Abstract
Background
Knowledge on optimal electrical stimulation (ES) modalities and region-specific functional effects of colonic neuromodulation is lacking. We aimed to map the regional colonic motility in response to ES of i) the colonic tissue and ii) celiac branch of the abdominal vagus nerve (CBVN) in an anesthetized porcine model.
Methods
In male Yucatan pigs, direct ES (10Hz, 2ms, 15mA) of proximal (pC), transverse (tC) or distal (dC) colon was done using planar flexible multi-electrode array panels and CBVN ES (2Hz, 0.3–4ms, 5mA) using pulse-train (PT), continuous (10 min) or square-wave (SW) modalities, with or without afferent nerve block (200Hz, 0.1ms, 2mA). The regional luminal manometric changes were quantified as area under the curve of contractions (AUC) and luminal pressure maps generated. Contractions frequency power spectral analysis was performed. Contraction propagation was assessed using video animation of motility changes.
Key Results
Direct colon ES caused visible local circular (pC, tC) or longitudinal (dC) muscle contractions, and increased luminal pressure AUC in pC, tC and dC (143.0±40.7%, 135.8±59.7% and 142.0±62% respectively). The colon displayed prominent phasic pressure frequencies ranging from 1–12 cpm. Direct pC and tC ES increased the dominant contraction frequency band (1–6 cpm) power locally. CBVN ES (PT, 2Hz, 4ms, 5mA) triggered pancolonic contractions, reduced by concurrent afferent block. Colon contractions propagated both orally and aborally in short distances.
Conclusion and Inferences
In anesthetized pigs, the dominant contraction frequency band is 1–6 cpm. Direct colonic ES causes primarily local contractions. The CBVN-induced pancolonic contractions involve central neural network.
Keywords: Celiac branch of the abdominal vagus nerve, colon, electroceuticals, functional mapping, manometry, motility
1 |. INTRODUCTION
The colon plays a critical role in the absorption of water, electrolytes and nutrients, storage of digesta as well as fermentation and generation of useful neuroactive molecules, elimination of gas, fluid and solid waste with a remarkable selectivity. Colonic motility is key in these complex functions and dysmotility is associated with a variety of severe diseases such as gastroparesis adynamic ileus, chronic constipation and diarrhea 1–6. On the other hand, diseases such as Hirschsprung’s disease, spinal cord injuries, brain trauma and multiple sclerosis cause motility disorders that lead to chronic neurogenic colonic dysfunction with devastating emotional and quality of life impacts 3, 7, 8. Although prokinetic or inhibitory pharmacological agents influencing motility or dietary interventions provide relief to several of these conditions in some patients, they suffer from low efficiency and side effects, while surgical removal of gut segments affects the overall gut functions 5, 9.
In light of this therapeutic gap, interest in electroceutical-neuromodulation is increasing, as an alternative to mainstay therapies 10–12. The rationale for the use of neuromodulation to treat refractory gut diseases is further strengthened by the fact that the gut is highly controlled by the electroceutically-accessible autonomic nerves that impinge on the enteric nervous system 13. Electrical stimulation can therefore be applied directly to the organ or to target nerves of the autonomic nervous system, which have specific innervation patterns 10, 14, 15. While enthusiasm is high for this new therapeutic approach to alleviate colonic disorders, the optimal approach, neuromodulation modalities, effects and mechanisms are still not well known. Clinical and preclinical neuromodulation studies done thus far, on gut functions, have mainly targeted the stomach 16–18. In past years however, a number of electrical interventions have been tested for treating colonic motility disorders both in experimental models and clinical settings as recently reviewed 10,14. These include colonic electrical stimulation, sacral nerve stimulation, transcutaneous or percutaneous tibial nerve stimulation, transcutaneous electroacupuncture, and transabdominal electrical stimulation 10, 14. To date, the few clinical studies that have addressed the effect of the different types of neuromodulation on the lower gut function either by directly targeting the organ 19, 20 or by stimulating the autonomic nervous system, 14, 21, 22 showed mixed outcomes of success and failures 14. Reasons for this outcome inconsistency may be multiple including: 1) the diversity of the targeted pathology (fecal incontinence, slow transit constipation, irritable bowel syndrome-constipation predominant, colonic inertia), 2) the gap in knowledge related to the colonic region specific effects in response to ES of different nerves, 3) the lack of adequate evidence on the optimal stimulation parameters which are, for the most part, adopted from those established to be effective in urinary system dysfunction, 4) the variable and unpredictable motility pattern of the colon 23–26, 5) the lack of knowledge about potential colonic pacemakers 27, and 6) the limitations in the translatability of most pre-clinical models used (rodents, cat, dogs) to human colon in terms of colon structure, innervation, motility patterns and diet. To address the latter, interest has grown over the past decade, on the use of pigs as relevant preclinical models to study the pathophysiology of different organs due to their anatomical and physiological similarities with humans 28–30. In particular, the pig colon, similar to humans, possess unique tenia and sacculations 31 (not present in dogs, cats, rats and mice). Both species are colon fermenters and have similar colonic microbial composition 32, 33. Thus, the porcine model has both high face and construct validity to gain insight for better electroceutical therapy development. However, the influence of colonic neuromodulation on healthy pigs has been the object of a limited number of reports so far. Most of these studies involve acute direct ES on anesthetized and cleansed descending colon 34–37 or cecum 38, 39 using a broad range of stimulation parameters (5–130 Hz, 0.03–3 ms, 7–30 mA). These protocols induce local contractions (single point of stimulation),35, 39 propulsive contractions, 37–39 luminal content movement, 36, 39 or acceleration of colonic transit in response to sequential stimulations 36, 37, 40. These data confirmed or expanded other preclinical studies performed in healthy 41–44 or constipation models 45, 46 in rodents, cats and dogs. A few additional studies in pigs addressed the influence of the autonomic nervous system on the anorectal function and fecal evacuation by assessing the impact of neuromodulation targeting the pelvic nerve, hypogastric nerve and sacral nerves 47–50. While providing some insight into the potential beneficial effects of neuromodulation, the majority of those studies focused on distal colon and the response of the different colonic regions to a direct or selective nerve stimulation has not been simultaneously studied. This is of importance because, there is mounting evidence of clear regional differences in the colon both at a structural and functional level 51. In addition, while the pelvic nerve, hypogastric nerve and sacral nerves are well known to innervate the colon, the extent to which other extrinsic nerves, such as the vagus nerve, impinge on the colon is unclear. For instance, in humans, studies reported that vagal innervation extends from the upper gut up to the splenic flexure while others reported that it is continuing up to include the rectal ampulae (see 52, 53). Despite the ubiquity and key role of the vagus nerve in the brain-gut interactions, the neuromodulation of colonic motility by the vagus nerve has received limited interest thus far 54–56.
