Abstract
Most disease-modifying drugs (DMDs) are contraindicated in pregnancy. Management of MS is especially challenging for pregnant patients, as withdrawal of DMDs leave the patient at risk of increased disease activity. We, a group of experts in MS care from countries in the Arab Gulf, present our consensus recommendations on the management of MS in these patients. Where possible, a patient planning pregnancy can be switched to a DMD considered safe in this setting. Interferon β now can be used during pregnancy, where there is a clinical need to maintain treatment, in addition to glatiramer acetate. Natalizumab (usually to 30 weeks’ gestation for patients with high disease activity at high risk of relapse and disability progression) may also be continued into pregnancy. Cladribine tablets and alemtuzumab have been hypothesised to act as immune reconstitution therapies (IRTs). These drugs provide a period of prolonged freedom from relapses for many patients, but the patient must be prepared to wait for up to 20 months from initiation of therapy before becoming pregnant. If a patient becomes pregnant while taking fingolimod, and requires continued DMD treatment, a switch to interferon β or natalizumab after a variable washout period may be prescribed, depending on the level of disease activity. Women who wish to breastfeed should be encouraged to do so, and interferon β may also be used during breastfeeding. There is a lack of data regarding the safety of using other DMDs during breastfeeding.
Keywords: Breastfeeding, Disease-modifying drugs, Family planning, Multiple sclerosis, Pregnancy
Key Summary Points
| The management of MS is especially challenging for pregnant patients, as most disease-modifying drugs (DMDs) are contraindicated at this time |
| We, a group of experts in MS care from countries in the Arab Gulf, present our consensus recommendations on the management of MS in these patients |
| Interferon β now can be used during pregnancy and breastfeeding, where there is a clinical need to maintain treatment, in addition to glatiramer acetate |
| Natalizumab (usually to 30 weeks’ gestation for patients with high disease activity at high risk of relapse and disability progression) may also be continued into pregnancy |
| Pharmacological immune reconstitution therapies (currently cladribine tablets and alemtuzumab) provide prolonged freedom from relapses for many patients, but pregnancy should not occur for up to 20 months from initiation of therapy |
| Consider a switch to interferon β or natalizumab after an appropriate washout period for women who become pregnant on fingolimod |
Introduction
Multiple sclerosis (MS) is diagnosed around 30 years of age, on average, a figure which has tended to decrease over time [1, 2]. A diagnosis of MS therefore commonly arises during women’s childbearing years. Indeed, MS affects about 2–3 times as many women as men during these years of life [1]. The majority of people who develop MS are women, and a marked increase in the prevalence of MS in most countries has further increased the number of women who develop MS at a time that they are likely to consider planning a family [2–4].
These trends in the epidemiology of MS are evident in the authors’ countries in the Gulf region, as elsewhere [5–7]. Large families are the norm in the Middle East, and cultural issues relating to contraception (and termination of a pregnancy exposed to a potentially unsafe therapy) must be discussed carefully [8, 9]. MS has no adverse impact per se on a woman’s fertility, or on a pregnancy; conversely, pregnancy has no long-term impact on the course of MS (aside from short-term changes in relapse rates during and after a pregnancy, which are discussed in more detail below) [10]. Nevertheless, the onset of MS can have a profound influence on patients’ reproductive choices: studies have shown that the fear and uncertainty provoked by the diagnosis resulted in these women having fewer pregnancies than they expected to have, had they not developed MS [11, 12]. Pregnancy also impacts on the management of MS. For example, women with MS commonly stop taking their disease-modifying drug (DMD) treatment for this reason because of concerns relating to the potential for adverse effects of the treatment on the pregnancy [13, 14]. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Methods and Objectives
An expert group in the UK has provided guidance on the management of people with MS who are planning a family [15], but otherwise guidance is limited. Prescribing practices in Gulf countries are influenced by international labelling, e.g. from the European medicines Agency or the US Food and Drug Administration, but are not constrained by them [16]. Accordingly, international guidelines for the management of MS may not fully support local practice in the Gulf region. This article provides consensus recommendations on the application of DMD-based therapy for MS during pregnancy from a group of specialists in the management of MS in countries in the Arabian Gulf, considering in particular the impact of low or high levels of MS disease activity on management. Efficacy and the safety/tolerability and monitoring burdens of individual DMDs are key aspects to consider when prescribing a DMD, irrespective of pregnancy status or plans. A full account of these is beyond the scope of this review and has been reviewed elsewhere [17–19].
The expert consensus described in this article arose from a closed meeting (in Muscat, Oman, November 2019) in which all authors participated; delegates from Kuwait, Oman, Qatar, and the United Arab Emirates participated. The expert consensus is supported by a narrative review, based on presentations at this meeting, supplemented by additional literature searches and material provided by co-authors. The level of consensus on recommendations within the expert group was explored by open voting: a “high” level of consensus was defined arbitrarily as supported by at least 7–8/9 experts, moderate consensus was defined as being supported by 4–6/9 experts, and lower support was defined as a “low” level of consensus.
Principles of Management of MS Before, During, and After Pregnancy
Preconception Counselling
Misconceptions relating to the practicability of completing a pregnancy are common among patients with MS [1–12], and patients should be counselled carefully that their MS has no impact on their ability to conceive, to carry the pregnancy to term, and to give birth to a healthy neonate [20]. In the authors’ experience, patients are often concerned that they will pass on their MS to the children. Although there is familial clustering in MS, this is not considered to be a hereditary disease per se, as the overall risk of developing MS depends on interactions between genetic and environmental factors [21]. Patients may be counselled that the risk of MS in their children is low.
Need for Active Treatment of MS During Pregnancy?
On average, the frequency of relapses in a woman with MS declines during pregnancy, especially during the second and third trimesters, but then increases markedly during the 3 months following delivery [22, 23]. These observations and concerns regarding possible adverse effects to the foetus have led most women to discontinue DMD therapy during pregnancy. This position is reinforced by current (2018) European guidelines for the management of MS [24, 25] and an expert position statement from Italy [26], which have supported continued use of certain DMDs (interferon, glatiramer acetate, natalizumab) into pregnancy, within a multidisciplinary approach, especially for women with persistent high disease activity or who are at high risk of disease reactivation. US guidelines (also 2018) recommend that women may defer use of DMDs until after their pregnancy [19, 25]. A study from two pregnancy registries in the Middle East found a higher rate of relapses than reported previously in a population of women with MS who had mostly received “platform” or “first-line” therapies in the year before conception, such as glatiramer acetate or interferon, perhaps indicating a greater need for continued treatment at this time that was considered previously [27]. Importantly, we have little information on the rate of relapses during pregnancy in women with higher pre-conception levels of MS disease activity.
