Abstract
Although the concentrations of Alzheimer’s disease (AD) biomarkers Aβ1–40, Aβ1–42 and tau protein are very low in human plasma, ultrasensitive assays such as immunomagnetic reduction (IMR) are able to precisely quantify them. Review articles have described the detailed working mechanism of IMR and revealed the feasibility of detecting early-stage AD by assaying these plasma biomarkers with IMR. In this review, we aimed to compare the significance of these plasma biomarkers in predicting cognitive decline in patients with Down syndrome, stroke, or amnestic mild cognitive impairment based on findings in the literature. We found that plasma Aβ1–42 might play the predominant role in predicting cognitive decline in these patients.
Keywords: Cognitive decline, Down syndrome, Immunomagnetic reduction, Plasma biomarkers, Stroke
Key Summary Points
| A convenient examination such as blood tests that can evaluate the progression to dementia or cognitive decline in population at-risk is urgently needed. |
| The results of reported plasma biomarkers relevant to Alzheimer’s disease, such as Aβ1–40, Aβ1–42 and Tau, in patients with Down syndrome, stroke and amnestic mild cognitive impairment are discussed |
| The levels of plasma Aβ1–42 are possibly the most dominant to predict the cognitive decline in these patients. |
| It would be hopeful to stop the development of dementia or delay cognitive decline by eliminating the abnormal changes in the level of plasma Aβ1–42. |
Digital Features
This article is published with digital features to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.12982070.
Introduction
Pathological evidence of Alzheimer’s disease (AD) includes the presence of amyloid plaques and tau protein tangles in the brain [1–4], which are typically detected with positron emission tomography (PET) [5–8]. Although amyloid or tau PET has been approved for clinical use, its high cost and low availability seriously limit its utility in clinical practice. Instead, the detection of amyloid β (Aβ) and tau protein in cerebrospinal fluid (CSF) has been suggested as a method to probe for pathological evidence of AD [9–12]. However, it has not been easy to implement CSF biomarker detection more widely in clinical practice because of the need to perform a lumbar puncture. The detection of Aβ and tau protein in other body fluids such as blood, urine, or saliva has been proposed as an alternative method. Compared to those in the CSF, the levels of Aβ and tau protein in such body fluids are extremely low, on the order of picograms per milliliter [13–15]. Thus, ultrasensitive assays are needed to precisely quantify the levels of Aβ and tau proteins in blood, urine, or saliva. At present, several ultrasensitive assaying technologies have been developed [16–20], but there is no consistency in the measured concentrations of plasma Aβ and tau protein among the different assaying technologies. For example, the levels of plasma amyloid β 1–42 (Aβ1–42) were found to be reduced in AD patients as compared to normal controls using single-molecule array (SIMOA), whereas they were found to be higher using immunomagnetic reduction assay (IMR). The discrepancies in measured results among assaying technologies could be attributable to several factors, including antibodies, assay methodology, and sample preparation. Hence, it would be better to concentrate on the results obtained by assay with one of these technologies. In this review, the assay results obtained with superconducting quantum interference device-based immunomagnetic reduction (SQUID IMR) are the subject of interest [21, 22].
Most studies utilizing SQUID IMR have focused on exploring the associations between Aβ or tau protein in plasma and in CSF [15], the results of amyloid or tau PET [23–25] or magnetic resonance imaging [24, 26], or clinical diagnosis [27–29]. These studies have yielded promising results regarding the feasibility of detecting AD in patients by assaying blood Aβ and tau protein. We will not summarize such works in this review, because previous review articles have already investigated the working mechanism and clinical impact of SQUID IMR [30, 31]. Instead, according to published papers [32–35], we will aim to discuss the possible dominance of plasma Aβ and tau protein in the progression to dementia in at-risk patients, such as those with Down syndrome, stroke, or amnestic mild cognitive impairment (aMCI). Such research is so novel that few papers have been published. In this review, five studies are referenced to show the roles of plasma Aβ and tau in the progression to dementia in these at-risk patients. This is a review article that is based on reported studies and does not contain any new studies with human participants or animals performed.
Prediction of Dementia in Patients with Down Syndrome
Down syndrome (DS) is a common chromosomal abnormality [36]. Due to the presence of a triplicated chromosome 21, DS patients usually suffer from amyloid deposition in the brain, which increases the risk of developing dementia. The onset age of dementia in DS patients is earlier than that in normal controls by 30 years. Thus, a high prevalence of dementia has been observed in DS patients younger than 35 years of age [37]. However, in clinical practice it is very difficult to diagnose dementia in a DS patient, regardless of the results of magnetic resonance imaging, amyloid PET, neuropsychological tests, or CSF biomarker detection. Therefore, a delayed diagnosis of dementia for DS patients often occurs in the clinic. On the other hand, drawing blood is relatively easy for DS patients. This has motivated studies exploring the feasibility of discriminating dementia in DS patients via plasma biomarkers.