In view of these gaps, it is critical to understand the functional sphere of influence of specific nerves to develop efficient neuromodulation. Therefore, in the current study, we aimed to map the region specific (proximal/ascending, pC, transverse, tC and distal/descending colon, tC) motility response to selective ES applied directly on each of the colonic pC, tC, DC segments or the abdominal vagus nerve (specifically the celiac branch) in a healthy porcine anesthetized model. Part of these data were previously reported in abstract form 57.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Yucatan minipigs, male castrated at 7 days of age (~7 months old, 25–36 kg, total of 35), were obtained from S&S Farms (Ramona, CA) and group housed in pens (either bedding or grate floor, depending on housing availabilities − 2 pigs/pen, 42 ft2) in an environmentally controlled room (lights on/off 6AM/6PM, 61–81°F) under specific pathogen free conditions. All pigs received ad libitum access to diet (5p94 Prolab mini pig diet, PMI nutrition) and filtered tap water. All husbandry practices and procedures were conformed to the NIH Guide for the Care and Use of Laboratory Animals (8th edition) and were reviewed and approved by the UCLA Animal Research Committee (Institutional Animal Care and Use Committee) under protocol # 2018–074-01. All efforts were made to minimize any suffering as well as the number of animals used.
2.2 |. Equipment
Electrodes, leads, and stimulator
The planar electrode arrays were produced on 8-μm thick polyimide substrates, providing the mechanical flexibility and durability to withstand the motion of the intestine. As previously described 58–60, the electrodes were fabricated using e-Beam evaporation deposition to create layers with a thickness of 200-nm platinum and 10-nm titanium. Each electrode had a geometric size of 500 μm × 200 μm and a surface roughness process was applied to increase the effective surface area, and thus to increase the charge storage capacity. Each planar electrode array contained six rows of three individual electrodes (18 channels total) that could be potentially used for stimulation and recording as in our previous report 58.
2.3 |. Experimental protocol
A detailed experimental protocol is available through Protocols.io at https://www.protocols.io/view/tache-mulugeta-ot2od024899-colon-tissue-electrical-3rmgm46.
For all the experiments, pigs were fasted for at least 12 h prior to surgery with free access to water. On the day of surgery, pigs were pre-medicated with midazolam (1 mg/kg, cat # 067595, Covetrus, Dublin, OH), ketamine (15 mg/kg, cat # 068317, Covetrus) and meloxicam (0.3 mg/kg, #049755, Covetrus) injected intramuscularly. They were then intubated, connected to a respirator for ventilation (13–16 breaths/min), and maintained under general anesthesia with 1–3% inhaled isoflurane. Maintenance fluids (Lactated Ringers, cat # 059380, Covetrus) were administered at 10 ml/kg/h.
During the surgical procedure, pigs were positioned on a heating pad (32°C) in supine position. ECG electrodes and a femoral arterial line was placed and a midline abdominal incision was performed. Three colonic regions of interest - pC, tC and dC - were identified and externalized. Flexible solid-state-manometry probes (Mikro-Cath™ diagnostic pressure catheter, cat # 825–0101, Millar Inc., Houston, TX) were inserted into the different segments of the colon via a small incision and maintained in position with a loophole silk ligature. For the pC, 4 manometric probes were inserted about 10 cm below the ceco-colic junction, at 10, 13, 16 and 19 cm from the point of entry. For the tC, 4 manometric probes were inserted at the end of the pC at 10, 13, 16 and 19 cm from the point of entry. Distal probes were inserted in the dC through the anus with sensors at 10, 13, 16 and 19 cm proximal to the anal verge (Fig. 1A). For each region, four single sensor probes were mounted together in stagger such that the sensors were spaced 3 cm apart. Pigs were euthanized at the end of the experiment with an intravenous injection of pentobarbital (100 mg/kg, cat # 009444, Covetrus).
