Abstract
The association between fetal gender and rare pregnancy complications has not been extensively investigated, and no studies have examined this association in Japanese women. Thus, we used a large Japanese birth registry database to investigate the extent to which fetal gender affects various pregnancy outcomes. We analyzed 1,098,268 women with a singleton delivery with no congenital anomaly at 22 weeks or later between 2007 and 2015. Women carrying a male fetus had a significantly higher risk of placental abruption (adjusted risk ratio [aRR] 1.15, 95% confidence interval (CI) 1.10–1.20)], preterm delivery (aRR 1.20, 95% CI 1.19–1.22), instrumental delivery (aRR 1.27, 95% CI 1.26–1.29), and cesarean delivery (aRR 1.01, 95% CI 1.00–1.02). In contrast, they had a significantly lower risk of preeclampsia (aRR 0.92, 95% CI 0.89–0.94), placenta accreta (aRR 0.90, 95% CI 0.85–0.96), atonic hemorrhage (aRR 0.95, 95% CI 0.93–0.96), and maternal blood transfusion (aRR 0.95, 95% CI 0.92–0.99). Our findings demonstrate a significant association between fetal gender and various pregnancy complications and delivery outcomes among Japanese women.
Subject terms: Medical research, Risk factors
Introduction
Some studies have investigated the association between fetal gender and pregnancy outcomes1,2. While these studies consistently showed that women carrying a male fetus were more likely to have an increased risk of preterm delivery3–5, premature rupture of membranes6,7, gestational diabetes mellitus (GDM)8–10, fetal macrosomia6,9,10, cord complications9,10, and instrumental and cesarean delivery6,8–11, studies of preeclampsia risk have shown inconsistent results8–10,12. Furthermore, the association between fetal gender and certain important outcomes, such as placenta accreta and abruption, has not been extensively studied, possibly due to their rarity.
Most studies to date have been primarily conducted in Western countries, although the association between fetal gender and pregnancy outcomes may differ by ethnicity13. Two studies enrolling Chinese subjects had non-negligible limitations, including a higher rate of cesarean section and distortion of the male:female ratio14,15. These are the only studies to date that have been conducted in Asia. Thus, replicative studies on an Asian population are required using other databases with minimum bias.
Our study used a large Japanese birth registry database to examine the extent to which fetal gender affects outcomes.
Methods
Study population
We used data from the Japan Society of Obstetrics and Gynecology (JSOG) perinatal database, which has been described elsewhere in detail16. Briefly, the database is an ongoing registry that began in 2001 and is based on the collaboration of 149 tertiary hospitals (as of 2012), covering nearly 15% of the annual births in Japan. The database contains transcribed data, including maternal demographics, pregnancy complications, and delivery outcomes extracted from the medical records at each hospital. The registry of the JSOG perinatal database was conducted in accordance with the guidelines of the Declaration of Helsinki and other nationally valid regulations. Informed consent was obtained in the form of an opt-out on the web site.
Our sample population was restricted to women who delivered singletons with no congenital anomaly between 2007 and 2015, because the effect of fetal gender may differ in cases of multiple pregnancies and fetal malformations. Women with missing data on fetal gender and gestational age at birth as well as women with unreliable data on the combination of birth weight and gestational age were excluded according to criteria proposed by a previous study17. Our main analysis of pregnancy complications was based on the women remaining after excluding those with missing or unreliable data on maternal age, parity, maternal height, pre-pregnancy body mass index (BMI), conception method, delivery mode, and fetal presentation at birth. We considered “missing” as an independent status for maternal smoking during pregnancy as these data were missing in a large number of women. Women who experienced intrauterine fetal death were also excluded from our analysis of delivery outcomes. Our analysis of instrumental deliveries excluded those with a cesarean section.
We conducted a sensitivity analysis to assess for any possible bias due to missing data based on all women regardless of missing or unreliable data. Missing or unreliable data were replaced with imputations for maternal age, parity, maternal height, pre-pregnancy BMI, conception method, delivery mode, and fetal presentation at birth. We created 30 sets of imputed datasets using multivariate imputation by chained equations.