Thus, the choice of DMD for a female patient of childbearing potential will be influenced by her plans for starting a family, as becoming pregnant may provoke an immediate change in treatment. Decisions on maintaining or switching treatment are complex and will be influenced by the patient’s individual circumstances. Important considerations at this time include the risks to the foetus of the patient’s current DMD, the level of disease activity experienced by the patient, the required washout period for the current DMD, and the time needed for the new DMD’s efficacy to build up.
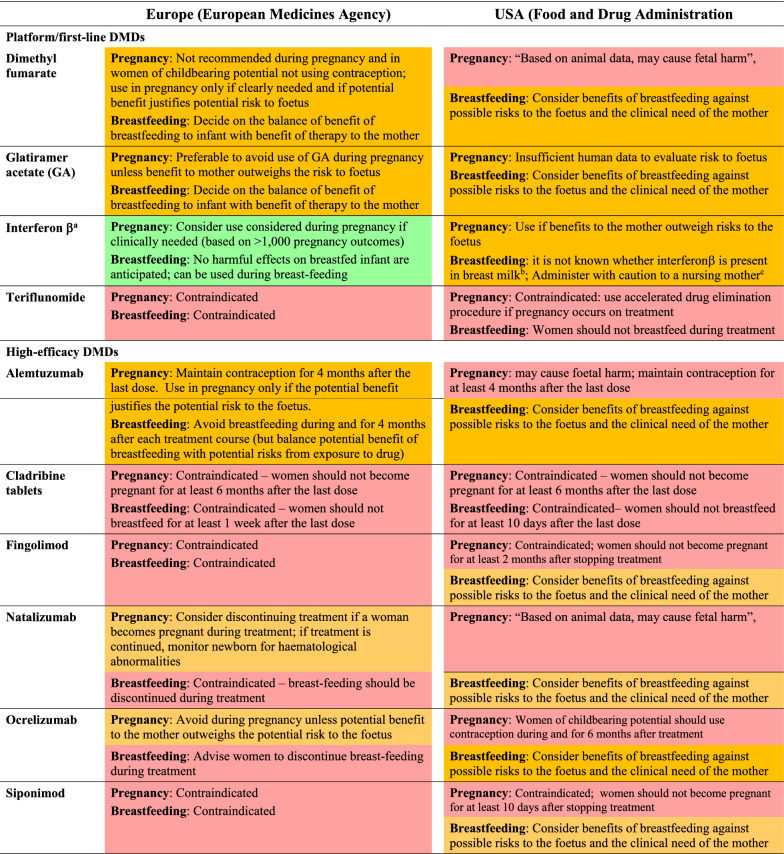
Research into the use of DMDs by women who are either pregnant or planning to become pregnant has continued, with a particular focus on gathering outcomes from registries dedicated to this purpose (see below), and statements such as “DMDs are not licensed during pregnancy, except glatiramer acetate” from the European MS guidelines are no longer clearly consistent with prescribing recommendations (Table 1). Indeed, the support for use of individual DMDs during pregnancy within their labelling varies widely between individual agents (Table 1). Teriflunomide, cladribine tablets, fingolimod, and siponimod have absolute contraindications for use during pregnancy in both the USA and Europe. Glatiramer acetate may be continued into pregnancy according to both US and European labels, as long as this is supported by an assessment of risks to the foetus and benefits to the mother. Dimethyl fumarate, alemtuzumab, natalizumab, and ocrelizumab may be used in Europe, according to such a risk:benefit assessment, but their use in pregnancy is discouraged (with warnings of potential “foetal harm”; thier use is contraindicated in the USA. The use of all preparations of interferon β in pregnancy is more strongly supported in Europe.
Table 1.
Current restrictions on the use of disease-modifying drugs (DMDs) relevant to pregnancy and breastfeeding
Abstracted from European Summaries of Product Characteristics and US Prescribing Information. Colours are applied arbitrarily according to strength of support for use during pregnancy and breastfeeding (red = contraindicated, amber = warning/precaution, green = indicated). Recommendations shown here are paraphrased for brevity: always consult your local labelling
aIncludes interferon β1a (formulations for both s.c. and i.m. injections), and interferon β1b (s.c. injections)
bStatement made for all formulations of interferon β
cSimilar statements are made in US Prescribing Information for s.c. interferon β1a and interferon β1b, but no such statement is made for i.m. interferon β1a
Current Data on DMDs and Pregnancy
Nature of the Evidence Base
The current evidence base from clinical studies regarding the use of individual DMDs in pregnancy is summarised below. For convenience we have divided the DMDs arbitrarily into the commonly used categories of “first-line” or “platform” DMDs (which usually include dimethyl fumarate, glatiramer acetate, interferon β, and teriflunomide) and “high-efficacy” DMDs (alemtuzumab, cladribine tablets, fingolimod, natalizumab, ocrelizumab) [17]. We have sought to cite papers from peer-reviewed journals here. However, the application of DMD-based therapy in the setting of pregnancy is an evolving science and many of the reports considered below are recent presentations at major congresses. These are included to provide the best snapshot possible of the current state of the science.
Platform DMDs
Glatiramer acetate This treatment has no teratogenic effects, according to data from national registries. In the Italian Multiple Sclerosis Register, analysis of data from 427 pregnancies in mothers with MS from 21 centres found no additional risk of spontaneous abortion or other adverse maternal or foetal outcomes [28, 29]. A total of 151 women with MS in Germany had been taking glatiramer acetate before the pregnancy, of whom 148 discontinued treatment in the first trimester and 3 discontinued treatment in the second trimester; 95 pregnancies unexposed to DMDs served as a control group [30]. There was no difference between groups for the proportions of live births or the risk of spontaneous abortion, any congenital anomaly, major congenital anomaly, preterm birth or need for caesarean section. In another study, evaluation of 5042 pregnancies exposed to glatiramer acetate demonstrated low and comparable rates of adverse pregnancy outcomes compared with data from two control databases of birth outcomes that together include > 1.7 million births each year [31]. Glatiramer acetate therefore appears to be safe with regard to use in pregnancy, at least during the first trimester.
Dimethyl fumarate Analysis of 63 pregnancies in women enrolled in clinical trials and of 135 pregnancies arising from post-marketing reports revealed no adverse effects on pregnancy outcomes [32]. An international registry is tracking pregnancies in women exposed to dimethyl fumarate; a recent report from this database (194 pregnancies with known outcome) showed that the rate of premature loss of the foetus was 9%, with live births occurring in the remainder, and a rate of birth defects of 4% [33]. To date, therefore, dimethyl fumarate has not been associated with adverse pregnancy outcomes.