Fang et al. investigated the difference in levels of plasma biomarkers between DS individuals without and with dementia [32]. They enrolled 35 DS patients without dementia and 16 DS patients with dementia, using the Adaptive Behavior Dementia Questionnaire (ABDQ) score to discriminate between the two groups. SQUID IMR was used to assay plasma Aβ1–42, amyloid β 1–40 (Aβ1–40), and tau protein (tau) in the enrolled subjects. All three biomarkers were reported to be moderately associated with the ABDQ score, as illustrated in Table 1. Notably, the plasma Aβ1–42 level showed the most significant correlation with the ABDQ score in DS patients. The results of the receiver operating characteristic (ROC) curve analysis for discriminating dementia in DS using the plasma concentrations of Aβ1–42, Aβ1–40, and tau are listed in Table 2. It was observed that the plasma Aβ1–42 level was the predominant indicator for the early detection of neurodegeneration in DS. These results reveal that the plasma Aβ1–42 level might be the most powerful parameter for predicting the progression of dementia in DS.
Table 1.
Correlation coefficient r between plasma biomarkers and the ABDQ score in DS patients (all p < 0.05) [32]
| Biomarker | Aβ1–42 | Aβ1–40 | Tau |
|---|---|---|---|
| r | 0.621 | −0.556 | −0.410 |
Table 2.
Results of ROC curve analysis for determining dementia in DS using plasma biomarkers [32]
| Biomarker | Aβ1–42 | Aβ1–40 | Tau |
|---|---|---|---|
| Cutoff value | 12.36 pg/ml | 92.2 pg/ml | 26.04 pg/ml |
| Sensitivity | 0.875 | 0.813 | 0.636 |
| Specificity | 0.943 | 0.943 | 0.883 |
Identification and Prediction of Vascular Cognitive Impairment in Stroke Patients
Stroke causes serious brain damage or death in afflicted patients. It can also lead to cerebral ischemia, which triggers the deposition of Aβ, the hyperphosphorylation of tau, and neuroinflammation in the brain [38–40]. Hence, post-stroke patients are at risk for the progression of dementia. It has been reported that post-stroke dementia occurs in 7% of patients 1 year after a stroke [41], which increases to 52% at 6 months if mild post-stroke cognitive impairment is included in the diagnosis [42]. Thus, management for both stroke and dementia is needed in patients post-stroke. However, such patients usually experience impairment in activities of daily living, language, etc. [43, 44]. It is difficult to evaluate neurodegeneration in post-stroke patients using neuroimaging or neuropsychological tests. Blood tests, on the other hand, would be a more accessible method.
Tang et al. utilized an IMR assay for plasma Aβ1–40, Aβ1–42 and tau in post-stroke patients with (n = 34, age = 76.9 ± 6.2 years) or without (n = 27, age = 71.0 ± 6.6 years) dementia [33]. Neuropsychological tests, including the clinical dementia ranking (CDR) scale, the Mini-Mental State Examination (MMSE), and the Montreal Cognitive Assessment (MoCA), were performed for every participant. The clinical diagnosis of vascular dementia was made in accordance with the criteria for dementia from the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN). The results of the study showed values of CDR ≥ 1, MMSE = 13.1 ± 4.2, and MoCA = 7.3 ± 4.7 for post-stroke patients with dementia, and CDR ≤ 0.5, MMSE = 28.7 ± 0.9, and MoCA = 23.6 ± 3.4 for those without dementia. Among the measured plasma biomarkers, only the Aβ1–42 level was significantly different between post-stroke patients with (17.6 ± 2.6 pg/ml) and without dementia (15.4 ± 1.8 pg/ml, p < 0.01). Receiver operating characteristic curve analysis determined a cutoff value of 15.59 pg/ml for discriminating non-dementia from dementia in post-stroke patients, resulting in clinical sensitivity, specificity, and area under the curve of 0.765, 0.741, and 0.760, respectively, as shown in Fig. 1. Furthermore, plasma Aβ1–42 showed a moderately negative correlation with the MMSE (r = −0.46, p < 0.01) and MoCA scores (r = −0.44, p < 0.01) in post-stroke patients, as shown in Table 3. The levels of plasma Aβ1–40 and tau were not associated with the MMSE or MoCA scores. Hence, the plasma Aβ1–42 level is a promising index for determining neurodegeneration in post-stroke patients.
