Abstract
Background:
Fear responding in posttraumatic stress disorder (PTSD) is sexually heterogeneous and varies with hormonal fluctuations throughout the menstrual cycle. While research suggests that estrogen levels affect PTSD symptoms among women, there is a dearth of research on modulatory effects of estrogen on fear responding among women with PTSD, and neural outcome measures are lacking.
Methods:
Forty women with PTSD underwent two consecutive, alternating blocks of fear conditioning and extinction training, during which a CS+, but not CS−, predicted occurrence of an electric shock in acquisition, but not extinction, contexts. Assayed saliva determined estradiol levels. Skin conductance response (SCR) and whole-brain voxelwise activity during fMRI were outcome variables in linear mixed effects models, with estradiol level, PTSD severity, and task contrasts as predictors.
Results:
SCR exhibited a significant Estradiol x PTSD severity x Habituation interaction (t=3.180, p=.002), such that PTSD severity was correlated with increased arousal responding between training blocks among women with lower (t=−3.985, p<.001) but not higher (t=0.550, p=0.583) Estradiol. Voxelwise activity additionally demonstrated an identical three-way interaction within dorsomedial prefrontal cortex and anterior insula clusters. The SCR and imaging interactions between PTSD severity and estradiol were not specific to CS type or Context.
Conclusions:
Estradiol moderated the relationship between PTSD severity and arousal response habituation between fear conditioning and extinction training sessions, such that high estradiol protected against the negative impact of severe PTSD symptoms on fear habituation. These findings suggest that estrogen enhances habituation among women with severe PTSD, potentially influencing the efficacy of extinction-based therapies.
Keywords: PTSD, fear extinction, habituation, estrogen, hormones, imaging
Introduction
Posttraumatic Stress Disorder (PTSD) is costly and highly impairing[1]. Development of PTSD following a traumatic experience is highly discrepant between sexes, with an estimated lifetime prevalence ratio of 2:1 (females to males)[2,3]. Women are more likely to be exposed to certain types of traumatic experiences (e.g., intimate partner violence, sexual violence) than men, yet, psychosocial factors, such as the severity of trauma exposure, do not fully explain sex differences in PTSD prevalence rates[4]. Sex-linked factors, such as differences in gonadal hormone levels, appear to contribute to this discrepancy[5,6]. Major reproductive hormone fluctuations (such as postpartum and perimenopause) are temporally linked with pronounced vulnerability to anxiety and mood dysregulation in women, possibly underpinning mood-related outcomes in females[7,8]. Additionally, hormonally cycling women with PTSD commonly report more severe re-experiencing, anxiety, and depressive symptoms during phases in the menstrual cycle characterized by low estrogen levels in comparison to high estrogen level timepoints of the menstrual cycle[9–11]. Furthermore, administering exogenous estradiol to ovariectomized rodents and postmenopausal adult women has been shown to reduce anxious behavior and enhance memory[12], suggesting a protective role of estrogen in regulatory cognitive-emotional processes. Overall, extant research suggests that estrogen levels confer either protection from (when high estrogen) or vulnerability to (when low estrogen) posttraumatic and related symptoms, which may partially explain disproportionate risk among women for developing PTSD following a traumatic event. The link between ovarian hormones and specific cognitive-emotional mechanisms linked with posttraumatic psychopathology, however, remain underexplored.
Fear and safety learning processes have emerged as a set of etiologically relevant mechanisms related to PTSD with which estrogen may interact in women. PTSD is canonically related to impairments in two distinct mechanisms of associative safety learning: fear extinction learning, i.e. the within-session process of associating safety with a previously threatening conditioned stimulus (CS+), and fear extinction recall, i.e. between-session retention of safety cue associations and decreased fear responding [13–18]. Estrogen appears to interact with fear extinction processes among women with PTSD, however, estrogen may instead exert generalized effects on overall arousal by influencing non-associative safety learning, such as arousal habituation [19]. Failure-to-habituate in PTSD is conceptualized as a failure to diminish persistent arousal responding with repeated presentation of both conditioned stimuli (CS)[16]. Failure-to-habituate also implicates stress psychophysiology and fear neurocircuitry related to re-experiencing and hyperarousal symptoms of PTSD [20–23]. Notably, aberrant fear extinction mechanisms in PTSD are complex and are not likely explained by a single impaired learning mechanism [16]. Additionally, fear extinction analyses do not always account for the influence of overall arousal habituation. In order to understand the mechanism through which estradiol interacts with PTSD, one must statistically distinguish fear and arousal measurement. Whether fear extinction occurs specifically toward the CS+ or overall arousal habituation toward both CS, fear reduction abnormalities characterize PTSD as a disorder involving persistent impaired physiological and neural fear responding with which estrogen may interact.
Neurocircuitry models of PTSD often implicate hypoactive blood-oxygen-level-dependent (BOLD) activation within the ventromedial prefrontal cortex (vmPFC) and hippocampus, accompanied by exaggerated responsivity of the anterior insular cortex (AIC), dorsal anterior cingulate cortex / dorsomedial PFC (dmPFC) and amygdala [24–27]. In these models, the hypoactive vmPFC fails to exert inhibitory control over the amygdala, resulting in a hyperactive fear response, while disrupted hippocampal and AIC gating fails to modulate this inhibitory pathway, resulting in disrupted fear regulation [24,28]. Notably, fear extinction circuitry hubs that are commonly dysregulated among individuals with PTSD, including the vmPFC, hippocampus, and amygdala, contain highly expressed estrogen receptors, sensitive to fluctuating estradiol levels [29,30]. Additionally, cycling women exhibit greater activation in neural regions associated with fear conditioning and extinction, specifically, the vmPFC, hippocampus, and amygdala, during low estrogen menstrual cycle phases in comparison to high estrogen phases, suggesting that estrogen influences fear and safety learning processes in women through interactions with fear and inhibition neurocircuitry [31].
Although relationships between estrogen and fear acquisition remain unclear [32,33], interactions with fear conditioning processes consistently emerge in fear extinction recall. For example, female humans and non-human animals learning fear extinction during high estrogen timepoints of the menstrual/estrus cycle exhibit better extinction recall than when extinction learning occurs during low estrogen timepoints [34–38]. Additionally, estradiol administration during fear extinction learning demonstrates that estradiol enhances extinction memory recall [29,38,39]. Women and animal models consistently demonstrate effects of estrogens on cognitive-behavioral responding; however, directionality of functional magnetic resonance imaging (fMRI) findings exploring the same modulatory relationship is less clear [40]. Some evidence suggests that high estrogen is related to enhanced fear extinction and habituation learning, as demonstrated through attenuated functional activation of limbic neurocircuitry [31] coupled with increased vmPFC activity [39]. Together, findings from diverse scientific disciplines suggest that estrogen influences extinction and inhibition learning and corresponding neurocircuitry in healthy women, which may explain variations in PTSD symptomology across the menstrual cycle.