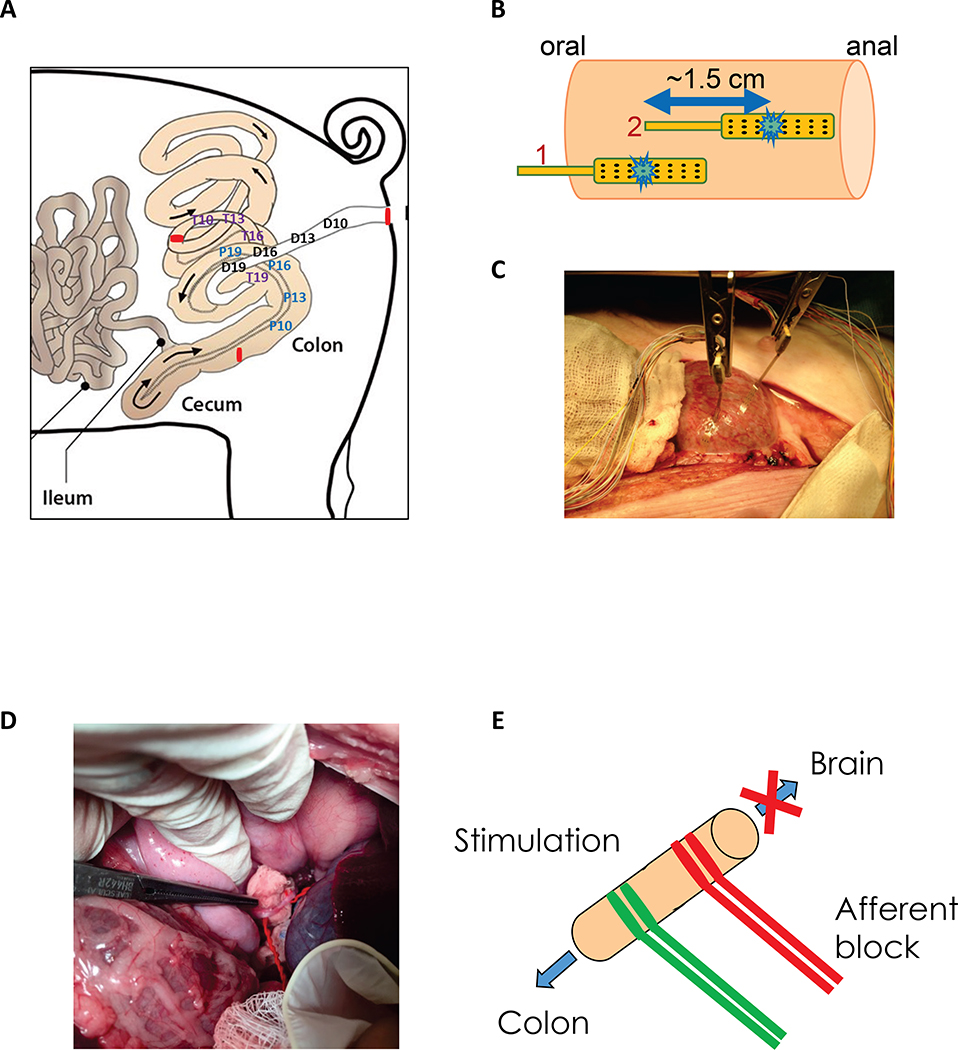
Figure 1. Schematic representation of colonic manometry recordings in overnight fasted and anesthetized adult male castrated Yucatan pigs.
A) Solid state manometry sensors were positioned at 10, 13, 16 and 19 cm from the ceco-colic junction (P10, P13, P16, P19), from the distal end of the proximal/ascending colon (T10, T13, T16, T19) and from the anal verge (D10, D13, D16, D19). B-C) Direct electrical stimulation of the colon tissue was performed using 5 cycles of alternating stimulation of electrodes #1 and #2 (total of 10 stimulations) at 10 Hz, 2 ms, 15 mA, 30 s ON, 60 s OFF as shown in B). D-E) The celiac branch of the abdominal vagus nerve was stimulated using hook electrodes (2 Hz, 0.3 or 4 ms, 5 mA) with or without afferent anodal block (200 Hz, 0.1 ms, 2 mA) using 5 cycles of 30 s ON and 90 s OFF.
Direct colonic tissue electrical stimulation
Planar electrode arrays were placed onto the serosal surface of the colonic region of interest (pC, tC or dC) for stimulation as described in previous studies (Fig. 1B, 1C) 58, 60. The electrodes were positioned to lie on the serosal side of the first 3 luminally placed pressure sensors (10–16 cm). A baseline was established for a period of 30 min, and stimulation was initiated using the customized stimulation device 59, 60. Two stimulation electrodes, configurated as bipolar pair and (1.5 apart), were stimulated alternatively. The protocol of direct colon stimulation was done as follows: pulse-train, 10 Hz, 2 ms, 15 mA, 30 s ON and 60 s OFF for 10 consecutive cycles and a total duration of 15 min. At the end of ES, the recording continued for another 30 min. The stimulation parameters were chosen based on sweep trial studies using diverse stimulation parameters performed under our conditions of experimentation (2–100 Hz, 2–4 ms, 4–15 mA) (https://doi.org/10.26275/ajkk-l7xd) and reported in prior studies 58, 60 to cause contractions or stimulate propulsion.
Abdominal vagus nerve electrical stimulation
The posterior branch of the abdominal vagus nerve was exposed through a midline abdominal incision. The celiac branch of the abdominal vagus nerve (CBVN) was identified and hook electrodes placed at 5–10 mm apart (Fig. 1D). Nerve block electrodes were placed 1–2 cm proximal from the stimulating electrodes (Fig. 1E). The posterior branch was chosen since it arises from the right cervical vagus nerve and innervates the small intestine and the colon avoiding the major hepatic branches that leave off the anterior branch of the abdominal vagus 61. The CBVN was stimulated with the following protocols: pulse-train, 2 Hz, 0.3 or 4 ms, 5 mA, 5 cycles of 30 s ON and 90 s OFF for a total duration of 10 min and a at a frequency of. In addition, the effect of different protocols of electrical stimulation including continuous and square-wave were evaluated. For nerve block, a 200 Hz, 0.1 ms, 2 mA parameters was used, concurrent with the nerve stimulation. Stimulation parameters were determined based on sweep trial study with different combination of parameters.