Interferon β Pregnancy outcomes with interferon β have been collected in major registries, namely the Italian Multiple Sclerosis Register (88 exposed and 308 unexposed pregnancies) [28, 34], the German Multiple Sclerosis and Pregnancy Registry (251 exposed and 194 unexposed pregnancies) [35], the Merck KGaA Global Drug Safety Database (1022 exposed pregnancies) [36], and a Nordic Pregnancy Registry (875 exposed pregnancies, 1831 unexposed pregnancies) [37]. Together, these studies showed that there was no excess risk to the foetus resulting from exposure in utero to interferon β, with regard to rates of live births, spontaneous abortions, or congenital abnormalities; the frequency of these outcomes was comparable to those observed in the general population. Mean birth weight and birth length were also consistent between neonates exposed or not exposed to interferon β in utero [35, 37].
Teriflunomide and leflunomide An analysis of the global pharmacovigilance database for this agent found a rate of spontaneous abortion of 19% among 70 pregnancies with known exposure to teriflunomide, which was described as being within the range of rates expected for the general population (40% of these women underwent elective terminations of the pregnancy) [38]. There were no congenital abnormalities in 26 live births. Most of the women who carried the pregnancy to term underwent the rapid elimination procedure for teriflunomide (23/26, 88%). A more recent (up to December 2017) survey of 437 teriflunomide-exposed pregnancies (220 with known outcomes) found a rate of spontaneous abortion of 21% [39]. There were four birth defects (one considered major). These outcomes were again considered consistent with those expected from the general population, without demonstration of a teratogenic signal for teriflunomide.
The analysis from the global pharmacovigilance database for teriflunomide also documented no adverse birth outcomes from 22 pregnancies of partners of male patients taking teriflunomide [38]. Similar results were found from a recent analysis of 232 pregnancies exposed to teriflunomide or leflunomide [40]. The ongoing International Teriflunomide Pregnancy Exposure Registry is aiming to recruit 196 women with pregnancies exposed to teriflunomide (with at least 104 live births) from 17 countries [41]. This population will provide 80% power to detect a 3.95-fold increase in the risk of birth defects associated with teriflunomide exposure compared with rates from the EUROCAT database.
Leflunomide, which acts as a prodrug for teriflunomide, has been in clinical use for arthritis for about 2 decades. Of 587 leflunomide-exposed pregnancies with known outcome, the rate of birth defects was 7%, with the majority being minor birth defects [42]. According to the authors, these data suggested a lack of teratogenic potential for leflunomide, consistent with experience with teriflunomide, discussed above.
“High-efficacy” DMDs
Alemtuzumab A total of 179 pregnancies occurred in 131 women treated with alemtuzumab in randomised clinical trials in a report from 2015 [43]. Although the number of pregnancies was limited, these data suggested a similar frequency of spontaneous abortions between women who had received alemtuzumab and women in the general population. No congenital abnormalities were observed in these offspring. An updated report from 2017 (248 pregnancies) provided similar conclusions [44].
Cladribine tablets Forty-four pregnancies occurred in women exposed to cladribine tablets and 20 pregnancies occurred in placebo-treated women during the clinical development of cladribine tablets in MS. Outcomes were similar in each group for live births (41% vs. 45%, respectively) and spontaneous abortion (21% vs. 25%); rates of elective termination were 32% vs. 20%, respectively [45].
Fingolimod A recent study of prospective data was reported from the Multinational Gilenya® Pregnancy Exposure Registry (prospective) and the Novartis Safety database (NSDB), which includes the PRegnancy outcomes Intensive Monitoring (PRIM) programme [46]. Rates of congenital malformations in 1586 fingolimod-exposed pregnancies were within the expected range for the general population. The rate of spontaneous abortion was 7.4% in the Registry, 8.1% in the NDSB, and 13.1% in PRIM. These were all within the expected range for this outcome cited for the general population of 7–20%, and the authors emphasised methodological differences likely contributed to the differences between the three databases. However, a recent survey of data by the European Medicines Agency has found a doubling of rates of major congenital malformations in offspring of mothers exposed vs. not exposed to fingolimod, which underpins the current contraindication for fingolimod in pregnancy [47]. It is important to avoid reactivation of disease activity in patients who discontinue fingolimod (see below).
Natalizumab Data from a registry in Germany were used to compare pregnancy outcomes among three groups of women: 101 women with RRMS with pregnancy exposed to natalizumab in the first trimester (72 live births), 78 women with RRMS with pregnancies not exposed to natalizumab, although they may have received treatment with other DMDs (69 live births), and a healthy control group of 97 women without MS (92 live births) [48]. There were no significant differences between groups for rates of major malformations, birth weight < 2500 g, or premature birth. There were significantly more miscarriages in the groups with MS and average birth weights were lower compared with the healthy group, although there were no significant differences between patients with RRMS who had been exposed or not exposed to natalizumab. In another observational study, a multivariable analysis reported an increased rate of miscarriage in women who had received natalizumab compared with other DMDs (odds ratio 3.9, p < 0.001), although the observed rate did not exceed the range of rates reported for the general population; there was no increased risk of foetal malformations with natalizumab [49]. However, the global Tysabri Pregnancy Exposure Registry did not find excess miscarriages or birth defects associated with pregnancies exposed to natalizumab, compared with the general population and based on 376 pregnancies [50]. A recent retrospective chart review that included data on 15 infants born to 13 mothers with MS who were treated with natalizumab in the third trimester concluded that haematological abnormalities may occur in one-third of infants exposed to natalizumab during the third trimester, but that the treatment was generally safe [51]. Withdrawal of natalizumab is also associated with potential for disease reactivation (see below).
Ocrelizumab Outcomes from 267 pregnancies in women with MS who became pregnant while receiving ocrelizumab have been reported [52]. For exposed vs. unexposed pregnancies, there were similar rates of spontaneous abortion (4% vs. 3%), and the proportion of live births was similar once a higher proportion of elective terminations in the ocrelizumab-exposed group was considered.
Consensus Recommendations on the use of DMDS During Pregnancy and Breastfeeding
Women Planning a Pregnancy
The evidence summarised above has demonstrated a low risk of adverse pregnancy outcomes in patients who became pregnant while taking most DMDs. This information is useful for counselling and reassuring patients who become pregnant while taking a DMD who decide to take their pregnancy to term. Nevertheless, the number of exposed pregnancies is low for most DMDs at this time, except for the large database of clinical experience gained with interferon β in this setting during decades of therapeutic use; in general, the current contraindications in the labelling for current DMDs should be respected.
