Abstract
Low-dose aspirin, which selectively inhibits thromboxane synthesis, is now standard of care for the prevention of preeclampsia in at risk women, but some women still develop preeclampsia despite an aspirin regimen. To explore the “aspirin failures”, we undertook a comprehensive evaluation of placental lipids to determine if abnormalities in non-aspirin sensitive lipids might help explain why some women on low-dose aspirin develop preeclampsia. We studied placentas from women with normal pregnancies and women with preeclampsia. Placental villous explants were cultured and media analyzed by mass spectrometry for aspirin-sensitive and non-aspirin-sensitive lipids. In women who developed severe preeclampsia and delivered preterm, there were significant elevations in non-aspirin-sensitive lipids with biologic actions that could cause preeclampsia. There were significant increases in 15- and 20-hydroxyeicosantetraenoic acids and sphingolipids: D-e-C18:0 ceramide, D-e-C18:0 sphingomyelin, D-e-sphingosine-1-phosphate and D-e-sphinganine-1-phosphate. With regard to lipids sensitive to aspirin, there was no difference in placental production of thromboxane or prostacyclin, but prostaglandins were lower. There was no difference for isoprostanes, but surprisingly, anti-inflammatory omega 3 and 6 PUFAs were increased. In total, 10 of 30 eicosanoids and 5 of 42 sphingolipids were abnormal in women with severe early onset preeclampsia. Lipid changes in women with mild preeclampsia who delivered at term were of lesser magnitude with few significant differences. Conclusions: 1) The placenta produces many aspirin-sensitive and non-aspirin-sensitive lipids; 2) Abnormalities in eicosanoids and sphingolipids not sensitive to aspirin might explain why some aspirin- treated women develop preeclampsia.
Keywords: low-dose aspirin, preeclampsia, eicosanoids, sphingolipids, placenta
Introduction
The first clinical trial of low-dose aspirin to prevent preeclampsia was published in 1986 by Wallenburg et al [1]. It was based on the finding of increased thromboxane and decreased prostacyclin production by placentas of women with preeclampsia [2]. At the time, low-dose aspirin was being used to prevent recurrent myocardial infarction and other thrombotic events based on its ability to selectively inhibit platelet thromboxane synthesis without affecting endothelial prostacyclin synthesis [3–6]. With the discovery that there was an imbalance between thromboxane and prostacyclin in preeclampsia, it was reasonable to evaluate if aspirin would be effective for preeclampsia prevention. A plethora of clinical trials followed with varying degrees of effectiveness. However, when meta-analyses were performed, it was evident that in almost all trials of low-dose aspirin, the incidence of preeclampsia decreased [7–10]. Low-dose aspirin is now the standard of care to prevent preeclampsia in at risk women [11–13].
Although low-dose aspirin reduces the risk of preeclampsia, some women on low-dose aspirin still develop the disorder. To explore the biochemical phenotype of aspirin failures, we undertook a comprehensive evaluation of placental lipids in women with normal pregnancy and women who developed preeclampsia on low-dose aspirin therapy. We were most interested in severe preeclampsia with premature delivery because it is clinically most significant with regard to morbidity and the mortality of the mother and baby. We evaluated cyclooxygenase eicosanoids that would be affected by aspirin and non-cyclooxygenase eicosanoids and sphingolipids that would not be affected by aspirin. We tested the hypothesis that abnormalities in non-aspirin-sensitive lipids might help explain why some women on low-dose aspirin therapy develop preeclampsia.
Materials and Methods
Study Subjects
As part of the NICHD Human Placenta Project we recruited pregnant women from our obstetrics clinics at MCV Hospitals, Virginia Commonwealth University Medical Center. We collected placentas after delivery from women who had normal pregnancies and women who developed preeclampsia. Women at risk for preeclampsia were prescribed 81 mg of aspirin per day as standard of care according to the guidelines of the American College of Obstetrics and Gynecology [11, 12]. Preeclampsia risk factors for our patients included: history of preeclampsia, chronic hypertension and pre-gestational diabetes. Patients were contacted by phone following their pregnancy to assess their compliance. Subjects were divided into four groups: 1) Women who had normal pregnancies with no risk factors and were not prescribed aspirin (NP, n = 35); 2) women who had normal pregnancies with risk factors and were prescribed aspirin (NP ASA, n = 14); 3) women who had risk factors, were prescribed aspirin and developed mild preeclampsia at term (MPE ASA, n = 12); 4) women who had risk factors, were prescribed aspirin and developed severe preeclampsia at < 37 weeks’ gestation (SPE ASA, n = 13). Preeclamptic patients had blood pressures of ≥140/90 mm Hg on 2 occasions at least 4 hours apart after 20 weeks’ gestation and proteinuria (≥0.3 gm/24 hours or protein/creatinine ratio ≥0.3). Normal pregnant patients had maternal blood pressures ≤110/70 mm Hg, no proteinuria and no other complications. The Office of Research Subjects Protection of Virginia Commonwealth University approved this study (HM20005160). All subjects gave informed consent, and the procedures followed were in accordance with institutional guidelines.
Placental Explant Culture
Placentae were collected at delivery and processed as previously described and validated [14, 15]. Cell viability is greater than 95%, cell morphology and integrity are intact, the tissue is metabolically active and synthesizes proteins [14, 15]. Placental villous tissue was collected from various cotyledons excluding chorionic and basal plates and washed repeatedly with phosphate buffered saline to remove blood. Tissues were minced and cultured (500 mg) under sterile conditions in 5 ml of Medium 199 in 6- well 35 mm polystyrene tissue culture plates for 48 hours in an incubator at 37° C, 5% CO2. Media and cell pellets were collected after 48 hours and frozen at −80° C until analysis.
Analysis of Eicosanoids and Sphingolipids by UPLC ESI-MS/MS
Placental explant media were extracted and analyzed for eicosanoids as previously described [16, 17] and for sphingolipids using a modified Bligh Dyer Extraction as previously described [18, 17, 19]. Each sample was spiked with 1.5 pmol/μl of eicosanoid standards (Cayman Chemical, Ann Arbor, MI) or 250 pmol of sphingolipid standards (Avanti Polar Lipids, Alabaster, AL). Eicosanoids and sphingolipids were separated using a Shimadzu Nexera X2 LC-30AD coupled to a SIL-30AC auto injector, coupled to a DGU-20A5R degassing unit. A reversed phase LC method utilizing an Acentis Express C18 column (50mm x 2.1mm, 2.7μm) was used to separate eicosanoids from sphingolipids. Eicosanoids and sphingolipids were analyzed by mass spectrometry using an AB Sciex Triple Quad 5500 Mass Spectrometer.
