Abstract
Objective:
We performed a systematic literature review to identify all reports of immune checkpoint inhibitor–associated inflammatory arthritis to describe it phenotypically and serologically.
Methods:
PubMed, Embase, and Cochrane databases were searched for reports of musculoskeletal immune-related adverse events secondary to ICI treatment. Publications were included if they provided individual patient level data regarding the pattern of joint involvement. Descriptive statistics were used to summarize results.
Results:
A total of 4339 articles were screened, of which 67 were included, encompassing 372 patients. The majority of patients had metastatic melanoma (57%), and they were treated with anti-PD1 or anti-PDL1 therapy (78%). Median time to onset of arthritis was 4 months (range, 1 day to 53 months). Forty-nine percent had polyarthritis, 17% oligoarthritis, 3% monoarthritis, 10% arthralgia, and 21% polymyalgia rheumatica. More than half of patients were described as having a “rheumatoid arthritis– like” presentation. Nine percent tested positive for rheumatoid factor or anti–cyclic citrullinated peptide antibodies. Seventy-four percent required corticosteroids, and 45% required additional medications. Sixty-three percent achieved arthritis control, and 32% were ultimately able to discontinue antirheumatic treatments. Immune checkpoint inhibitors were continued in 49%, transiently withheld in 11%, and permanently discontinued due to musculoskeletal immune-related adverse events in 13%.
Conclusions:
Half of reported immune checkpoint inhibitor–associated arthritis cases present with polyarthritis (often RA-like), but only 9% are seropositive. Polymyalgia rheumatica is also common. Most patients respond to steroids alone, but about half require additional medications. Further studies are needed to determine long-term musculoskeletal outcomes in these patients, and the impact of arthritis treatment on cancer survival.
Keywords: checkpoint inhibitor, inflammatory arthritis, immune-related adverse event, rheumatoid arthritis, immunotherapy
The use of immune checkpoint inhibitors (ICIs) has dramatically changed the landscape of cancer therapeutics. These agents have been shown to provide significant survival benefits for a wide range of malignancies where traditional chemotherapies have failed. At this time, there are 7 ICIs targeting either cytotoxic T-lymphocyte–associated protein-4 (CTLA-4), programed cell death protein 1 (PD-1), or programed death ligand 1 (PD-L1) that have been approved by the US Food and Drug Administration to treat a large and ever-increasing variety of malignancies. Some of these malignancies include melanoma, non–small cell lung cancer, renal cell carcinoma, urothelial cancers, Merkel cell carcinoma, and Hodgkin lymphoma1; however, the indications for use of ICIs are continuing to grow and evolve. Immune checkpoint inhibitors are monoclonal antibodies that function by blocking the immune checkpoints, CTLA-4 and PD-1/PD-L1, which normally downregulate T-cell activation, preventing autoreactivity and protecting “self” tissues from damage. Tumor cells exploit these T-cell tolerance mechanisms in order to evade detection and proliferate. Immune checkpoint inhibitor treatment blocks inhibitory signaling molecules so that T cells can mount effective antitumor response.2
This enhanced T-cell response may also lead to increased inflammation of various organ systems, including the skin, gastrointestinal tract, liver, and thyroid,3,4 manifesting as inflammatory skin conditions, diarrhea/colitis, hepatitis, and hypothyroid/hyperthyroid states, respectively. These events have been termed immune-related adverse events (irAEs) and have been well-documented and studied in large cohorts given the relatively high frequency of their occurrence.1,3 Immune checkpoint inhibitor arthritis, defined as inflammatory joint pain that results as a consequence of ICI treatment, has also been reported,5–7 but cohort sizes are relatively small and the condition remains poorly characterized. In a systematic literature review of ICI clinical trials, Cappelli et al estimated the incidence of arthralgia as 1% to 43% and of arthritis 1% to 7%. This wide estimate range suggests significant heterogeneity in the ascertainment of musculoskeletal symptoms within oncology clinical trials.8 The prevalence of rheumatoid factor (RF) and/or anti–cyclic citrullinated peptide (CCP) antibody seropositivity also varies widely in published series of ICI-associated arthritis.5–7 The objective of this study was to analyze all published cases of ICI arthritis in order to describe the condition phenotypically. Secondary aims were to determine the frequency of RF and/or anti-CCP positivity and to describe treatment strategies that have been used.