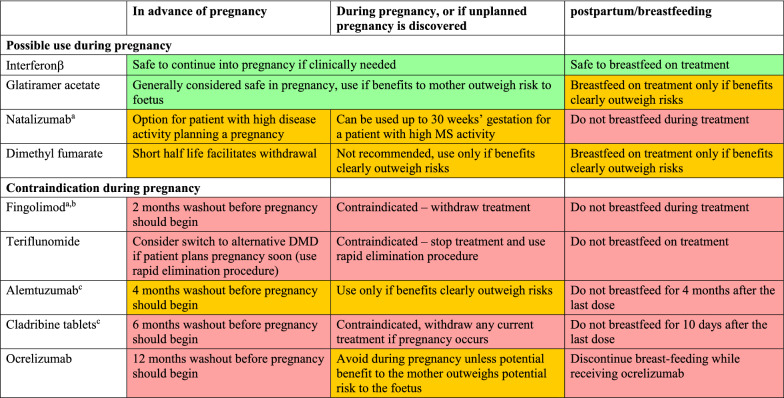
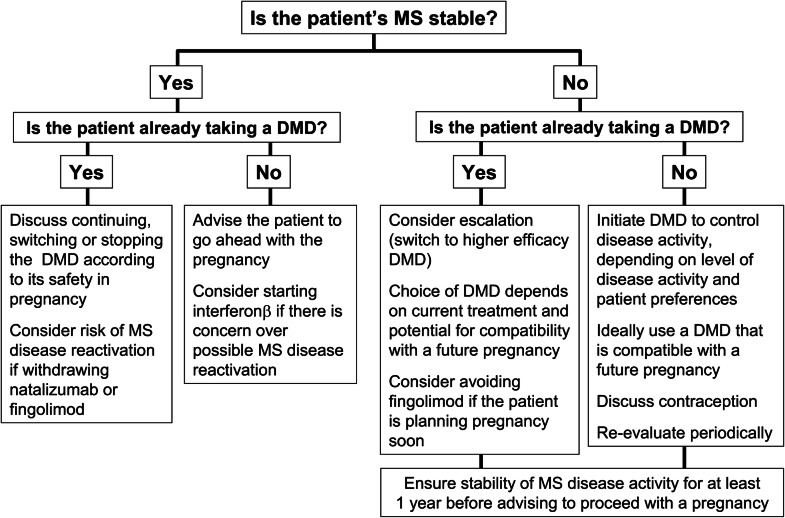
General principles for making treatment decisions when a woman with MS is planning a family is planning a family are shown in Table 2 and Fig. 1, and our consensus on which DMDs are suitable for patients with different levels of MS disease activity is shown in Table 3. The group recommends that a patient needs to be in remission for at least 1 year prior to pregnancy planning to avoid any potential risk of disease reactivation when DMDs are discontinued. A patient with active MS disease requires treatment. Interferon β is a rational option at this time, especially for a patient with low disease activity, given its indication for use in pregnancy. Glatiramer acetate is an alternative option, although its labelling does not provide strong support for use during pregnancy (see Table 1). A weaker consensus supported the use of dimethyl fumarate in a woman planning pregnancy, as the short half life of this agent facilitates switching at the time a pregnancy occurs. Alternatively, a patient with a low level of disease activity has the option of delaying treatment for a year to complete a pregnancy.
Table 2.
Summary of authors’ recommendations for the use of DMDs in advance of, during, and in the postpartum period
aRisk of rebound activation of MS disease activity if treatment is withdrawn; consider bridging with another DMD that is safe to use in pregnancy, e.g. interferon β
bContraindications also apply to siponimod, which is not indicated for use in relapsing–remitting multiple sclerosis in Europe (the washout period for siponimod is 10 days)
cAlemtuzumab and cladribine tablets are hypothesised to act as immune reconstitution inhibitors, which may provide an opportunity for longer-term planning of a pregnancy free of DMD treatment or MS disease activity for the majority of patients (see text). Recommendations are compiled from labelling of DMDs, published articles, (see text for references) and authors’ clinical experience
Fig. 1.
Practical considerations relating to reviewing treatment of a woman with MS who is planning pregnancy
Table 3.
Expert consensus recommendations on the use of individual disease-modifying drugs (DMDs) in women planning a pregnancy according to disease activity
| Patient with active MS | Patient with highly active MS |
|---|---|
|
High consensus Interferon β Glatiramer acetate |
High consensus Cladribine tablets Natalizumab Ocrelizumab |
|
Moderate consensus Dimethyl fumarate Cladribine tablets |
Moderate consensus Alemtuzumab |
|
Low consensus Natalizumab Ocrelizumab |
Low consensus Dimethyl fumarate |
Consensus levels were as follows: high, 8 or more physicians; moderate, 4–7 physicians; low, 1–3 physicians
The care of women with high disease activity, which usually requires a high-efficacy DMD, involves a trade-off between protecting the patient from relapses and MS progression and minimising the risk of exposure of an unplanned pregnancy to treatment. For women with high MS disease activity planning pregnancy, the consensus supported the use of natalizumab with no washout period, while different washout periods from the last dose are required when using alemtuzumab, ocrelizumab, and cladribine tablets for women with high MS disease activity planning pregnancy (below, and Table 1). The absolute contraindications to the use of teriflunomide and fingolimod (and siponimod) during pregnancy should be respected for a patient with any level of disease activity in the absence of new data confirming their safety (Tables 1, 2).
It is important to note that alemtuzumab and cladribine tablets are hypothesised to act as immune reconstitution therapies (IRT), where short treatment courses given over 2 years provide durable freedom from disease activity for a substantial proportion of patients [53]. Alemtuzumab can, in principle, be administered during pregnancy, according to its European label, although safety concerns unrelated to pregnancy have now limited its use [54], and treatment with cladribine tablets is contraindicated during pregnancy (Table 1). If the patient is prepared to delay her pregnancy for 4–6 months after the last dose of the IRT, this approach may provide a window of opportunity to complete a pregnancy uncomplicated by either recurrence of disease activity or concomitant DMD administration.
Unplanned Pregnancy While Taking a DMD for MS
About half of all pregnancies worldwide are unplanned [55]. Most DMDs should be discontinued immediately on discovering a pregnancy, with the following possible exceptions, based on the literature and our clinical experience. Treatment with interferon β (and perhaps glatiramer acetate) can be continued if required clinically (see above). Where active treatment must be maintained, bridging between prior and subsequent higher activity DMDs with interferon β may be an option. A patient already taking natalizumab is likely to have high disease activity prior to natalizumab institution and will be at risk of reactivation of MS disease activity if treatment is withdrawn (see above). We recommend the use of natalizumab in a pregnant patient with high disease activity requiring continued treatment, usually continuing until up to week 30 of the pregnancy, based on individual patient considerations (the use of natalizumab in the third trimester has been associated with transient mild-to-moderate thrombocytopenia and anaemia in the neonate [56]). Natalizumab can be given at 6-weekly intervals, instead of the usual 4-weekly doing interval, if reducing the intensity of treatment is considered helpful.