Data Analysis
Demographic data are presented as means ± SD and experiment data are presented as box and whisker plots with error bars representing the 5 and 95 % confidence limits. Data were analyzed for significance by one-way ANOVA with the Kruskal-Wallis test and Dunn’s multiple comparisons test using a statistical software program (Prism 4, GraphPad software, San Diego, CA). Outlier analysis was performed using ROUT with Q = 0.5%. A p value of < 0.05 was considered statistically significant. Radar graphs for % changes in preeclamptic eicosanoids and sphingolipids as compared to normal pregnancy were created using Microsoft Excel for Mac (Version 16).
Results
Study Subjects
Demographic information for the study subjects is shown in Table 1. Most of the study subjects were overweight or obese. Blood pressures were significantly higher for preeclamptic patients. Gestational age at birth and infant birth weight were significantly lower for patients with severe preeclampsia. There were 36 patients with risk factors for preeclampsia who were prescribed aspirin. We were able to contact 32 patients by phone, 21 reported compliance with aspirin therapy (66%) and 11 reported noncompliance (34%). We were not able to contact 4 patients. Of those patients reporting compliance with aspirin, 12 began at ≤ 16 weeks’ gestation. Three of the patients who developed mild preeclampsia did not have risk factors and were not prescribed aspirin.
Table 1:
Clinical Characteristics of Study Subject Groups
| Variable | NP n = 35 |
NP ASA n = 14 |
MPEASA n = 12 |
SPE ASA n = 13 |
|---|---|---|---|---|
| Maternal age (years) | 27.4 ± 5.3 | 28.6 ± 4.7 | 30.0 ± 8.8 | 29.7 ± 4.3 |
| Pre-pregnancy BMI (kg/m2) | 29.1 ± 6.6 | 32.2 ± 12 | 28.4 ± 5.7 | 36.4 ± 15.4 |
| BMI at sample collection (kg/m2) | 34.6 ± 7.0 | 36.5 ± 9.9 | 33.9 ± 5.8 | 41.3 ± 15.0 |
| Systolic blood pressure (mmHg) | 121 ± 12 | 129 ± 13 | 151 ± 19*** | 154 ± 18*** |
| Diastolic blood pressure (mmHg) | 73 ± 11 | 81 ± 10 | 92 ± 8*** | 98 ± 9*** |
| Proteinuria (mg/24 h) | ND | 3.1 | 214 ±305 | 636 ± 784 |
| Protein/creatinine Ratio | ND | 0.1 | 0.6 ± 0.4 | 2.6 ± 3.5 |
| Primigravida | 7 | - | 2 | 4 |
| Multigravida | 28 | 14 | 10 | 9 |
| Race | ||||
| White | 11 | 4 | 1 | 3 |
| Black | 20 | 9 | 6 | 8 |
| Hispanic | 2 | 1 | 5 | 2 |
| Other | 2 | - | - | - |
| Delivery method | ||||
| Vaginal | 24 | 8 | 7 | 6 |
| C-section | 11 | 6 | 5 | 7 |
| Gestational age (weeks) | 38.6 ± 1.5 | 37.7 ± 1.8 | 38.0 ± 1.6 | 32.9 ± 3.7**** |
| Infant birth weight (grams) | 3243 ± 479 | 3019 ± 580 | 2757 ± 1038 | 1822 ± 854*** |
Values are means ± SD
p < 0.001
p < 0.0001 compared to other groups
NP, normal pregnancy without aspirin and no risk factors; NP ASA, normal pregnancy with risk factors and aspirin; MPE ASA, mild preeclampsia with risk factors and aspirin; SPE ASA, severe preeclampsia with risk factors and aspirin; BMI, body mass index; ND, not determined
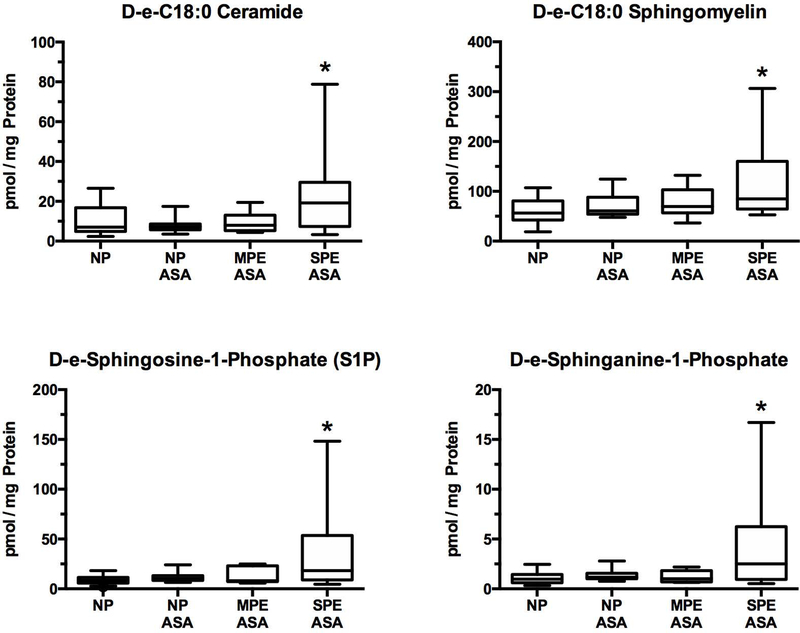
Sphingolipids
Sphingolipids are major constituents of the cell membranes involved in cell signaling. They are not metabolites of cyclooxygenase, and so, are not affected by aspirin. The placenta produced 42 sphingolipids, 5 of which were abnormal in women with severe preeclampsia. All sphingolipids that were abnormal in women with severe preeclampsia were significantly increased as compared to women with normal pregnancy who did not have risk factors for preeclampsia, but not as compared to women with normal pregnancy or mild preeclampsia who had risk factors for preeclampsia. Abnormal sphingolipids included the major C:18 sphingolipids: D-e-C18:0 ceramide, D-e-C18:0 sphingomyelin, D-e-sphingosine-1-phosphate (S1P), and D-e-sphinganine-1-phosphate (Fig. 1). Data for all 42 sphingolipids are given in the Supplemental Figures.
Fig.1.