The fear- and anxiety-related elements of PTSD psychopathology are primarily characterized by a marked failure to consolidate and recall extinction memories [16]; yet, it is unclear whether findings suggesting protective effects of estrogen on fear extinction learning among healthy women generalize to women with PTSD. In a psychologically healthy sample of women exposed to a psychosocial stressor, women with lower estradiol exhibited deficits in fear response inhibition compared to women with higher estradiol [41–43]. This suggests that stressful experiences, such as a trauma, interact with estrogen’s protective effects on fear responding, and thus, high estrogen might confer unique benefits to a clinical population characterized by impaired fear extinction processes. Indeed, emerging research among women with PTSD demonstrates that during fear extinction training, women with low estradiol levels show higher conditioned responding (i.e. worse extinction memory learning) in comparison to women with higher estradiol [32,44,45]. These studies suggest a potentially deleterious effect of low estradiol (and a protective effect of high estradiol) on fear inhibition among women with PTSD. Investigating the role of estrogen on posttraumatic stress physiology and neurocircuitry during fear and safety learning processes would continue to clarify hormonal mechanisms of increased risk for PTSD related to biological sex. Clinically, investigations inform the potential to use hormones as cognitive enhancers to improve effectiveness of extinction-based treatments (i.e. prolonged exposure) for PTSD, which rely upon fear and safety learning mechanisms including non-associative habituation processes.
The current study aims to address this dearth of research by exploring a modulating role of estradiol on neurocircuitry of fear and safety learning among women with PTSD. Participants underwent a cued contextual fear conditioning paradigm involving acquisition and extinction phases during fMRI. Fear acquisition, extinction learning, and habituation were measured through skin conductance responding (SCR) and BOLD activation of brain regions canonically involved in fear extinction and PTSD, such as the vmPFC, AIC, dmPFC, amygdala, and hippocampus[27,46]. We hypothesized that throughout fear and extinction learning, salivary estradiol levels would moderate the relationship between fear responding and PTSD severity such that high estradiol levels would be related to overall lower fear responding throughout the fear and extinction learning task blocks. Specifically, we predicted that estradiol would be related to enhanced context-cued extinction memory learning, resulting in lower responding to the CS+ (as opposed to CS−) among women with higher estradiol. Furthermore, we predicted that among women with higher estradiol levels, women with more severe PTSD symptoms would exhibit a greater fear response difference between fear and extinction learning task blocks, in comparison to women with less severe PTSD symptoms.
Methods
Participants
Forty-three biologically-female women between the ages of 21–50 with a current PTSD diagnosis related to interpersonal violence exposure were recruited through advertisement from the local community. The sample was sourced from a larger clinical trial, selected based on extant estradiol data collected from a subset of the clinical trial sample. Participants were stable on psychiatric medication for at least four weeks prior to participation, and all participants prescribed acute-acting medications (e.g., benzodiazepines, stimulants) were required to forgo that medication on the fMRI scan day. Participants were not excluded for contraception use and were recruited to participate across all stages of the menstrual cycle. Menstrual cycle stage was not estimated due to high rates of menstrual cycle irregularity in the current sample, which is a common occurrence among trauma-exposed females [47]. Before analyzing effects of estradiol level on SCR and imaging data, one participant was excluded from all analyses due to having an outlier estradiol level. Five participants whose galvanic skin response showed excessive artifact or flat responding were excluded from SCR analyses, and two participants with excessive head motion during scanning were excluded from fMRI analyses. This resulted in a final sample of 42 participants free of major medical disorders, neurological disorders, active substance use disorders, psychotic symptoms, and MRI contraindications. See Table 1 for sample demographics, medication use, and clinical diagnoses. All procedures were approved by the university Institutional Review Board and all participants provided informed consent.
Table 1.
Demographics, contraceptive use, smoking status, and psychiatric medication use correlated with salivary estradiol level. All participants prescribed acute-acting psychotropic medications (stimulants, benzodiazepines, etc.) refrained from taking that medication on the day of the scan. All scans occurred between the hours of 1:00 — 7:00pm. The racial categories used by the US Census (African-American, Asian-American, Native-American, Latinx, and Pacific Islander) are included in the category “non-White.” Digit span is from the Wechsler Adult Intelligence Scale-IV. GAD = Generalized Anxiety Disorder. CAPS = Clinician Administered PTSD Scale. A value followed by s indicates a statistically significant Spearman correlation (p < .05).
| Variable | Participants N=42 | p values |
|---|---|---|
| Age (yr) | 30.8 (8.2) | .721 |
| Education (yr) | 16.2 (2.4) | .258 |
| BMI | 27.2 (6.4) | .908 |
| Non-White Ethnicity (%) | 21.4 | .502 |
| Digit span | 10.0 (2.7) | .709 |
| Direct assault types (#) | 4.6 (2.5) | .187 |
| Sexual assault (%) | 92.9 | .636 |
| Physical assault (%) | 78.6 | .363 |
| Physical abuse (%) | 50.0 | .732 |
| Age first assault (yr) | 10.2 (6.4) | .317 |
| Age last assault (yr) | 25.5 (8.5) | .285 |
| Time since last assault (yr) | 5.4 (6.7) | .067 |
| Current mood disorder (%) | 35.7 | .262 |
| Current anxiety disorder (%) | 76.2 | .756 |
| Current GAD (%) | 57.1 | .364 |
| CAPS total severity | 41.5 (10.8) | .818 |
| Any | 52.4 | .805 |
| Daily cigarette smoker (%) | 11.9 | .450 |
| Any psychotropic med | 45.2 | .514 |
Assessments
Prior to participating in the fear conditioning task, participants completed structured clinical interviews with trained research staff, including the Clinician Administered PTSD Scale for DSM-5 (CAPS-5) [48] to assess PTSD symptom severity, the trauma assessment portion of the National Women Survey (NWS) [38–40] to assess history of interpersonal trauma, and Structural Clinical Interview for DSM-IV (SCID-IV) [52] to assess current comorbid Axis I disorders and psychotic symptoms. Participants also reported menstrual cycle regularity and contraceptive types.
Estrogen Assays
Salivary samples were collected between 3–8:00pm, one hour following fear conditioning and extinction training. Samples were assayed using a Salimetrics enzyme-linked immunosorbent assay kit to determine concentration of a predominant estrogen, 17β-estradiol, within each sample. See Supplemental Material for additional details. Spearman correlations were conducted to examine relationships between estradiol levels and demographic and clinical variables, shown in Table 1.