Discontinuing a DMD and Avoiding Rebound MS Activation
Washout periods vary between different DMDs. As a general guide, based on labelling and clinical experience, we recommend the following intervals between withdrawal of DMDs and becoming pregnant (this list does not include those agents which can be continued into pregnancy if needed, see Table 2):
Alemtuzumab 4 months (but can be used in pregnancy if clinically justified).
Cladribine tablets 6 months.
Fingolimod 2 months.
Ocrelizumab 6–12 months.
Siponimod 10 days.
Teriflunomide: Plasma levels of teriflunomide must be < 0.02 mg/l before pregnancy can be initiated. Unaided, this takes 8 months, on average, but can be achieved in 11 days using the rapid elimination procedure [57, 58].
Marked increases in MS disease activity (clinical relapses and MRI lesions) have been observed in patients who have discontinued treatment with fingolimod or natalizumab, including when these drugs have been discontinued to facilitate a pregnancy [59–62]. A report from the pregnancy registry for fingolimod in Germany found that relapses during pregnancy were common among women who discontinued fingolimod before they became pregnant (28% of 46 women) and among women who discontinued fingolimod after a positive pregnancy test (24% of 110 women) [63]. Data from a registry based in Kuwait showed that the risk of relapses during pregnancy and postpartum was higher than had been assumed previously (17% and 14%, respectively) and that the highest rate of relapses occurred in patients previously managed on fingolimod and natalizumab [64].
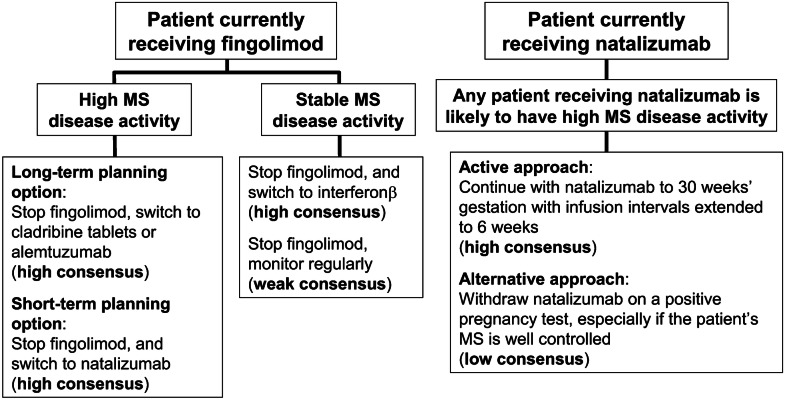
Lymphopenia occurring during the first 3 months of treatment with fingolimod has been proposed as a possible marker of patients at most risk of MS reactivation following withdrawal of this agent [60]. Higher disease activity and longer duration of MS were described as risk factors for disease reactivation following withdrawal of natalizumab [65]. A longer washout period before pregnancy predicted a higher risk of relapse following withdrawal of fingolimod or natalizumab [60, 64]. Figure 2 shows consensus recommendations for avoiding disease reactivation in patients receiving fingolimod or natalizumab who are or intend to become pregnant based on the considerations discussed above.
Fig. 2.
Consensus recommendations on avoiding rebound MS disease activity in patients receiving fingolimod or natalizumab who are or intend to become pregnant. “High” or “stable” disease activity is defined arbitrarily based on the presence or absence of MS disease activity during the preceding year
Breastfeeding
Women are at increased risk of relapses during the postpartum period, but this risk must be balanced with the need to support and encourage women who wish to breastfeed their child. In the authors’ experience, most neurologists are willing to maximise options for breastfeeding, providing disease activity can be controlled (see Table 2). The absence of disease activity during pregnancy and in MRI follow-up performed following delivery may encourage a period of breastfeeding if the mother is willing to do so. If a relapse occurred during pregnancy or radiological activity appeared, it is recommended to resume the DMD after delivery. The European label for interferon β supports its use during breastfeeding, with qualified support for glatiramer acetate, and alemtuzumab only at this time (Table 1). US labels for all DMDs provide a general instruction to balance benefits and risks, except for contraindications for teriflunomide and cladribine tablets.
Managing Relapses During Pregnancy
Corticosteroids are the mainstay of treatment for MS relapses, including during pregnancy [15], but are weakly teratogenic, with an increased risk of cleft palate when used in the first trimester (odds ratio 3.5) [66]. Intravenous immunoglobulin (IVIg) is considered safe for use during breastfeeding, according to the European label for an IVIg product, as immunoglobulins pass naturally from mother to child via breast milk [67]. There is some evidence that IVIg may reduce the rate of relapses during pregnancy and the postpartum period [68], although the recent consensus statement from the UK does not support [15]. Plasma exchange may be considered for severe or disabling relapses that do not respond to corticosteroid treatment [15, 69]. Evidence supporting the use of plasma exchange in less severe cases is lacking, however, and this intervention may increase the risk of hemodynamic instability and central line infection and thrombosis [69].
Conclusions and Summary of Recommendations
Managing MS in women considering, or already embarked on, a pregnancy is a challenging balancing act between protecting the patient from MS activity and progression and avoiding unnecessary exposure of the foetus to DMDs. In addition, data on the efficacy and safety of most individual DMDs are limited for this population. Exceptions to this include interferon β and glatiramer acetate (which are relatively well studied and likely to be safe for use in pregnancy) and DMDs with outright contraindications in this setting (which should never be used in pregnancy). Table 2 summarises our recommendations for DMD treatment for a patient in advance of pregnancy, during pregnancy, and postpartum. These were based to an important extent on US and European labelling. This is a fast-moving field, however, and regulatory labels may be slow to catch up with the clinical evidence base. We have also brought our clinical experience to bear here, as leading physicians in Gulf states. Guidance on the selection of DMD treatment according to disease activity for this population has been particularly lacking in current guidance, and we have sought to provide pragmatic recommendations on this.
Care should be taken to withdraw a DMD that is unsafe for use in pregnancy in good time for its potentially adverse effects to dissipate. Where possible, switching a patient to a DMD that is known to be relatively safe during pregnancy provides a rational option for these patients, especially as many pregnancies are unplanned. The recent change to the indication for interferon β, permitting use in pregnancy where clinically needed and removing obstacles to its use during breastfeeding, has broadened the options for maintaining DMD therapy at this time beyond glatiramer acetate. Natalizumab (for patients with high disease activity) may also be continued into a pregnancy, although a patient taking natalizumab should not breastfeed. The use of DMDs hypothesised to act as IRTs (currently alemtuzumab and cladribine tablets) may provide a period of stable disease without maintenance treatment that provides an opportunity to embark on long-term planning for a pregnancy. However, the patient should be prepared to wait for up to 20 months (cladribine tablets) before becoming pregnant.