Assessment of Colonic Motor Function
Intracolonic pressure (manometry) recording procedure
Each pressure transducer catheter was connected via pressure cables (PEC-10D, cat # 850–5090, Millar Inc.) to a transducer (PCU-2000, cat # 880–0129, Millar Inc.). The signal was acquired via a Micro1401 analog-to-digital interface (Cambridge Electronic Design, Cambridge, UK) at 100 samples/s, and recorded with Spike 2 version 7.10 data acquisition software (Cambridge Electronic Design). The system was calibrated by using known pressures at 0, 20, 40, and 60 mmHg at the start of each experiment to convert voltage output to intraluminal pressure. Abdominal contractions and breathing artifacts were excluded by smoothing the original trace with a time constant of 2 s. Recording of motility data began after surgery was completed and continued for at least 30 min for stabilization of baseline motility, and then for at least another 30 min following completion of the stimulation experiment.
Intracolonic pressure data analysis
Motility index
Colonic contractile pressure changes were quantified by measuring the area under the curve of the phasic component of the intraluminal pressure trace (pAUC) every minute. The phasic component of intracolonic pressure was extracted from the original trace as previously reported 24 by removing the direct current (DC) component with a time constant of 10 s from the 2-s smoothed original trace.
Spectral analysis of pressure changes
Spectral power analysis of luminal pressure changes in the pC, tC and dC at basal and in response to direct colon ES and post stimulation periods were done for each of the pressure sensor probes. After removing the DC component, fast Fourier transform (FFT) was applied to obtain the frequency spectra. The integrated power of the prominent 0–12 cpm frequency band and the dominant frequency band (1–6 cpm) were then calculated by using a lab-written MATLAB code. The pressure wave data has frequency components that can be unveiled using FFT. The conditional relationship between frequency resolution and time window at low frequency (1–12 cpm) was carefully chosen such that accurate FFT analysis was obtained. The power of the frequency component indicates 1) the amplitude of the activity in a specific frequency band, and 2) how much activity in that frequency band were present during the period of analysis.
Generation of colonic motility pressure maps (colon luminal pressure and contraction frequency power map) and video animation
For visual representation of the strength of pressure changes and their regional distribution across the colon, luminal pressure changes (motility index/min), at basal and in response to ES were processed as heat map images using MATLAB code. Likewise, the contraction frequency band power changes are generated using the same software. In addition, the motility index data was used to generate video animation to view the propagative nature of pressure changes.
Statistical analysis
Normality, differences in variance and the presence of outliers were determined via the Kolmogorov–Smirnov test, F test and Grubbs’ testing, respectively, prior to further statistical analysis. There were no outliers removed. GraphPad Prism V.5.01 (GraphPad Prism, La Jolla, CA, USA) software was used to perform statistical analysis. Time course response of the motility index were analyzed using repeated measure 2-way ANOVA and Bonferroni post-hoc test. Mean motility index expressed in % baseline data were analyzed using repeated measure one-way ANOVA and Tukey’s or Dunn’s post hoc test. Motility index expressed as mean change from baseline were analyzed using Wilcoxon paired or Mann-Whitney unpaired t tests as appropriate. Contraction frequency power changes were analyzed using repeated measures 2-way ANOVA and Sidak’s post hoc test. Data are presented as mean ± SEM. P< 0.05 indicates a significant difference.