Fig. 1.
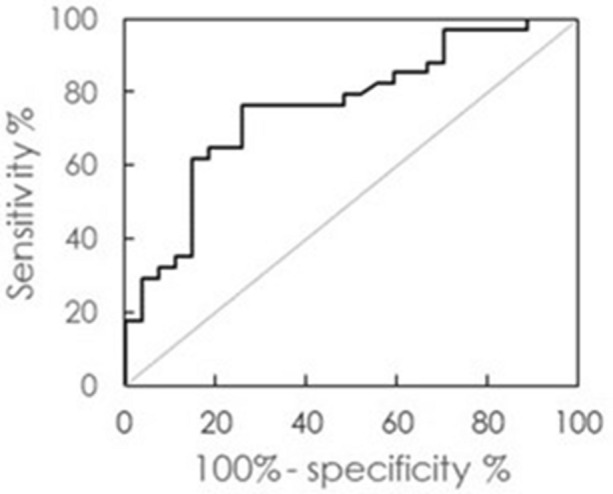
Receiver operating characteristic curve analysis using plasma Aβ1–42 concentration as an index to discriminate non-dementia from dementia in post-stroke patients
Table 3.
Correlations between levels of plasma biomarkers Aβ1–40, Aβ1–42, tau, and MMSE and MoCA scores in post-stroke patients [33]
| Biomarker | Aβ1–42 | Aβ1–40 | Tau |
|---|---|---|---|
| MMSE | |||
| r | −0.46 | 0.07 | −0.04 |
| p value | 0.002 | 0.631 | 0.751 |
| MoCA | |||
| r | −0.44 | 0.17 | −0.02 |
| p value | 0.001 | 0.251 | 0.878 |
Chi et al. investigated the prediction of cognitive impairment in post-stroke patients 1 year after stroke using levels of plasma biomarkers 3 months after stroke [34]. Forty-two post-stroke patients with and 13 post-stroke patients without cognitive impairment after 1 year were enrolled. The plasma Aβ1–42, Aβ1–40, and tau levels 3 months after stroke were assayed. The level of plasma Aβ1–42 at 3 months was the most significant biomarker in predicting post-stroke cognitive impairment 1 year after stroke. Therefore, plasma Aβ1–42 is a promising index for both predicting and determining cognitive impairment in post-stroke patients.
Prediction of Cognitive Decline in aMCI patients
Amnestic mild cognitive impairment (aMCI) is defined as a transition from normal aging to Alzheimer’s disease (AD). Approximately 30% of aMCI patients progress to AD in 1–1.5 years, and more than 50% progress to AD in 3 years [45, 46]. One of the symptoms during this progression is cognitive decline. In the clinic, the objective is to predict cognitive decline in aMCI patients. Tsai et al. investigated the predictive power for cognitive decline of the baseline levels of plasma biomarkers at a 1.2-year follow-up in aMCI patients [34]. Twenty-four aMCI patients were enrolled. The value of Pearson’s correlation coefficient r between the baseline levels of the plasma biomarkers and the annual changes in the MMSE score are listed in Table 4. Among the three different plasma biomarkers, Aβ1–42 showed a significant and moderate negative correlation with the annual change in the MMSE score.
Table 4.
Correlations between baseline levels of plasma biomarkers and the annual change in MMSE score in aMCI patients with an average follow-up period of 1.2 years [34]
| Biomarker | Aβ1–42 | Aβ1–40 | Tau |
|---|---|---|---|
| aMCI (n = 24) | |||
| Pearson’s r | −0.51 | 0.003 | −0.28 |
| p value | 0.012 | 0.992 | 0.189 |
Similar results were reported independently by Chen et al. and are listed in Table 5. Twenty-two aMCI patients were enrolled [35], and changes in the MMSE scores were measured at the 1–1.5-year follow-up. The value of Pearson’s r between the baseline levels of the plasma biomarkers and the annual change in the MMSE score was analyzed. Compared to the levels of Aβ1–40 and tau, the level of plasma Aβ1–42 at baseline showed the strongest association with the annual change in the MMSE score. The two independent studies by Tsai et al. and Chen et al. illustrate that plasma Aβ1–42 at baseline is a useful parameter for predicting cognitive decline in aMCI.
Table 5.