Variables of interest
The primary outcomes of interest were pregnancy complications and delivery outcomes. Preeclampsia, hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome, placenta previa, placenta accreta, placental abruption, fetal death in utero, chorioamnionitis (CAM), and amniotic fluid embolism were defined as pregnancy complications, whereas preterm birth, fetal macrosomia, fetal presentation at birth, hypotonic and hypertonic uterine inertia, atonic hemorrhage, blood transfusion at birth, and instrumental or cesarean delivery were defined as delivery outcomes. Unfortunately, the information of GDM was not included in our database. Thus, we were unable to include GDM as a pregnancy complication of interest. Preterm delivery, very preterm delivery, and extremely preterm delivery were defined as less than 37, 32, and 28 completed weeks of gestation, respectively. Macrosomia and low birth weight were defined as a birth weight ≥ 4000 g and < 2500 g18, respectively. Preeclampsia was diagnosed when a systolic/diastolic blood pressure > 140/90 mmHg emerged after 20 weeks’ gestation with significant proteinuria (≥ 300 mg/day) in accordance with the national guidelines19. Similarly, obstetricians clinically diagnosed HELLP syndrome and CAM according to the criteria in the national guidelines. HELLP syndrome was diagnosed by a combination of elevated liver enzymes (both aspartate transaminase > 70 IU/L and lactate dehydrogenase > 600 IU/L), thrombocytopenia (< 100,000/µL), and decreased anti-thrombin III activity (< 60%)20. CAM was diagnosed by the presence of maternal fever with one or more of the following symptoms: maternal tachycardia (≥ 100 times/min), uterine tenderness, maternal white blood cell count > 15,000/mm3, and purulent discharge19. Obstetricians at each hospital reported the other variables by checking against the items in the JSOG database based on their clinical diagnosis.
Among the other covariates, parity was classified as either “0” or “1 or above.” The conception method was categorized as “with assisted reproductive technology (ART)” or “without ART,” and smoking status during pregnancy was categorized as “yes,” “no,” or “missing.”
Statistical analysis
First, the maternal demographics and birth year by fetal gender were compared using Student’s t-test for continuous variables and the chi-square test for categorical variables. We used linear regression analysis to calculate the mean difference for continuous variables. Second, we used multivariate Poisson regression analysis for categorical variables and linear regression analysis for continuous variables to estimate the association between fetal gender and pregnancy complications as well as delivery outcomes using the female fetus as a reference. Maternal age, maternal height, pre-pregnancy BMI, parity, conception method, and smoking status during pregnancy were considered potential confounding factors in the multivariate analysis. Although some studies have shown that sex selection may be influenced by environmental factors, such as the conception method21 and maternal periconceptional smoking status22, there is a lack of studies on significant associations between maternal factors (such as maternal age, height, and BMI) and fetal sex. However, we considered that the adjustment of these factors was important as there is insufficient evidence showing that they are independent of fetal sex. To confirm the results of our analyses after excluding women with missing or unreliable data, we conducted a sensitivity analysis of the same cohort and imputed the missing or unreliable data.
All descriptive and statistical analyses were performed using STATA v15 (STATA Corp, College Station, TX). Each result was presented as a risk ratio and 95% confidence interval (CI). A p-value < 0.05 was considered statistically significant.
Study approval
The study protocol was approved by the Institutional Review Board of the National Center for Child Health and Development on November 29, 2018 (no. 1983).
Results
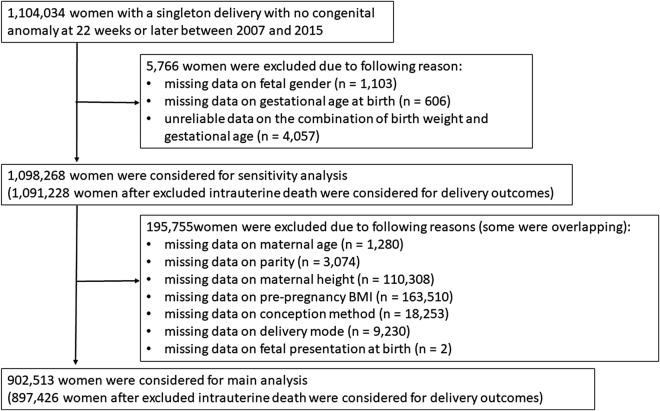
We analyzed 1,104,034 women with a singleton delivery with no congenital anomaly at 22 weeks or later between 2007 and 2015. We excluded 1103 (0.10%) with missing data on fetal gender, 606 (0.05%) with missing data on gestational age at birth, and 4057 (0.37%) with unreliable data on the combination of birth weight and gestational age. The main analysis was conducted on 902,513 of the remaining 1,098,268 women after excluding those with missing or unreliable data on maternal age, parity, maternal height, pre-pregnancy BMI, conception method, delivery mode, and fetal presentation at birth (Fig. 1).
Figure 1.
Flow chart showing the study population selection. The main analyses were conducted based on 902,513 women (897,426 were used for delivery outcomes, after excluding those with intrauterine death) after excluding those with missing data on fetal gender and gestational age at birth and unreliable data on the combination of birth weight and gestational age. Sensitivity analyses were conducted based on 1,098,268 women (1,091,228 for delivery outcomes) after excluding missing data on maternal age, parity, maternal height, pre-pregnancy BMI, conception method, delivery mode, and presentation at birth.
There were 464,075 (51.4%) women carrying a male fetus and 438,438 (48.6%) carrying a female fetus. Table 1 shows the maternal characteristics stratified by fetal gender. Non-significant differences by fetal gender were observed in terms of maternal age, parity, conception method, maternal height, and smoking status. However, women carrying a male fetus had a significantly higher pre-pregnancy BMI (p = 0.005) with a mean difference of 0.02 kg/m2 (mean pre-pregnant BMI among women carrying a male fetus vs. female fetus was 21.24 kg/m2 vs. 21.22 kg/m2).