Fear Conditioning and Fear Extinction Task
During fMRI and SCR acquisition, participants completed a context-cued fear learning paradigm modeled in two prior studies and depicted in Figure S1 [53]. The unconditioned stimulus (US) was a mild, yet aversive electrotactile stimulation administered to the participant’s left shin. Participants calibrated their individualized level of “maximally uncomfortable, but not painful” stimulation prior to task administration. Stimulation workup consisted of delivering up to four stimulations gradually increasing in intensity until an individual gave a rating of 7/10 discomfort [54]. Conditioned stimuli (CS) were two different geometric shapes, each alternating in display for 3s with a jittered inter-trial interval of 2–6s. Colored backgrounds served as cues distinguishing acquisition and extinction contexts. Shapes serving as CS+ versus CS− and background colors distinguishing contexts were counterbalanced across participants. An initial baseline phase consisted of 6 presentations of each stimulus with no US onsets. The task then alternated between acquisition (A) and extinction (E) phases two times each (AEAE) with each complete acquisition and extinction learning phase (AE) representing one learning block. The difference in responding between the first and second learning block will be referred to as “Habituation.” During acquisition, each CS was presented 18 times, with a 50% probability of US delivery 2.5s after CS+ presentation (per the Partial Reinforcement Extinction Effect), but while CS+ remained displayed [55]. The extinction phase presented each stimulus 18 times with no US deliveries. Across two blocks of Acquisition and Extinction learning, participants completed 156 total trials. Participants’ instruction on the task was to identify the stimulus presented on each trial through button-press, and they were not explicitly informed about contingencies between stimuli and shocks. Self-reported expectancy ratings were not included for reasons detailed in Supplemental Material. Thus, analyses focus on SCR and imaging data collected throughout the task.
MRI and SCR Acquisition and Processing
SCR Analysis
SCR measured autonomic responses to cued-contextual fear conditioning and extinction. SCR data were analyzed with linear mixed-effects (LME) models, including factors for Estradiol level, PTSD severity, CS (CS+ versus CS−), Context (Acquisition versus Extinction), Slope (a linear habituation regressor for repeated stimuli presentation within a block), and Habituation (Learning Block 1 versus Learning Block 2) interactions. Age, Education, Ethnicity, and Contraceptive Use were included as covariates. In order to validly interpret interaction effects across variables, analyses centered raw scores of Estradiol level, PTSD severity, Age, and Education through z-scoring [56]. A categorical Contraceptive Use variable was determined by coding either a “1” or “−1” if the participant endorsed using or not using any hormonal contraceptive, respectively.
Voxelwise Activation Analyses during Fear Acquisition and Extinction Training
We assessed neural activation during fear conditioning and extinction using whole-brain voxelwise BOLD activation for fMRI. Individual voxelwise generalized linear models (GLMs) were implemented in AFNI (3dDeconvolve, 3dREMLfit), computing mean images corresponding to task condition phases of interest: CS+/− during acquisition and extinction, excluding images acquired during baseline and shock administration[57]. The resulting within-subject β coefficients were carried forward into a group-level LME model including factors for Estradiol level, PTSD severity, CS (CS+ versus CS−), Context (Acquisition versus Extinction), and Habituation (Learning Block 1 versus Learning Block 2) interactions. Age, Education, Contraceptive Use, and Head Motion (mean number of TRs included after censoring) were used as covariates, again computing raw score of Head Motion, Estradiol level, PTSD severity, Age, and Education into Z-scored values. Cluster-level thresholding controlled for voxelwise comparisons using an uncorrected p = 0.001 and cluster size k >= 18 [57,58].
Results
Estradiol and clinical factors
Estradiol level was significantly correlated with SNRI use, but no other clinical or demographic variables (Table 1). The impact of Estradiol level on SCR when considering possible confounding effect of participants taking psychotropic medication is described in Table S4.
Fear conditioning and SCR
The LME model verified the expected CS x Context interaction, t(272) = 5.630, p < .001. Contrary to expectation, we observed no statistically significant Estradiol x CS x Context interaction, t(272) = .441, p = .660. SCR was not directly related to Estradiol level, t(272) = −0.024, p = .981. Contraceptive Use was not significantly related to SCR, t(272) = −0.709, p = .479. All main effects and interactions resulting from the omnibus LME model are detailed in Table S1. Omnibus LME modeling demonstrated a significant Habituation (Learning Block 1 vs Learning Block 2) x Estradiol x PTSD severity interaction, t(272) = 3.180, p = 0.002.
Estradiol and SCR habituation
We probed the omnibus interaction with subsequent tests to determine the nature of the three-way interaction, conducting separate analyses for low Estradiol (L-EST) and high Estradiol (H-EST), dividing participants by median Estradiol level. Mean SCR between L-EST and H-EST participants for each stimulus presentation (CS+ versus CS−) within each Context (Acquisition versus Extinction) is depicted in Figure 1a.
Figure 1.