METHODS
This systematic review was designed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) framework, and registered with the International Register of Prospective Reviews (PROSPERO) (registration CRD42019138288).
We reviewed English-language full-length articles as well as abstracts in order to define arthritis phenotypes seen after treatment with ICI.
Inclusion criteria were as follows: (1) publications must describe arthritis as an adverse effect after treatment with checkpoint inhibitors (anti-CTLA-4 and/or anti-PD-1/PD-L1), and (2) data must be provided regarding arthritis phenotype, specifically describing the number and/or pattern of joints involved. Abstracts, short reports, and-full length articles were eligible for inclusion.
Exclusion criteria were as follows: (1) non-English manuscripts; (2) animal studies; (3) clinical trials (which do not describe arthritis phenotypes); (4) reviews without original data; (5) editorials; (6) reports of patients with preexisting systemic autoimmune disease; (7) ICI cohort studies describing all irAEs that lacked a description of arthritis phenotypes; and (8) cases of mechanical (noninflammatory) joint pain in patients who received an ICI.
Information Sources and Search Strategy
A medical librarian searched the MEDLINE database in PubMed, the EMBASE database, and the Cochrane Library (including the Cochrane Central Register of Controlled Trials, Health Technology Assessment Database, and NHS Economic Evaluation Database). The search strategy can be found in Supplemental Document 1, http://links.lww.com/RHU/A187. Screening of titles and abstracts was performed using Covidence software. Publications were included up until the end date of May 31, 2019.
Publication Selection
Titles, abstracts, and full-texts were all screened for inclusion by at least 2 authors (A.B., N.G., and/or K.C.) for relevance and inclusion of original data (Fig.). Discrepancies were discussed and mediated by the third reviewer. Abstracts and letters or brief correspondences were included if an arthritis phenotype was provided. Three reviewers (N.G., M.T., and C.S.) independently abstracted data from manuscripts. Discrepancies were resolved by consensus. In publications where inflammatory arthritis pheno-types were alluded to but not explicitly mentioned, an attempt to reach the corresponding author via electronic mail for phenotypic description was made, and the studies were included if additional information was provided. Manuscripts or abstracts with duplicate cohorts were excluded, and when necessary, the duplication was confirmed upon correspondence with the authors.