Table1.
Demographics of pregnant women stratified by fetal sex. (n = 902,513).
| Variables | Fetal sex | Mean difference mean (95% CI) | p-valuea | |
|---|---|---|---|---|
| Male (%) (n = 464,075) | Female (%) (n = 438,438) | |||
| Continuous variables [Mean (n)] | ||||
| Maternal age (years) | 32.0 (5.3) | 32.1 (5.4) | 0.00 (− 0.03 to 0.02) | 0.165 |
| Pre-pregnant body mass index (kg/m2) | 21.24 (3.47) | 21.22 (3.47) | 0.02 (0.01 to 0.04) | 0.005 |
| Maternal height (cm) | 158.3 (5.5) | 158.3 (5.5) | − 0.01 (− 0.03 to 0.01) | 0.394 |
| Categorical variables [n (%)] | ||||
| Year | 0.273 | |||
| 2007–2009 | 76,087 (51.6) | 71,337 (48.4) | ||
| 2010–2012 | 136,261 (51.4) | 128,856 (48.6) | ||
| 2013–2015 | 251,727 (51.4) | 238,245 (48.6) | ||
| Parity | 0.066 | |||
| Primiparous | 240,523 (51.5) | 226,388 (48.5) | ||
| Multiparous | 223,552 (51.3) | 212,050 (48.7) | ||
| Conception method | 0.928 | |||
| Without ART | 445,831 (51.4) | 421,218 (48.6) | ||
| With ART | 182,44 (51.4) | 17,220 (48.6) | ||
| Maternal smoking during pregnancy | 0.488 | |||
| Yes | 21,223 (51.7) | 19,833 (48.3) | ||
| No | 353,727 (51.4) | 334,502 (48.6) | ||
| Missing | 89,125 (51.5) | 84,103 (48.6) | ||
All mean differences are calculated using females as the reference.
CI confidence interval, BMI body mass index, ART assisted reproductive technology.
aStudent's t-test was used for continuous variables and the chi-squared test for categorical variables.
Table 2 shows the association between fetal gender and pregnancy complications. Women carrying a male fetus had a significantly higher risk of placental abruption (0.85%, male and 0.74%, female; adjusted risk ratio [aRR] = 1.15, 95% CI 1.10–1.20). In contrast, women carrying a male fetus had a significantly lower risk of preeclampsia (2.00%, male and 2.17%, female; aRR 0.92, 95% CI 0.89–0.94) and placenta accreta (0.42%, male and 0.47%, female; aRR 0.90, 95% CI 0.85–0.96). We did not detect any significant association between fetal gender and other pregnancy complications, including HELLP syndrome, placenta previa, intrauterine fetal death, CAM, and amniotic fluid embolism.
Table 2.
Association between fetal sex and pregnancy complications. (n = 902,513).
| Outcomes | Male (%) (n = 464,075) | Female (%) (n = 438,438) | Crude RR (95% CI) | Adjusted RRa (95% CI) |
|---|---|---|---|---|
| Preeclampsia | 9265 (2.00) | 9502 (2.17) | 0.92 (0.89–0.95) | 0.92 (0.89–0.94) |
| HELLP syndrome | 565 (0.18) | 509 (0.17) | 1.05 (0.93–1.18) | 1.05 (0.93–1.18) |
| Placental abruption | 3946 (0.85) | 3250 (0.74) | 1.15 (1.10–1.20) | 1.15 (1.10–1.20) |
| Placenta accreta | 1941 (0.42) | 2040 (0.47) | 0.90 (0.84–0.96) | 0.90 (0.85–0.96) |
| Placenta previa | 7312 (1.58) | 6876 (1.57) | 1.00 (0.97–1.04) | 1.01 (0.97–1.04) |
| Intrauterine fetal death | 2641 (0.57) | 2446 (0.56) | 1.02 (0.97–1.08) | 1.02 (0.96–1.08) |
| Chorioamnionitis | 4187 (0.90) | 3843 (0.88) | 1.03 (0.99–1.08) | 1.03 (0.98–1.07) |
| Cord prolapse | 136 (0.04) | 104 (0.04) | 1.24 (0.96–1.60) | 1.24 (0.96–1.60) |
| Amniotic fluid embolism | 50 (0.01) | 51 (0.01) | 0.93 (0.63–1.37) | 0.93 (0.63–1.37) |
All risk ratios are calculated with females set as the reference.
RR risk ratio, CI confidence interval, HELLP hemolysis, elevated liver enzymes, low platelet count.
aAdjusted for maternal age, maternal height, maternal pre-pregnant body mass index, parity, conception method, and maternal smoking status during pregnancy.