Increases in sphingolipids related to the development of preeclampsia. Placental production of sphingolipids would not be affected by low-dose aspirin therapy. D-e-C18:0 ceramide, D-e-C18:0 sphingomyelin, D-e-sphingosine-1-phosphate (S1P), and D-e- sphinganine-1-phosphate were significantly increased in SPE ASA as compared to NP. There were no differences among NP, NP ASA and MPE ASA for any of the sphingolipids. (*P < 0.05; NP - normal pregnancy without aspirin and no risk factors; NP ASA - normal pregnancy with risk factors and aspirin; MPE ASA - mild preeclampsia with risk factors and aspirin; SPE ASA - severe preeclampsia with risk factors and aspirin)
Eicosanoids
The placental explants produced 30 eicosanoids in the following classes: prostaglandins (PGs), thromboxanes (TX), isoprostanes (8-iso PGF2a, 5-iPF2a), hydroxyeicosatetraenoic acids (HETEs), leukotrienes (LTs), lipoxins, resolvins, epoxyeicosatrienoic acids (EETs), dihydroxyeicosatrienoic acids (DHETs), omega-3 and omega-6 polyunsaturated fatty acids (PUFAs, eicosapentaenoic acid, EPA and docosahexaenoic acid, DHA), dihomo-gamma-linolenic acid (DHGLA) and arachidonic acid (AA). A total of 10 were abnormal in women with severe preeclampsia. Data for all 30 eicosanoids are given in the Supplemental Figures.
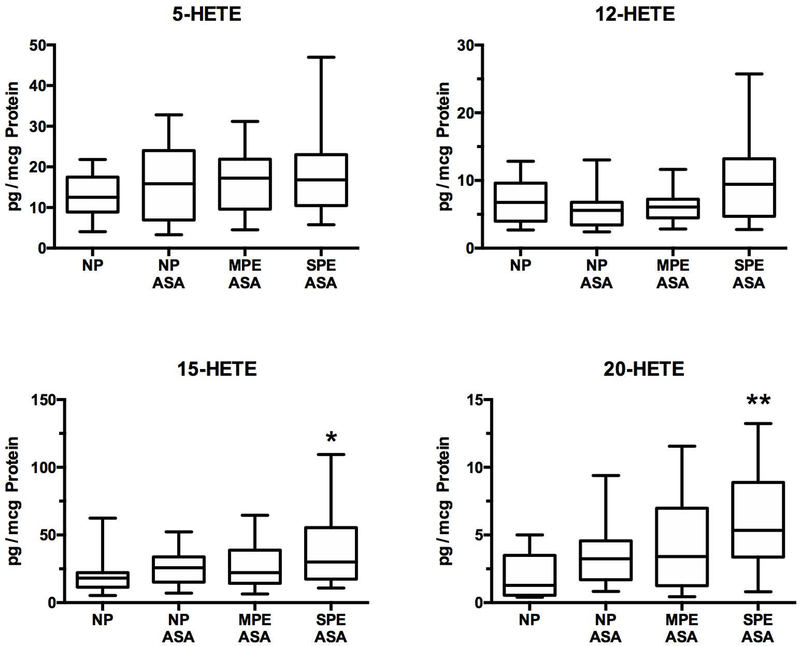
Lipoxygenase metabolites
Figure 2 shows placental production of the HETEs, which are lipoxygenase metabolites of arachidonic acid, and therefore, not affected by aspirin. The placental produced 5- HETE, 12-HETE, 15-HETE and 20-HETE. 15-HETE and 20-HETE were significantly increased in women with severe preeclampsia as compared to women with normal pregnancies who did not have risk factors for preeclampsia, but not as compared to women with normal pregnancies or mild preeclampsia who had risk factors.
Fig.2.
Increases in HETEs related to the development of preeclampsia. Placental production of lipoxygenase metabolites would not be affected by low-dose aspirin. There were no differences for 5-HETE or 12-HETE among groups and there were no differences among NP, NP ASA and MPE ASA for 15-HETE and 20-HETE, however 15-HETE and 20-HETE were significantly increased in women with SPE ASA as compared to women with NP. (*P < 0.05; ** P < 0.01, HETE, hydroxyeicosatetraenoic acid)
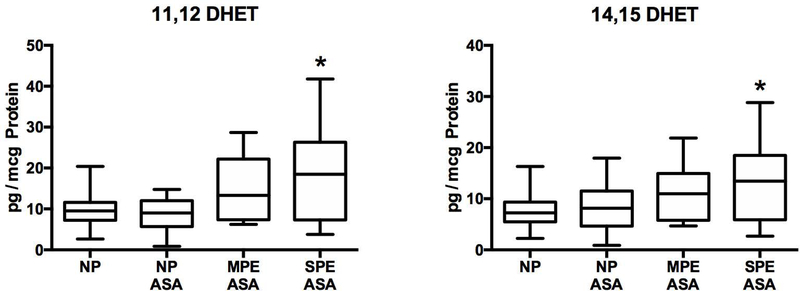
Anti-inflammatory eicosanoids
The placenta produced three DHETs, two EETs, two resolvins and one lipoxin. Only two were altered. 11,12 DHET and 14,15 DHET were significantly increased in women with severe preeclampsia as compared to women with normal pregnancies who did not have risk factors (Fig. 3). Placental production of resolvins and lipoxins was very low or non-detectable.
Fig.3.