FIGURE.
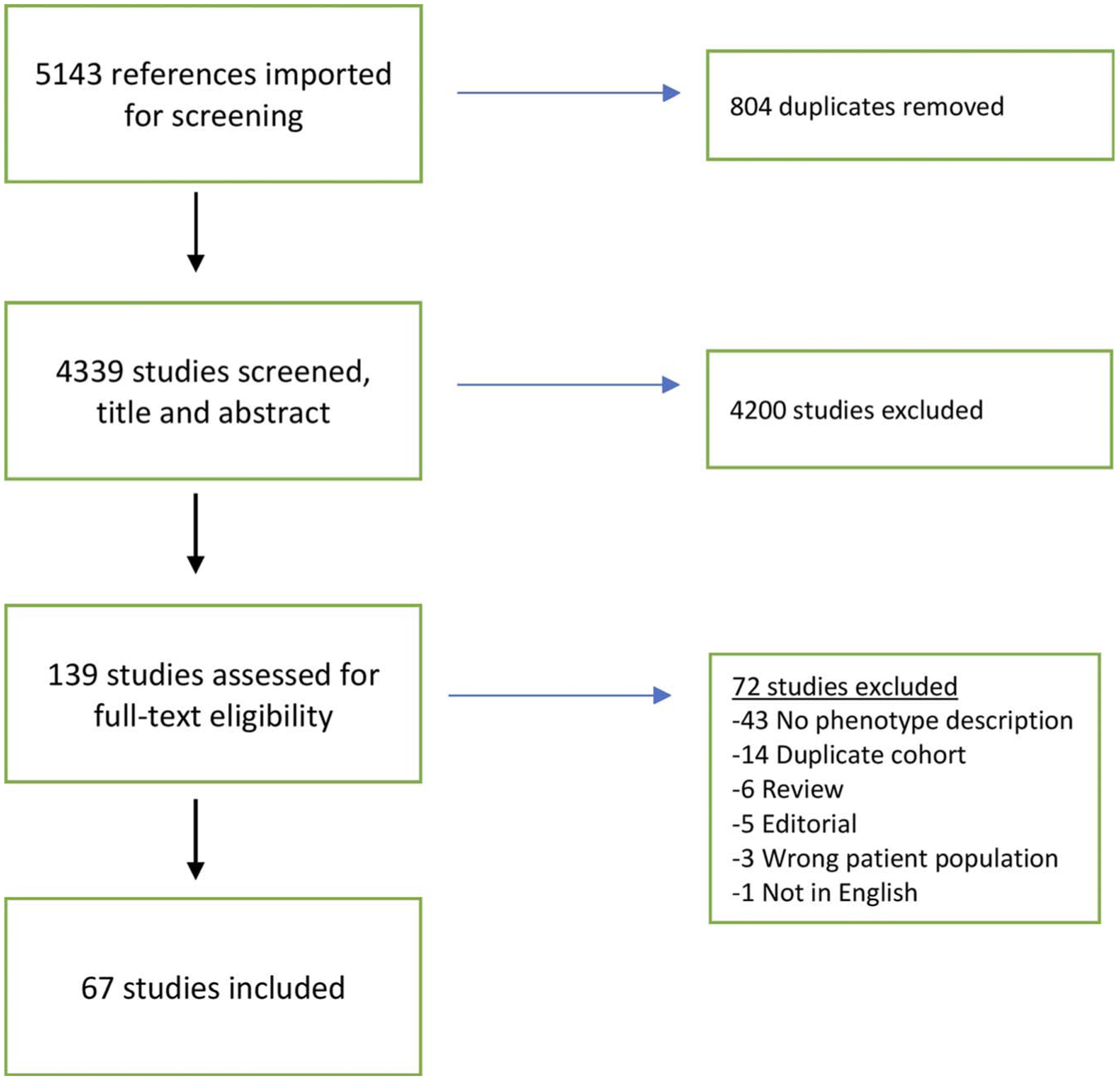
Flow diagram of publication identification. Color online-figure is available at http://www.jclinrheum.com.
Arthritis phenotype was characterized as 1 of the 5 entities: polyarticular (>4 joints), oligoarticular (2–4 joints), monoarticular, arthralgia (inflammatory joint pain without evidence of arthritis), or polymyalgia rheumatica (PMR). If reported, note was also made of any similarity to de novo rheumatologic conditions (rheumatoid arthritis, psoriatic arthritis, spondyloarthritis). Additional information such as the presence of preexisting osteoarthritis, tendon involvement, dactylitis, crystal disease, or pitting edema were noted. We also documented the number of patients in each study, age, sex, race, cancer type, ICI given, time to onset of arthritis, grade of adverse event according to Common Terminology Criteria for Adverse Events9 (CTCAE) classification, serologies, family history of rheumatic disease, arthritis treatments, arthritis outcomes, oncologic outcomes according to Response Evaluation Criteria in Solid Tumors,10 and other irAE experienced. Missing data were marked as not reported, and the total number of patients with reportable data was calculated for each data element in order to provide denominators.
Quality Assessment
All included studies provided level 4 or 5 evidence level (grade D) according to the Oxford Center for Evidence-Based Medicine levels of evidence.11
Statistical Analysis
Descriptive statistics were used to summarize the data.
Sources of Funding
Carolyn Stewart received funding from the Weill Cornell Work Study Program (Cornell Student Employment) and a grant from the Hospital for Special Surgery, Department of Rheumatology. Michael Tiongson received funding from Albany Medical College 2019 Summer Research Fellowship. Laura Cappelli, MD, has received funding from NIAMS K23 AR075872.
RESULTS
A total of 4339 titles/abstracts were screened, 139 full-texts were reviewed, and 67 publications were selected for inclusion (Fig.), encompassing 372 patients (Table 1). Forty-two publications were case reports, 15 were case series, and 10 were retrospective chart reviews. Thirty-seven were full-length manuscripts, 10 were abstracts, 16 were letters to editors, and 4 were brief communications (Supplemental Document 1, Table 2, http://links.lww.com/RHU/A187).
