Abstract
Bariatric surgery is currently the most efficacious and durable intervention for severe obesity. The most commonly performed procedures in the U.S. are the Roux-en-Y Gastric Bypass (RYGB) and the Sleeve Gastrectomy (SG), which involve significant anatomical and physiological alterations that lead to changes in behavior and biology. Unfortunately, many patients experience sub-optimal weight loss and/or substantial weight regain. Eating and physical activity/sedentary behaviors, mood, cognition and the gut microbiome all change postoperatively and have an association with weight change. The longitudinal relationship between changes in the gut microbiome and postoperative weight trajectory has not been explored thoroughly, and the interactive associations among the gut microbiome and the other variables that impact weight have been similarly understudied.
The following is a methods and design description for a prospective, 24-month longitudinal study of 144 bariatric surgery patients, at two sites, that aims to identify predictors of weight loss trajectories over 24 months following RYGB and SG. Specifically, the study will examine the relationships between empirically supported behavioral and biological variables and their combined impact on postoperative weight trajectories. Novel data collection will include intensive measurement of problematic eating behaviors and dietand physical activity postoperatively, which may be altered in parallel with, or in response to, changes observed in the gut microbiota. Identifying postoperative predictors of weight loss and comorbidity resolution should inform development of novel interventions that that are tailored to individual patients’ risk profiles to optimize and sustain more favorable weight trajectories.
Keywords: bariatric surgery, weight loss, microbiome, gut-brain axis, eating behavior, physical activity
Introduction
The most effective treatment for severe obesity is bariatric surgery (1), with the Roux-en-Y Gastric Bypass (RYGB) and the Sleeve Gastrectomy (SG) being the most commonly performed procedures in the US (2). However, the multi-site Longitudinal Assessment of Bariatric Surgery (LABS) study found that a significant subset of patients (~25%), do not achieve expected weight loss outcomes (3). Reviews also suggest that most patients regain some weight with approximately 20 % regaining the majority of lost weight (4). These sub-optimal outcomes can result in lack of improvement or recurrence of medical comorbidity (5-6).
Prior research has shown significant associations between weight loss following bariatric surgery and variables involving eating pathology such as “loss of control” eating (5, 7-9), “grazing”, eating when not hungry (5, 10-11), nocturnal eating and stress-induced eating (5, 10-12). Residual depressive symptoms also have been shown to negatively impact weight outcomes (13) as has lower physical activity and higher sedentary behavior (14-16). Differences in executive function may impact eating, adherence and physical activity (17).
Beyond behavioral variables, biological factors—namely the gut microbiome—have been a recent area of focus in the context of bariatric surgery. Human and animal work have established obesity-associated permutations in the gut microbiota with some studies demonstrating causal relationships between specific changes in the gut microbiota and weight loss (18). Numerous anatomical and physiological changes follow RYGB and SG that may contribute to changes in the microbial flora of the distal gut. Prospective research shows increases in specific phyla such as Proteobacteria after RYGB that consistently correlate with changes in body weight, serum leptin, and variably with inflammation markers including C-reactive protein (19). The ratio of Firmicutes to Bacteroidetes has been shown to decrease following RYGB after controlling for gender, age, and medication (19). Importantly, past studies have not adequately controlled for dietary intake. This is considered to be a significant deficiency of the extant literature (20). The collective body of evidence suggests there is bidirectional communication between the gut microbiome and brain function (i.e., the gut-brain axis) (21) which may impact behavior, but this interface has not been well studied in bariatric samples.
Aims of the BioBehavioral Trial
The contributors to suboptimal weight loss or regain following bariatric surgery are poorly understood and likely involve complex interactions between behavioral and biological variables. Our first aim is to describe the prospective relationships among weight change and variations in post-operative problematic eating behaviors, physical activity/sedentary behaviors, mood, and cognitive function, and whether these relationships are moderated by surgical procedure. We hypothesize that patients who report fewer problematic eating behaviors, greater levels of physical activity/lower levels of sedentary behavior, less depressive symptoms and better cognitive function, will experience a more favorable weight loss trajectory and greater percent excess weight loss (%EWL) at 24 months following surgery. Further, we hypothesize that these relationships will be stronger for participants receiving RYGB versus SG. Aim 2 will characterize the prospective relationships between postoperative weight trajectory and changes in the composition of the gut microbiome and further explore whether this relationship is moderated by surgical procedure. We hypothesize that the presence and abundance of specific taxa within the intestinal microbiota will be demonstrated in patients who experience the most favorable weight outcomes and greatest 24-month %EWL, and that these relationships will be moderated by surgical procedure. A final exploratory aim will assess whether the microbiome gut-brain axis is a mechanistic determinant of weight trajectory following bariatric surgery. We hypothesize that changes in the composition of the gut microbiome will mediate the relationship between weight trajectory following RYGB or SG and behavioral variables of interest. This aim, within a longitudinal study, allows us to begin to better understand the interaction between biological and behavioral variables on explaining weight loss trajectories.
Research Designs and Methods
This study is designed as a prospective, longitudinal assessment of behavioral, psychological, and biological factors temporally related to weight loss trajectory over two years following RYGB or SG. It was submitted to NIDDK in response to RFA-DK-16-017 (Psychosocial and Behavioral Mechanisms in Bariatric Surgery). Data collection occurs at two sites REDACTED FOR BLIND REVIEW, in collaboration with REDACTED FOR BLIND REVIEW, in REDACTED FOR BLIND REVIEW; and REDACTED FOR BLIND REVIEW, in REDACTED FOR BLIND REVIEW).
