcal | 888 ± 22.8 | 864 ± 30 | 0.52 |
| Gastric emptying solids (T-50%), min | 108 ± 2.2 | 105 ± 3 | 0.46 |
| Gastric emptying liquids (T-50%), min | 21 ± 1.1 | 18 ± 1.5 | 0.14 |
| Fasting ghrelin, pg/ml | 69.36 ± 3.3 | 90.80 ± 9.2 | 0.03* |
| Postprandial ghrelin (AUC), pg/ml | 42.11 ± 1.7 | 57.54 ± 7.1 | 0.038* |
| Males (mean ± SEM) | |||
| Variables | Non-PA | PA | P value |
| N | 38 | 45 | |
| Body weight, kg | 106.08 ± 2.6 | 97.60 ± 2.4 | 0.017* |
| Height , cm | 179.97 ± 1.1 | 177.42 ± 1 | 0.086 |
| BMI, kg/m2 | 32.9 ± 0.7 | 30.9 ± 0.7 | 0.048* |
| Waist circumference, cm | 107.81 ± 1.9 | 102.89 ± 1.8 | 0.06 |
| Hip circumference, cm | 113.9 ± 1.4 | 112.1 ± 1.3 | 0.32 |
| Usual satiation (VTF), ml | 861.7 ± 53 | 874 ± 48 | 0.86 |
| Maximal satiation (MTV), ml | 1572± 71 | 1557± 64 | 0.88 |
| Ad libitum buffet meal intake, Kcal | 1199 ± 49.8 | 1159 ± 46.2 | 0.55 |
| Gastric emptying solids (T-50%), min | 86.5 ± 3.9 | 81.6 ± 3.5 | 0.35 |
| Gastric emptying liquids (T-50%), min | 18.5 ± 1.2 | 16.3 ± 1.0 | 0.15 |
| Fasting ghrelin, pg/ml | 69.76 ± 14.4 | 71.37 ± 6.9 | 0.4707 |
| Postprandial ghrelin (AUC), pg/ml | 43.22 ± 4.8 | 45.99 ± 3.7 | 0.6484 |
Description: Anthropometric and ghrelin data for males and females when grouped by activity
Men who report being physically active had a significantly lower bodyweight (97.60 ± 2.51 kg vs 106.08 ± 2.39 kg, p=0.0165) and BMI (30.88 ± 0.65 kg/m2vs 32.88 ± 0.75 kg/m2, p=0.0483), and borderline lower waist circumference (102.89 ± 1.85 cm vs 107.81 ± 1.80cm, p=0.0608) than those who reported not being physically active. There was no difference in VTF, MTV, energy intake, gastric emptying of solids, gastric emptying of liquids, fasting or postprandial ghrelin (Table 2).
Outcome Measures Based on Waist Circumference
Those with normal waist circumference who reported regular PA have no difference in body weight, BMI, VTF, MTV, energy intake, gastric emptying of solid solids or liquids, postprandial ghrelin (AUC), or fasting ghrelin compared to those who did not (Table 3).
Table 3:
Results grouped by Waist Circumference
| Normal Waist Circumference (Mean ± SEM) | |||
|---|---|---|---|
| Variables | Non-PA | PA | P value |
| N | 22 | 35 | |
| Body weight, kg | 83.19 ± 2.3 | 78.75 ± 1.8 | 0.13 |
| Height , cm | 170.5 ± 1.2 | 168.57 ± 1.5 | 0.42 |
| BMI, kg/m2 | 28.52 ± 0.4 | 27.58 ± 0.3 | 0.06 |
| Waist circumference, cm | 87.29 ± 1.8 | 88.14 ± 1.2 | 0.69 |
| Hip circumference, cm | 107.84 ± 1.5 | 106.36 ± 1 | 0.42 |
| Usual satiation (VTF), ml | 690 ± 58 | 659 ± 46 | 0.69 |
| Maximal satiation (MTV), ml | 1345.89 ± 85 | 1221.63 ± 68 | 030 |
| Ad libitum buffet meal intake, Kcal | 986.56 ± 75.5 | 934.21 ± 49.8 | 0.57 |
| Gastric emptying solids (T-50%), min | 107.96 ± 5.8 | 96.071 ± 4.6 | 0.13 |
| Gastric emptying liquids (T-50%), min | 26.89 ± 3.4 | 19.06 ± 2.7 | 0.15 |
| Fasting ghrelin, pg/ml | 93.24 ± 21.9 | 79.50 ± 7.68 | 0.56 |
| Postprandial ghrelin (AUC), pg/ml | 48.92 ± 6.5 | 46.97 ± 3.5 | 0.79 |
| Abnormal Waist Circumference (Mean ± SEM) | |||
| Variables | Non-PA | PA | P value |
| N | 126 | 74 | |
| Body weight, kg | 98.34 ± 1.4 | 98.14 ± 1.9 | 0.93 |
| Height , cm | 168.37 ± 0.8 | 169.65 ± 1.1 | 0.35 |
| BMI, kg/m2 | 34.73 ± 0.43 | 33.96 ± 0.55 | 0.27 |
| Waist circumference, cm | 104.66 ± 0.9 | 105.43 ± 1.2 | 0.60 |
| Hip circumference, cm | 119.15 ± 0.9 | 117.70 ± 1.1 | 0.29 |
| Usual satiation (VTF), ml | 687.80 ± 25.4 | 773.58 ± 40.1 | 0.07 |
| Maximal satiation (MTV), ml | 1236.19 ± 36.3 | 1391.68 ± 51.4 | 0.01* |
| Ad libitum buffet meal intake, Kcal | 966.58 ± 25.7 | 981.87 ± 33.2 | 0.71 |
| Gastric emptying solids (T-50%), min | 102.22 ± 2.4 | 94.79 ± 2.7 | 0.04* |