TABLE 1.
Immunotherapy Arthritis Patient Characteristics (Total n = 372)
| Variable (No. Cases Reporting) | Proportion % (95% CI) |
|---|---|
| Mean age (SD), y (n = 234) | 63 ± 11 |
| Male (n = 291) | 61 (55–66) |
| Cancer types (n = 322) | |
| Melanoma | 57 (51–62) |
| NSCLC | 22 (18–27) |
| Renal cell carcinoma | 7 (5–10) |
| Head and neck | 2 (1–4) |
| Hodgkin lymphoma | 2 (1–4) |
| Merkel cell carcinoma | 1 (0–3) |
| Other | 9 (7–13) |
| Cancer treatment (n = 372) | |
| Anti-PD-1/anti-PD-L1 | 78 (73–82) |
| Anti-CTLA-4 | 5 (4–8) |
| Combination | 17 (13–21) |
| Combination then anti-PD-1/anti-PD-L1 | 10 |
| Combination then anti-CTLA-4 | 1 |
| Months to onset, median (IQR), (n = 162)* | 4 (1–6) |
| Months to onset, range | 0–53 |
| Joint involvement (n = 372) | |
| Polyarthritis | 49 (44–54) |
| Oligoarthritis | 17 (14–21) |
| Monoarthritis | 3 (2–6) |
| Arthralgia | 10 (7–13) |
| PMR† | 21 (17–25) |
| Arthritis joint pattern (n = 181) | |
| Rheumatoid arthritis | 65 (58–72) |
| Large joint | 22 (17–29) |
| PsA/spondyloarthritis | 13 (9–18) |
| Serologies | |
| RF(n = 251) | 9 (6–13) |
| CCP (n = 248) | 8 (5–12) |
| RF and/or CCP (n = 270) | 9 (6–13) |
| ANA ≥1:40 (n = 193) | 30 (24–36) |
| Systemic steroid usage (any) (n = 353) | 74 (69–79) |
| Steroid dose (n = 162) | |
| ≥20 mg prednisone | 45 (37–52) |
| <20 mg prednisone | 54 (47–62) |
| “Pulse” methylprednisolone | 1 (0–4) |
| Additional arthritis treatment (n = 350)‡ | |
| None | 55 (50–61) |
| NSAIDs | 20 (16–24) |
| DMARDs | 31 (27–36) |
| Methotrexate | 12 (9–16) |
| Hydroxychloroquine | 11 (8–15) |
| Sulfasalazine | 5 (3–8) |
| TNF inhibitor | 5 (3–8) |
| IL-6R inhibitor | 3 (2–6) |
| Arthritis outcomes (n = 260) | |
| Ongoing and uncontrolled | 4 (2–7) |
| Improving with treatment | 17 (13–22) |
| Controlled with treatment | 46 (40–52) |
| Recovered off treatment | 32 (27–38) |
| Recovered but with lasting damage | 1 (0–3) |
| Cancer status at last follow-up (n = 209) | |
| Complete response | 20 (15–26) |
| Partial response | 33 (27–40) |
| Stable | 21 (16–27) |
| Progression | 27 (21–33) |
| Other irAE, any grade (n = 248) | 52 (45–58) |
| ICI continuation/discontinuation (n = 286)§ | |
| Continued | 49 (43–55) |
| Transiently held, then rechallenged | 11 (8–15) |
| Permanently discontinued, any reason | 38 (32–44) |
| Permanently discontinued, arthritis | 13 (9–17) |
NSCLC, non–small cell lung carcinoma; PMR, polymyalgia rheumatica; PsA, psoriatic arthritis; NSAIDs, nonsteroidal anti-inflammatory drugs; TNF, tumor necrosis factor; IL-6R, interleukin 6 receptor.
Time from initiation of checkpoint inhibitor(s). Only available from publications with individual-level data reporting time to onset.
15/78 PMR patients had hand involvement.
Some patients were on multiple medications; thus, totals do not fully add up.
7 patients (2%) had completed ICI therapy before symptom onset.