3 |. RESULTS
3.1 |. Direct colonic tissue electrical stimulation induces region specific changes in the colonic motility index and power of contraction frequency band in anesthetized pigs
Colon motility was recorded for over 3 hours without any ES in a few pigs (n=3). After the initial 30 min post-surgery, the colon motility remains stable throughout the experimental recording. Effective delivery of electrical current to the colon was confirmed by the direct measurement of electrode overpotential. Activation of the electrode also corresponded with an immediate local contraction of the colonic segment directly under the electrode. Data associated with this study, Larauche et al (2020) 62, were collected as part of the Stimulating Peripheral Activity to Relieve Conditions (SPARC; RRID:SCR_017041) project and are available through the SPARC Data Portal (RRID:SCR_017041) under a CC-BY 4.0 license.
Proximal colon ES
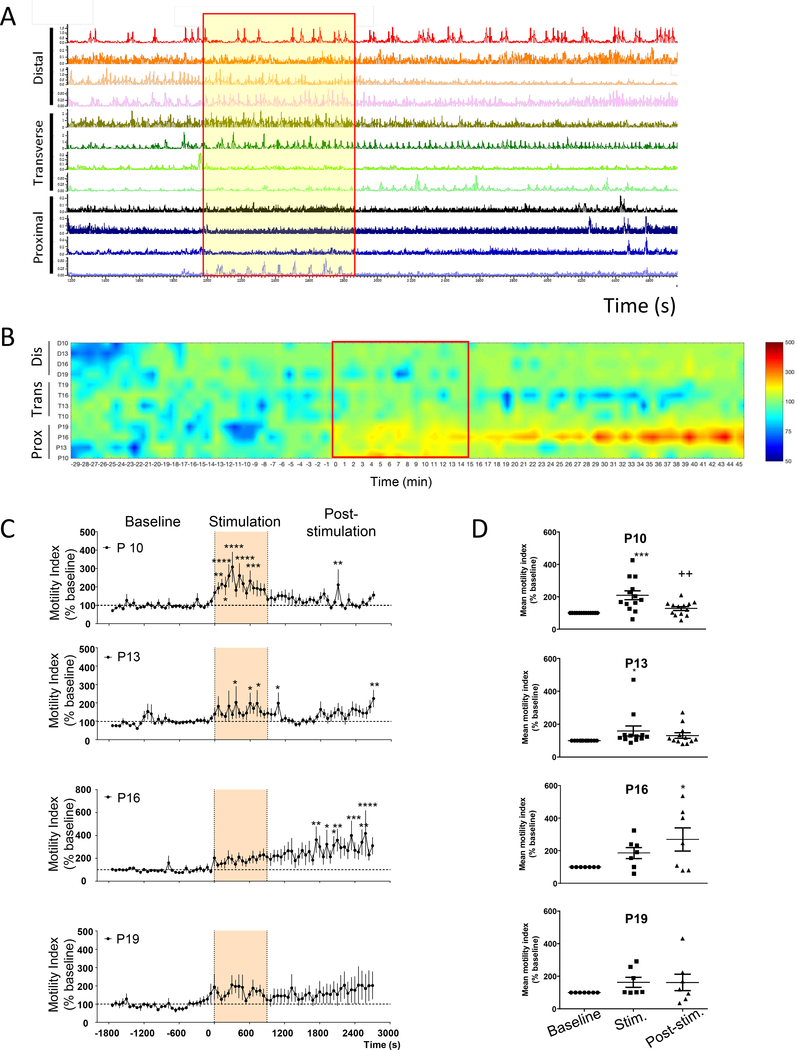
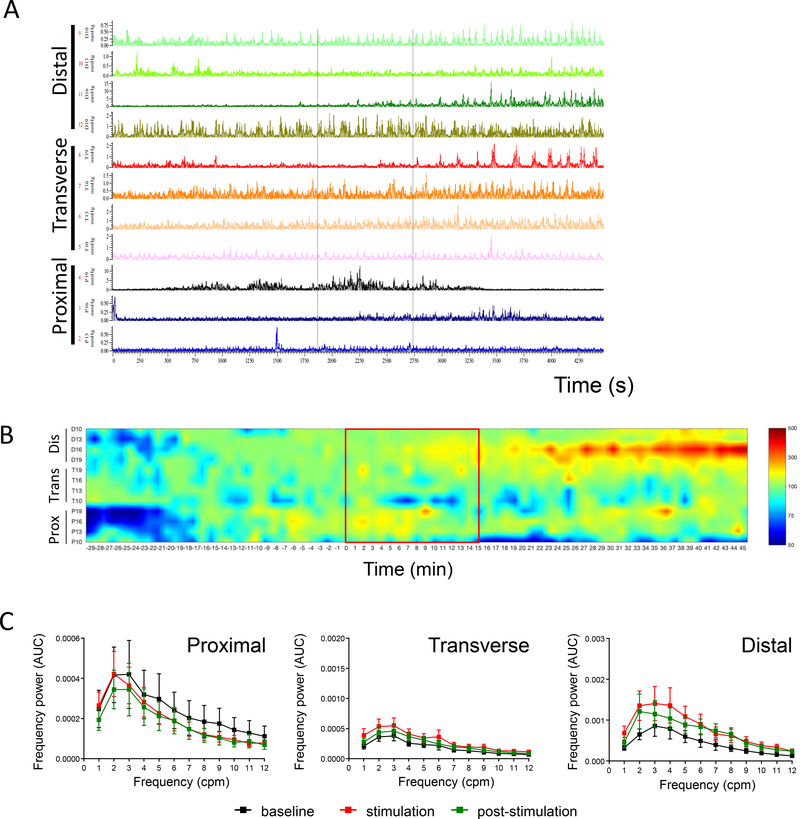
Stimulation of the pC induced a very strong circular muscular contraction observed visually (Suppl. Fig. 1A). This contraction was also detected on manometry recordings (Fig. 2A and Table 1). This was followed by a marked activation of the pC for the next 30 min, and an overall trend to decrease the motility in the tC as shown on the pressure map (Fig. 2B), which did not reach statistical significance (Table 1). The dC motility remained unchanged throughout the recording period (Fig. 2B and Table 1). Time course response of the motility index of the pC at each of the 4 proximal sensors (Fig. 2C) showed increased motility index (% change of baseline) during stimulation (P10 and P13) and post-stimulations (P16), suggesting a possible propagation from P10-P13 sites to the P16 site. Similarly, comparison of the mean motility index of the basal vs the stimulation or post stimulation period showed an increased mean motility index at P10 and 16 (during stimulation) and P16 (during the post stimulation period) (Fig. 2D).
Figure 2. Influence of direct proximal colon electrical stimulation.
(10 Hz, 2 ms, 15 mA, pulse train protocol) as monitored by manometry in anesthetized pigs (n=7–15 each). A) Representative trace of direct proximal colon stimulation; B) Pressure map representation; C) Time course response of the motility response recorded by the different proximal probes (P10, P13, P16, P19) at basal, during and post direct proximal colon electrical stimulation (motility index, % baseline) (n=7–13). Traces show the baseline response (30 min), stimulation (15 min) and post stimulation (30 min) periods. Data are mean ± SEM, 2-way ANOVA and Bonferroni’s post hoc test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs baseline. D) Mean motility index change (% baseline) during baseline (30 min), stimulation (15 min) and post stimulation (30 min) in proximal (n=7–13 pigs) regions. Data are mean ± SEM, repeated measures one-way ANOVA and Tukey’s post hoc test, *p<0.05, ***p<0.001 vs baseline, ++p<0.01 vs stimulation. Channels represent the position of the different manometry probes in the different colonic parts (P: proximal, T: transverse and D: distal).
Table 1:
Colonic mean motility index (in % baseline) during stimulation (15 min) and post stimulation (30 min) in response to direct proximal, transverse and distal colonic stimulation in anesthetized male Yucatan pigs.