Participants and Recruitment
Participants who are in the evaluation process for RYGB and SG are offered the opportunity to volunteer for this study. A total of 144 participants undergoing bariatric surgery (~50% RYGB and 50% SG) will be enrolled in this study. In anticipation of a rate of attrition of approximately 30%, this enrolled sample is expected to yield a final sample size of 100, which will provide adequate power to address the stated hypotheses. Enrolled participants are assessed at baseline (pre-surgery) and specified time-points (1, 6, 12, 18, 24 months) following surgery (Figure 1).
Figure 1:
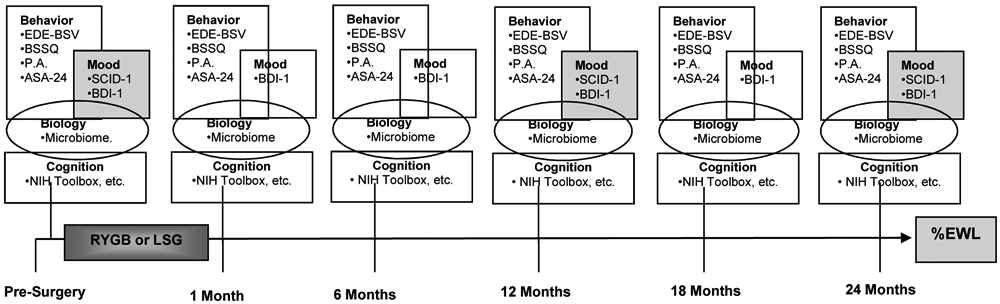
Schedule of Study Assessments
EDE-BSV=Eating Disorders Examination—Bariatric Surgery Version
BSSQ= Bariatric Surgery Self-Management Questionnaire
PA=Physical Activity
ASA-24=The ASA24 Automated Self-administered 24-Hour
SCID-1=Structured Clinical Interview for DSM-5)
BDI-1=The Beck Depression Inventory 1
Recruitment takes place through REDACTED FOR BLIND REVIEW in REDACTED FOR BLIND REVIEW, and through the REDACTED FOR BLIND REVIEW. Criteria for enrollment were carefully considered and chosen to balance generalizability with the need to reduce some confounding variables (presented in Table 1). Additional data that could influence the microbiome are collected and will be used as covariates in analyses if appropriate (e.g., comorbid medical conditions, medication use, surgical history (especially of cholecystectomy/appendectomy), nutritional supplement use, etc.).
Table 1:
Study Enrollment Criteria
| Inclusion Criteria | Method of Assessment |
|---|---|
| Male or Female between the ages of 18-65 years, inclusive | Photo Identification |
| In evaluation for Roux-en-Y Gastric Bypass or Sleeve Gastrecomy as primary procedure | Surgery Department |
| Planning to be available for two years of follow-up after surgery | Self-report |
| Exclusion Criteria | Method of Assessment |
| Alcohol or substance use disorder in the past year | Structured Interview |
| Severe psychiatric disorder that may affect the ability of the participant’s ability to comply with the protocol | Structured interview |
| Tobacco use in the prior year | Self-report |
| Current medication known to significantly influence gastrointestinal transit time (e.g., opioids, glp-1 agonists, metoclopramide) or is being taken routinely for weight loss (e.g., phentermine, topiramate) | Medical History |
| Use of any oral or injectable antibiotic in the past month | Medical History |
| Use of commercially available prebiotic or probiotic in the past month | Medical History |
| History of significant intestinal disease or disorder that would influence the microbiome (e.g., Crohn’s Disease, etc.) | Medical History |
| History of significant gastrointestinal surgery that would influence the microbiome, not including cholecystectomy or appendectomy | Medical History |
| Inability to engage in physical activity or dietary monitoring or any medical condition that would put the participant at risk in the study | Self-report |
| Positive urine drug screen for a non-prescribed medication | Urine drug screen |
| Pregnancy or breast feeding | Self-report |
Study Assessments and Timeline
Screening Visit.
Participants who provide informed consent at a pre-surgical evaluation visit in the bariatric clinic are screened for study eligibility by research staff according to the list of inclusion and exclusion criteria (Table 1). All criteria are in place to protect participant safety and to avoid introducing unnecessary confounding variables into the study data. At screening, each participant completes a medical history to assess for inclusion/exclusion criteria. Participants also have the first Structured Clinical Interview (SCID-5) (22) administered during the screening visit to assess for psychopathology that may affect protocol compliance. Participants who meet all eligibility crtieria are invited to enroll in the study and complete study assessements according to the schedule described below. Participants are required to complete study baseline assessments prior to beginning any prescribed pre-surgical diet.
Study Visits (pre-surgery; 1, 6, 12, 18, 24 months post-surgery).
With the exception of the stuctured clinical interview for psychological disorders, all assessments are completed at each study visit (Figure 1). The following assessments are completed.
Measures and Variables of Interest
Body Weight, %EWL, BMI.
Height and weight data are collected at each study visit. Participants are weighed in light clothing without shoes. Participants’ Ideal Body Weight is calculated based upon a BMI=25 at baseline to facilitate determination of percentage excess body weight loss (%EWL) following surgery. To facilitate comparison between studies, data is recorded in weight (lbs, kg), body mass index (BMI) units (kg/m2), and %EWL.
Dietary Recall.
The ASA24 Automated Self-administered 24-Hour. The ASA24 dietary recall was developed by the National Cancer Institute (NCI). The ASA24 has been validated for research use and offers a web-based platform for the collection of automated, self-administered, 24 hour dietary recalls (food, fluids, vitamins and supplements). The ASA24 is based upon the USDA Automated Multiple-Pass Method (AMPM) which is validated and provides accurate estimations of mean total energy and protein consumption in biomarker testing (23-24). The system has been used widely in nutrient research and is considered the gold standard method of assessment of food intake. Participants completed 3 days of dietary recall at each study visit.