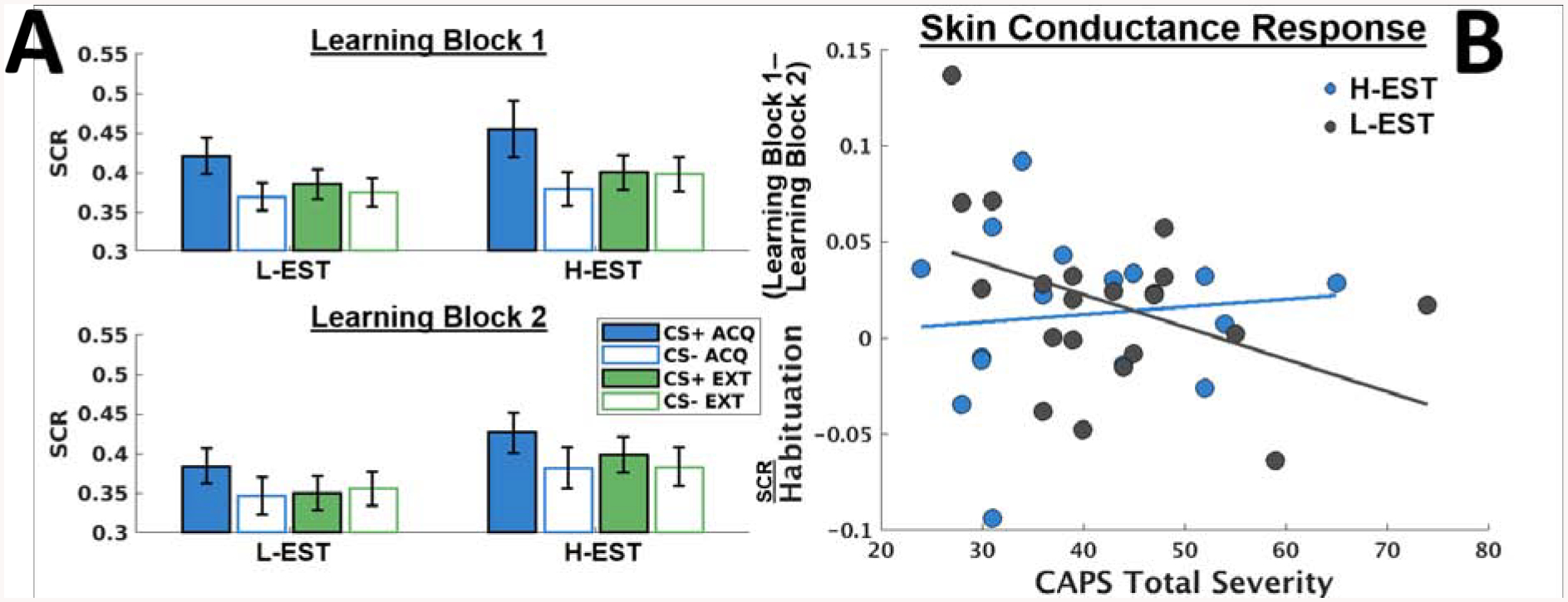
A) Bar graphs indicating skin conductance responses (SCR) throughout two Learning Blocks of a contextual fear conditioning task during fMRI, with each Learning Block consisting of two alternating blocks of fear Acquisition (ACQ) and fear Extinction (EXT) phases, respectively. ACQ and EXT task phases were administered using different background colors indicating distinct contexts. In order to probe direction of interactions, mean skin conductance responses across stimulus type (CS+ versus CS−) are reported separately, distinguishing between low salivary estradiol level (L-EST) versus high salivary estradiol level (H-EST), determined by median split. Error bars denote standard errors. B) Scatterplot depicting relationships between Habituation (difference between SCR throughout Learning Block 1 and Learning Block 2) and PTSD severity (determined with the Clinician-Administered PTSD Scale for DSM-5 [CAPS]), plotted separately for L-EST and H-EST participants. Habituation between Learning Blocks is negatively related to PTSD severity among L-EST participants, t(132)=−3.985, p<.001, but not among H-EST participants, t(136)=0.550, p=0.583.
PTSD severity was inversely related to SCR Habituation (Habituation x PTSD severity interaction) among L-EST women, t(132) = −3.985, p < .001, but not H-EST, t(136) = 0.550, p = 0.583. This interaction among L-EST participants is attributed to less Habituation from Learning Block 1 to Learning Block 2 among L-EST participants with more severe PTSD, t(64) = 4.622, p < .001, whereas PTSD severity was not related to Habituation among H-EST participants (t(68)=.92, p = .36). Mean SCR as a function of PTSD severity between Learning Blocks for H-EST versus L-EST groups is shown in Figure 1b.
In order to aid interpretation, we explored whether Estradiol level interacts with the relationship between Habituation and PTSD symptom clusters (Habituation x Estradiol x PTSD symptom cluster severity). Estradiol was most strongly related to decreased fear responding as a function of PTSD Re-experiencing Symptom Severity (Habituation x Estradiol x Re-experiencing Severity), t(272) = 4.511, p < .001. Estradiol was also related to decreased fear responding as a function of PTSD Avoidance Symptom Severity (Habituation x Estradiol x Avoidance Severity), t(272) = 3.657, p < .001, and PTSD Negative Cognitions/Mood (Habituation x Estradiol x Negative Cognitions/Mood Severity), t(272) = 2.412, p = .017, but not Hyperarousal/reactivity (Habituation x Estradiol x Hyperarousal Severity), t(272) = 0.455, p = .649.
The Supplemental Material describes an additional analysis including a linear slope regressor to test for a relationship between estradiol and within-Block habituation, distinct from between-Learning Block Habituation, within the same LME model.
Estradiol and whole-brain voxelwise activation
Corroborating the SCR results, the omnibus voxelwise LME demonstrated significant clusters of activation in the left AIC, dmPFC, and left hippocampus (Figure 2A), among other clusters that withstood thresholding (Figure S2; Table 2), for the Habituation x Estradiol x PTSD severity interaction.
Figure 2.
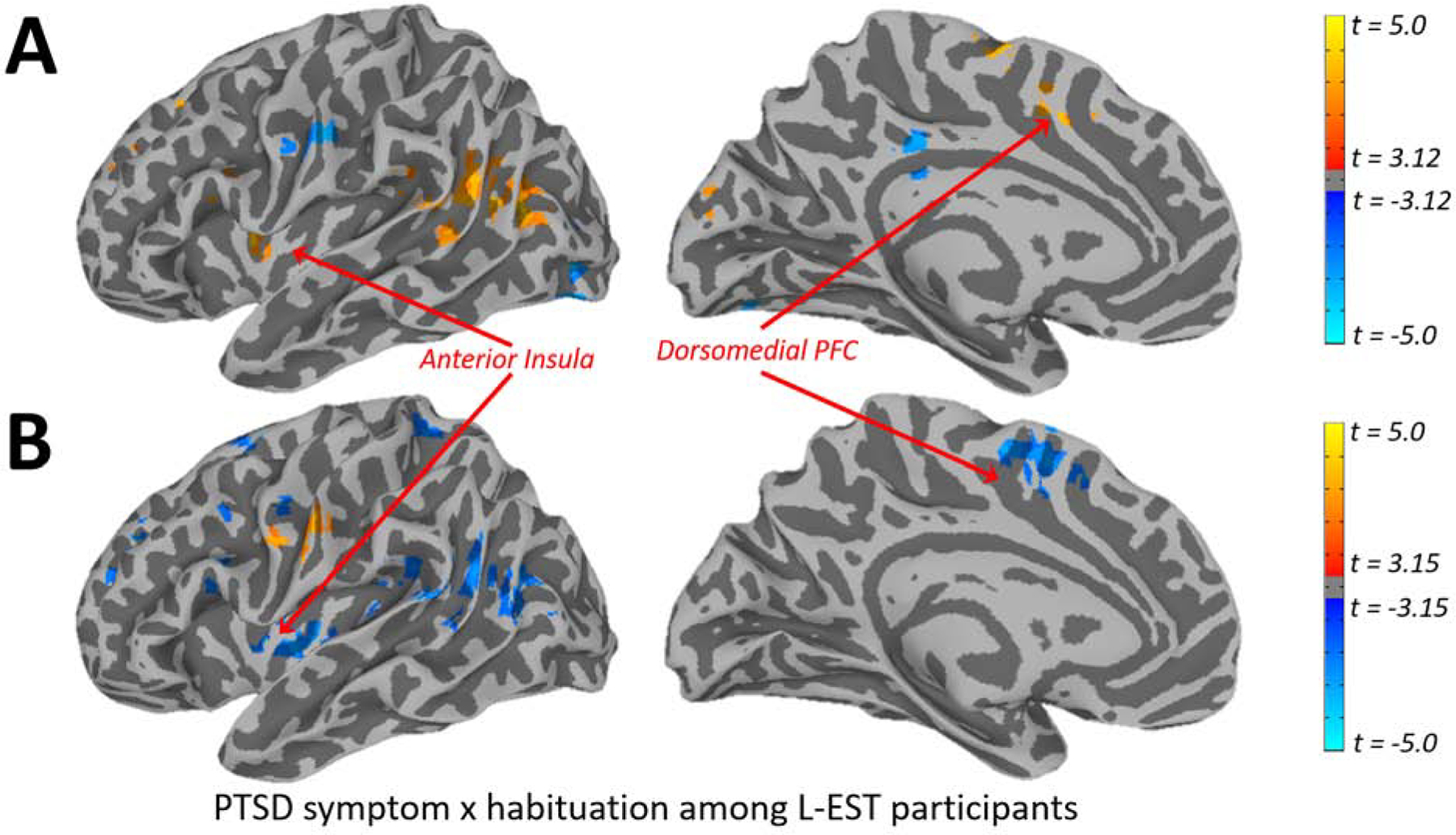
A) Depiction of whole-brain voxelwise clusters exhibiting a Habituation x PTSD severity x Estradiol level interaction during two learning blocks of a context-cued fear conditioning and extinction task, identified using Linear Mixed Effects modeling. Habituation was determined using the difference between BOLD activation throughout Learning Block 1 and Learning Block 2, PTSD severity was determined using the CAPS for DSM-5, and salivary estradiol level was determined using ELISA assay. Table 2 provides sizes and coordinates of significant clusters of activation. B) Corresponding depiction of whole-brain voxelwise clusters exhibiting a Habituation x PTSD severity interaction specifically among low estradiol (L-EST) participants, identified via median split. As can be seen, the analysis among only the L-EST participants demonstrates overlap with omnibus LME (Figure 2A) in the left anterior insula and dmPFC, among other regions. This analysis also demonstrates the direction of the effect in the omnibus LME: that increasing PTSD symptom severity is associated with lower Habituation.