Alterations in placental metabolism of eicosanoids with anti-inflammatory properties. 11, 12 DHET and 14, 15 DHET were significantly increased in women with SPE ASA as compared to NP. There were no significant differences among NP, NP ASA and MPE ASA for DHETs. (*P < 0.05; DHET, dihydroxyeicosatrienoic acids)
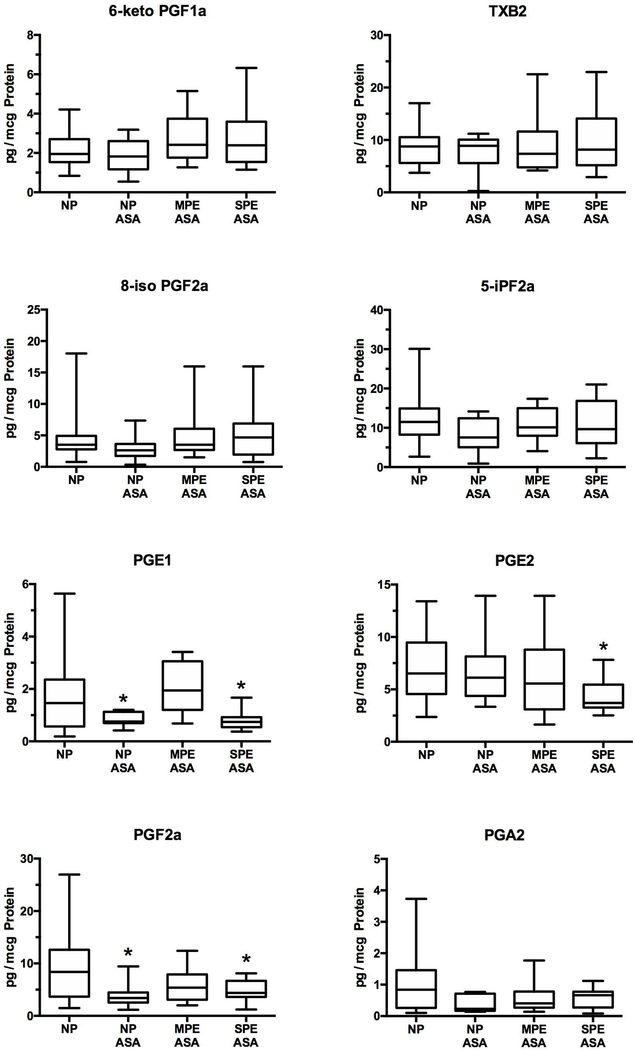
Cyclooxygenase metabolites
Thromboxane, prostacyclin and prostaglandins are cyclooxygenase metabolites of arachidonic acid, and so subject to effects of aspirin. There were no differences among groups for 6-keto PGF1α or TXB2. PGE1, PGE2 and PGF2α were significantly lower for severe preeclampsia as compared to normal pregnancy without aspirin and PGE1 and PGF2α were also significantly lower for normal pregnancy with aspirin as compared to normal pregnancy without aspirin (Fig. 4). Prostaglandin levels were not lower for mild preeclampsia, but three of these women were not prescribed aspirin because they did not have risk factors for preeclampsia.
Fig.4.
Placental production of cyclooxygenase metabolites and isoprostanes. Cyclooxygenase metabolites are aspirin-sensitive lipids. There were no differences among groups for 6-keto PGF1a, TXB2, 8-iso PGF2a or 5-iPF2a. PGE1, PGE2 and PGF2a were significantly lower than NP for SPE ASA and PGE1 and PGF2a were significantly lower than NP for NP ASA as well. (*P < 0.05; TXB2, thromboxane B2; 6- keto PGF1a, 6-keto prostaglandin F1a; 8-iso PGF2a, 8-isoprostane; 5-iPF2a, 5- isoprostane; PGE1, prostaglandin E1; PGE2, prostaglandin E2; PGF2a, prostaglandin F2α)
Isoprostanes
The placenta produced two isoprostanes. There were no significant differences in the placental production rates of 8-iso PGF2α or 5-iPF2α among groups (Fig. 4).
Dietary anti-inflammatory PUFAs
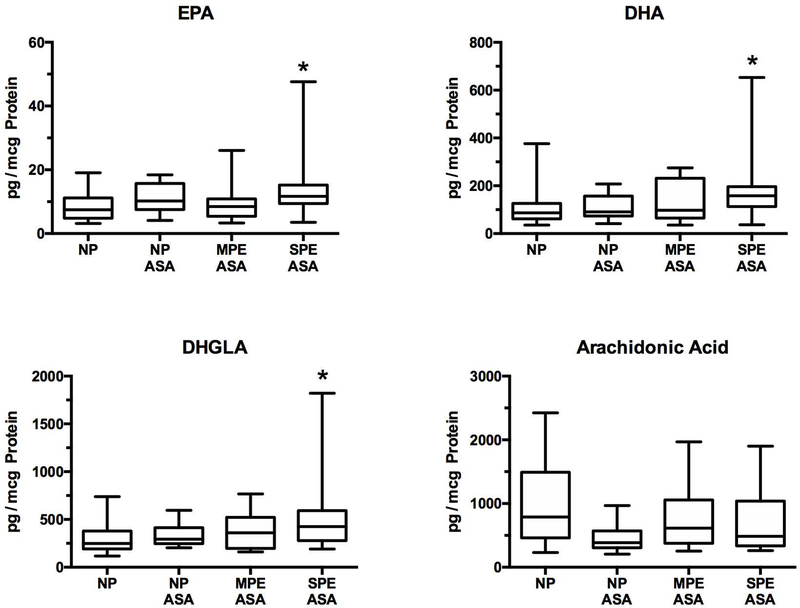
Figure 5 shows the results for dietary anti-inflammatory PUFAs. EPA, DHA and DHGLA were significantly elevated in women with severe preeclampsia as compared to women with normal pregnancies not on aspirin.
Fig.5.
Anti-inflammatory omega-3 and omega-6 PUFAs in the placenta. There were no significant differences among NP, NP ASA and MPE ASA for EPA, DHA or DHGLA, however the SPE ASA group was significantly higher than NP for all three PUFAs. There were no significant differences for arachidonic acid. (*P < 0.05; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DHGLA, dihomo-gamma-linolenic acid)
Leukotrienes
Placental production of leukotrienes was very low or non-detectable and there were no significant differences among groups.