Psychological Assessment.
Structured Clinical Interview for DSM-5 (SCID-I) (22). This is a structured interview that is used as a diagnostic tool for psychiatric disorders following DSM-5 criteria. Psychological assessors on this protocol have regular meetings to ensure high inter-rater reliability on administration of the SCID. The Beck Depression Inventory (BDI) (25). The BDI is a 21-item self-report measure that assesses current depression level and symptoms of depression. It is a widely used and established measure with demonstrated reliability and validity (26). Higher scores on the BDI reflect higher levels of depression and, more broadly, negative affect (27). There is a detailed protocol and safety plan for any acute psychiatric symptoms or suicidality.
Problematic Eating Behaviors and Adherence to Recommendations.
Eating Disorders Examination-Bariatric Surgery Version (EDE-BSV) (7). The EDE-BSV is a clinical interview measure adapted from the EDE interview (28) for use in bariatric surgery samples. The EDE is considered to have the best validity and reliability in assessing eating disordered behavior and the EDE-BSV contains additional items that are specific to the concerns of weight loss surgery patients (e.g., dumping, plugging, etc.). Similar to the SCID, assessors meet regularly to ensure high inter-rater reliability. Bariatric Surgery Self-Management Questionnaire (BSSQ) (29). Participants complete the BSSQ, a 33-item questionnaire regarding adherence to prescribed behaviors specific to bariatric surgery. This measure asks about adherence over the previous week in eating behaviors, physical activity, dumping syndrome management, intake of fluids, supplements, fruits/vegetables/whole grains, and protein. Subscales for each domain, as well as a total score, are converted to a 0-99 range. Higher scores indicate better adherence. Internal consistency of the BSSQ in bariatric samples is α=.83 (29).
Physical Activity – Objective Measurement of PA.
Participants’ daily time spent in physical activity (PA) and sedentary behavior (SB) at the different pre- and postoperative assessment periods is measured using the newest generation Actigraph monitor—i.e. ActiGraph GT9X Link (AG; Actigraph, LLC, Pensacola, FL, USA). The AG device is worn on the waist and combines a rigorously validated triaxial accelerometer with several advanced new features including a wear time sensor (to detect if the device has been removed) and a secondary accelerometer, gyroscope, and magnetometer that together provide highly advanced data on movement, rotation, and body position (note: the AG’s LCD window display for providing real-time participant feedback will be deactivated during assessments). The AG is shown to reliably measure PA and SB in adults over 7 days in free-living conditions (30). Data from the AG is processed using ActiLife software version 6.11.3 (ActiGraph, LLC, Pensacola, FL, USA). Participants are asked to wear the AG on the waist during all waking hours, exclusive of bathing and swimming, for seven consecutive days at each assessment period (31). Participants are required to provide ≥ four days of data (including one weekend day), defined as ≥600 min of wear time during the hours of 7 am to 11 pm, to be included in analyses. Variables of primary interest will include: 1) total and bout-related moderate-to-vigorous PA and 2) % of daily time spent in SB overall and that accumulated in bouts (e.g., ≥ 30 min) to capture the prolonged nature of SB and related metabolic health risks (32-33).
Cognitive Assessment.
The NIH Toolbox for the Assessment of Neurological and Behavioral Function (34) was developed to assess (via a computer platform) cognitive function across the lifespan and promote generalizability of study findings across researchers. Specific measures utilized are consistent with those found in our past work to predict weight loss outcomes in bariatric surgery patients (35-36) and include the following assessments.
Attention/Executive Function:
The Dimension Change Card Sort Test is similar to the Wisconsin Card Sort Task and assesses hypothesis testing and ability to change mental set in the face of new contingencies. The Flanker Inhibitory Control and Attention Test is similar to the Erikson flanker task and require participants make rapid decisions about the direction of target stimuli. The List Sorting Working Memory Test requires attending to a string of visual and auditory information and reciting them back in pre-determined order.
Memory:
The Picture Sequence Memory Test asks individuals to learn and remember pictured objects and their actions on the screen.
Processing Speed:
The Pattern Comparison Processing Speed Test asks participants to quickly identify if the two visual patterns are the same or different.
Language:
The Picture Vocabulary Test is an adaptive measure of receptive vocabulary and asks individuals to match words to target pictures among a collection of foils.
Gut Microbiome Assessment:
Fecal Collection.
Fecal material is collected by each individual enrolled in our study. At the commencement of our study, and at each study visit, participants are provided a fecal sample collection kit and study cooler. For each study visit participants have the option to provide a fecal sample at the time of the study visit or may do so at home if desired. If home sample collection is preferred, participants are asked to bring the sample to the research facility, on ice/ice-packs, within 24 hours of collection. Samples are labeled and stored at −80°C until analysis. Each sample is transported to the molecular microbiology laboratory on dry ice where it is mechanically homogenized using a sterile spatula and aliquoted into as many 2 mL cryo-tubes as can be filled. The tubes are then labeled and immediately stored in a −80°C freezer. The same procedures are used to obtain subsequent longitudinal samples from individuals 1, 6, 12, 18, and 24 months post-surgery.
Isolation of Microbial DNA.
Microbial DNA are isolated from intestinal tissue and fecal material using a phenol/chloroform extraction method combined with physical disruption of bacterial cells and a DNA clean-up kit (Qiagen DNeasy® Blood and Tissue extraction kit [Qiagen, Valencia, CA]) as previously described (37). The composition of the microbiome will be assessed through 16S high-throughput sequencing as well as through a more comprehensive metagenomic shotgun sequencing approach.