Table 2.
Omnibus whole-brain voxelwise LME across all participants. Significant clusters of activation for the Estradiol x Habituation (Learning Block 1 vs Learning Block 2) x PTSD severity interaction from the linear mixed-effects analysis on imaging data during the fear learning and extinction paradigm. Cluster thresholding was implemented using AFNIs 3dClustSim, in which a voxel-level uncorrected p < .001 was used with a cluster size of k>=18 and a corrected threshold of t=3.120. Images used Orig space and RPI orientation.
| Region | MNI Center-of-Mass Coordinates | Peak t | Cluster Size | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left temporoparietal junction | −46 | −59 | 16 | 7.33 | 176 |
| Right supramarginal gyrus | 54 | −23 | 38 | −6.05 | 128 |
| Left middle insula | −39 | 6 | 6 | 5.88 | 110 |
| Right precentral gyrus | 42 | 5 | 45 | −6.69 | 68 |
| Right V4/inferior occipital gyrus | 30 | −86 | −4 | −6.75 | 59 |
| Right precuneus | 10 | −67 | 34 | −4.70 | 55 |
| Left temporoparietal junction | −60 | −43 | 10 | 4.96 | 54 |
| Right inferior parietal lobule | 50 | −46 | 40 | −5.30 | 49 |
| Right precentral gyrus | 21 | −28 | 60 | 5.40 | 48 |
| Right superior frontal gyrus | 21 | 22 | 53 | −5.62 | 46 |
| Left posterior cingulate | 0 | −31 | 33 | −4.37 | 34 |
| Left supplementary motor area | −4 | 9 | 45 | 5.20 | 34 |
| Left postcentral gyrus | −59 | −11 | 33 | −4.85 | 33 |
| Left hippocampus | −26 | −10 | −10 | 5.44 | 31 |
| Left V4/middle occipital gyrus | −29 | −84 | 1 | −6.23 | 28 |
| Right inferior parietal lobule | 42 | −55 | 50 | −5.73 | 28 |
| Left V4/lingual gyrus | −26 | −85 | −16 | −5.19 | 27 |
| Left cerebellum | −7 | −60 | −11 | 5.31 | 26 |
| Left frontal eye fields/middle frontal gyrus | −27 | 29 | 42 | 5.55 | 26 |
| Right V2/cuneus | 4 | −90 | 18 | 4.60 | 25 |
| Right cerebellum | 45 | −59 | −32 | 4.26 | 24 |
| Right temporal pole | 43 | 15 | −19 | 5.36 | 24 |
| Left dorsolateral prefrontal cortex (dlPFC) | −24 | 46 | 28 | 4.57 | 24 |
| Right cerebellum | 2 | −46 | −49 | 4.42 | 23 |
| Left supplementary motor area | −7 | −7 | 62 | 5.75 | 23 |
| Left cerebellum | −16 | −75 | −26 | 5.91 | 22 |
| Right temporoparietal junction | 40 | −59 | 14 | −5.19 | 22 |
| Right caudate | 15 | 14 | 19 | −4.77 | 22 |
| Left interior frontal gyrus | −49 | 14 | 21 | 4.38 | 19 |
| Right middle frontal gyrus | 33 | 0 | 61 | −5.63 | 19 |
| Cerebellum | 11 | −74 | −36 | 4.16 | 18 |
We probed the three-way interaction in whole-brain voxelwise analyses identically to SCR analyses, dividing participants into L-EST and H-EST groups determined by a median split. Among L-EST participants, analyses identified a significant Habituation x PTSD symptom severity interaction (Figure 3) in the left AIC, dmPFC, and additional regions (Figure 2B; Table S2), such that L-EST participants with more severe PTSD symptoms demonstrated less Habituation. Contrary to expectations from the omnibus LME, this analysis among the L-EST participants did not demonstrate a Habituation x PTSD symptom severity interaction in the left hippocampus. The whole-brain voxelwise analysis did not demonstrate significant clusters in the left AIC, dmPFC, or left hippocampus with respect to the Habituation x PTSD symptom severity interaction among H-EST participants (Table S3), suggesting a unique relationship between Habituation and PTSD symptom severity only among L-EST participants.