| n | stimulation | post-stimulation | ||
|---|---|---|---|---|
| Direct proximal colon stimulation | P10 | 13 | 209.0 ± 28.1*** | 127.2 ± 11.8 ++ |
| P13 | 12 | 159.5 ± 30.9 * | 131.4 ± 17.0 | |
| P16 | 7 | 186.5 ± 34.1 | 270.1 ± 71.6 * | |
| P19 | 7 | 163.6 ± 31.3 | 162.0 ± 51.0 | |
| T10 | 14 | 118 ± 12.1 | 125.6 ± 20.5 | |
| T13 | 13 | 112.6 ± 16.3 | 109.8 ± 25.9 | |
| T16 | 15 | 102.1 ± 10.5 | 89.03 ± 12.2 | |
| T19 | 15 | 114.5 ± 7.0 | 109.7 ± 24.9 | |
| D10 | 14 | 111.4 ± 10.7 | 114.7 ± 13.5 | |
| D13 | 13 | 111.3 ± 7.0 | 128.1 ± 17.7 | |
| D16 | 14 | 103.5 ± 6.7 | 121.6 ± 14.5 | |
| D19 | 12 | 96.0 ± 10.7 | 129.7 ± 24.3 | |
| Direct transverse colon stimulation | P10 | 5 | 95.6 ± 12.7 | 117.3 ± 26.1 |
| P13 | 7 | 92.2 ± 10.5 | 131.9 ± 27.1 | |
| P16 | 7 | 102.5 ± 14.0 | 85.2 ± 10.6 | |
| P19 | 8 | 125.2 ± 17.9 | 109.6 ± 28.8 | |
| T10 | 6 | 240.2 ± 26.8 * | 123.2 ± 24.4 | |
| T13 | 7 | 124.9 ± 21.7 | 104.9 ± 18.0 | |
| T16 | 8 | 125.2 ± 14.8 | 105.7 ± 16.7 | |
| T19 | 8 | 118.9 ± 14.7 | 99.8 ± 10.3 | |
| D10 | 8 | 125.7 ± 19.7 | 137.3 ± 24.7 | |
| D13 | 7 | 139.5 ± 21.5 | 171.0 ± 30.9 | |
| D16 | 8 | 153.9 ± 26.9 | 192.8 ± 29.5 * | |
| D19 | 7 | 141.8 ± 28.2 | 119.3 ± 20.2 | |
| Direct distal colon stimulation | P10 | 2 | 86.4 ± 13.2 | 73.8 ± 12.7 |
| P13 | 6 | 143.4 ± 9.4 * | 118.4 ± 16.4 | |
| P16 | 5 | 142.9 ± 17.6 | 131.6 ± 33.1 | |
| P19 | 5 | 142.7 ± 28.3 | 141.0 ± 53.6 | |
| T10 | 5 | 92.5 ± 9.8 | 106.6 ± 3.7 | |
| T13 | 5 | 106.0 ± 12.1 | 97.7 ± 21.1 | |
| T16 | 6 | 109.0 ± 12.0 | 123.5 ± 15.5 | |
| T19 | 6 | 135.8 ± 24.4 | 157.1 ± 21.0 | |
| D10 | 4 | 115.5 ± 9.7 | 152.0 ± 41.1 | |
| D13 | 6 | 131.4 ± 20.2 | 149.1 ± 40.7 | |
| D16 | 5 | 129.5 ± 15.9 ** | 192.8 ± 23.7 + | |
| D19 | 6 | 147.3 ± 23.8 | 193.2 ± 77.7 | |
Data are mean ± SEM of n as indicated for each probe/set. Repeated measure one-way ANOVA and Tukey’s post hoc test
p<0.05
p<0.01 and
p<0.001 vs baseline (100.0 ± 0.0%)
p<0.05
p<0.01 vs stimulation.
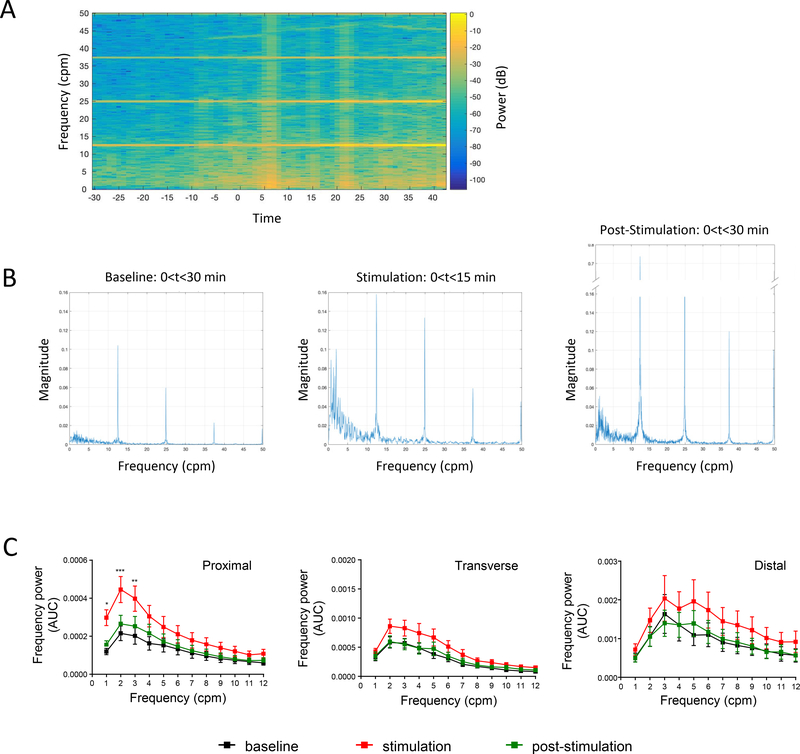
Spectral power analysis of contractions at baseline was characterized by prominent frequency power of 0 to 12 cycles/min (cpm) band in all the colonic regions. Frequency band of 13–17 cpm were overwhelmed by the high power of the ventilator/breathing frequency while higher frequency bands had too low or negligible power (Fig. 3A, 3B). The frequency band-to-frequency power (Fig. 3C) show a hump (dominant power) in the range of 1–6 cpm band. Direct proximal ES significantly increased the mean power of the 1–3 cpm frequencies and caused an overall increase in the 0–12 cpm frequency band (Fig. 3C, left panel). A trend to increase in the power of frequency spectrum was also observed in the tC (Fig. 3C, middle panel) and dC (Fig. 3C, right panel), but these did not reach statistical significance. The integrated spectra showed a return to baseline levels in all regions within the 30 min post-stimulation analysis.