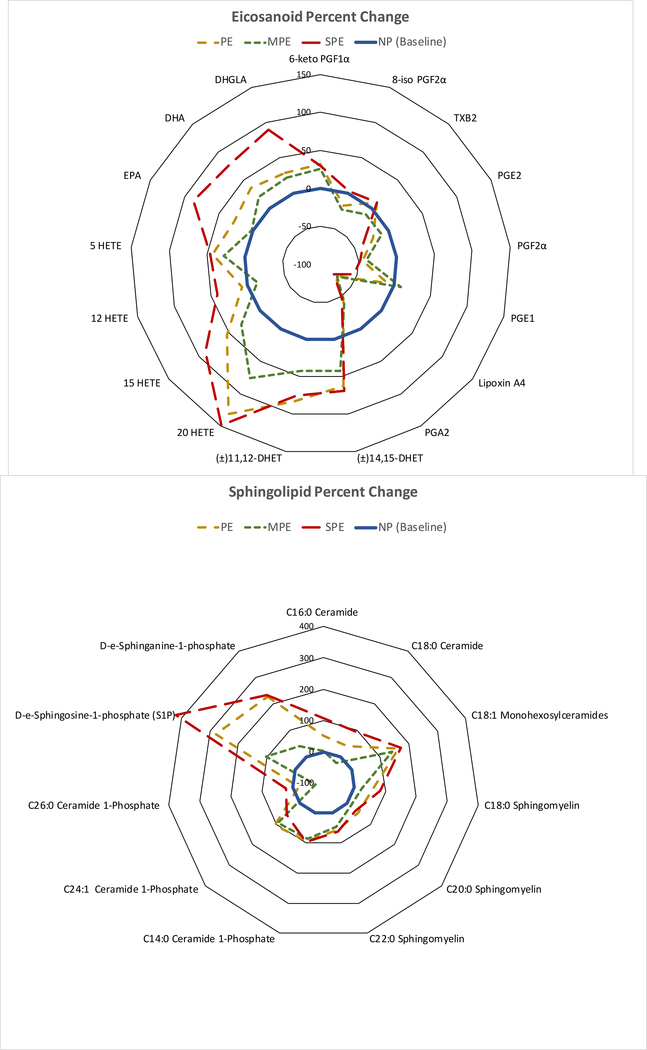
Eicosanoid and sphingolipid fingerprints
Figure 6 shows radar graphs of the % changes for selected eicosanoids and sphingolipids in women with SPE as compared to women with NP or women with MPE. Percent changes in women with MPE followed the same pattern as for those of SPE women, but the % changes were not as great.
Fig.6.
Radar graphs showing percent changes of selected eicosanoids and sphingolipids in women with SPE (red long dashed line) as compared to women with normal pregnancies (blue inner circle solid line) and women with MPE (green short dashed line). Women with MPE showed changes similar to SPE, but the changes were not as great. PE (tan medium dashed line) is the average of SPE and MPE. Prostaglandins and thromboxane on the right-hand side of the eicosanoid plot for PE patients were less than or no different than NP, whereas HETEs, EPA, DHA and DHGLA on the left-hand side were greater than NP. All sphingolipids that were abnormal in SPE were significantly elevated as compared to normal pregnancy. Placental S1P, which would be secreted into the maternal circulation, was over 5-fold higher for SPE than NP. (PE, preeclampsia; MPE, mild preeclampsia; SPE, severe preeclampsia; NP, normal pregnancy; 6-keto PGF1α, 6-keto prostaglandin F1α; 8-iso PGF2α, 8-isoprostane; TXB2, thromboxane B2; PGE2, prostaglandin E2; PGF2a, prostaglandin F2a; PGE1, prostaglandin E1; PGA2, prostaglandin A2; DHET, dihydroxyeicosatrienoic acids; HETE, hydroxyeicosatetraenoic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DHGLA, dihomo-gamma-linolenic acid; S1P, sphingosine-1-phosphate)
Discussion
Low-dose aspirin reduces the risk of preeclampsia, but it does not prevent the disease in all women. Our results may explain why. In the women who developed severe preeclampsia and delivered preterm, there were significant elevations in non-aspirin sensitive sphingolipids and HETEs with biologic actions that could cause preeclamptic symptoms. Elevations in these non-cyclooxygenase lipids could explain why these women developed preeclampsia despite aspirin therapy.
Sphingolipids are long-chain fatty acids that contain a backbone of sphingosine. They include sphingomyelin, ceramide, sphingosine and sphingosine-1-phosphate. Sphingolipids are bioactive lipids involved in inflammatory signaling pathways and implicated in cardiovascular disease [20–25]. Sphingolipids would not be affected by low-dose aspirin therapy. All sphingolipids that were abnormal in severe preeclampsia were significantly elevated as compared to normal pregnancy, including the major C:18 forms. D-e-C18:0 ceramide, D-e-C18:0 sphingomyelin, D-e-sphingosine-1-phosphate (S1P), and D-e-sphinganine-1-phosphate were increased 2-fold to over 5-fold as compared to placentas of normal pregnant women.
Abnormal placental sphingolipid production may contribute to a number of preeclamptic problems. Ceramide induces apoptosis and so may contribute to placental apoptosis [26], whereas S1P inhibits extravillous trophoblast migration [27], and so may contribute to failure of extravillous trophoblast to effectively erode the spiral arteries in preeclampsia. S1P is also involved in inflammation, angiogenesis, vascular permeability and immune response. S1P is an intracellular second messenger, but it is also a blood borne lipid mediator, and as such has extracellular actions by binding to S1P receptors. Placental secretion of S1P could be responsible for abnormalities in the maternal circulation. Very little information is available for sphingolipids in pregnancy, but maternal levels of ceramide and S1P have been reported to be elevated in preeclampsia and linked to a placental source [28, 29].
The placenta produced four HETEs, two of which, 15-HETE and 20-HETE, were significantly elevated in women delivering preterm with severe preeclampsia. Both of these HETEs cause inflammation [30–37] and placental pathologic features of preterm preeclampsia are consistent with chronic inflammation [38]. In addition to inflammation, 20-HETE promotes hypertension, vasoconstriction and vascular dysfunction [37, 35, 36]. Our finding of increased placental production of 20-HETE confirms a recent report [39]. Intrauterine production of 20-HETE by the placenta could contribute to reduced uterine blood flow and placental vasoconstriction in preeclampsia, whereas placental release into the maternal circulation could contribute to maternal hypertension. In this regard, 20-HETE is not only a vasoconstrictor, but it also enhances vascular reactivity to angiotensin II.
Sphingolipids and HETEs for women with severe preeclampsia were not significantly higher than for women with normal pregnancies or mild preeclampsia who had risk factors for preeclampsia and were prescribed aspirin, but this may not be surprising because the women receiving aspirin are not really normal. They are receiving aspirin because they have conditions associated with inflammation and oxidative stress that put them at risk, but they do not develop severe preeclampsia perhaps because their inflammatory lipids are not elevated sufficiently to cause severe symptoms.
There were alterations in the placental metabolism of other eicosanoids that would not be affected by aspirin. 11,12 DHET and 14,15 DHET were significantly increased in severe preeclampsia as compared to normal pregnancy. These DHETs are also increased in the urine of preeclamptic women [40]. DHETs have anti-inflammatory actions [41,42] so these may be compensatory increases to counteract inflammation.