Figure 3.
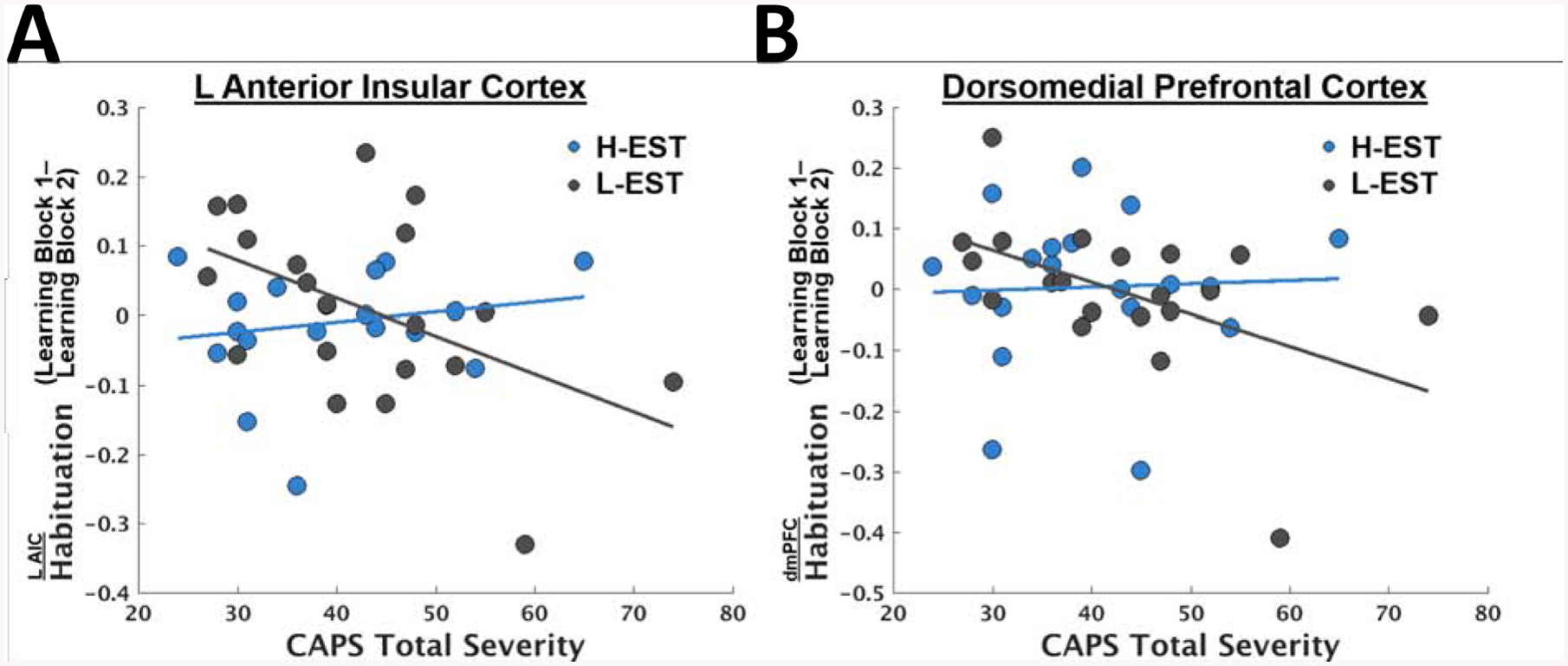
Scatterplots depicting relationships between PTSD symptom severity and Habituation (difference in responding between Learning Block 1 and Learning Block 2) in the left anterior insular cortex (AIC) (A) and dorsomedial prefrontal cortex (dmPFC) (B). These ROIs were taken from the conjunction of clusters identified in the omnibus Habituation x PTSD symptom x Estradiol analysis (Figure 2A above) and the Habituation x PTSD symptom analysis among just the participants with low estradiol, determined by a median split.
Finally, we conducted post-hoc tests of whether significant clusters from the above analyses (i.e., emerging in both the omnibus whole-brain LME interactions and the whole-brain LME interaction among the L-EST group) were predictive of the SCR measure of Habituation. Here, we focused on the left AIC and dmPFC clusters, as these are consistent with canonical fear extinction circuitry[46]. Controlling for the same covariates, SCR Habituation was significantly related to Habituation in the left AIC, t(32) = 2.36, p = .0245, but not the dmPFC, t(32) = 1.69, p = .10.
Discussion
The present study examined a modulatory role of a predominant estrogen, estradiol, on psychophysiological and neural responsivity during fear conditioning and extinction training among women with PTSD. We found that estradiol modulated the negative relationship between PTSD severity and SCR habituation, such that participants with high estradiol and severe PTSD symptoms exhibited SCR habituation across learning trial blocks, whereas participants with low estradiol and severe PTSD symptoms failed to exhibit SCR habituation. Furthermore, estradiol demonstrated an similar modulatory role in the relationship between PTSD severity and habituation in the left AIC and dmPFC, key nodes implicated in fear extinction and PTSD neurocircuitry[27,46]. Specifically, increasing PTSD severity was associated with less habituation among L-EST participants but not H-EST participants, suggesting a protective effect of estradiol on the deleterious impact of PTSD symptoms on fear response habituation. Surprisingly, estradiol did not significantly augment SCR or BOLD responsivity toward the fear or safety cue (CS+ versus CS−) nor context cue (acquisition versus extinction context). Instead, estradiol modulated overall (i.e., stimulus and context non-specific) SCR and neural responsivity among women with PTSD. The lack of specificity towards stimulus or context suggests that estradiol influences responding through a mechanism more related to arousal rather than fear specifically; accordingly, we discuss the results in the context of arousal rather than fear.
Prior literature demonstrates that PTSD is accompanied by impaired habituation, and that failure-to-habituate may be specifically related to re-experiencing symptoms [21]. Current findings suggest that estradiol enhances habituation to conditioned stimuli, especially among individuals with more severe PTSD symptoms, and most strongly related to re-experiencing symptoms. Differential relationships between habituation and PTSD severity by estradiol level suggest that among participants with severe PTSD, low estradiol confers vulnerability to sustained arousal responding, whereas high estradiol confers protection against failure-to-habituate [44]. Although trial-by-trial learning (indexed via linear responding within testing blocks) was not related to estradiol in the current study, SCR differences between learning blocks appear to reflect habituation to the presentation of previously learned stimuli as a function of estradiol. That is, habituation to stimuli, contexts, and US contingencies with which the participant is already familiar. Altogether, psychophysiological findings demonstrate a protective role of estradiol against failure-to-habituate in severe PTSD.