Figure 3. Influence of direct proximal colon electrical stimulation on the regional colonic frequency power spectrum.
A) Representative spectrogram of the proximal colon frequency power spectrum in an anesthetized male Yucatan minipig in response to direct proximal colon electrical stimulation (10 Hz, 2 ms, 15 mA, pulse train protocol). Note that most of the frequency power comes from the ~0–12 cpm band B) Representative periodogram showing dominant frequency band (~1–6 cpm) during baseline, stimulation and post stimulation periods. Note that the power of the 1–6 cpm band is increased during stimulation and post-stimulation periods. Note also (in A and B), the breathing artifact and its harmonic waves are straight and stable. Breathing artifact (~12.5 cpm in this example) is the original frequency. The harmonic waves (~25, 37.5, 50 cpm in this example) represent artifact frequency that is a positive integer multiple of the frequency of the original wave (breathing artifact). C) Aggregated data on frequency power spectrum in the proximal, transverse and distal colon in response to direct proximal stimulation (cumulative recordings from 4 probes per colonic region in n=7–15 pigs). Direct ES of the proximal colon increased the power of the 1–12 cpm frequencies in all regions with a statistical significant difference in the 1–3 cpm frequencies in the proximal colon. Data are mean ± SEM, repeated measures two-way ANOVA and Sidak’s post hoc test, * p<0.05, ** p<0.01, *** p<0.001, vs baseline in respective groups.
Transverse colon ES
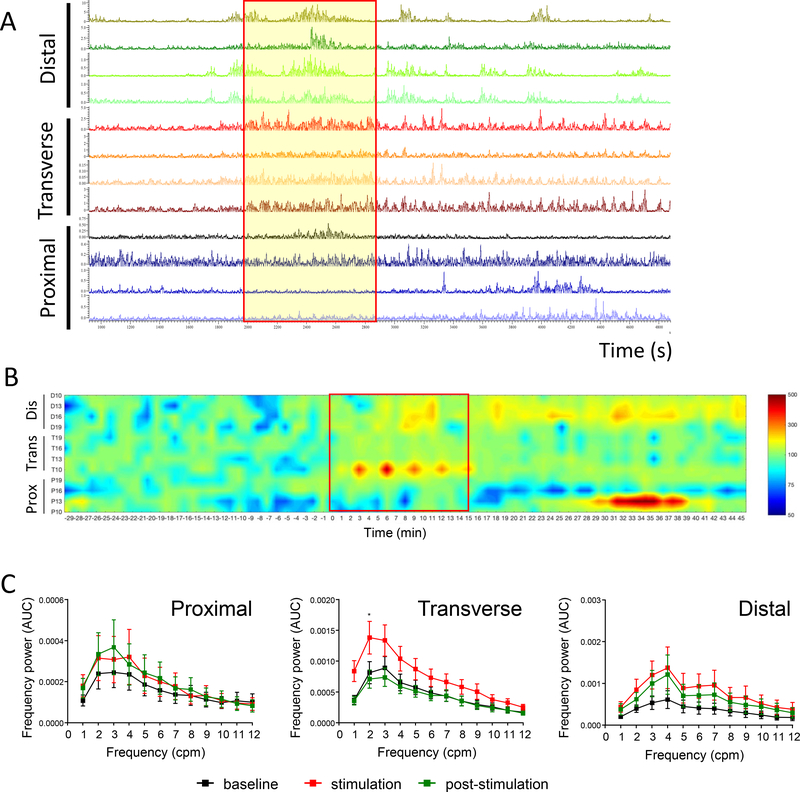
Stimulation of the tC also induced a circular muscular contraction observed visually, which could also be detected on manometric recordings in anesthetized pigs (Fig. 4A). As in pC response, stimulation of tC showed increased luminal pressure heat map primarily occurring in the tC (Fig 4B) although it was associated with some activation in the dC (Fig. 4B). After the end of the tC stimulation period, the motility index was increased in the dC and with a non-significant increased trend in the pC while tC motility was back to baseline levels (Fig. 4B; Table 1). Spectral analysis showed, like the pC, a prominent 0–12 cpm band power with the 1–6 cpm being the dominant range as shown in the frequency band-to-frequency power curves (Fig. 4C). ES of the tC increased the power of 1–6 cpm band in the tC reaching statistical significance for the 2 cpm frequencies (p<0.05) (Fig. 4C). The tC post stimulation response was characterized by the decrease in the power to the level of the corresponding basal time. The responses of pC and dC to the tC stimulation displayed variable changes, a reflection of the motility index and pressure map images.
Figure 4. Influence of direct transverse colon stimulation.
(10 Hz, 2 ms, 15 mA, pulse train protocol) as monitored by manometry in anesthetized pigs (n=5–8 each). A) Representative trace of direct transverse colon stimulation; B) Pressure map representation. Channels represent the position of the different manometry probes in the different colonic parts (P: proximal, T: transverse and D: distal). C) Frequency power spectrum in the proximal, transverse and distal colon in response to direct transverse stimulation (cumulative recordings from 4 probes per colonic region in n=5–8 pigs). Direct ES of the transverse colon increased the power of the 1–12 cpm frequencies in the transverse and distal colon with a statistical significant difference in the 2 cpm frequencies in the transverse colon. Data are mean ± SEM, repeated measures two-way ANOVA and Sidak’s post hoc test, * p<0.05 vs baseline in respective groups.