Thromboxane, prostacyclin and prostaglandins are cyclooxygenase metabolites, and therefore, aspirin-sensitive lipids. Prior to the use of low-dose aspirin, numerous investigators reported that thromboxane is increased and prostacyclin is decreased in placentas of women with preeclampsia by measuring production rates, tissue levels and the expression and activity of enzymes involved in their synthesis [43–49, 2, 50–52]. We did not find this in the present study. There was no difference in the placental production rate for either thromboxane or prostacyclin between women with normal pregnancies and women with preeclampsia. It is possible maternal aspirin corrected the placental imbalance previously reported because, as in the maternal circulation, thromboxane and prostacyclin are compartmentalized to different cell types. Thromboxane is produced by the trophoblast cells on the maternal side, whereas prostacyclin is produced by the endothelial cells on the fetal side [14, 53, 54]. Since only 34% of aspirin crosses from the maternal to the fetal side, it is possible for maternal ingestion of aspirin to selectively inhibit thromboxane, while sparing prostacyclin [55–57].
The placenta produced two isoprostanes, both of which have been shown to be increased in placentas of preeclamptic women [58, 59]. We found no difference in placental production of either 8-isoprostane or 5-isoprostane for women with normal pregnancies or women with preeclampsia. Low-dose aspirin might have attenuated the generation of isoprostanes indirectly because cyclooxygenase generates reactive oxygen species [60]. Therefore, inhibition of cyclooxygenase could have removed the source of free radicals to generate isoprostanes from arachidonic acid. This idea is consistent with previous reports that low-dose aspirin inhibits lipid peroxides along with thromboxane in the maternal circulation and in the placenta [55–57, 61].
Surprisingly, anti-inflammatory dietary omega-3 and omega-6 PUFAs, EPA, DHA and DHGLA, were elevated in women with severe preeclampsia on low-dose aspirin. All of these contain three double bonds, so they are highly susceptible to oxidation. The inhibition of cyclooxygenase might have spared their oxidation by decreasing the generation of reactive oxygen species, although other factors are probably involved. EPA and DHA are metabolized to prostaglandins of the 3-series and DHGLA to prostaglandins of the 1-series. Prostaglandins of the 1- and 3- series are not biologically active, except for PGI3. Prior to aspirin use, low levels of maternal omega-3 PUFAs were reported to be associated with development of preeclampsia [62].
A visual representation or fingerprint of these placental lipid changes is shown by plotting the data as radar graphs, which show the % changes of the preeclamptic patients from normal pregnant patients (Fig. 6). It is evident that cyclooxygenase metabolites for preeclamptic women on the right-hand side of the eicosanoid plot are lower or not different from normal pregnancy. Also evident are the fold increases of the HETEs and sphingolipids, especially S1P, on the left-hand sides of the plots for preeclamptic women. The changes for mild preeclampsia were similar to those for severe preeclampsia, but of less magnitude and with few significant differences.
The failure of low-dose aspirin to prevent preeclampsia in our study could be related to several factors. A dose of 81 mg/day may have been sufficient in the 1980s when the first clinical trials were started, but since then the United States and other countries have experienced an obesity epidemic. Most of our study subjects were overweight or obese. Recent meta-analysis studies have suggested that doses higher than 81 mg are more effective [63–66]. Meta-analysis studies have also shown that aspirin is more effective when begun ≤ 16 weeks’ gestation [8, 64, 9]. Only 57% of our patients who reported taking aspirin began at ≤ 16 weeks. Whether a woman is compliant in taking her aspirin every day is another important factor. Only 66% of our patients reported being compliant. Regardless of the reason for aspirin failure, our study provides a biological explanation related to significant increases in non-aspirin-sensitive lipids with biological actions that can cause clinical symptoms of preeclampsia.
In summary, we conducted a comprehensive analysis of aspirin-sensitive and non-aspirin -sensitive lipids produced by the placenta to explore why some women on low-dose aspirin therapy develop preeclampsia. We conclude that elevations in placental eicosanoids and sphingolipids not affected by aspirin, but that have biologic actions that can cause preeclampsia, may explain why some women on low-dose aspirin therapy develop preeclampsia.
Supplementary Material
Acknowledgments
This work was presented at the 66th Annual Scientific Meeting of the Society for Reproductive Investigation, March 12–16, 2019, Paris, FRANCE
Sources of financial support: This work was supported by Eunice Kennedy Shriver National Institute of Child Health & Human Development Grant 1U01 HD087198 (SWW, CEC) and by the Office of the Director, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Appendix A. Supplemental Data
All eicosanoid and sphingolipid data for normal pregnancy, mild preeclampsia and severe preeclampsia are presented in the Supplemental Figures.
Conflict of interest: The authors declared they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Office of Research Subjects Protection of Virginia Commonwealth University (HM20005160) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Wallenburg HCS, Makovitz JW, Dekker GA, Rotmans P. Low-dose aspirin prevents pregnancy-induced hypertension and pre-eclampsia in angiotensinsensitive primigravidae. Lancet. 1986;1(8471):1–3. [DOI] [PubMed] [Google Scholar]
- 2.Walsh SW. Preeclampsia: An imbalance in placental prostacyclin and thromboxane production. Am J Obstet Gynecol. 1985;152(3):335–40. [DOI] [PubMed] [Google Scholar]
- 3.Bunting S, Moncada S, Vane JR. The prostacyclin--thromboxane A2 balance: pathophysiological and therapeutic implications. Br Med Bull. 1983;39(3):271–6. [DOI] [PubMed] [Google Scholar]
- 4.Lewis HD Jr., Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;309(7):396–403. doi: 10.1056/nejm198308183090703. [DOI] [PubMed] [Google Scholar]