Arthritis Phenotypes
Of the 372 cases, 49% had polyarthritis (>4 joints involved), 17% oligoarthritis, 3% monoarthritis, and 10% arthralgia. The remaining 78 cases (21%) had a PMR phenotype, of whom 15 also had small joint arthritis. Other arthritis descriptors were also used by authors in half the cases (n = 181). Of these, 65% had “rheumatoid arthritis– (RA-) like” disease, 22% had exclusively “large joint arthritis,” and 13% had spondyloarthritis/psoriatic arthritis. Three of the patients with RA-like disease were also described as having remitting seronegative symmetrical synovitis with pitting edema (RS3PE). Recurrent crystalline disease, both gout and pseudogout, was noted in 2 patients that appeared to be temporally related to ICI therapy. Arthritis CTCAE grade was documented in only 114 patients. Of these, 36% were grade 1, 49% grade 2, and 15% grade 3. Median time to arthritis onset, reported in 162 patients, was 4 months (range, 1 day to 53 months).
Serologies
Rheumatoid factor and/or anti-CCP antibody testing was positive in 9% (25/270) of cases (9% CCP, 7% RF positive). Six patients were documented to have had autoantibodies prior to initiation of ICI (4 CCP, 2 RF); 5 remained seropositive after ICI therapy while the sixth did not have antibodies rechecked. ANA was positive (≥1:40) in 30% (57/193) of cases, and HLA-B27 was positive in 4/32 patients tested.
Other Patient Characteristics
Demographics
Mean age was 63 ± 11 years, 61% (176/291) were male, and there was a family history of autoimmune disease in 20 of 86 patients in whom it was reported (23%). The most common cancer types were melanoma (57%) and non–small cell lung cancer (22%). The majority of patients received monotherapy with either an anti–PD-1 or anti–PD-L1 antibody (78%). Fifty-two percent of patients experienced other irAE.
Treatment
Of 353 patients with treatment reported, 74% received systemic steroids, often high-dose (>20 mg daily prednisone). Of the 350 patients with other additional treatments reported, 31% (109/350) received 1 or more disease-modifying antirheumatic drugs (DMARDs), either conventional or biologic, such as methotrexate (12%), hydroxychloroquine (11%), sulfasalazine (5%), TNF inhibitors (5%), and/or IL-6R inhibitors (3%). Many individuals required more than one of these additional treatments, sometimes simultaneously. Arthritis outcomes were described in 260 cases, of which 63% improved and/or achieved control, 32% recovered completely off treatment and 4% had ongoing/uncontrolled symptoms at the time of publication. Two patients recovered from their arthritis but suffered lasting structural damage (ie, erosions) that caused functional impact.
Checkpoint Inhibitor Discontinuation
Of 286 cases reporting, 49% continued the ICI, 11% transiently held the ICI and were then rechallenged, and 38% had their ICI permanently discontinued. A third of ICI discontinuations were due to ICI arthritis. Reasons for ICI discontinuation other than arthritis included other irAE, cancer progression, or cancer remission. In 2% of cases, the ICI had already been discontinued at the time of arthritis onset.
Cancer Status
Of 209 cases with cancer status documented at the last reported visit, 20% had a complete response, 33% partial response, 21% stable disease, and 27% disease progression.
DISCUSSION
In this systematic review of cases and case series, we aimed to describe inflammatory arthritis phenotypes that arise as a result of ICI treatment. We demonstrate that 49% of reported cases are polyarthritis, often described as “RA-like,” 17% oligoarthritis and 21% PMR-like. A small group of cases was described specifically as having “spondyloarthritis.” Only 9% of patients tested had a positive RF and/or CCP antibody, whereas 30% had a positive ANA; however, titers were not consistently reported. The majority of cases (74%) were treated with systemic corticosteroids, and 31% required a conventional or biologic DMARDs such as hydroxychloroquine, methotrexate, and/or a TNF inhibitor. Thirty-eight percent of cases had to discontinue ICI therapy, 13% due to their arthritis.