Acknowledgements
Funding
Merck Serono Middle East FZ-Ltd, United Arab Emirates, funded the Rapid Service Fee for this review.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Dr Mike Gwilt of GT Communications, funded by Merck Serono Middle East FZ-Ltd, United Arab Emirates, an affiliate of Merck KGaA, Darmstadt, Germany.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
All authors participated in the closed meeting that gave rise to this article, which was organised by Merck Serono Middle East FZ-Ltd, United Arab Emirates, an affiliate of Merck KGaA, Darmstadt, Germany. Jihad Inshasi, Abdullah Al-Asmi, and Jaber Alkhabouri declared no additional duality of interest. Additional duality of interest declarations are as follows. Raed Alroughani received honoraria as a speaker and for serving in scientific advisory boards from Bayer, Biogen, Merck, Novartis, Roche, and Sanofi. AA-A received honoraria for serving on scientific advisory boards from Merck, Novartis, Roche, and Sanofi, and also received travel reimbursement from, Biologix, Sanofi, Merck, Roche, Bayer and Novartis. Ahmed Shatila received honoraria for lectures from Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, Boehringeringer Ingelheim, and Biologix, and for advisory boards from Sanofi-Genzyme, Roche, Novartis, Pfizer, and Biologix, and received financial support for registration, accommodation and travel for conferences provided by Sanofi-Genzyme, Merck, Genpharm, Roche, Novartis, and Biologix. Beatriz Canibano has received travel, speaker and consultant honoraria from Merck, Novartis, Biologix, Roche and Sanofi. Taoufik Alsaadi has received speaker fees from Newbridge, Novartis, Eli Lilly, GlaxoSmithKline, Janssen-Cilag, LivaNova, Lundbeck, Merck, Pfizer, Hikma, Sanofi, has received consultancy fees from Cyberonics, Eli Lilly, LivaNova, Lundbeck, Merck, Pfizer, Hikma, Novartis, Sanofi; and has received research grants from Novartis, Biogen, GlaxoSmithKline, Cyberonics, Schwartz, Pfizer, UCB, Astra, Merck, and Elan Pharmaceuticals; Taoufik Alsaadi holds no shares in, nor has any ongoing financial relationship with, any pharmaceutical company. Samar Farouk Ahmed reported no additional duality of interest. Amir Boshra is an employee of Merck Serono Middle East FZ-Ltd, United Arab Emirates, an affiliate of Merck KGaA, Darmstadt, Germany.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data AvailabilityData Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12459572
References
- 1.Multiple Sclerosis International Federation. Atlas of MS 2013. Mapping multiple sclerosis around the world. https://www.msif.org/about-us/who-we-are-and-what-we-do/advocacy/atlas/. Accessed Apr 2020.
- 2.GBD 2016 Multiple Sclerosis Collaborators Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:269–285. doi: 10.1016/S1474-4422(18)30443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–532. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 4.Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler. 2014;20:520–526. doi: 10.1177/1352458513519181. [DOI] [PubMed] [Google Scholar]
- 5.Al Tahan AM, Alsharoqi I, Bohlega SA, et al. Characteristics of multiple sclerosis in the Middle East with special reference to the applicability of international guidelines to the region. Int J Neurosci. 2014;124:635–641. doi: 10.3109/00207454.2013.865620. [DOI] [PubMed] [Google Scholar]
- 6.Heydarpour P, Khoshkish S, Abtahi S, Moradi-Lakeh M, Sahraian MA. Multiple sclerosis epidemiology in Middle East and North Africa: a systematic review and meta-analysis. Neuroepidemiology. 2015;44:232–244. doi: 10.1159/000431042. [DOI] [PubMed] [Google Scholar]
- 7.Nasr Z, Etemadifar M, Khalili N. Epidemiology of multiple sclerosis in the Middle East: a systematic review and meta analysis. Mult Scler Relat Disord. 2014;3:744. doi: 10.1016/j.msard.2014.09.164. [DOI] [Google Scholar]
- 8.Habibzadeh F. Editor's Page. Contraception in the Middle East. Lancet Middle East Edition. https://download.thelancet.com/flatcontentassets/pdfs/Sep12_MiddleEastEd.pdf. Accessed Apr 2020.
- 9.Roudi-Fahimi F, Abdul Monem A, Ashford L, El-Adawy M. United Nations Population Fund. Women’s need for family planning in Arab countries. https://www.who.int/evidence/resources/policy_briefs/UNFPAPBunmentneed2012.pdf. Accessed Feb 2020.
- 10.Mendibe Bilbao M, Boyero Durán S, Bárcena Llona J, Rodriguez-Antigüedad A. Multiple sclerosis: pregnancy and women's health issues. Neurologia. 2019;34:259–269. doi: 10.1016/j.nrl.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Smeltzer SC. Reproductive decision making in women with multiple sclerosis. J Neurosci Nurs. 2002;34:145–157. doi: 10.1097/01376517-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho AT, Veiga A, Morgado J, et al. Multiple sclerosis and motherhood choice: an observational study in Portuguese women patients. Rev Neurol. 2014;59:537–542. [PubMed] [Google Scholar]
- 13.Riñon A, Buch M, Holley D, Verdun E. The MS Choices Survey: findings of a study assessing physician and patient perspectives on living with and managing multiple sclerosis. Patient Prefer Adherence. 2011;5:629–643. doi: 10.2147/PPA.S26479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicks P, Brandes D, Park J, Liakhovitski D, Koudinova T, Sasane R. Preferred features of oral treatments and predictors of non-adherence: two web-based choice experiments in multiple sclerosis patients. Interact J Med Res. 2015;4(1):e6. doi: 10.2196/ijmr.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobson R, Dassan P, Roberts M, Giovannoni G, Nelson-Piercy C, Brex PA. UK consensus on pregnancy in multiple sclerosis: 'Association of British Neurologists' guidelines. Pract Neurol. 2019;19:106–114. doi: 10.1136/practneurol-2018-002060. [DOI] [PubMed] [Google Scholar]
- 16.Alroughani R, Inshasi J, Al-Asmi A, et al. Expert consensus from the Arabian Gulf on selecting disease-modifying treatment for people with multiple sclerosis according to disease activity. Postgrad Med. 2020 doi: 10.1080/00325481.2020.1734394. [DOI] [PubMed] [Google Scholar]
- 17.Alroughani R, Inshasi JS, Deleu D, et al. An overview of high-efficacy drugs for multiple sclerosis: Gulf region expert opinion. Neurol Ther. 2019;8:13–23. doi: 10.1007/s40120-019-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AlJumah M, Marwan Alkhawajah M, Qureshi S, et al. Cladribine tablets and relapsing–remitting multiple sclerosis: a pragmatic, narrative review of what physicians need to know. Neurol Ther. 2020;9:11–23. doi: 10.1007/s40120-020-00177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Academy of Neurology. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. https://www.aan.com/Guidelines/home/GuidelineDetail/898. Accessed Apr 2020.