- 5.Marcus AJ. Aspirin as an antithrombotic medication. N Engl J Med. 1983;309(24):1515–7. doi: 10.1056/nejm198312153092410. [DOI] [PubMed] [Google Scholar]
- 6.Salzman EW. Aspirin to prevent arterial thrombosis. N Engl J Med. 1982;307(2): 113–5. doi: 10.1056/nejm198207083070209. [DOI] [PubMed] [Google Scholar]
- 7.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575): 1791–8. [DOI] [PubMed] [Google Scholar]
- 8.Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402–14. doi: 10.1097/AOG.0b013e3181e9322a [doi] 00006250–201008000-00023 [pii]. [DOI] [PubMed] [Google Scholar]
- 9.Roberge S, Villa P, Nicolaides K, Giguere Y, Vainio M, Bakthi A et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141–6. doi:000336662 [pii] 10.1159/000336662 [doi]. [DOI] [PubMed] [Google Scholar]
- 10.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110–20.e6. doi: 10.1016/j.ajog.2016.09.076. [DOI] [PubMed] [Google Scholar]
- 11.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5): 1122–31. doi: 10.1097/01.AOG.0000437382.03963.88 [doi] 00006250–201311000-00036 [pii]. [DOI] [PubMed] [Google Scholar]
- 12.ACOG Committee Opinion No. 743: Low-Dose Aspirin Use During Pregnancy. Obstet Gynecol. 2018;132(1):e44–e52. doi: 10.1097/aog.0000000000002708. [DOI] [PubMed] [Google Scholar]
- 13.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-Dose Aspirin for Prevention of Morbidity and Mortality From Preeclampsia: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014. doi:1859300 [pii] 10.7326/M13-2844 [doi]. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DM, Walsh SW. Thromboxane and prostacyclin production by different compartments of the human placental villus. J Clin Endocrinol Metab. 1989;68(3):676–83. [DOI] [PubMed] [Google Scholar]
- 15.Walsh SW, Behr MJ, Allen NH. Placental prostacyclin production in normal and toxemic pregnancies. Am J Obstet Gynecol. 1985;151:110–5. [DOI] [PubMed] [Google Scholar]
- 16.Blaho VA, Buczynski MW, Brown CR, Dennis EA. Lipidomic analysis of dynamic eicosanoid responses during the induction and resolution of Lyme arthritis. J Biol Chem. 2009;284(32):21599–612. doi: 10.1074/jbc.M109.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simanshu DK, Kamlekar RK, Wijesinghe DS, Zou X, Zhai X, Mishra SK et al. Non-vesicular trafficking by a ceramide-1-phosphate transfer protein regulates eicosanoids. Nature. 2013;500(7463):463–7. doi: 10.1038/nature12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA et al. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50(8):1692–707. doi: 10.1194/jlr.D800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijesinghe DS, Allegood JC, Gentile LB, Fox TE, Kester M, Chalfant CE. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res. 2010;51(3):641–51. doi: 10.1194/jlr.D000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mihanfar A, Nejabati HR, Fattahi A, Latifi Z, Pezeshkian M, Afrasiabi A et al. The role of sphingosine 1 phosphate in coronary artery disease and ischemia reperfusion injury. J Cell Physiol. 2019;234(3):2083–94. doi: 10.1002/jcp.27353. [DOI] [PubMed] [Google Scholar]
- 21.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781(9):424–34. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1- phosphate, a novel lipid, involved in cellular proliferation. The Journal of cell biology. 1991;114(1):155–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norris GH, Blesso CN. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients. 2017;9(11). doi: 10.3390/nu9111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coant N, Sakamoto W, Mao C, Hannun YA. Ceramidases, roles in sphingolipid metabolism and in health and disease. Advances in biological regulation. 2017;63:122–31. doi: 10.1016/j.jbior.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerage D, Brindley DN, Hemmings DG. Review: novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta. 2014;35 Suppl:S86–92. doi: 10.1016/j.placenta.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Huppertz B, Kadyrov M, Kingdom JC. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006;195(1):29–39. [DOI] [PubMed] [Google Scholar]
- 27.Westwood M, Al-Saghir K, Finn-Sell S, Tan C, Cowley E, Berneau S et al. Vitamin D attenuates sphingosine-1-phosphate (S1P)-mediated inhibition of extravillous trophoblast migration. Placenta. 2017;60:1–8. doi: 10.1016/j.placenta.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charkiewicz K, Goscik J, Blachnio-Zabielska A, Raba G, Sakowicz A, Kalinka J et al. Sphingolipids as a new factor in the pathomechanism of preeclampsia - Mass spectrometry analysis. PLoS One. 2017;12(5):e0177601. doi: 10.1371/journal.pone.0177601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melland-Smith M, Ermini L, Chauvin S, Craig-Barnes H, Tagliaferro A, Todros T et al. Disruption of sphingolipid metabolism augments ceramide-induced autophagy in preeclampsia. Autophagy. 2015;11(4):653–69. doi: 10.1080/15548627.2015.1034414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell WS, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo- ETEs) derived from arachidonic acid. Biochim Biophys Acta. 2015;1851(4):340–55. doi: 10.1016/j.bbalip.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Rao J, Liu Y, Cao Y, Zhang Y, Zhang Q et al. 15-Lipoxygenase promotes chronic hypoxia-induced pulmonary artery inflammation via positive interaction with nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2013;33(5):971–9. doi: 10.1161/atvbaha.113.301335. [DOI] [PubMed] [Google Scholar]
- 32.Stenson WF, Parker CW. Leukotrienes. Advances in internal medicine. 1984;30:175–99. [PubMed] [Google Scholar]
- 33.Rubin P, Mollison KW. Pharmacotherapy of diseases mediated by 5- lipoxygenase pathway eicosanoids. Prostaglandins Other Lipid Mediat. 2007;83(3): 188–97. doi: 10.1016/j.prostaglandins.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Goetzl EJ, Goldman DW, Naccache PH, Sha’afi RI, Pickett WC. Mediation of leukocyte components of inflammatory reactions by lipoxygenase products of arachidonic acid. Adv Prostaglandin Thromboxane Leukot Res. 1982;9:273–82. [PubMed] [Google Scholar]