High-throughput Sequencing of 16S rRNA Genes.
Bacterial community composition in isolated DNA from intestinal tissue and fecal material are characterized by amplification of the V6 variable region of the 16S rRNA gene by polymerase chain reaction (PCR) (forward: 5'-CAACGCGARGAACCTTACC-3'; reverse: 5'-CAACACGAGCTGACGAC-3') and sequencing both 5' and 3' ends using the Illumina HiSeq 2500 platform at the high throughput sequencing (HTS) core at REDACTED FOR BLIND REVIEW as previously described (38).
Analysis of 16S rRNA Sequences using the DADA2 Pipeline. 16S rRNA sequence data generated by the Illumina HiSeq 2500 platform will be processed by the Divisive Amplicon Denoising Algorithm 2 (DADA2) pipeline (39), which identifies sequence variants at a 100% identity threshold. Sequence variants will be assigned to a taxonomy using a DADA2-formatted reference database (silva_nr_v128_train_set.fa.gz). Read counts at each taxonomic level will be normalized using a formula as previously described to account for different sequencing depths across samples (40). Shannon diversity index, a measurement of within-sample diversity (α-diversity), will be calculated using the "diversity" function from the vegan package in R. Principal coordinate analysis (PCoA) will be used to visualize between-sample diversity (β-diversity) in the microbial composition. Principal coordinate analysis will be performed using Bray-Curtis disimilarity indices and the "capscale" function from the vegan package in R.
Analysis of 16S rRNA Sequences using the QIIME Pipeline.
16S rRNA sequence data generated by the Illumina HiSeq 2500 platform will be processed by the quantitative insights into microbial ecology (QIIME) pipeline as previously described (41). Sequences will be clustered into Operational Taxonomic Units (OTUs) based on a 97% sequence similarity (similar to species level) using UCLUST (42-43). OTUs will be assigned to a taxonomy using the Ribosomal Database Project (RDP) Naive Bayes classifier (44). The percentages of specific bacterial taxa in each sample will be summarized at 6 levels (phylum, class, order, family, genus, and species). Microbial richness measures will be generated by rarefaction of 16S rRNA sequences and expressed as (i) the number of observed OTUs in each sample and (ii) the Shannon index of diversity. Principal coordinates (PCoA) for each sample using un-weighted and weighted UniFrac distances (45) will also be generated. PCoAs define the similarities or dissimilarities of variables that best represent the pair-wise distances between samples.
Metagenomic Shotgun Sequencing.
We will use the Illumina HiSeq 4000 platform at the REDACTED FOR BLIND REVIEW high throughput sequencing facility to complete whole genome shot-gun sequencing. This approach will be used to characterize the microbiome of all participants at each time-point of data collection. Metagenomic data analysis will occur in collaboration with the REDACTED FOR BLIND REVIEW. Metagenomic DNA sequences will be decontaminated from human genomes using KneadData (http://huttenhower.sph.harvard.edu/kneaddata). The decontaminated sequences will be taxonomically classified using the Kraken2 (46) through an automated pipeline BiolockJ (https://github.com/msioda/BioLockJ). Microbial read counts at each taxonomic level will be normalized as described for the 16S rRNA sequencing data. Microbial gene families and gene pathways will be characterized using the pipeline HUManN2 and abundances will be normalized using the HUMAnN2 tool renorm table (47). Similar to the 16S rRNA sequencing data, α- and β-diversity will be calculated by Shannon Diversity Index and Bray-Curtis dissimilarity, respectively. Principal coordinate analysis will be used to visualize dissimilarities between samples using the "capscale" function in R.
Collection of Additional Information that May Influence the Microbiome.
At study visits, additional data is recorded which may influence the microbiome, including the use of all medications, gastrointestinal surgery history, and menstrual cycle status.
Ethics and informed consent
The study protocol was reviewed and approved by the institutional review board at both sites. Written informed consent from the patient is obtained prior to beginning the initial study visit. The current protocol has been registered on the National Institutes of Health’s clinicaltrials.gov website.
Statistical Analyses and Sample Size Estimation
Preliminary analyses.
Descriptive statistics and frequency distributions will be examined for all screening data, as well as key measures obtained at each assessment. Descriptive statistics will be compiled for the overall sample, as well as separately by site. Distribution diagnostics and outlier analyses will be performed on key variables to inform decisions about the transformation of measures for subsequent analyses. Proportional hazards regression analyses (48) will be used to evaluate whether baseline demographic characteristics or clinical characteristics are associated with study retention. Missing value analyses will be performed to identify missing data patterns and evaluate possible mechanisms for missing data (i.e., MCAR: missing completely at random, MAR: missing at random, MNAR: missing not at random). Variables that are associated with early dropout or missing data will be considered for use as covariates in subsequent analyses to minimize potential biases associated with missing data. Analyses will be performed to compare key baseline and clinical characteristics by site and surgical procedure. Significant differences between groups will help to inform the specifics of the analyses below as well as the use of covariates.
Hypothesis Testing.
Hypothesis 1a:
Patients who report fewer problematic eating behaviors, greater levels of physical activity, less mood symptoms and better cognitive function, will experience a more favorable weight loss trajectory longitudinally following RYGB or SG and greater %EWL from preoperative baseline to 24-months postoperatively. Linear mixed-effects time-lagged models with random intercepts and slopes will be used to evaluate the prospective predictors of weight trajectory. The longitudinal portion of the model will be constructed following the guidelines of Singer and Willet (49), where alternative methods for modeling time (e.g., unconditional means model, unconditional linear growth model, and various unconditional non-linear models) will be compared using the Akaike Information Criterion (AIC). Predictors will then be added to the model as time-varying effects and will be time-lagged such that predictors at time 1 will be used to predict weight at time 2, controlling for time 1 weight. Models will include both the main effects for predictors, as well as the predictor x time interaction(s). Preliminary models will evaluate predictors separately. A multivariate model will then be constructed using significant univariate predictors.