Correlations between baseline levels of plasma biomarkers and the annual change in MMSE score in aMCI patients with an average follow-up period of 1–1.5 years [35]
| Biomarker | Aβ1–42 | Aβ1–40 | Tau |
|---|---|---|---|
| aMCI (n = 22) | |||
| Pearson’s r | −0.512 | NA | −0.376 |
| p value | 0.015 | NA | 0.085 |
Conclusions
In populations at risk for developing dementia, such as patients with Down syndrome, stroke, and amnestic mild cognitive impairment, it is important to be able to predict the progression to dementia or cognitive decline. In clinical practice, a convenient examination that can evaluate progression to dementia or cognitive decline is urgently needed. According to this review, plasma Aβ1–42 may play a more key role in this prediction than Aβ1–40 or tau. This implies that abnormal levels of Aβ1–42 might be a critical trigger of the onset of dementia or cognitive decline. Therefore, there may be hope for stopping the development of dementia or delaying cognitive decline by eliminating the abnormal changes in the level of plasma Aβ1–42. Nevertheless, there are limitations in this work that should be mentioned. Notably, because of the novelty of such research, only five studies were available for this review. Additional independent studies should be performed to further explore the promising results described in this review.
Acknowledgements
Funding
The study, the Rapid Service Fee, and/or the Open Access fee were funded by MagQu Co., Ltd.
Authorship
All named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosures
Shieh-Yueh Yang and Huei-Chun Liu are employees of MagQu Co., Ltd. Wen-Ping Chen is an employee of MagQu LLC. Shieh-Yueh Yang is a shareholder of MagQu Co., Ltd.
Compliance with Ethics Guidelines
This is a review article that is based on reported studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.
References
- 1.Mena R, Edwards P, Pérez-Olvera O, Wischik CM. Monitoring pathological assembly of tau and β-amyloid proteins in Alzheimer's disease. Acta Neuropathol. 1995;89:50–56. doi: 10.1007/BF00294259. [DOI] [PubMed] [Google Scholar]
- 2.An TH, Termont A, Merchiers P, Schilling S, Demuth HU, Scrocchi L, et al. Pathological hallmarks, clinical parallels, and value for drug testing in Alzheimer’s disease of the APP[V717I] London transgenic mouse model. Int J Alzheimer Dis. 2010;2010:417314. doi: 10.4061/2010/417314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak J, Tredici KD. The preclinical phase of the pathological process underlying sporadic Alzheimer's disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- 4.He Z, Guo JL, McBride JD, Narasimhan S, Kim H, Changolkar L, et al. Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med. 2018;24:29–38. doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordberg A, Rinn JO, Kadir A, Långström B. The use of PET in Alzheimer disease. Nature Rev Neurol. 2010;6:78–87. doi: 10.1038/nrneurol.2009.217. [DOI] [PubMed] [Google Scholar]
- 6.Adlard PA, Tran BA, Finkelstein DI, Desmond PM, Johnston LA, Bush AI, et al. A review of β-amyloid neuroimaging in Alzheimer’s disease. Front Neurosci. 2014;8:327. doi: 10.3389/fnins.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Wiste HJ, Schwarz CG, Lowe VL, Senjem ML, Vemuri P, et al. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain. 2018;141:1517–1528. doi: 10.1093/brain/awy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Leon MJ, Desanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, et al. MRI and CSF studies in the early diagnosis of Alzheimer’s disease. J Int Med. 2004;256:205–223. doi: 10.1111/j.1365-2796.2004.01381.x. [DOI] [PubMed] [Google Scholar]
- 10.Bibl M, Mollenhauer B, Esselmann H, Lewczuk P, Klafki HW, Sparbier K, et al. CSF amyloid-β-peptides in Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease dementia. Brain. 2006;129:1177–1187. doi: 10.1093/brain/awl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anoop A, Singh PK, Jacob RS, Maji SK. CSF biomarkers for Alzheimer’s disease diagnosis. Int J Alzheimer’s Dis. 2010;2010:606802–606812. doi: 10.4061/2010/606802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal R, Chhillar N, Mishra VN, Tripathi CB. CSF tau and amyloid β42 levels in Alzheimer’s disease—a meta-analysis. Adv Alzheimer’s Dis. 2012;1:30–44. [Google Scholar]