| Gastric emptying liquids (T-50%), min | 18.75 ± 0.8 | 16.41 ± 0.6 | 0.016* |
| Fasting ghrelin, pg/ml | 66.37 ± 3.3 | 84.18 ± 8.5 | 0.05* |
| Postprandial ghrelin (AUC), pg/ml | 41.70 ± 1.7 | 56.33 ± 6.51 | 0.03* |
Description: Anthropometric, satiation, satiety, and ghrelin results grouped by waist circumference as defined by a waist measurement > 102 cm for men and >88 cm for women.
statistically significant.
Those with abnormal waist circumference who reported regular PA had significantly higher MTV (1391.68 ± 51.39 ml vs 1236.19 ± 36.25 ml, p=0.0147) and significantly faster gastric emptying of both liquids (16.41 ± 0.57 min vs 18.75 ± 0.79 min, p=0.0164) and solids (94.79 ± 2.69 vs 102.22 ± 2.43min, p=0.0419). They also had significantly higher postprandial ghrelin (AUC) (56.33 ± 6.51 pg/ml vs 41.70 ± 1.72 pg/ml, p=0.0329) and higher fasting ghrelin that approaches significance (84.18 ± 8.54 pg/ml vs 66.37 ± 3.23 pg/ml, p=0.0544) (Table 3).
DISCUSSION
The purpose of this study was to explore the possible associations between physical activity and gastrointestinal physiology of relevance to obesity. While using this questionnaire to group participants by activity level is limiting, the results indicate that those who reported being physically active had significant differences in gastric function compared to those who did not. In all-comers, PA is associated with a lower BMI, but faster GE and higher postprandial ghrelin levels, with no difference in satiation variables or energy intake at the buffet meal. This indicates that PA appears to have a protective effect on BMI and weight, despite faster GE and increasing ghrelin. Additionally, our findings may suggest that PA may have a compensatory effect to increased ghrelin which may also accelerate gastric emptying to increase caloric intake and compensate for the physical activity and associated increased energy expenditure due to the exercise.
Unexpectedly, there was no difference in hip circumference or waist circumference. This could be due to the method of assessing PA, as PA has previously been associated with lower hip and waist measurements.23 However, men and women tend to accumulate adipose tissue in different patterns.24,25 Thus, the groups were separated by gender. Women who get regular PA have a significantly lower hip circumference, than women who don’t. Men who get regular PA have a lower waist circumference then men who do not. Both men and women who exercise have significantly lower weight and BMI.
While it appears this explains why the original waist and hip measures were not different amongst exercise groups, when other outcome measures were assessed, only women who exercised maintained the significantly different ghrelin results compared to those who don’t exercise. However, the gastric emptying values were no longer significant. As previously stated, women who were physically active also had significantly lower waist circumference. This led us to wonder if the gastric differences observed were driven by waist circumference as opposed to gender.
When separated by waist circumference, those with abnormal waist circumference who exercised had faster gastric emptying and higher ghrelin both fasting and postprandially. It is difficult to differentiate if this is truly due to visceral adiposity or simply due to increased adiposity in general. Previous literature demonstrates that increased waist circumference26 and visceral adiposity27,28 increases risk of cardiovascular disease, and therefore it is reasonable to suspect visceral adiposity may play a role in gastric function as well.