Hypothesis 1b.
Surgical procedure (RYGB vs. SG) will moderate the relationship between eating behavior, physical activity, mood, cognitive function, and weight outcome such that these relationships will be stronger for participants receiving RYGB compared to those receiving SG. The moderating effects of surgical procedure will be evaluated by adding a main effect for surgical procedure, as well as procedure x predictor and procedure x predictor x time interaction(s) to the models above.
Hypothesis 2a.
The presence and abundance of specific taxa within the intestinal microbiota will be associated with patients who experience the most favorable weight outcomes and greatest 24-month %EWL post-surgery. The prospective association between intestinal microbiome predictors and weight trajectories will be evaluated using linear mixed-effects time-lagged models comparable to those described for Hypothesis 1.
Hypothesis 2b.
Surgical procedure (RYGB vs. SG) will moderate the relationship between the composition of the intestinal microbiome and post-surgical weight outcomes wherein RYGB will more strongly influence the relationship between the microbiome and weight outcome compared with SG. The moderating effects of surgical procedure will be evaluated by adding a main effect for surgical procedure, as well as procedure x predictor and procedure x predictor x time interaction(s) to the models above.
Hypothesis 3.
Changes in the composition of the gut microbiome will mediate the relationship between weight trajectory following RYGB or SG and eating and physical activity behaviors, mood, and cognitive function.
Multilevel structural equation modeling (50) with random slopes will be used to evaluate the mediating effects of changes in the gut microbiome. A 1-1-1 MSEM model (referring to the measurement level of the independent, mediator, and dependent variable, respectively) will be constructed with predictors at time 1 as the independent variable, changes in gut microbiome from time 1 to time 2 as the mediator, weight at time 2 as the dependent variable, and weight at time 1 as a covariate. This model provides non-conflated estimates of both the between- and within-subjects indirect effects.
For exploratory analyses, linear mixed-effects time-lagged models with random intercepts and slopes will be used to evaluate whether weight trajectories differ as a function of baseline age, gender, surgergy type (RYGB vs. SG), or the presence or absence of Type 2 Diabetes mellitus.
Power analysis for Hypotheses 1a and 2a were based upon multi-level Monte Carlo simulations using Mplus version 6 software (51). Simulations assumed an unconditional linear model, 6 assessment points, a 30% attrition rate over the 24-month period, and a two-tailed alpha of .05. The magnitude of the predictor main effect was then varied until the null hypothesis of no indirect effect was rejected on 80% of the 10,000 replications (i.e., .80 power). The proposed enrolled sample size of N = 144 (with approximately 100 completing the study) provides a statistical power of .80 to detect a standardized predictor effect of .21. Sample size estimates for Hypotheses 1b and 2b were obtained by adding a predictor x surgery procedure interaction to the simulation models described above and then systematically varying the magnitude of the interaction effect until a power of .80 was obtained. The proposed sample size of 144 provides a statistical power of .80 to detect a standardized interaction effect of .34. Power analyses for Hypothesis 3 were based upon the methods described by Fritz and MacKinnon for determining a required sample size to detect a mediated effect (52). Using the bias-corrected bootstrap method, a sample of size of 71 would provide adequate power (.80) to detect medium effects (.39) for both thea path (IV to mediator) and the b path (mediator to DV). Thus, the proposed enrolled sample of 144 (approximately 100 study completers) provides sufficient power to evaluate the mechanistic role of the gut-brain microbiome.
Trial Status
The trial was successfully registered on clinicaltrials.gov on 2/27/2017 and the IRB’s approved the study on 2/9/2017 (REDACTED FOR BLIND REVIEW) and 12/19/2016 (REDACTED FOR BLIND REVIEW). Recruitment began on 6/20/17 in REDACTED FOR BLIND REVIEW and 6/1/17 in REDACTED FOR BLIND REVIEW. 79 participants at REDACTED FOR BLIND REVIEW and 70 participants at REDACTED FOR BLIND REVIEW have been enrolled (over-enrollment is due to participants having baseline visits but not proceeding to surgery). The final 24 month assessments for all participants are anticipated to occur by the spring of 2022.
Discussion
Although bariatric surgery is the most effective and durable treatment for severe obesity, outcomes are variable and many experience weight regain. Research has yet to establish mechanisms for weight regain although a number of behavioral and biological variables have been posited. The study’s overarching goal is to optimize bariatric surgery weight loss outcomes through identification of key mechanisms that can be targeted for intervention.
This will be the first prospective, longitudinal study to carefully examine the biobehavioral contributors to bariatric surgery weight loss trajectory. Most studies have been limited by short follow-up periods and small sample sizes. In contrast, the current sufficiently powered study includes postoperative follow-up assessments that capture multiple points before weight nadir (1, 6 months), approaching weight nadir (12 months), and after weight nadir when weight regain may occur (18, 24 months)(3). Additionally, the majority of extant data involves either behavioral or biological etiologies rather than utilizing an integrated approach. Limited existing evidence suggests bidirectional communication between the microbiome and brain function both in times of homeostasis and disease (21). This communication seems to occur primarily through complex interactional effects in the hypothalamic-pituitary-adrenal (HPA) axis and structures in the central nervous system (CNS) that can affect cognition, mood, and emotion. There are currently very few data addressing the relationship between post-bariatric surgery changes in the gut microbiota on behavior, and this prospective study will allow us to investigate the gut-brain axis through detailed and comprehensive assessments of the gut microbiota, psychopathology, eating behavior, food intake, and cognition.