Table 3 shows the association between fetal gender and delivery outcomes among women with live birth. The analyses on delivery outcomes were based on 897,426 women after excluding those with intrauterine fetal death. The analysis of instrumental deliveries was restricted to 632,659 women, excluding those with a cesarean section. Women carrying a male fetus had a higher risk of preterm delivery (12.27%, male and 10.20%, female; adjusted odds ratio (aOR): 1.20, 95% CI 1.19–1.22), very preterm delivery (2.37%, male and 2.13%, female; aOR 1.11, 95% CI 1.08–1.14), and extremely preterm delivery (0.77%, male and 0.72%, female; aOR 1.08, 95% CI 1.02–1.13). They also had a higher risk of fetal macrosomia (0.99%, male and 0.54%, female; aOR 1.83, 95% CI 1.73–1.91), hypotonic uterine inertia (9.50%, male and 8.89%, female; aOR 1.07, 95% CI 1.05–1.08), hypertonic uterine inertia (0.11%, male and 0.09%, female; aOR 1.21, 95% CI 1.06–1.37), instrumental delivery using vacuum and forceps (11.17%, male and 8.79%, female; aOR 1.27, 95% CI 1.26–1.29), and cesarean delivery (29.63%, male and 29.37%, female; aOR 1.01, 95% CI 1.00–1.02). However, women carrying a male fetus had a lower risk of low birth weight (14.55%, male and 16.87%, female; aOR 0.84, 95% CI 0.83–0.85), non-cephalic position at birth (5.36%, male and 6.16%, female; aOR 0.87, 95% CI 0.85–0.88), atonic hemorrhage (5.18%, male and 5.47%, female; aOR 0.95, 95% CI 0.93–0.96), and maternal blood transfusion (1.09%, male and 1.14%, female; aOR 0.95, 95% CI 0.92–0.99). The analysis of continuous variables showed that the gestational age at birth was lower (adjusted mean difference: − 0.18 weeks, 95% CI − 0.19 weeks to − 0.17 weeks) and the birth weight was higher (adjusted mean difference: 77.3 g, 95% CI 75.1–79.5 g) among women carrying a male fetus.
Table 3.
Association between fetal sex and delivery outcomes among women with live birth. (n = 897,426).
| Outcomes | Male (%) (n = 461,434) |
Female (%) (n = 435,992) |
Crude RR (95% CI) | Adjusted RRa (95% CI) |
|---|---|---|---|---|
| Categorized variables [n (%)] | ||||
| Preterm delivery (< 37 weeks) | 56,604 (12.27) | 44,476 (10.20) | 1.20 (1.19–1.22) | 1.20 (1.19–1.22) |
| Very preterm delivery (< 32 weeks) | 10,929 (2.37) | 9279 (2.13) | 1.11 (1.08–1.14) | 1.11 (1.08–1.14) |
| Extremely preterm delivery (< 28 weeks) | 3561 (0.77) | 3126 (0.72) | 1.08 (1.03–1.13) | 1.08 (1.02–1.13) |
| Macrosomia | 4575 (0.99) | 2369 (0.54) | 1.82 (1.74–1.92) | 1.83 (1.73–1.91) |
| Low birth weight | 67,151 (14.55) | 73,561 (16.87) | 0.84 (0.83–0.85) | 0.84 (0.83–0.85) |
| Non-cephalic position at birth | 24,715 (5.36) | 26,868 (6.16) | 0.87 (0.85–0.88) | 0.87 (0.85–0.88) |
| Hypotonic uterine inertia | 43,834 (9.50) | 38,753 (8.89) | 1.07 (1.05–1.08) | 1.07 (1.05–1.08) |
| Hypertonic uterine inertia | 512 (0.11) | 401 (0.09) | 1.21 (1.06–1.37) | 1.21 (1.06–1.37) |
| Atonic hemorrhage | 23,915 (5.18) | 23,869 (5.47) | 0.95 (0.93–0.96) | 0.95 (0.93–0.96) |
| Blood transfusion | 5032 (1.09) | 4983 (1.14) | 0.95 (0.92–0.99) | 0.95 (0.92–0.99) |
| Instrumental deliveryb | 36,260 (11.17) | 27,056 (8.79) | 1.27 (1.25–1.29) | 1.27 (1.26–1.29) |
| Cesarean delivery | 136,728 (29.63) | 128,039 (29.37) | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) |
| Mean difference (95% CI) | Adjusted mean differencea (95% CI) | |||
|---|---|---|---|---|
| Continuous variables (mean ± SD) | ||||
| Gestational age at birth (weeks) | 38.1 ± 2.4 | 38.3 ± 2.35 | − 0.18 (− 0.19 to − 0.17) | − 0.18 (− 0.19 to − 0.17) |
| Birth weight (g) | 2934 ± 546 | 2857 ± 522 | 77.6 (75.4 to 79.8) | 77.3 (75.1 to 79.5) |
All risk ratios and mean differences are calculated with females set as the reference.