Our findings suggest that regular physical activity may be associated with a brain-gut axis pro-appetite phenotype as well as healthier anthropometric measurements. While we cannot confirm causation, it is reasonable to associate the anthropometric differences with increased energy expenditure due to regular physical activity. Physical activity appears to have a protective effect on BMI and weight, despite faster GE and increasing ghrelin. However, the effect on acceleration of GE, despite the statistical difference, is only 7 (7%) minutes for solids and 3 (15%) minutes for liquids; while the same GE difference is seen in participants with abnormal circumference, in these participants, there is also an increase in consumption of 155 calories prior to reaching maximal fullness in those physically active. This investigation demonstrates that at a single time point, physical activity is associated with both a lower weight and BMI and with increased ghrelin and gastric emptying.
Further investigation is necessary to determine the long-term effects of physical activity on satiety and satiation variables and the contribution of these variables to body weight and body composition in physically active individuals. Specifically, in this era of precision medicine, is essential to identify which patients with obesity may experience higher levels of ghrelin and acceleration of GE when attempting weight loss with increased physical activity, and, while more studies are needed, maybe those patients should focus in a reduce calorie diet with minimal changes to their PA for successful weight loss.
This study has several limitations: 1) The results presented in this paper were not the primary endpoints of this study. They resulted from a posthoc analysis of the results based on a questionnaire. 2) The questionnaire gives very limited information about the physical activity habits of those who participated and presented a recall bias which might be altered in obesity. Even with these limitations, the results of this study were significant. This indicates that some aspect of physical activity impacts both motility and GI hormones, and this warrants further investigation into the specific modalities and intensities of physical activity and their effect on this system in obesity. Specially to consider whether there could be a dose-effect of intensity and frequency of physical activity and exercise in gastric emptying and further studies are needed to address these questions. Future investigations should quantify both physical activity and physical fitness to determine the correlations that may exist between these variables and variables associated with obesity.
In conclusion, physical activity is associated with lower BMI, but faster GE and higher postprandial ghrelin levels, two factors that are also associated with obesity. The benefit of physical activity for weight management in obesity may be tampered by accelerated gastric emptying and increased ghrelin, which may increase the desire for food intake.
Key Point Message.
Rapid gastric emptying, increased food intake, and alterations in gastrointestinal hormones are associated with obesity. Physical activity and exercise are key aspects in the management of obesity. The effect of regular physical activity (PA) on food intake, gastric emptying (GE), gastric accommodation and gastrointestinal (GI) hormones in adults with obesity remains unclear.
In a cohort of 270 participants with obesity, those physically active had lower BMI and body weight; and a paradoxical rapid GE of solids and liquids and higher levels of postprandial ghrelin.
The benefit of physical activity for weight management in obesity may be tampered by accelerated gastric emptying and increased ghrelin, which may increase the desire for food intake.
Acknowledgements, Funding and Disclosures
This study was supported by funds from the National Institute of Health and the American Neurogastroenterology and Motility Society. The authors report no conflict of interest and the results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
Funding support: Dr. Camilleri is supported by NIH RO1-DK67071. Dr. Acosta is supported by NIH (C-Sig P&F NIDDK P30DK084567, K23, NIDDK114460) and ANMS Career Development Award
Footnotes
Disclosures:
There are no conflicts of interest related to this manuscript. Dr. Acosta is a stockholder in Gila Therapeutics, and Phenomix Sciences; he serves as a consultant for Rhythm Pharmaceuticals, General Mills, Gila Therapeutics. Dr. Camilleri is a stockholder in Phenomix Sciences and serves as a consultant to Takeda, Allergan, Rhythm, Salix, Arena, Enterin.