This study will help answer whether the gut microbiome changes in response to diet and whether there are associations between the gut microbiome and problematic eating behaviors. The dramatic changes in nutrient intake that follow bariatric surgery are expected to alter the composition of the gut microbiome. Dietary intake over long periods of time plays a major role in shaping the composition and determining the diversity of the intestinal microbiota. The combined approach of 16S and metagenomic shotgun sequencing will allow us to examine both taxonomic and functional changes to the microbiome that occur in response to surgery.
An increasing literature is exploring the likely bi-directional relationship between eating behavior and the gut microbiome. Among animal studies, yo-yo dieting may be as unhealthy as a steadily poor diet (53). To date, there are limited data concerning the effects of anorexia nervosa on the microbiome (54-56), but the influence of binge eating on the intestinal microbiome remains an empirical question, and one that is being addressed in the current study.
The gut microbiome also appears to be sensitive to changes in physical activity. The majority of studies examining the effects of exercise on the intestinal microbiota, however, have been conducted in animal models and shows that exercise affects the diversity of the intestinal microbiome, especially the Bacteroidetes and Firmicutes phyla (57-60) and have not assessed this relationship in a bariatric sample.
Further, a growing number of studies have suggested that the gut microbiome plays a role in depression (61-63). Inducing depression-like states in mice, has been found to result in measurable changes to the intestinal microbiome (64). It appears that the gut microbiome also has the capacity to influence mood and anxiety symptoms (21) Using six data collection time-points spanning from before to 24 months after surgery, we will be able to characterize the temporal relationships between changes in the microbiota and changes in mood. Necessary future studies will be able to build upon data collected in this study, with the aim of exploring the gut-brain-axis in greater detail among bariatric surgery patients. In particular, given the high rates of mood disorders present in the bariatric surgery population and the recurrence of mood symptoms that often occurs distal to surgery, it is particularly important to focus on clarifying mechanisms responsible for enduring or relapsing symptoms.
A growing number of studies also suggest that the gut microbiome is an important contributor to cognitive function. Though the mechanisms remain incompletely understood, gut-brain communication is mediated through multiple systems, including numerous cytokines (65). Mice assigned to a high sucrose diet exhibit a greater reduction in Bacteroidates and poorer performance on a measure of cognitive flexibility (66). Similarly, mice receiving microbiome transplantation from those prescribed a high fat diet show poorer learning of new information and exploratory behavior than controls (67). Few studies have directly examined the relationship between the gut microbiome and cognitive function in humans. An initial study in persons with cirrhosis compared those with and without hepatic encephalopathy (HE) and revealed a pathway from dysbiosis of microbiome to inflammatory processes to cognitive dysfunction in those with HE (68). We have recently generated additional evidence of a positive association between cognition and the gut microbiome (69).
The current study will help elucidate the interaction between biological and behavioral variables and may be used as a framework from which to begin additional more detailed mechanistic assessments between specific significant associations with the gut microbiome and variables of interest after bariatric surgery.
This study will address limitations in previous, smaller investigations of the gut microbiome in bariatric surgery patients that may contribute to inconsistency among findings. Inconsistent results of prior studies may be attributable to several factors. There have been variations in study design, laboratory techniques used to characterize the microbiome, and sample sizes have generally been small. Additionally, limited attention has been given to controlling variables that may confound study results, such as dietary intake (20). The consistent identification of changes in the microbiota of the gut following bariatric surgery, coupled with the inconsistent findings with respect to the downregulation or proliferation of specific bacterial species, supports the need for additional well-controlled research in this area. Each of these limitations will be strengthened by the current study proposal which involves detailed collection of dietary intake information.
The data captured in this study will be instrumental in moving toward precision medicine in bariatric surgical care. We will use these data to identify predictors of post-surgical weight loss outcome so that at-risk patients can be identified and preventative and therapeutic interventions can be implemented in a timely fashion. These data will also serve as the basis for designing subsequent psychotherapeutic and pharmacological weight regain prevention and treatment options to help optimize bariatric surgery outcomes. Ultimately, these data may help to elucidate the mechanism(s) responsible for weight change and weight recidivism following bariatric surgery, which may help in the future development of nonsurgical, less invasive treatment options for severe obesity.
Challenges and Design Considerations.
This study provides one approach to collecting data on the gut microbiome in the bariatric surgery population. This patient population presents numerous challenges to the collection of data on the gut microbiome, and requires a careful balance between limiting confounds and maintaining generalizability. Among the most significant design considerations was whether or not to exclude common disease states or surgical histories that are relatively common but may influence the gut microbiota, and importantly, may also change following surgery (e.g., diabetes, hypertension, history of cholecystectomy). Ultimately, we elected to include all disease states except those which are relatively rare among this patient population and expected to dramatically influence the gut microbiota (e.g., colon resection). Other major design considerations surrounded which medications to include as exclusionary criteria. Ultimately the study team established a list of medications which would be expected to have a profound influence on the gut microbiota when taken routinely, and which may not be controllable analytically due to low base rates of use and possible changes from pre-to-post-surgery in use, such as glucagon-like-peptide agonists, opioids, potent anticholinergics, and a few others with empirical or theoretical impact on the microbiome. Acid-reducing medications, despite their known impact on the microbiota, were permitted because of their pervasive use among this population. An additional major variable we elected to control for by excluding participants or carefully timing baseline data collection was pre-surgery weight loss medications and dietary prescriptions (i.e., liquid diet prior to surgery) which we expected to have a major impact on weight trajectory and the gut microbiota.