RR risk ratio, CI confidence interval, SD standard deviation.
aAdjusted for maternal age, maternal height, maternal pre-pregnant body mass index, parity, and maternal smoking status during pregnancy.
bAnalysis for instrumental delivery in 63,316 women (excludes those who received cesarean section).
We conducted a sensitivity analysis based on all 1,098,268 women with an imputed database including maternal age (n = 1280), parity (n = 3074), maternal height (n = 110,308), pre-pregnancy BMI (n = 163,510), conception method (n = 18,253), delivery mode (n = 9230), and fetal presentation at birth (n = 2). We conducted an analysis of delivery outcomes after excluding those with intrauterine fetal death (n = 1,091,228). The association between fetal gender and pregnancy complications and delivery outcomes was similar to that observed using the imputed data (Supplementary Tables S1 and S2 online).
Discussion
Our study demonstrated that women carrying a male fetus had a significantly higher risk of preterm birth, abnormal uterine inertia, instrumental or cesarean delivery, and placental abruption. Those carrying a female fetus had a higher risk of preeclampsia, placenta accreta, a non-cephalic position at term, atonic hemorrhage, and maternal blood transfusion. Ours is the largest study showing an association between fetal gender and pregnancy outcomes as well as being the first study of Japanese women.
In line with previous studies7–9,23, we demonstrated that male fetal gender was associated with an increased risk of preterm delivery, very preterm delivery, and extremely preterm delivery. Although the etiology of this association remains unclear, several hypotheses have been proposed. One posits a difference in immune response according to fetal gender, with women carrying a male fetus being more likely to have chronic placental inflammation accompanied by incomplete remodeling of the spiral arterioles, leading to preterm birth24–26. This hypothesis is supported by a report of women carrying a male fetus having higher circulating levels of pro-inflammatory cytokines (e.g., granulocyte colony-stimulating factor, interleukin-21, and interleukin-33), which are responsible for excessive maternal inflammatory responses27. In an alternative hypothesis based on the findings of a study demonstrating a significant association between male fetal gender and increased CAM risk28, CAM contributed to an increased risk of preterm birth. However, our study failed to reveal a significant association between fetal gender and CAM, suggesting that another mechanism was responsible.
Studies of the association between fetal gender and preeclampsia are inconsistent. Some showed that the risk of preeclampsia was higher in women carrying a female fetus29,30, but others showed that the risk was higher in those carrying a male fetus12,31. While our finding of a higher risk of preeclampsia in women carrying a female fetus is reliable due to the large sample size (the largest study to date of this nature), further research is necessary to confirm the association.
Several mechanisms could explain why women carrying a female fetus had a higher preeclampsia risk. One possible explanation involves the gender-dependent differences in the renin–angiotensin system during early gestation. Reports have shown that among women carrying a female fetus, those with preeclampsia had a higher angiotensin level at early pregnancy than those without preeclampsia, while the angiotensin level was not elevated in women carrying a male fetus32. Another possible explanation is that the levels of human chorionic gonadotropin (hCG) vary by fetal gender. Several recent studies have reported that elevated hCG was a predictive marker of preeclampsia33,34 and that hCG levels were higher in women carrying a female fetus35,36. Although our study showed that women carrying a female fetus had a higher risk of preeclampsia, a previous study showed women carrying a male fetus have higher circulating levels of pro-inflammatory cytokines27, which are associated with an increased risk of preeclampsia and preterm birth. Thus, the underlying mechanisms may be complex, and future research focusing on pathophysiology is warranted.
We demonstrated that women carrying a male fetus had an increased risk of placental abruption, while those carrying a female fetus had an increased risk of placenta accreta. These findings correlate with the few studies that have examined the association between fetal gender and placental abruption or placenta accreta37,38. While several have demonstrated that women carrying a male fetus are likely to have higher circulating levels of pro-inflammatory cytokines, leading to the failure of spiral artery remodeling4,26,27, other studies have suggested that women carrying a male fetus also have a higher risk of placental abruption due to poor placental vascularization and deficient anchor in the matrix tissue39,40. In contrast, women carrying a male fetus were unlikely to have placenta accreta due to insufficient remodeling.
In line with previous findings6,8–11,14,15, we demonstrated that male fetal gender was an independent risk factor for instrumental and cesarean delivery. This may be explained by fetal gender-dependent differences in adaptation to distress during labor. Women carrying a male fetus are prone to having abnormal second-stage fetal heart rate patterns41–43; abnormal labor progress, including hyper- and hypotonic labor as previously demonstrated44; and macrosomia; which are all risk factors of instrumental and cesarean delivery45,46. Although the female fetus is likely to be in a non-cephalic position at birth, other factors, including abnormal fetal heart rate patterns, abnormal labor progress, and macrosomia, may be more influential contributors to an increased risk of cesarean section. In addition to the traditional complications of cesarean section, such as infection, surgical injury, and abnormal placentation in future pregnancies47–49, cesarean section could cause long-term risks in children, such as allergic disorders, childhood/adolescent obesity, autism spectrum disorders, and attention deficit hyperactivity disorder50–52. A higher rate of cesarean section among women carrying a male fetus could play some role in these associations.