The distribution of arthritis phenotypes in the ICI setting demonstrated in this study is somewhat similar to that in the general rheumatic disease population. For example, the DANBIO registry, a Danish quality and research registry of inflammatory arthritis patients, is composed of ~26,000 (70%) RA and 9400 (27%) psoriatic/spondyloarthritis patients.12 The RISE registry, a quality and research registry of patients from academic rheumato-logic practices in the United States, is composed of ~60,000 (66%) RA, 23,500 (26%) psoriatic/spondyloarthritis, and 8000 (9%) PMR patients.13 Similar phenotypic frequencies in the ICI setting suggest that ICI may trigger actual RA and/or spondyloarthropathies in genetically predisposed individuals. In fact, a genetic evaluation comparing ICI arthritis patients to RA patients and healthy controls of European descent found that ICI arthritis patients had a higher prevalence of the HLA class II shared epitope (HLA-DRB1), which is responsible for the presentation of anticitrullinated peptides that is vital in the RA pathogenesis model, as compared with healthy controls.14 When compared with RA patients, the probability of ICI arthritis patients having at least one shared epitope allele did not statistically differ, although all but one of the ICI-induced arthritis patients were seronegative. The same study also showed no association between the presence of HLA B27 with the development of ICI arthritis when compared with controls. Polymyalgia rheumatica was reported in a higher percentage of patients in our study of ICI-treated patients (~21%) than in the RISE registry (9%). This could suggest that ICI is a potent trigger of PMR. Alternatively, PMR may be underrepresented in RISE (a registry of rheumatology practices) because PMR patients are often cared for by their primary care physician.
The proportion of patients that are RFand/or CCP positive in ICI arthritis (9%) is much lower than the roughly 70% seen in RA,15 and the prevalence of low titer ANA (30%) was similar to that in the general population.16 In RA, patients can have positive serologies for years prior to arthritis onset,17,18 unlike the rapid onset of arthritis in ICI-treated patients (median of 4 months from the time of ICI initiation in this study). We hypothesize that patients who develop ICI arthritis within days of treatment were “primed” immunologically and would have developed arthritis eventually, regardless of ICI treatment, but this remains unproven. Future studies should look for biomarkers of arthritis in patients initiating ICI therapy, as well as evidence of eventual seroconversion in patients with persistent ICI arthritis.
The pathophysiology of ICI arthritis is poorly understood, but 2 case reports describe synovial histology similar to RA. One case, from a patient treated with anti–PD-1 antibodies revealed a chronic proliferative lymphoplasmacytic synovitis with equal numbers of B cells and T cells (mixture of CD4+ and CD8+).19 Another demonstrated similar B cell, T cell, and macrophage populations in the synovium compared with seropositive early RAwith the exception of higher TNF staining in the ICI synovium than in the RA controls.20 Of note, in one case, PD-1 staining could not be done due to continued receptor occupancy by nivolumab (PD-1) 7 months after ICI discontinuation,20 which may explain the long duration of arthritis seen in this setting.7 Another study analyzed synovial fluid from ICI arthritis patients and found CD4+ T cells to be in the highest proportions, although CD8+ were also present, primarily as effector memory cells.21 Among the CD4+ T cells, both regulatory T cells (Treg) and non–Treg-produced IL-17, and non–Treg IL-17 production were enhanced in patients with persistent arthritis despite steroid treatment. To date, no correlations have been made between synovial findings and clinical arthritis phenotypes, but this is a potential future direction of research. In RA, PD-L1 expression on synovial lining cells is associated with disease activity22; however, its role in disease pathogenesis is not fully elucidated.