References
- 1.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2013. doi: 10.1161/01.cir.0000437739.71477.ee [published Online First: 2013/11/14] [DOI] [Google Scholar]
- 2.Acosta A, Streett S, Kroh MD, et al. White Paper AGA: POWER - Practice Guide on Obesity and Weight Management, Education, and Resources. Clin Gastroenterol Hepatol 2017;15(5):631–49 e10. doi: 10.1016/j.cgh.2016.10.023 [published Online First: 2017/03/01] [DOI] [PubMed] [Google Scholar]
- 3.Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43(7):1334–59. doi: 10.1249/MSS.0b013e318213fefb [published Online First: 2011/06/23] [DOI] [PubMed] [Google Scholar]
- 4.Exercise Stensel D., appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab 2010;57 Suppl 2:36–42. doi: 10.1159/000322702 [published Online First: 2010/01/01] [DOI] [PubMed] [Google Scholar]
- 5.King JA, Wasse LK, Broom DR, et al. Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med Sci Sports Exerc 2010;42(3):485–92. doi: 10.1249/MSS.0b013e3181ba10c4 [DOI] [PubMed] [Google Scholar]
- 6.Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 2015;148(3):537–46 e4. doi: 10.1053/j.gastro.2014.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horner KM, Byrne NM, Cleghorn GJ, et al. Influence of habitual physical activity on gastric emptying in healthy males and relationships with body composition and energy expenditure. Br J Nutr 2015;114(3):489–96. doi: 10.1017/S0007114515002044 [DOI] [PubMed] [Google Scholar]
- 8.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology 2006;131(6):1717–24. doi: 10.1053/j.gastro.2006.10.025 [DOI] [PubMed] [Google Scholar]
- 9.Glasbrenner B, Pieramico O, Brecht-Krauss D, et al. Gastric emptying of solids and liquids in obesity. Clin Investig 1993;71(7):542–6. [DOI] [PubMed] [Google Scholar]
- 10.Buchholz V, Berkenstadt H, Goitein D, et al. Gastric emptying is not prolonged in obese patients. Surg Obes Relat Dis 2013;9(5):714–7. doi: 10.1016/j.soard.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 11.Evans GH, Shirreffs SM, Watson P, et al. Gastric emptying rate and perceived hunger after rest and exercise in man. British Journal of Sports Medicine 2010;44(14):i20–i21. doi: 10.1136/bjsm.2010.078972.61 [DOI] [Google Scholar]
- 12.Horner KM, Schubert MM, Desbrow B, et al. Acute exercise and gastric emptying: a meta-analysis and implications for appetite control. Sports Med 2015;45(5):659–78. doi: 10.1007/s40279-014-0285-4 [DOI] [PubMed] [Google Scholar]
- 13.Hazell TJ, Islam H, Townsend LK, et al. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016;98:80–8. doi: 10.1016/j.appet.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 14.Carrio I, Estorch M, Serra-Grima R, et al. Gastric emptying in marathon runners. Gut 1989;30(2):152–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiper JB, Broad NP, Maughan RJ. Effect of intermittent high-intensity exercise on gastric emptying in man. Med Sci Sports Exerc 2001;33(8):1270–8. [DOI] [PubMed] [Google Scholar]
- 16.Broom DR, Miyashita M, Wasse LK, et al. Acute effect of exercise intensity and duration on acylated ghrelin and hunger in men. J Endocrinol 2017;232(3):411–22. doi: 10.1530/JOE-16-0561 [DOI] [PubMed] [Google Scholar]
- 17.Broom DR, Batterham RL, King JA, et al. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol 2009;296(1):R29–35. doi: 10.1152/ajpregu.90706.2008 [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Aros S, Camilleri M, Castillo EJ, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clin Gastroenterol Hepatol 2005;3(10):997–1006. [published Online First: 2005/10/20] [DOI] [PubMed] [Google Scholar]
- 19.Breen M, Camilleri M, Burton D, et al. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterol Motil 2011;23(4):308–15. doi: 10.1111/j.1365-2982.2010.01660.x [published Online First: 2011/01/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chial H, Camilleri C, Delgado-Aros S, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2002;14(3):249–53. [DOI] [PubMed] [Google Scholar]
- 21.Sarkin JA, Johnson SS, Prochaska JO, et al. Applying the transtheoretical model to regular moderate exercise in an overweight population: validation of a stages of change measure. Prev Med 2001;33(5):462–9. doi: 10.1006/pmed.2001.0916 [published Online First: 2001/10/26] [DOI] [PubMed] [Google Scholar]
- 22.Organization WH. Waist circumference and waist-hip ration: report of a WHO expert consultation. . Geneva, Switzerland: WHO; 2008 [Google Scholar]
- 23.Choi J, Guiterrez Y, Gilliss C, et al. Physical activity, weight, and waist circumference in midlife women. Health Care Women Int 2012;33(12):1086–95. doi: 10.1080/07399332.2012.673658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karastergiou K, Smith SR, Greenberg AS, et al. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ 2012;3(1):13. doi: 10.1186/2042-6410-3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaak E Gender differences in fat metabolism. Curr Opin Clin Nutr Metab Care 2001;4(6):499–502. [DOI] [PubMed] [Google Scholar]
- 26.de Koning L, Merchant AT, Pogue J, et al. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J 2007;28(7):850–6. doi: 10.1093/eurheartj/ehm026 [DOI] [PubMed] [Google Scholar]
- 27.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 1994;73(7):460–8. [DOI] [PubMed] [Google Scholar]
- 28.Numan Ahmad M, Halim Haddad F. Suitability of Visceral Adiposity Index as a Marker for Cardiometabolic Risks in Jordanian Adults. Nutr Hosp 2015;32(6):2701–9. doi: 10.3305/nh.2015.32.6.9543 [DOI] [PubMed] [Google Scholar]