- 35.Hoopes SL, Garcia V, Edin ML, Schwartzman ML, Zeldin DC. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2015;120:9–16. doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldman M, Peterson SJ, Arad M, Hochhauser E. The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins Other Lipid Mediat. 2016;125:108–17. doi: 10.1016/j.prostaglandins.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Garcia V, Schwartzman ML. Recent developments on the vascular effects of 20-hydroxyeicosatetraenoic acid. Current opinion in nephrology and hypertension. 2017;26(2):74–82. doi: 10.1097/mnh.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 38.Salafia CM, Pezzullo JC, Lopez-Zeno JA, Simmens S, Minior VK, Vintzileos AM. Placental pathologic features of preterm preeclampsia. Am J Obstet Gynecol. 1995;173(4): 1097–105. [DOI] [PubMed] [Google Scholar]
- 39.Plenty NL, Faulkner JL, Cotton J, Spencer SK, Wallace K, LaMarca B et al. Arachidonic acid metabolites of CYP4A and CYP4F are altered in women with preeclampsia. Prostaglandins Other Lipid Mediat. 2018;136:15–22. doi: 10.1016/j.prostaglandins.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Catella F, Lawson JA, Fitzgerald DJ, FitzGerald GA. Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci U S A. 1990;87(15):5893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang X, Hu S, Xu B, Snyder GD, Harmon S, Yao J et al. 14,15-Dihydroxyeicosatrienoic acid activates peroxisome proliferator-activated receptor-alpha. Am J Physiol Heart Circ Physiol. 2006;290(1):H55–63. doi: 10.1152/ajpheart.00427.2005. [DOI] [PubMed] [Google Scholar]
- 42.Yang T, Peng R, Guo Y, Shen L, Zhao S, Xu D. The role of 14,15-dihydroxyeicosatrienoic acid levels in inflammation and its relationship to lipoproteins. Lipids Health Dis. 2013;12:151. doi: 10.1186/1476-511x-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowen RS, Zhang Y, Gu Y, Lewis DF, Wang Y. Increased phospholipase A2 and thromboxane but not prostacyclin production by placental trophoblast cells from normal and preeclamptic pregnancies cultured under hypoxia condition. Placenta. 2005;26(5):402–9. [DOI] [PubMed] [Google Scholar]
- 44.Bussolino F, Benedetto C, Massobrio M, Camussi G. Maternal vascular prostacyclin activity in pre-eclampsia. Lancet. 1980;2(8196):702. [DOI] [PubMed] [Google Scholar]
- 45.Cervar M, Kainer F, Jones CJ, Desoye G. Altered release of endothelin-1,2 and thromboxane B2 from trophoblastic cells in pre-eclampsia. Eur J Clin Invest. 1996;26(1):30–7. [DOI] [PubMed] [Google Scholar]
- 46.Ding ZQ, Rowe J, Sinosich MJ, Saunders DM, Gallery EDM. In-vitro secretion of prostanoids by placental villous cytotrophoblasts in pre-eclampsia. Placenta. 1996;17(7):407–11. [DOI] [PubMed] [Google Scholar]
- 47.Downing I, Shepherd GL, Lewis PJ. Reduced prostacyclin production in preeclampsia. Lancet. 1980;2(8208–8209):1374. [DOI] [PubMed] [Google Scholar]
- 48.Johnson RD, Sadovsky Y, Graham C, Anteby EY, Polakoski KL, Huang X et al. The expression and activity of prostaglandin H synthase-2 is enhanced in trophoblast from women with preeclampsia. J Clin Endocrinol Metab. 1997;82(9): 3059–62. [DOI] [PubMed] [Google Scholar]
- 49.Remuzzi G, Marchesi D, Zoja C, Muratore D, Mecca G, Misiani R et al. Reduced umbilical and placental vascular prostacyclin in severe preeclampsia. Prostaglandins. 1980;20(1): 105–10. [DOI] [PubMed] [Google Scholar]
- 50.Walsh SW, Wang Y, Jesse R. Placental production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. Hypertens Pregn. 1996;15(1):101–11. [Google Scholar]
- 51.Wang Y, Walsh SW, Kay HH. Placental lipid peroxides and thromboxane are increased and prostacyclin is decreased in women with preeclampsia. Am J Obstet Gynecol. 1992;167:946–9. [DOI] [PubMed] [Google Scholar]
- 52.Woodworth SH, Li X, Lei ZM, Rao CV, Yussman MA, Spinnato JA, 2nd et al. Eicosanoid biosynthetic enzymes in placental and decidual tissues from preeclamptic pregnancies: increased expression of thromboxane-A2 synthase gene. J Clin Endocrinol Metab. 1994;78(5):1225–31. [DOI] [PubMed] [Google Scholar]
- 53.Thorp JA, Walsh SW, Brath PC. Low-dose aspirin inhibits thromboxane, but not prostacyclin, production by human placental arteries. Am J Obstet Gynecol. 1988;159(6):1381–4. [DOI] [PubMed] [Google Scholar]
- 54.Walsh SW, Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J Clin Endocrinol Metab. 1995;80:1888–93. [DOI] [PubMed] [Google Scholar]
- 55.Nelson DM, Walsh SW. Aspirin differentially affects thromboxane and prostacyclin production by trophoblast and villous core compartments of human placental villi. Am J Obstet Gynecol. 1989;161(6 Pt 1):1593–8. [DOI] [PubMed] [Google Scholar]
- 56.Walsh SW, Wang Y. Maternal perfusion with low-dose aspirin preferentially inhibits placental thromboxane while sparing prostacyclin. Hypertens Pregn. 1998;17(2):203–15. [Google Scholar]
- 57.Wang Y, Walsh SW. Aspirin inhibits both lipid peroxides and thromboxane in preeclamptic placentas. Free Radic Biol Med. 1995;18(3):585–91. [DOI] [PubMed] [Google Scholar]
- 58.Bilodeau JF. Review: maternal and placental antioxidant response to preeclampsia - impact on vasoactive eicosanoids. Placenta. 2014;35 Suppl:S32–8. doi:S0143–4004(13)00833–3 [pii] 10.1016/j.placenta.2013.11.013 [doi]. [DOI] [PubMed] [Google Scholar]
- 59.Walsh SW, Vaughan JE, Wang Y, Roberts LJ, II. Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14(10):1289–96. [DOI] [PubMed] [Google Scholar]
- 60.Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986;59(6):612–9. [DOI] [PubMed] [Google Scholar]
- 61.Walsh SW, Wang Y, Kay HH, McCoy MC. Low-dose aspirin inhibits lipid peroxides and thromboxane but not prostacyclin in pregnant women. Am J Obstet Gynecol. 1992;167(4 Pt 1):926–30. [DOI] [PubMed] [Google Scholar]
- 62.Williams MA, Zingheim RW, King IB, Zebelman AM. Omega-3 fatty acids in maternal erythrocytes and risk of preeclampsia. Epidemiology. 1995;6(3):232–7. [DOI] [PubMed] [Google Scholar]
- 63.Finneran MM, Gonzalez-Brown VM, Smith DD, Landon MB, Rood KM. Obesity and laboratory aspirin resistance in high-risk pregnant women treated with low- dose aspirin. Am J Obstet Gynecol. 2019;220(4):385.e1–e6. doi: 10.1016/j.ajog.2019.01.222. [DOI] [PubMed] [Google Scholar]
- 64.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218(3):287–93.e1. doi: 10.1016/j.ajog.2017.11.561. [DOI] [PubMed] [Google Scholar]
- 65.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med. 2017;377(7):613–22. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 66.Seidler AL, Askie L, Ray JG. Optimal aspirin dosing for preeclampsia prevention. Am J Obstet Gynecol. 2018;219(1):117–8. doi: 10.1016/j.ajog.2018.03.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.