- 20.Coyle PK. Management of women with multiple sclerosis through pregnancy and after childbirth. Ther Adv Neurol Disord. 2016;9:198–210. doi: 10.1177/1756285616631897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyment DA, Sadovnick AD, Ebers GC. Genetics of multiple sclerosis. Hum Mol Genet. 1997;6:1693–1698. doi: 10.1093/hmg/6.10.1693. [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 23.Hellwig K, Gold R. Family planning and multiple sclerosis. Akt Neurol. 2010;37:292–303. doi: 10.1055/s-0030-1248564. [DOI] [Google Scholar]
- 24.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24:96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 25.Ghezzi A. European and American guidelines for multiple sclerosis treatment. Neurol Ther. 2018;7:189–194. doi: 10.1007/s40120-018-0112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amato MP, Bertolotto A, Brunelli R, et al. Management of pregnancy-related issues in multiple sclerosis patients: the need for an interdisciplinary approach (published correction appears in Neurol Sci. 2017 Dec 26) Neurol Sci. 2017;38:1849–1858. doi: 10.1007/s10072-017-3081-8. [DOI] [PubMed] [Google Scholar]
- 27.Alroughani R, Akhtar S, Zeineddine M, et al. Risk of relapses during pregnancy among multiple sclerosis patients. Mult Scler Relat Disord. 2019;34:9–13. doi: 10.1016/j.msard.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Trojano M, Bergamaschi R, Amato MP, et al. The Italian multiple sclerosis register. Neurol Sci. 2019;40:155–165. doi: 10.1007/s10072-018-3610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannini M, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after Glatiramer acetate exposure in patients with multiple sclerosis: a prospective observational multicentric study. BMC Neurol. 2012;12:124. doi: 10.1186/1471-2377-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbstritt S, Langer-Gould A, et al. Glatiramer acetate during early pregnancy: a prospective cohort study. Mult Scler. 2016;22:810–816. doi: 10.1177/1352458515623366. [DOI] [PubMed] [Google Scholar]
- 31.Sandberg-Wollheim M, Neudorfer O, Grinspan A, et al. Pregnancy outcomes from the branded glatiramer acetate pregnancy database. Int J MS Care. 2018;20:9–14. doi: 10.7224/1537-2073.2016-079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold R, Phillips JT, Havrdova E, et al. Delayed-release dimethyl fumarate and pregnancy: preclinical studies and pregnancy outcomes from clinical trials and postmarketing experience. Neurol Ther. 2015;4:93–104. doi: 10.1007/s40120-015-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hellwig K, Rog D, McGuigan C, Chen K, Parks B, Jones CC. An international registry tracking pregnancy outcomes in women treated with dimethyl fumarate. Abstract (P1147) at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278349/kerstin.hellwig.an.international.registry.tracking.pregnancy.outcomes.in.women.html. Accessed Apr 2020.
- 34.Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-β exposure in multiple sclerosis. Neurology. 2010;75:1794–1802. doi: 10.1212/WNL.0b013e3181fd62bb. [DOI] [PubMed] [Google Scholar]
- 35.Thiel S, Langer-Gould A, Rockhoff M, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis—a prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler. 2016;22:801–809. doi: 10.1177/1352458516634872. [DOI] [PubMed] [Google Scholar]
- 36.Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler. 2011;17:423–430. doi: 10.1177/1352458510394610. [DOI] [PubMed] [Google Scholar]
- 37.Hellwig K, Geissbuehler Y, Sabidó M, et al. Pregnancy and infant outcomes with interferon beta: data from the European Interferon Beta Pregnancy Registry and Population Based Registries in Finland and Sweden. Abstract (A-0950-0000-02658) and poster at the ECTRIMS 2018 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2018/ectrims-2018/228131/kerstin.hellwig.pregnancy.and.infant.outcomes.with.interferon.beta.data.from.html. Accessed Apr 2020.
- 38.Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing–remitting multiple sclerosis. Neurol Ther. 2014;3:133–138. doi: 10.1007/s40120-014-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vukusic S, Coyle PK, Jurgensen S, et al. Pregnancy outcomes in patients with multiple sclerosis treated with teriflunomide: Clinical study data and 5 years of post-marketing experience. Mult Scler. 2019 doi: 10.1177/1352458519843055. [DOI] [PubMed] [Google Scholar]
- 40.Vukusic S, Hellwig K, Truffinet P, et al. Pregnancy outcomes in female partners of male patients treated with teriflunomide or leflunomide (an in vivo precursor of teriflunomide). Abstract (P1146) at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278348/sandra.vukusic.pregnancy.outcomes.in.female.partners.of.male.patients.treated.html. Accessed Apr 2020.
- 41.Lebrun-Frenay C, Rog D, Benamor M, Jurgensen S, Truffinet P, Ghezzi A. Teriflunomide (Aubagio®) International Pregnancy Registry: Enrollment Update (P4.371). Neurology 2018;90 (15 Supplement). Abstract available at https://n.neurology.org/content/90/15_Supplement/P4.371. Accessed Apr 2020.
- 42.Truffinet P, Afsar S, Davenport L, Purvis A, Poole EM, Henson LJ. Pregnancy outcomes in patients treated with leflunomide, an in vivo precursor of the multiple sclerosis drug teriflunomide. Abstract (P1148) at the ECTRIMS 2019 congress, available at https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278350/lily.j.henson.pregnancy.outcomes.in.patients.treated.with.leflunomide.an.in.html. Accessed Apr 2020. [DOI] [PubMed]
- 43.Achiron A, Chambers C, Fox EJ, et al. Pregnancy outcomes in patients with active RRMS who received alemtuzumab in the clinical development program. Abstract (P1120) and poster at the ECTRIMS 2015 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2015/31st/116041/anat.achiron.pregnancy.outcomes.in.patients.with.active.rrms.who.received.html. Accessed Apr 2020.