- 13.Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 14.Pereira JB, Westman E, Hansson O, ADNI Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol Aging. 2017;58:14–29. doi: 10.1016/j.neurobiolaging.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Teunissen CE, Chiu MJ, Yang CC, Yang SY, Scheltens P, Zetterberg H, et al. Plasma amyloid-β (Aβ42) correlates with cerebrospinal fluid Aβ42 in Alzheimer’s disease. J Alzheimer’s Dis. 2018;62:1857–1863. doi: 10.3233/JAD-170784. [DOI] [PubMed] [Google Scholar]
- 16.Xia W, Yang T, Shankar G, Smith IM, Shen Y, Walsh DM, et al. A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch Neurol. 2009;66:190–199. doi: 10.1001/archneurol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh ES, Mielke MM, Rosenberg PB, Jain A, Fedarko NS, Lyketsos CG, et al. Comparison of conventional ELISA with electrochemiluminescence technology for detection of amyloid-β in plasma. J Alzheimer’s Dis. 2010;21:769–773. doi: 10.3233/JAD-2010-100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkmann E, Henke F, Weinmann N, Dumpitak C, Groschup M, Funke A, et al. Counting of single prion particles bound to a capture-antibody surface (surface-FIDA) Vet Microbiol. 2007;123:294–304. doi: 10.1016/j.vetmic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Ahn HS, Cho SM, Lee JE, Kim YS, Lee C. Detection and quantification of plasma amyloid-β by selected reaction monitoring mass spectrometry. Anal Chim Acta. 2014;840:1–9. doi: 10.1016/j.aca.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 20.Mondello S, Buki A, Barzo P, Randall J, Provuncher G, Hanlon D, et al. CSF and plasma amyloid-β temporal profiles and relationships with neurological status and mortality after severe traumatic brain injury. Sci Rep. 2014;4:6446–6451. doi: 10.1038/srep06446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chieh JJ, Yang SY, Jian ZF, Wang WC, Horng HE, Yang HC, et al. Hyper-high-sensitivity wash-free magnetoreduction assay on biomolecules using high-Tc superconducting quantum interference devices. J Appl Phys. 2008;103:014703. [Google Scholar]
- 22.Chiu MJ, Horng HE, Chieh JJ, Liao SH, Chen CH, Shih BY, et al. Multi-channel SQUID-based ultra-high-sensitivity in-vitro detections for bio-markers of Alzheimer’s disease via immunomagnetic reduction. IEEE Trans Appl Supercond. 2011;21:477–480. [Google Scholar]
- 23.Tzen KY, Yang SY, Chen TF, Cheng TW, Horng HE, Wen HP, et al. Plasma Aβ but not tau is related to brain PiB retention in early Alzheimer’s disease. ACS Chem Neurosci. 2014;5:830–836. doi: 10.1021/cn500101j. [DOI] [PubMed] [Google Scholar]
- 24.Chiu MJ, Chen YF, Chen TF, Yang SY, Yang FPG, Tseng TW, et al. Plasma tau as a window to the brain—negative associations with brain volume and memory function in mild cognitive impairment and early Alzheimer’s disease. Hum Brain Mapp. 2014;35:3132–3142. doi: 10.1002/hbm.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SY, Lin KJ, Lin PC, Huang CC, Chang CH, Lee YC, et al. Plasma amyloid assay as a pre-screening tool for amyloid positron emission tomography imaging in early stage Alzheimer’s disease. Alzheimer’s Res Ther. 2019;11:111. doi: 10.1186/s13195-019-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan LY, Tzen KY, Chen YF, Chen TF, Lai YM, Yen RF, et al. The relation between brain amyloid deposition, cortical atrophy, and plasma biomarkers in amnesic mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci. 2018;10:175. doi: 10.3389/fnagi.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu MJ, Yang SY, Chen TF, Chieh JJ, Huang TZ, Yip PK, et al. New assay for old markers-plasma beta amyloid of mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer’s Res. 2012;9:1142–1148. doi: 10.2174/156720512804142967. [DOI] [PubMed] [Google Scholar]
- 28.Lue LF, Sabbagh MN, Chiu MJ, Jing N, Snyder NL, Schmitz C, et al. Plasma levels of Aβ42 and tau identified probable Alzheimer’s dementia: findings in two cohorts. Front Aging Neurosci. 2017;9:226. doi: 10.3389/fnagi.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang CC, Chiu MJ, Chen TF, Chang HL, Liu BH, Yang SY. Assay of plasma phosphorylated tau protein (Threonine 181) and total tau protein in early-stage Alzheimer’s disease. J Alzheimer’s Dis. 2018;61:1323–1332. doi: 10.3233/JAD-170810. [DOI] [PubMed] [Google Scholar]