We also observed a corroborative interaction between estradiol and neural attenuation in women with severe PTSD, evidenced through impaired BOLD attenuation within the left AIC and dmPFC among L-EST participants, but not H-EST participants. One role of dmPFC and AIC activity might be stimulus novelty and salience, in which case, failure-to-habituate in L-EST individuals might reflect sustained novelty and salience processing [59]. An alternative explanation is that prolonged AIC and dmPFC activity might reflect sustained allocation of attentional resources, in which case prolonged responding among L-EST individuals might reflect sustained allocation of attention to the task stimuli. Relatedly, extinction failure may involve sustained dmPFC and AIC activation during conditioned threat exposure[46]. Indeed, evidence suggests links between increased AIC activity and re-experiencing symptoms in individuals with PTSD [60]. Estradiol did not demonstrate a robust interaction with amygdala or vmPFC activity during fear extinction learning, consistent with recent meta-analyses suggesting that neither the amygdala nor vmPFC are recruited during fear extinction learning in [28]. This suggests that during fear extinction learning, estradiol is related to decreased responding through a different mechanism than inhibitory signaling via the vmPFC. Together, our findings extend previous findings of aberrant neural habituation implicated in anxiety disorders, identifying estradiol as an interacting variable within PTSD-related neural correlates.
Overall, the current results characterize interactions between estradiol and arousal responding during fear conditioning and extinction among women with PTSD using a multi-modal approach. Limited prior studies have demonstrated that estradiol may enhance threat contingency learning in PTSD as measured by BOLD activation or SCR [29,39,44,61]; however, our data do not demonstrate a link between estradiol levels and enhanced CS+/CS− discrimination in either neural activation nor SCR during fear acquisition. Prior literature also demonstrates estrogens interacting with associative fear extinction in women with PTSD, yet, the current data also do not support estradiol enhancing fear extinction [34,40,45]. Instead, the data suggest a link between estradiol levels and enhanced non-associative arousal habituation. Further research should clarify discrepant results across studies measuring estradiol and fear extinction in women with PTSD. Future studies should also test fear extinction recall, retention, and reinstatement to assess whether estradiol modulates long-term extinction retention in broader clinical populations characterized by aberrant extinction learning. Protective effects of endogenous estradiol may have implications for improving efficacy of extinction-based therapies that focus on reducing fear responding, like prolonged exposure therapy. Both associative fear extinction and non-associative arousal habituation (between-session) are implicated as key mechanisms involved in decreasing fearful responding throughout extinction-based therapies [62,63]. Whether estradiol promotes fear extinction or arousal habituation, either mechanism might encourage overall decreased fear/arousal responding, perhaps increasing the effectiveness of extinction-based therapies [62].
The current study has several limitations. Importantly, there is wide individual variation in response to estradiol depending on a person’s underlying hormonal milieu. Prior studies tracking anxiogenic patterns throughout the menstrual cycle demonstrate that relative hormone increases are more informative than absolute hormone levels [7,8,54]. Yet, because trauma exposure is highly comorbid with menstrual cycle irregularity, tracking menstrual cycles of trauma-exposed women can be practically difficult and contribute to research participation burden. Thus, because we directly measured estradiol levels, rather than relying upon menstrual cycle staging, we cannot determine whether estradiol demonstrates phasic vulnerability. Additionally, approximately half of the current sample were using hormonal contraceptives, and therefore not regularly cycling. Therefore, although analyses controlled for contraceptive use, the current study could not address potential differences between low estradiol levels due to cycling versus artificially low levels of estradiol induced by contraceptives. Unfortunately, inability to control for menstrual phase neither a priori nor post hoc warrants the possibility that other circulating sex steroid hormones, such as progesterone, may also influence fear responsivity. However, prior research suggests that estrogens predominantly modulate behavioral and neural activation rather than progesterone and other circulating gonadal hormones [34,37,39]. Yet, potential confounding effects of other circulating estrogens, gonadal hormones, or neurotropic factors cannot be excluded. Finally, the current study does not address how relationships between estradiol and fear extinction may differ in a comparison group (e.g. trauma-exposed controls), which is an important question for future research. Therefore, although the study is limited in generalizability to a trauma-exposed control group, current findings are clinically relevant for women with symptoms of PTSD.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Patrick H. Roseboom for valuable help training and coordinating estradiol ELISA assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Portions of this work were supported by NIH grants MH106860, MH108753, and the Brain and Behavior Foundation. All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. J Clin Psychiatry. 2000;61:4–14. [PubMed] [Google Scholar]
- 2.Shansky RM. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress. 2015;1:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebron-Milad K, Abbs B, Milad MR, Linnman C, Rougemount-Bücking A, Zeidan MA, et al. Sex differences in the neurobiology of fear conditioning and extinction: a preliminary fMRI study of shared sex differences with stress-arousal circuitry. Biol Mood Anxiety Disord. 2012;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G, et al. Trauma and PTSD in the WHO World Mental Health Surveys. Eur J Psychotraumatology. 2017;8:1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslau N The epidemiology of posttraumatic stress disorder: What is the extent of the problem? J Clin Psychiatry. 2001;62:16–22. [PubMed] [Google Scholar]
- 6.Kessler RC. Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048. [DOI] [PubMed] [Google Scholar]
- 7.Rapkin AJ, Mikacich JA, Moatakef-Imani B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. 2002;4:419–428. [DOI] [PubMed] [Google Scholar]
- 8.Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: A continuum of vulnerability? J Psychiatry Neurosci. 2008;33:331–343. [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant RA, Felmingham KL, Silove D, Creamer M, O’Donnell M, McFarlane AC. The association between menstrual cycle and traumatic memories. J Affect Disord. 2011;131:398–401. [DOI] [PubMed] [Google Scholar]
- 10.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen Levels Are Associated with Extinction Deficits in Women with Posttraumatic Stress Disorder. Biol Psychiatry. 2012;72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeng LY, Milad MR. Sex Differences in Anxiety Disorders: Interactions between Fear, Stress, and Gonadal Hormones. Horm Behav. 2015;76:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol Behav. 2010;99:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothbaum BO, Davis M. Applying Learning Principles to the Treatment of Post-Trauma Reactions. Ann N Y Acad Sci. 2003;1008:112–121. [DOI] [PubMed] [Google Scholar]