Interestingly, we found that women carrying a female fetus had a significantly higher risk of atonic hemorrhage and maternal blood transfusion, despite having a lower risk of macrosomia, abnormal labor, and instrumental delivery. Studies have also demonstrated a higher risk of atonic hemorrhage and blood transfusion in women carrying a female fetus15,53. Further research is necessary to clarify the etiology of this confusing association.
The main strength of our study lies in the large sample size, enabling a reliable assessment of the association between fetal gender and various pregnancy complications and delivery outcomes, including the accurate assessment of rare outcomes, such as placenta accreta and blood transfusion, as well as small differences in fetal gender-related risks. However, our study has several limitations. First, because it was based on records of deliveries at mainly tertiary obstetric centers, our sample was likely to include higher risk pregnancies than the general population. Thus, our results, especially those of the absolute prevalence of each outcome by fetal gender, may not apply to the general population. Future replicative studies using a population-based database are warranted. Second, GDM, an important pregnancy outcome, was not analyzed due to the lack of data. Third, maternal endometriosis, which is associated with pregnancy outcomes54, was not recorded in our database. As our database was based on a collaboration of tertiary hospitals, a non-negligible number women may have endometriosis. Finally, our study was limited to analyzing the association between fetal gender and the risk of adverse pregnancy outcomes and, therefore, did not investigate the etiology of the association. Moreover, as the differences in the risk of most of the associations observed in this study were small, the results must be carefully interpreted. Nonetheless, our findings lay the groundwork for elucidating the etiology of the association between fetal gender and various pregnancy outcomes, even if their impact on clinical practice at present is small.
Supplementary information
Acknowledgements
We thank the JSOG Perinatal Committee as well as all participating hospitals for providing the data used. We would also like to thank Mr. James R. Valera of the Department of Education for Clinical Research at the National Center for Child Health and Development for his assistance with editing this manuscript.
Author contributions
S.F., K.O., and N.O. initiated the concept. S.F., K.O., and N.O. designed the study. K.O. analyzed the data. S.F. wrote the first draft of the manuscript. N.M., A.O., and H.S. critically reviewed the study design and the statistical analysis results, interpreted the data, and revised the draft. All authors approved the final manuscript.
Funding
Kohei Ogawa was supported by a grant-in-aid for Scientific Research (C) from JSOG (17K10199). This work was partially supported by the Research Development Grant for Child Health and Development from the National Center for Child Health and Development (25-4).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-75969-8.
References
- 1.Al-Qaraghouli M, Fang YMV. Effect of fetal sex on maternal and obstetric outcomes. Front. Pediatr. 2017;5:144. doi: 10.3389/fped.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend. Med. 2007;4:19–30. doi: 10.1016/S1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 3.Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A. Fetal sex and preterm birth. Placenta. 2013;34:95–99. doi: 10.1016/j.placenta.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Brettell R, Yeh PS, Impey LW. Examination of the association between male gender and preterm delivery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008;141:123–126. doi: 10.1016/j.ejogrb.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Zeitlin J, Ancel PY, Larroque B, Kaminski M, Sturdy E. Fetal sex and indicated very preterm birth: results of the EPIPAGE study. Am. J. Obstet. Gynecol. 2004;190:1322–1325. doi: 10.1016/j.ajog.2003.10.703. [DOI] [PubMed] [Google Scholar]
- 6.Melamed N, Yogev Y, Glezerman M. Fetal gender and pregnancy outcome. J. Matern. Fetal Neonatal Med. 2010;23:338–344. doi: 10.3109/14767050903300969. [DOI] [PubMed] [Google Scholar]
- 7.Ingemarsson I. Gender aspects of preterm birth. BJOG. 2003;110(Suppl 20):34–38. doi: 10.1046/j.1471-0528.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 8.Khalil MM, Alzahra E. Fetal gender and pregnancy outcomes in Libya: a retrospective study. Libyan J. Med. 2013 doi: 10.3402/ljm.v8i0.20008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aibar L, Puertas A, Valverde M, Carrillo MP, Montoya F. Fetal sex and perinatal outcomes. J. Perinat. Med. 2012;40:271–276. doi: 10.1515/jpm-2011-0137. [DOI] [PubMed] [Google Scholar]