- 44.Rog D, Jiwon O, Chambers C, et al. Pregnancy outcomes in patients with RRMS treated with alemtuzumab from the clinical development program. Abstract (P749) and poster at the ECTRIMS 2017 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/200404/david.rog.pregnancy.outcomes.in.patients.with.rrms.treated.with.alemtuzumab.html. Accessed Apr 2020.
- 45.Galazka A, Nolting A, Cook S, et al. Pregnancy outcomes during the clinical development programme of cladribine in multiple sclerosis (MS): an integrated analysis of safety for all exposed patients. Abstract (P1874) at the ECTRIMS 2017 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/199894/vicky.john.pregnancy.outcomes.during.the.clinical.development.programme.of.html. Accessed Apr 2020.
- 46.Lopez Leon S, Geissbuehler Y, Moore A, et al. Effect of fingolimod on pregnancy outcomes in patients with multiple sclerosis. Abstract (P411) at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/278772/kerstin.hellwig.effect.of.fingolimod.on.pregnancy.outcomes.in.patients.with.html. Accessed Apr 2020.
- 47.European Medicines Agency. Updated restrictions for Gilenya: multiple sclerosis medicine not to be used in pregnancy. Press release 26/07/2019. https://www.ema.europa.eu/en/news/updated-restrictions-gilenya-multiple-sclerosis-medicine-not-be-used-pregnancy. Accessed Apr 2020.
- 48.Ebrahimi N, Herbstritt S, Gold R, Amezcua L, Koren G, Hellwig K. Pregnancy and fetal outcomes following natalizumab exposure in pregnancy. A prospective, controlled observational study. Mult Scler. 2015;21:198–205. doi: 10.1177/1352458514546790. [DOI] [PubMed] [Google Scholar]
- 49.Portaccio E, Annovazzi P, Ghezzi A, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: I: Fetal risks. Neurology. 2018;90:e823–e831. doi: 10.1212/WNL.0000000000005067. [DOI] [PubMed] [Google Scholar]
- 50.Friend S, Richman S, Bloomgren G, Cristiano LM, Wenten M. Evaluation of pregnancy outcomes from the Tysabri® (natalizumab) pregnancy exposure registry: a global, observational, follow-up study. BMC Neurol. 2016;16:150. doi: 10.1186/s12883-016-0674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Triplett JD, Vijayan S, Rajanayagam S, Tuch P, Kermode AG. Pregnancy outcomes amongst multiple sclerosis females with third trimester natalizumab use. Mult Scler Relat Disord. 2020;40:101961. doi: 10.1016/j.msard.2020.101961. [DOI] [PubMed] [Google Scholar]
- 52.Oreja-Guevara C, Wray S, Buffels R, Zecevik D, Vukusik S. Pregnancy outcomes in patients treated with ocrelizumab. Abstract (P780) and poster at the ECTRIMS 2019 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/279140/celia.oreja-guevara.pregnancy.outcomes.in.patients.treated.with.ocrelizumab.html. Accessed Apr 2020.
- 53.Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16:56–62. doi: 10.1038/s41582-019-0268-z. [DOI] [PubMed] [Google Scholar]
- 54.European Medicines Agency. Lemtrada. Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada (November 2019). https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada. Accessed Apr 2020.
- 55.Bearak J, Popinchalk A, Alkema L, Sedgh G. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: estimates from a Bayesian hierarchical model. Lancet Glob Health. 2018;6:e380–e389. doi: 10.1016/S2214-109X(18)30029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haghikia A, Langer-Gould A, Rellensmann G, et al. Natalizumab use during the third trimester of pregnancy. JAMA Neurol. 2014;71:891–895. doi: 10.1001/jamaneurol.2014.209. [DOI] [PubMed] [Google Scholar]
- 57.Miller AE. Teriflunomide: a once-daily oral medication for the treatment of relapsing forms of multiple sclerosis. Clin Ther. 2015;37:2366–2380. doi: 10.1016/j.clinthera.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 58.Genzyme Corporation. Elimination Procedure—AUBAGIO® (teriflunomide). https://www.aubagiohcp.com/content/pdf/drug_elimination_guide.pdf. Accessed Apr 2020.
- 59.Barry B, Erwin AA, Stevens J, Tornatore C. Fingolimod rebound: a review of the clinical experience and management considerations. Neurol Ther. 2019;8:241–250. doi: 10.1007/s40120-019-00160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Portaccio E, Moiola L, Martinelli V, et al. Pregnancy decision-making in women with multiple sclerosis treated with natalizumab: II: maternal risks. Neurology. 2018;90:e832–e839. doi: 10.1212/WNL.0000000000005068. [DOI] [PubMed] [Google Scholar]
- 61.Sepúlveda M, Montejo C, Llufriu S, et al. Rebound of multiple sclerosis activity after fingolimod withdrawal due to planning pregnancy: analysis of predisposing factors. Mult Scler Relat Disord. 2019;38:101483. doi: 10.1016/j.msard.2019.101483. [DOI] [PubMed] [Google Scholar]
- 62.Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. 2019;12:1756286419837809. doi: 10.1177/1756286419837809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hemat S. Disease activity during pregnancy after fingolimod withdrawal due to planning a pregnancy in women with multiple sclerosis. Abstract (207) at the ECTRIMS 2018 congress. https://onlinelibrary.ectrims-congress.eu/ectrims/2018/ectrims-2018/231956/spalmai.hemat.disease.activity.during.pregnancy.after.fingolimod.withdrawal.html. Accessed Apr 2020.
- 64.Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J. Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology. 2018;90:e840–e846. doi: 10.1212/WNL.0000000000005065. [DOI] [PubMed] [Google Scholar]
- 65.Ladeira F, Braz L, Salgado P, Vaz S. A multicenter, non-interventional study to evaluate the disease activity in multiple sclerosis after withdrawal of Natalizumab in Portugal. Clin Neurol Neurosurg. 2019;184:105390. doi: 10.1016/j.clineuro.2019.105390. [DOI] [PubMed] [Google Scholar]
- 66.Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385–392. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 67.KIOVIG 100 mg/L solution for infusion. European Summary of Product Characteristics. https://www.medicines.org.uk/emc/product/9198. Accessed Apr 2020.
- 68.Armon C, Baquis GD, Howard GF III, et al. Neurologic Disease and Pregnancy. Multiple Sclerosis. Updated: Aug 20, 2019. https://emedicine.medscape.com/article/1149405-overview#a7. Accessed Apr 2020.
- 69.Rolfes L, Pfeuffer S, Ruck T, et al. Therapeutic apheresis in acute relapsing multiple sclerosis: current evidence and unmet needs-a systematic review. J Clin Med. 2019;8:E1623. doi: 10.3390/jcm8101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.