Conclusions
Overall, this prospective, multi-center study will provide data from a large sample of bariatric surgery patients who are followed for two years post-operatively. Data will include comprehensive and validated assessments of behavior, along with assessment of the gut microbiome using contemporary shotgun sequencing methodology. We will be able to compare these relationships among patients who undergo RYGB with those who undergo SG. These data will provide an important framework from which to develop subsequent more targeted protocols exploring specific aspects of the gut microbiome and its mechanistic influence on bariatric surgery outcomes. Although the focus of this study is on post-bariatric surgery weight outcomes, data concerning obesity-associated comorbidity resolution and reoccurrence are also being collected. Increasingly, data suggest that the gut microbiome is an important regulator of obesity-associated comorbidities, such as diabetes and cardiovascular disease. Data collected through this study may pave the way to more tailored investigations exploring the mechanisms through which the gut microbiota impacts various disease states and psychopathology.
Suboptimal weight loss and weight regain following bariatric procedures affect a significant subset of patients.
Behavioral and psychological factors have been identified as risk factors. More recently the impact of gut microbiota have also been implicated. However, studies have not examined both of these in prospective trials.
The current trial employs novel data collection and may help inform development of novel interventions that that are tailored to individual patients’ risk profiles to optimize and sustain more favorable weight trajectories.
Acknowledgments
Supported by Grant NIH R01 DK112585-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors do not have any conflicts of interest.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. [DOI] [PubMed] [Google Scholar]
- 2.ASMBS. New Procedure Estimates for Bariatric Surgery: What the Numbers Reveal. 2014; http://connect.asmbs.org/may-2014-bariatric-surgery-growth.html, 2016.
- 3.Courcoulas AP, Christian NJ, Belle SH, et al. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310(22):2416–2425 2410p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight Recidivism Post-Bariatric Surgery: A Systematic Review. Obes Surg. 2013;23(11):1922–1933. [DOI] [PubMed] [Google Scholar]
- 5.Kofman MD, Lent MR, Swencionis C. Maladaptive Eating Patterns, Quality of Life, and Weight Outcomes Following Gastric Bypass: Results of an Internet Survey. Obesity. 2010;18(10):1938–1943. [DOI] [PubMed] [Google Scholar]
- 6.Diamantis T, Apostolou KG, Alexandrou A, Griniatsos J, Felekouras E, Tsigris C. Review of long-term weight loss results after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014;10(1):177–183. [DOI] [PubMed] [Google Scholar]
- 7.Meany G, Conceicao E, Mitchell JE. Binge eating, binge eating disorder and loss of control eating: effects on weight outcomes after bariatric surgery. Eur Eat Disord Rev. 2014;22(2):87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White MA, Kalarchian MA, Masheb RM, Marcus MD, Grilo CM. Loss of control over eating predicts outcomes in bariatric surgery patients: A prospective, 24- month follow-up study.J Clin Psychiatry. 2010;71(2):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheets CS, Peat CM, Berg KC, et al. Post-operative psychosocial predictors of outcome in bariatric surgery. Obes Surg. 2015;25(2):330–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colles SL, Dixon JB, O'Brien PE. Grazing and loss of control related to eating: Two high-risk factors following bariatric surgery. Obesity. 2008;16(3):615–622. [DOI] [PubMed] [Google Scholar]
- 11.Faria SL, Kelly E, Faria OP. Energy Expenditure and Weight Regain in Patients Submitted to Roux-en-Y Gastric Bypass. Obes Surg. 2009;19(7):856–859. [DOI] [PubMed] [Google Scholar]
- 12.Lanyon RI, Maxwell BM, Kraft AJ. Prediction of long-term outcome after gastric bypass surgery. Obes Surg. 2009;19(4):439–445. [DOI] [PubMed] [Google Scholar]
- 13.White MA, Kalarchian MA, Levine MD, Masheb RM, Marcus MD, Grilo CM. Prognostic Significance of Depressive Symptoms on Weight Loss and Psychosocial Outcomes Following Gastric Bypass Surgery: A Prospective 24-Month Follow-Up Study. Obes Surg. 2015;25(10):1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: a systematic review. 2010, 20(5): 657-65. Obes Surg. 2010;20(5):657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mundi MS, Lorentz PA, Swain J, Grothe K, Collazo-Clavell M. Moderate physical activity as predictor of weight loss after bariatric surgery. Obes Surg. 2013;23(10):1645–1649. [DOI] [PubMed] [Google Scholar]
- 16.Bond DS, Phelan S, Wolfe LG, et al. Becoming Physically Active After Bariatric Surgery is Associated With Improved Weight Loss and Health-related Quality of Life. Obesity. 2009;17(1):78–83. [DOI] [PubMed] [Google Scholar]
- 17.Galioto R, Gunstad J, Heinberg LJ, & Spitznagel MB (2013). Adherence and weight loss outcomes in bariatric surgery: Does cognitive function play a role? Obesity Surgery, 23, 1703–1710. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Practice & Research in Clinical Gastroenterology. 2014;28(4):727–740 714p.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husen Z, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani PD. Metabolism in 2013: The gut microbiota manages host metabolism. Nat Rev Endocrinol. 2014;10(2):74–76 73p. [DOI] [PubMed] [Google Scholar]
- 21.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Research Version ed. New York: : Biometrics Research, New York State Psychiatric Institue; November 2002. [Google Scholar]
- 23.Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–332. [DOI] [PubMed] [Google Scholar]
- 24.Kipnis V, Subar AF, Midthune D, et al. Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol. 2003;158(1):14–21; discussion 22–16. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA. Manual for Revised Beck Depression Inventory1987, New York: Psychology Corporation. [Google Scholar]
- 26.Beck AT, Steer RA. Psychometric Properties of the Beck Depression Inventory - 25 Years of Evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 27.Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- 28.Fairburn CG, Cooper Z, Wilson G. The Eating Disorder Examination In Fairburn CG (Ed.), Binge eating: Nature, assessment and treatment 12 ed. New York: Guilford Press; 1993:317–360. [Google Scholar]
- 29.Welch G, Wesolowski C, Piepul B, Kuhn J, Romanelli J, Garb J. Physical activity predicts weight loss following gastric bypass surgery: findings from a support group survey. Obes Surg. 2008;18(5):517–524. [DOI] [PubMed] [Google Scholar]
- 30.Aadland E, Ylvisåker E. Reliability of the Actigraph GT3X+ Accelerometer in Adults under Free-Living Conditions. PLoS ONE. 2015;10(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staudenmayer J, He S, Hickey A, Sasaki J, Freedson P. Methods to estimate aspects of physical activity and sedentary behavior from high-frequency wrist accelerometer measurements. J Appl Physio (1985). 2015;119(4):396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King WC, Chen J-Y, Bond DS, et al. Objective assessment of changes in physical activity and sedentary behavior: Pre- through 3 years post-bariatric surgery. Obesity. 2015;23(6):1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y, Welk GJ, Braun SI, Kang M. Extracting Objective Estimates of Sedentary Behavior from Accelerometer Data: Measurement Considerations for Surveillance and Research Applications. PLoS ONE. 2015;10(2): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toolbox N. Cognition. http://www.nihtoolbox.org/WhatAndWhy/Cognition/Pages/default.aspx. Accessed April 12, 2016.