- 10.Sheiner E, et al. Gender does matter in perinatal medicine. Fetal Diagn. Ther. 2004;19:366–369. doi: 10.1159/000077967. [DOI] [PubMed] [Google Scholar]
- 11.Dunn L, Prior T, Greer R, Kumar S. Gender specific intrapartum and neonatal outcomes for term babies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;185:19–22. doi: 10.1016/j.ejogrb.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Jaskolka D, Retnakaran R, Zinman B, Kramer CK. Fetal sex and maternal risk of pre-eclampsia/eclampsia: a systematic review and meta-analysis. BJOG. 2017;124:553–560. doi: 10.1111/1471-0528.14163. [DOI] [PubMed] [Google Scholar]
- 13.Wilms FF, et al. The impact of fetal gender and ethnicity on the risk of spontaneous preterm delivery in women with symptoms of preterm labor. J. Matern. Fetal Neonatal Med. 2016;29:3563–3569. doi: 10.3109/14767058.2016.1139566. [DOI] [PubMed] [Google Scholar]
- 14.Hou L, et al. Cross sectional study in China: fetal gender has adverse perinatal outcomes in mainland China. BMC Pregnancy Childbirth. 2014;14:372. doi: 10.1186/s12884-014-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Li G, Zhang W. Effect of fetal gender on pregnancy outcomes in Northern China. J. Matern. Fetal Neonatal Med. 2017;30:858–863. doi: 10.1080/14767058.2016.1189527. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa K, et al. Association of shorter height with increased risk of ischaemic placental disease. Paediatr. Perinat. Epidemiol. 2017;31:198–205. doi: 10.1111/ppe.12351. [DOI] [PubMed] [Google Scholar]
- 17.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet. Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 18.Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004;329:675–678. doi: 10.1136/bmj.329.7467.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minakami H, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) J. Obstet. Gynaecol. Res. 2014;40:1469–1499. doi: 10.1111/jog.12419. [DOI] [PubMed] [Google Scholar]
- 20.Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet. Gynecol. 2004;103:981–991. doi: 10.1097/01.AOG.0000126245.35811.2a. [DOI] [PubMed] [Google Scholar]
- 21.Supramaniam PR, et al. Secondary sex ratio in assisted reproduction: an analysis of 1376454 treatment cycles performed in the UK. Hum Reprod. Open. 2019 doi: 10.1093/hropen/hoz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda M, Fukuda K, Shimizu T, Andersen CY, Byskov AG. Parental periconceptional smoking and male: female ratio of newborn infants. Lancet. 2002;359:1407–1408. doi: 10.1016/S0140-6736(02)08362-9. [DOI] [PubMed] [Google Scholar]
- 23.Cooperstock M, Campbell J. Excess males in preterm birth: interactions with gestational age, race, and multiple birth. Obstet. Gynecol. 1996;88:189–193. doi: 10.1016/0029-7844(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 24.Ghidini A, Salafia CM. Gender differences of placental dysfunction in severe prematurity. BJOG. 2005;112:140–144. doi: 10.1111/j.1471-0528.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 25.Ghidini A, Salafia CM. Histologic placental lesions in women with recurrent preterm delivery. Acta Obstet. Gynecol. Scand. 2005;84:547–550. doi: 10.1111/j.0001-6349.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 26.Khong Y, Brosens I. Defective deep placentation. Best Pract. Res. Clin. Obstet. Gynaecol. 2011;25:301–311. doi: 10.1016/j.bpobgyn.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am. J. Reprod. Immunol. 2015;73:251–262. doi: 10.1111/aji.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGregor JA, Leff M, Orleans M, Baron A. Fetal gender differences in preterm birth: findings in a North American cohort. Am. J. Perinatol. 1992;9:43–48. doi: 10.1055/s-2007-994668. [DOI] [PubMed] [Google Scholar]
- 29.Shiozaki A, Matsuda Y, Satoh S, Saito S. Impact of fetal sex in pregnancy-induced hypertension and preeclampsia in Japan. J. Reprod. Immunol. 2011;89:133–139. doi: 10.1016/j.jri.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Elsmén E, Källén K, Marsál K, Hellström-Westas L. Fetal gender and gestational-age-related incidence of pre-eclampsia. Acta Obstet. Gynecol. Scand. 2006;85:1285–1291. doi: 10.1080/00016340600578274. [DOI] [PubMed] [Google Scholar]
- 31.Verburg PE, et al. Sexual dimorphism in adverse pregnancy outcomes: a retrospective Australian population study 1981–2011. PLoS ONE. 2016;11:e0158807. doi: 10.1371/journal.pone.0158807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sykes SD, et al. Fetal sex and the circulating renin-angiotensin system during early gestation in women who later develop preeclampsia or gestational hypertension. J. Hum. Hypertens. 2014;28:133–139. doi: 10.1038/jhh.2013.51. [DOI] [PubMed] [Google Scholar]