Most patients in our study were treated with corticosteroids often at much higher doses than are needed to treat RA. The relatively infrequent use of conventional DMARDs (eg, methotrexate, hydroxychloroquine) and biologics (eg, TNF inhibitor) in our study likely stemmed from questions regarding their impact on cancer control during ICI treatment. Although there have been no robust prospective, longitudinal studies assessing the impact of immunosuppression on oncologic outcomes, some studies have explored this question retrospectively, and results have been mixed. An earlier study of melanoma patients being treated with ipilimumab found no difference in overall survival (OS) or time to treatment failure (TTF) between those that received systemic immunosuppression versus those that did not for all irAEs collectively,23 although specific steroid dosing (high vs low) and duration was not reported. A study looking at steroid dosing (high vs low) for the treatment of hypophysitis in ipilimumab-treated melanoma patients found that high-dose steroids (average >7.5 mg/d prednisone) was associated with worse OS and TTF, even after taking into account burden of disease.24 One study looking at the role of baseline steroid use in non–small cell lung cancer patients, and found that patients on ≥10 mg of daily prednisone had worse outcomes.25 A recent study in a mouse model of ICI-induced colitis suggested that TNF inhibitor may not only be efficacious in controlling colitis, but may also improve anticancer response.26 Paralleling this, a study in patients with ICI colitis demonstrated no difference in OS between those that needed immunosuppression versus those that did not, and no difference in OS between those that received steroids only versus steroids and infliximab.27 Lastly, a case series of patients treated with multiple doses of infliximab along with their ICI after the initial development of gastrointestinal irAE showed no recurrence of symptoms, and restaging imaging did not show significant progression of disease.28
Our study has a number of strengths. To our knowledge, this is the largest systematic review of ICI arthritis cases. We provide information about the relative proportions of arthritis phenotypes, the prevalence of reported RF and CCP seropositivity, as well as treatment and prognosis. However, this review also has limitations. First, given that the included publications were case reports and case series, the quality of evidence is low. Data reporting was not standardized so there is significant missing data for variables other than arthritis phenotype. The included studies are heterogeneous, some enriching for phenotypes that the authors wanted to highlight (73–85) and some excluding entities such as PMR in their descriptions of “inflammatory arthritis”; thus, we wanted to take an aggregate of all the patients in order to more accurately describe phenotypic frequencies. Abstracts were included in this review in order to encompass as many patients as possible, although these publications often lack detail. We chose our approach because data from clinical trials, although rigorous, lacks granular detail about irAE presentations, such as arthritis, and may omit mild-moderate irAE such as arthritis altogether. Case reports and series can potentially fill these gaps. Once ICI arthritis is classified into distinct phenotypes, it should be easier to analyze it from both a pathogenetic and therapeutic perspective. However, case reports are likely skewed toward reporting more severe disease and possibly the use of more aggressive immunosuppression as well. Given the reporting bias inherent in case reports and case series, the phenotypic frequencies described in our study may not be accurate, and an assessment of arthritis phenotypes in large prospective cohorts is needed to confirm our findings. Since the date of our literature search, more publications pertaining to ICI arthritis have been published,29,30 highlighting the rapidly changing horizon in this field. Finally, we have limited our systematic review to patients treated with anti–PD-1/PD-L1 and anti–CTLA-4 antibodies, but other immune checkpoint-targeted therapies are under study (anti-TIM, −LAG, etc) and may also be associated with ICI arthritis.
In conclusion, ICI arthritis often presents as an RA-like or PMR-like disease, and much less frequently as a spondyloarthritis. Immune checkpoint inhibitor arthritis is also reported much more commonly in patients treated with anti–PD-1/PD-L1 monotherapy. Further work is needed to determine whether these forms of ICI arthritis represent true “RA,” “PMR,” or “spondyloarthritis.” Future translational studies will help to determine whether different “immunophenotypes” parallel the heterogenous clinical presentations of ICI arthritis and whether phenotypic differences mandate different treatment approaches. Ultimately, striking a balance between arthritis treatment to ensure satisfactory quality of life while allowing maximum immunotherapy efficacy is the goal, and for this, an assessment of the impact that arthritis treatment has on oncologic outcomes is needed.
Supplementary Material
KEY POINTS.
Immune checkpoint inhibitor inflammatory arthritis has been poorly characterized due to its clinical variability.
This study demonstrates that half of reported cases presented with a rheumatoid arthritis–like condition and a fifth with PMR.
Only 9% of reported ICI arthritis cases are CCP positive.
Many patients respond to steroid treatment; however, a large subset require additional therapies such as conventional DMARDs or biologics.
The distribution of ICI arthritis phenotypes is similar to that seen in the non-ICI setting, which raises the possibly of shared genetic factors predisposing to both sets of conditions.
Acknowledgments
We have not received any financial support from commercial sources. Michael Tiongson received a summer research grant from Albany Medical College, Carolyn Stewart received a summer research grant from Weill Cornell Medicine, and Laura Cappelli received funding from NIAMSK23 AR075872.