- 35.Spitznagel MB, Alosco M, Strain G, et al. Cognitive function predicts 24-month weight loss success after bariatric surgery. Surg Obes Relat Dis. 2013;9(5):765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitznagel MB, Alosco M, Galioto R, et al. The role of cognitive function in postoperative weight loss outcomes: 36-month follow-up. Obes Surg. 2014;24(7):1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;64(5):G799–G807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCafferty J, Mühlbauer M, Gharaibeh RZ, Arthur JC, Perez-Chanona E, Sha W, Jobin C, Fodor AA Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J. 2013;7:2116–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wood DE, Salzberg SL, Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014;15:R46–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franzosa EA, McIver LJ, Rahnavard G, Thompson LR, Schirmer M, Weingart G, Lipson KS, Knight R, Caporaso JG, Segata N, Huttenhower C Species-level functional profiling of metagenomes and metatranscriptomes. Nat Meth. 2018;15:962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons; 1980. [Google Scholar]
- 49.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY, US: Oxford University Press; 2003. [Google Scholar]
- 50.Preacher KJ, Zyphur MJ, Zhang Z. A general multilevel SEM framework for assessing multilevel mediation. Psychol Methods. 2010;15(3):209–233. [DOI] [PubMed] [Google Scholar]
- 51.Mplus Users’ Guide. Sixth Edition. [computer program]. Los Angeles, CA: Muthen & Muthen 1998-2011. [Google Scholar]
- 52.Fritz MS, MacKinnon DP. Required Sample Size to Detect the Mediated Effect. Psychol Sci. 2007;18(3):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaakoush NO, Martire SI, Raipuria M, et al. Alternating or continuous exposure to cafeteria diet leads to similar shifts in gut microbiota compared to chow diet. Mol Nutr Food Res. 2016; 61(1):1–17. [DOI] [PubMed] [Google Scholar]
- 54.Kleiman SC, Watson HJ, Bulik-Sullivan EC, et al. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom Med. 2015;77(9):969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleiman SC, Carroll IM, Tarantino LM, Bulik CM. Gut feelings: A role for the intestinal microbiota in anorexia nervosa? Int J Eat Disord. 2015;48(5):449–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carr J, Kleiman SC, Bulik CM, Bulik-Sullivan EC, Carroll IM. Can attention to the intestinal microbiota improve understanding and treatment of anorexia nervosa? Expert Rev Gastroenterol Hepatol. 2016:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121(6):725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLoS One. 2014;9(3):e92193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambert BS, Shimkus KL, Fluckey JD, et al. Anabolic responses to acute and chronic resistance exercise are enhanced when combined with aquatic treadmill exercise. Am J Physiol Endocrinol Metab. 2015;308(3):E192–200. [DOI] [PubMed] [Google Scholar]
- 60.Petriz BA, Castro AP, Almeida JA, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. 2013;16(3):240–245. [DOI] [PubMed] [Google Scholar]
- 62.Liang S, Wang T, Hu X, et al. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. [DOI] [PubMed] [Google Scholar]
- 63.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? J Neurogastroenterol Motil. 2013;25(9):713–719. [DOI] [PubMed] [Google Scholar]
- 64.Park AJ, Collins J, Blennerhassett PA, et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. J Neurogastroenterol Motil.2013;25(9):733–e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saulnier DM, Ringel Y, Heyman MB, et al. The intestinal microbiome, probiotics and 66. Magnusson KR, Hauck L, Jeffrey BM, et al. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. 2015;300:128–140. [DOI] [PubMed] [Google Scholar]
- 67.Bruce-Keller AJ, Salbaum JM, Luo M, et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry. 2015;77(7):607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bajaj JS, Hylemon PB, Ridlon JM, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manderino L, Carroll I, Azcarate-Peril MA, Rochette A, Heinberg L, Peat C, Steffen K, Mitchell J, Gunstad J. Preliminary Evidence for an Association Between the Composition of the Gut Microbiome and Cognitive Function in Neurologically Healthy Older Adults. J Int Neuropsychol Soc. 2017. September;23(8):700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]