- 30.Yang SY, Chiu MJ, Chen TF, Horng HE. Detection of plasma biomarkers using immunomagnetic reduction: a promising method for the early diagnosis of Alzheimer’s disease. Neurol Ther. 2017;6:S37–S45. doi: 10.1007/s40120-017-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lue LF, Kuo YM, Sabbagh MN. Advance in plasma AD core biomarker development: current findings from immunomagnetic reduction-based SQUID technology. Neurol Ther. 2019;8:S95–S111. doi: 10.1007/s40120-019-00167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee NC, Yang SY, Chieh JJ, Huang PS, Chang LM, Chiu YN, et al. Blood beta-amyloid and Tau in Down Syndrome: a comparison with Alzheimer’s disease. Front Aging Neurosci. 2017;8:316. doi: 10.3389/fnagi.2016.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang SC, Yang KC, Chen CH, Yang SY, Chiu MJ, Wu CC, et al. Plasma β-amyloids and tau proteins in patients with vascular cognitive impairment. Neuro Mol Med. 2018;20:498–503. doi: 10.1007/s12017-018-8513-y. [DOI] [PubMed] [Google Scholar]
- 34.Chi NF, Chao SP, Huang LK, Chan L, Chen YR, Chiou HY, et al. Plasma amyloid beta and tau levels are predictors of post-stroke cognitive impairment: a longitudinal study. Front Neurol. 2019;10:715. doi: 10.3389/fneur.2019.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai CL, Liang CS, Yang CP, Lee JT, Ho TH, Su MW, et al. Indicators of rapid cognitive decline in amnestic mild cognitive impairment: the role of plasma biomarkers using magnetically labeled immunoassays private. J Psychiatry Res. 2020;129:66–72. doi: 10.1016/j.jpsychires.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Lin SJ, Hu SC, Sheu SF, Ho JW, Chiou PC, Chao MC, et al. [Anthropometric study on Down syndrome in Taiwan] Zhonghua Min. Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1991;32:158–164. [PubMed] [Google Scholar]
- 37.Bush A, Beail N. Risk factors for dementia in people with Down syndrome: issues in assessment and diagnosis. Am J Ment Retard. 2014;109:83–97. doi: 10.1352/0895-8017(2004)109<83:RFFDIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Whitehead SN, Thiel A, Cechetto DF, Heiss WD, Hachinski V, Whitehead SN. Amyloid burden, neuroinflammation, and links to cognitive decline after ischemic stroke. Stroke. 2014;45:2825–2829. doi: 10.1161/STROKEAHA.114.004285. [DOI] [PubMed] [Google Scholar]
- 39.Mailliot C, Podevin-Dimster V, Rosenthal RE, Sergeant N, Delacourte A, Fiskum G, et al. Rapid tau protein dephosphorylation and differential rephosphorylation during cardiac arrest-induced cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2000;20:543–549. doi: 10.1097/00004647-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke. 2014;45:1531–1538. doi: 10.1161/STROKEAHA.113.004534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 42.Mijajlović MD, Pavlović A, Brainin M, Heiss W-D, Quinn TJ, Ihle-Hansen HB, et al. Post-stroke dementia—a comprehensive review. BMC Med. 2017;15:11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cumming TB, Brodtmann A, Darby D, Bernhardt J. The importance of cognition to quality of life after stroke. J Psychosom Res. 2014;77:374–379. doi: 10.1016/j.jpsychores.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Stephens S, Kenny RA, Rowan E, Kalaria RN, Bradbury M, Pearce R, et al. Association between mild vascular cognitive impairment and impaired activities of daily living in older stroke survivors without dementia. J Am Geriatr Soc. 2005;53:103–107. doi: 10.1111/j.1532-5415.2005.53019.x. [DOI] [PubMed] [Google Scholar]
- 45.Tripathi M, Parida GK, Kumar R, Dwivedi S, Nehra A, Bal C. Biomarker-based prediction of progression to dementia: F-18 FDG-PET in amnestic MCI. Neurol India. 2019;67:1310–1317. doi: 10.4103/0028-3886.271245. [DOI] [PubMed] [Google Scholar]
- 46.Jang H, Ye BS, Woo S, Kim SW, Chin J, Choi SH, et al. Prediction model of conversion to dementia risk in subjects with amnestic mild cognitive impairment: a longitudinal, multi-center clinic-based study. J Alzheimer’s Dis. 2017;60:1579–1587. doi: 10.3233/JAD-170507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.