- 33.Schalekamp-Timmermans S, et al. Fetal sex-specific differences in gestational age at delivery in pre-eclampsia: a meta-analysis. Int. J. Epidemiol. 2017;46:632–642. doi: 10.1093/ije/dyw178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asvold BO, Eskild A, Vatten LJ. Human chorionic gonadotropin, angiogenic factors, and preeclampsia risk: a nested case-control study. Acta Obstet. Gynecol. Scand. 2014;93:454–462. doi: 10.1111/aogs.12363. [DOI] [PubMed] [Google Scholar]
- 35.Olsen RN, Woelkers D, Dunsmoor-Su R, Lacoursiere DY. Abnormal second-trimester serum analytes are more predictive of preterm preeclampsia. Am. J. Obstet. Gynecol. 2012;207(228):e221–227. doi: 10.1016/j.ajog.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Steier JA, Myking OL, Bergsjø PB. Correlation between fetal sex and human chorionic gonadotropin in peripheral maternal blood and amniotic fluid in second and third trimester normal pregnancies. Acta Obstet. Gynecol. Scand. 1999;78:367–371. [PubMed] [Google Scholar]
- 37.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 38.Tikkanen M, et al. Male fetal sex is associated with earlier onset of placental abruption. Acta Obstet. Gynecol. Scand. 2010;89:916–923. doi: 10.3109/00016341003605685. [DOI] [PubMed] [Google Scholar]
- 39.Matthiesen L, et al. Immunology of preeclampsia. AChem. Immunol. Allergy. 2005;89:49–61. doi: 10.1159/000087912. [DOI] [PubMed] [Google Scholar]
- 40.Ananth CV, Getahun D, Peltier MR, Smulian JC. Placental abruption in term and preterm gestations: evidence for heterogeneity in clinical pathways. Obstet. Gynecol. 2006;107:785–792. doi: 10.1097/01.AOG.0000207560.41604.19. [DOI] [PubMed] [Google Scholar]
- 41.Sheiner E, et al. Clinical significance of fetal heart rate tracings during the second stage of labor. Obstet. Gynecol. 2001;97:747–752. doi: 10.1016/s0029-7844(01)01188-7. [DOI] [PubMed] [Google Scholar]
- 42.Bekedam DJ, Engelsbel S, Mol BW, Buitendijk SE, van der Pal-de Bruin KM. Male predominance in fetal distress during labor. Am. J. Obstet. Gynecol. 2002;187:1605–1607. doi: 10.1067/mob.2002.127379. [DOI] [PubMed] [Google Scholar]
- 43.Lieberman E, et al. The association of fetal sex with the rate of cesarean section. Am. J. Obstet. Gynecol. 1997;176:667–671. doi: 10.1016/S0002-9378(97)70567-2. [DOI] [PubMed] [Google Scholar]
- 44.Cahill AG, Roehl KA, Odibo AO, Zhao Q, Macones GA. Impact of fetal gender on the labor curve. Am. J. Obstet. Gynecol. 2012;206(335):e331–335. doi: 10.1016/j.ajog.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Mocanu EV, Greene RA, Byrne BM, Turner MJ. Obstetric and neonatal outcome of babies weighing more than 4.5 kg: an analysis by parity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000;92:229–233. doi: 10.1016/S0301-2115(99)00280-8. [DOI] [PubMed] [Google Scholar]
- 46.Sheiner E, et al. Failed vacuum extraction. Maternal risk factors and pregnancy outcome. J. Reprod. Med. 2001;46:819–824. [PubMed] [Google Scholar]
- 47.Franchi M, et al. Unintentional transvesical caesarean section: incidence, risk factors, surgical technique and post-operative management. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;236:26–31. doi: 10.1016/j.ejogrb.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 48.Hammad IA, Chauhan SP, Magann EF, Abuhamad AZ. Peripartum complications with cesarean delivery: a review of Maternal-Fetal Medicine Units Network publications. J. Matern. Fetal Neonatal Med. 2014;27:463–474. doi: 10.3109/14767058.2013.818970. [DOI] [PubMed] [Google Scholar]
- 49.Marshall NE, Fu R, Guise JM. Impact of multiple cesarean deliveries on maternal morbidity: a systematic review. Am. J. Obstet. Gynecol. 2011;205(262):e261–268. doi: 10.1016/j.ajog.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 50.Sandall J, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet. 2018;392:1349–1357. doi: 10.1016/S0140-6736(18)31930-5. [DOI] [PubMed] [Google Scholar]
- 51.Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin. Exp. Allergy. 2008;38:634–642. doi: 10.1111/j.1365-2222.2008.02939.x. [DOI] [PubMed] [Google Scholar]
- 52.Zhang T, et al. Association of cesarean delivery with risk of neurodevelopmental and psychiatric disorders in the offspring: a systematic review and meta-analysis. JAMA Netw. Open. 2019;2:e1910236. doi: 10.1001/jamanetworkopen.2019.10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malabarey O, Almog B, Brown R, Abenhaim HA, Shrim A. Postpartum hemorrhage in low risk population. J. Perinat. Med. 2011;39:495–498. doi: 10.1515/jpm.2011.059. [DOI] [PubMed] [Google Scholar]
- 54.Baggio S, et al. Delivery and pregnancy outcome in women with bowel resection for deep endometriosis: a retrospective cohort study. Gynecol. Surg. 2015;12:279–285. doi: 10.1007/s10397-015-0901-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.