- 14.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behav Res Ther. 2005;43:1391–1424. [DOI] [PubMed] [Google Scholar]
- 15.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. [DOI] [PubMed] [Google Scholar]
- 16.Lissek S, van Meurs B. Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int J Psychophysiol. 2015;98:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: Relation to symptom severity. Psychiatry Res. 2009;167:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers KM, Davis M. Behavioral and Neural Analysis of Extinction. Neuron. 2002;36:567–584. [DOI] [PubMed] [Google Scholar]
- 19.Bangasser DA, Eck SR, Ordoñes Sanchez E. Sex differences in stress reactivity in arousal and attention systems. Neuropsychopharmacology. 2019;44:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberzon I Neuroimaging Studies of Emotional Responses in PTSD. Ann N Y Acad Sci. 2006;1071:87–109. [DOI] [PubMed] [Google Scholar]
- 21.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear Extinction in Traumatized Civilians with Posttraumatic Stress Disorder: Relation to Symptom Severity. Biol Psychiatry. 2011;69:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pole N The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychol Bull. 2007;133:725–746. [DOI] [PubMed] [Google Scholar]
- 23.McCurry KL, Frueh BC, Chiu PH, King-Casas B. Opponent Effects of Hyperarousal and Re-experiencing on Affective Habituation in Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019. 25 September 2019. 10.1016/j.bpsc.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin LM, Liberzon I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology. 2010;35:169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauch SL, Shin LM, Phelps EA. Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research—Past, Present, and Future. Biol Psychiatry. 2006;60:376–382. [DOI] [PubMed] [Google Scholar]
- 26.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. [DOI] [PubMed] [Google Scholar]
- 27.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–2142. [DOI] [PubMed] [Google Scholar]
- 28.Fullana MA, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, et al. Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants. Neurosci Biobehav Rev. 2018;88:16–25. [DOI] [PubMed] [Google Scholar]
- 29.Chang Y-J, Yang C-H, Liang Y-C, Yeh C-M, Huang C-C, Hsu K-S. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor β. Hippocampus. 2009;19:1142–1150. [DOI] [PubMed] [Google Scholar]
- 30.Östlund H, Keller E, Hurd YL. Estrogen Receptor Gene Expression in Relation to Neuropsychiatric Disorders. Ann N Y Acad Sci. 2006;1007:54–63. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal Cycle Modulates Arousal Circuitry in Women Using Functional Magnetic Resonance Imaging. J Neurosci. 2005;25:9309–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeng LY, Milad MR. Sex Differences in Anxiety Disorders: Interactions between Fear, Stress, and Gonadal Hormones. Horm Behav. 2015;76:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia NM, Walker RS, Zoellner LA. Estrogen, progesterone, and the menstrual cycle: A systematic review of fear learning, intrusive memories, and PTSD. Clin Psychol Rev. 2018;66:80–96. [DOI] [PubMed] [Google Scholar]
- 34.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lonsdorf TB, Haaker J, Schümann D, Sommer T, Bayer J, Brassen S, et al. Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: the role of biological sex, contraceptives and menstrual cycle phases. J Psychiatry Neurosci JPN. 2015;40:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milligan-Saville JS, Graham BM. Mothers do it differently: reproductive experience alters fear extinction in female rats and women. Transl Psychiatry. 2016;6:e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White EC, Graham BM. Estradiol levels in women predict skin conductance response but not valence and expectancy ratings in conditioned fear extinction. Neurobiol Learn Mem. 2016;134 Pt B:339–348. [DOI] [PubMed] [Google Scholar]
- 38.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammoud MZ, Foa EB, Milad MR. Oestradiol, threat conditioning and extinction, post-traumatic stress disorder, and prolonged exposure therapy: A common link. J Neuroendocrinol;n/a:e12800. [DOI] [PubMed] [Google Scholar]
- 41.Antov MI, Stockhorst U. Stress exposure prior to fear acquisition interacts with estradiol status to alter recall of fear extinction in humans. Psychoneuroendocrinology. 2014;49:106–118. [DOI] [PubMed] [Google Scholar]
- 42.Cheung J, Chervonsky L, Felmingham KL, Bryant RA. The role of estrogen in intrusive memories. Neurobiol Learn Mem. 2013;106:87–94. [DOI] [PubMed] [Google Scholar]
- 43.Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem. 2014;116:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pineles SL, Nillni YI, King MW, Patton SC, Bauer MR, Mostoufi SM, et al. Extinction retention and the menstrual cycle: Different associations for women with posttraumatic stress disorder. J Abnorm Psychol. 2016;125:349–355. [DOI] [PubMed] [Google Scholar]
- 46.Fullana MA, Albajes-Eizagirre A, Soriano-Mas C, Vervliet B, Cardoner N, Benet O, et al. Fear extinction in the human brain: A meta-analysis of fMRI studies in healthy participants. Neurosci Biobehav Rev. 2018;88:16–25. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs MB, Boynton-Jarrett RD, Harville EW. Adverse childhood event experiences, fertility difficulties and menstrual cycle characteristics. J Psychosom Obstet Gynecol. 2015;36:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984. [DOI] [PubMed] [Google Scholar]
- 50.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National Estimates of Exposure to Traumatic Events and PTSD Prevalence Using DSM-IV and DSM-5 Criteria: DSM-5 PTSD Prevalence. J Trauma Stress. 2013;26:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. J Consult Clin Psychol. 2003;71:692–700. [DOI] [PubMed] [Google Scholar]
- 52.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 53.Haaker J, Gaburro S, Sah A, Gartmann N, Lonsdorf TB, Meier K, et al. Single dose of l-dopa makes extinction memories context-independent and prevents the return of fear. Proc Natl Acad Sci. 2013;110:E2428–E2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test). Nat Protoc. 2012;7:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bouton ME, Woods AM, Todd TP. Separation of Time-Based and Trial-Based Accounts of the Partial Reinforcement Extinction Effect. Behav Processes. 2014;101:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schumacker R, Robinson C, Schumacker RE. Interacting effects: Centering, variance inflation factor and interpretation issues. Multiple Linear Regression Viewpoints. 2009. [Google Scholar]
- 57.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res Int J. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 58.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avery SN, Blackford JU. Slow to warm up: the role of habituation in social fear. Soc Cogn Affect Neurosci. 2016;11:1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: Symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Trauma Stress. 2007;20:713–725. [DOI] [PubMed] [Google Scholar]
- 61.Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, et al. Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci JPN. 2013;38:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sripada RK, Rauch SAM. Between-session and within-session habituation in Prolonged Exposure Therapy for posttraumatic stress disorder: a hierarchical linear modeling approach. J Anxiety Disord. 2015;30:81–87. [DOI] [PubMed] [Google Scholar]
- 63.Cooper AA, Clifton EG, Feeny NC. An Empirical Review of Potential Mediators and Mechanisms of Prolonged Exposure Therapy. Clin Psychol Rev. 2017;56:106–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