Footnotes
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.jclinrheum.com).
REFERENCES
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (New York, NY). 2018;359:1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 5.Cappelli LC, Brahmer JR, Forde PM, et al. Clinical presentation of immune checkpoint inhibitor–induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum. 2018;48:553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostine M, Rouxel L, Barnetche T, et al. Rheumatic disorders associated with immune checkpoint inhibitors in patients with cancer—clinical aspects and relationship with tumour response: a single-centre prospective cohort study. Ann Rheum Dis. 2018;77:393–398. [DOI] [PubMed] [Google Scholar]
- 7.Smith MH, Bass AR. Arthritis after cancer immunotherapy: symptom duration and treatment response. Arthritis Care Res. 2019;71:362–366. [DOI] [PubMed] [Google Scholar]
- 8.Cappelli LC, Gutierrez AK, Bingham CO III, et al. Rheumatic and musculoskeletal immune-related adverse events due to immune checkpoint inhibitors: a systematic review of the literature. Arthritis Care Res. 2017;69:1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 2017. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Accessed March 4, 2020.
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 11.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford Cebm Evidence Levels of Evidence (Introductory Document). 2011. Available at: http://www.cebm.net/index.aspx?o=5653. Accessed March 4, 2020.
- 12.Ibfelt EH, Jensen DV, Hetland ML. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clin Epidemiol. 2016;8:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yazdany J, Bansback N, Clowse M, et al. Rheumatology informatics system for effectiveness: a national informatics-enabled registry for quality improvement. Arthritis Care Res (Hoboken). 2016;68:1866–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cappelli LC, Dorak MT, Bettinotti MP, et al. Association of hla-drb1 shared epitope alleles and immune checkpoint inhibitor–induced inflammatory arthritis. Rheumatology (Oxford). 2019;58:476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. [DOI] [PubMed] [Google Scholar]
- 16.Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997;40:1601–1611. [DOI] [PubMed] [Google Scholar]
- 17.Nielen MM, van Schaardenburg D, Reesink HW, et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. [DOI] [PubMed] [Google Scholar]
- 18.Sokolove J, Bromberg R, Deane KD, et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina HA, Eickhoff J, Edison JD. Thinking inside the box: synovial tissue biopsy in immune checkpoint inhibitor–induced arthritis. J Clin Rheumatol. 2019;1. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Murray-Brown W, Weedon H, Wilsdon T, et al. Nivolumab-induced synovial tissue T cell infiltration, sustained pd1 occupancy and resolution of severe synovitis with infliximab therapy [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). Available at: https://acrabstracts.org/abstract/nivolumab-induced-synovial-tissue-t-cell-infiltration-sustained-pd1-occupancy-and-resolution-of-severe-synovitis-with-infliximab-therapy/. [Google Scholar]
- 21.Kim S, Tayar J, Lu H, et al. Characterization of lymphoid cells in synovial fluid from cancer patients with immunotherapy-induced arthritis [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). Available at: https://acrabstracts.org/abstract/characterization-of-lymphoid-cells-in-synovial-fluid-from-cancer-patients-with-immunotherapy-induced-arthritis/. [Google Scholar]
- 22.Matsuda K, Miyoshi H, Hiraoka K, et al. Clinicopathological value of programmed cell death 1 (pd-1) and programmed cell death ligand 1 (pd-l1) expression in synovium of patients with rheumatoid arthritis. Clin Exp Med. 2018;18:487–494. [DOI] [PubMed] [Google Scholar]
- 23.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33: 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124: 3706–3714. [DOI] [PubMed] [Google Scholar]
- 25.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Ruiz E, Minute L, Otano I, et al. Prophylactic tnf blockade uncouples efficacy and toxicity in dual ctla-4 and pd-1 immunotherapy. Nature. 2019;569:428–432. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor–induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badran YR, Cohen JV, Brastianos PK, et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer. 2019;7:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braaten TJ, Brahmer JR, Forde PM, et al. Immune checkpoint inhibitor–induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2020;79:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan KK, Vitone G, Shanaj S, et al. Ab1079 checkpoint inhibitor–associated arthritis: phenotypes and cytokine associations. Ann Rheum Dis. 2019;78:2004–2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