Distal colon ES
The dC stimulation, unlike the pC and tC, induced a longitudinal contractile response (Suppl. Fig. 1B) and increased the manometric recording (Fig. 5A) and motility index (pressure heat map) in the dC during the stimulation period (Fig. 5B). This was followed in the post-stimulation period by a strong activation of contraction in the dC. Probes at pC and tC showed variable activation and inhibition of motility (Fig. 5B and Table 1). Spectral analysis of the dC motility displayed similar basal frequency band (0–12 cpm) as observed in the other colonic regions. Direct ES of the dC caused only a trend to increase locally the 1–6 cpm power during stimulation, while the other regions remained unaffected. The dC response during post stimulation period returned to basal power distribution.
Figure 5. Influence of direct distal colon stimulation.
(10 Hz, 2 ms, 15 mA, pulse train protocol) as monitored by manometry in anesthetized pigs (n=2–6 each). A) Representative trace of direct distal colon stimulation. B) Pressure map representation. Channels represent the position of the different manometry probes in the different colonic parts (P: proximal, T: transverse and D: distal). C) Frequency power spectrum in the proximal, transverse and distal colon in response to direct distal stimulation (cumulative recordings from 4 probes per colonic region in n=2–6 pigs). Direct ES of the distal colon induced a trend for increase in the power of the 1–12 cpm frequencies in distal colon exclusively. Data are mean ± SEM.
In all colonic regions, direct ES induced short distance anterograde and retrograde propagating contractions (see example in Suppl. Fig. 2).
At the end of the experiment, the proximal colon tissue was examined for damage in 2 pigs. H&E staining of the stimulated (under the electrodes) and the adjacent non-stimulated tissues showed no histological change (see Suppl. Fig. 4).
3.2. |. Abdominal vagus nerve stimulation: celiac branch
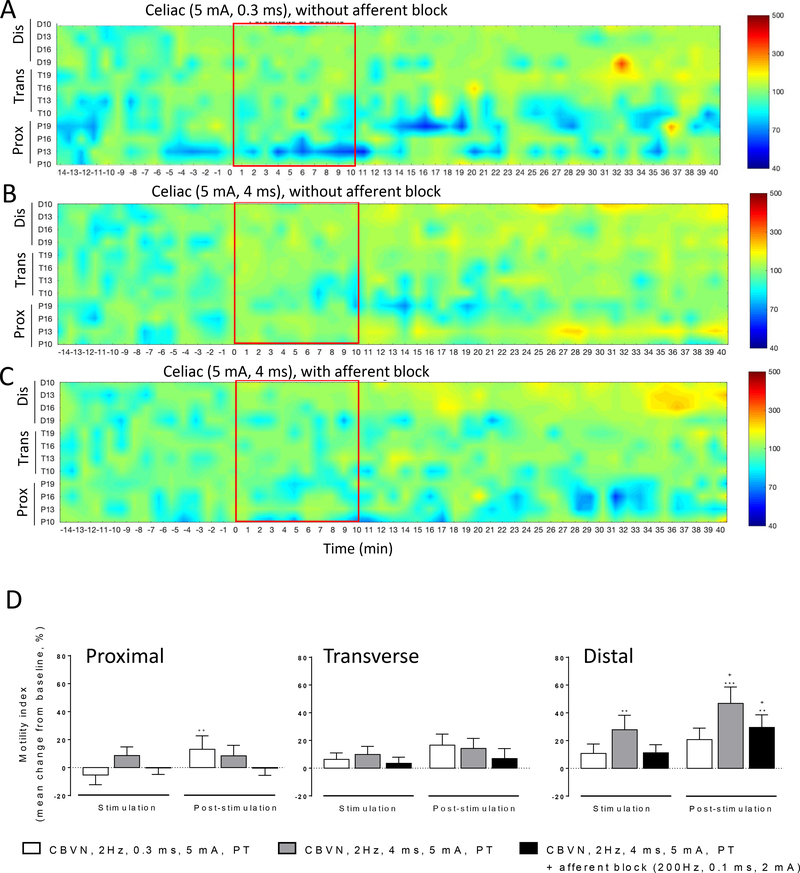
The stimulation of the CBVN using a pulse-train protocol induced a pancolonic motor response in anesthetized pigs that was more intense and widespread with the long pulse width (4 ms) (Fig. 6B) than short pulse width (0.3 ms) (Fig. 6A). The continuous and square-wave protocols were not as efficient as the pulse train protocol to produce motility changes (Suppl. Figs. 3A and 3B). The concomitant blockade of afferent fibers using an anodal afferent block attenuated the pancolonic activation in all regions (Fig. 6C). The CBVN ES induced pancolonic stimulation and its attenuation in the presence of afferent block is also reflected in the colonic motility index, expressed as AUC, particularly in the dC. (Fig. 6D).
Figure 6. Influence of different protocols of electrical stimulation of the abdominal vagus nerve, celiac branch on colonic motility in anesthetized pigs.
Pressure map of A) pulse train, 2 Hz, 0.3 ms, 5 mA, 10 min, without anodal block; B) pulse train, 2 Hz, 4 ms, 5 mA, 10 min, without anodal block; C) pulse train, 2 Hz, 4 ms, 5 mA, 10 min, with afferent anodal block 200 Hz, 2 ms, 0.1 mA. Channels represent the position of the different manometry probes in the different colonic parts (P: proximal, T: transverse and D: distal). Traces show the baseline response (15 min), stimulation (15 min) and post stimulation (30 min) periods. Data are mean ± SEM of recordings from n=7–14 pigs. D) Motility index changes (mean change from baseline %) in response to celiac branch vagus nerve stimulation (2 Hz, 5 mA) at pulse width of 0.3 ms (white bars), 4 ms (grey bars) and 4 ms with anodal block (black bars), during the stimulation (15 min) and post stimulation (30 min) in proximal, transverse and distal (n=7–14) regions. Data are mean ± SEM, Wilcoxon paired or Mann-Whitney unpaired t tests, *p<0.05, **p<0.01 vs baseline, +p<0.05 vs stimulation in respective groups.
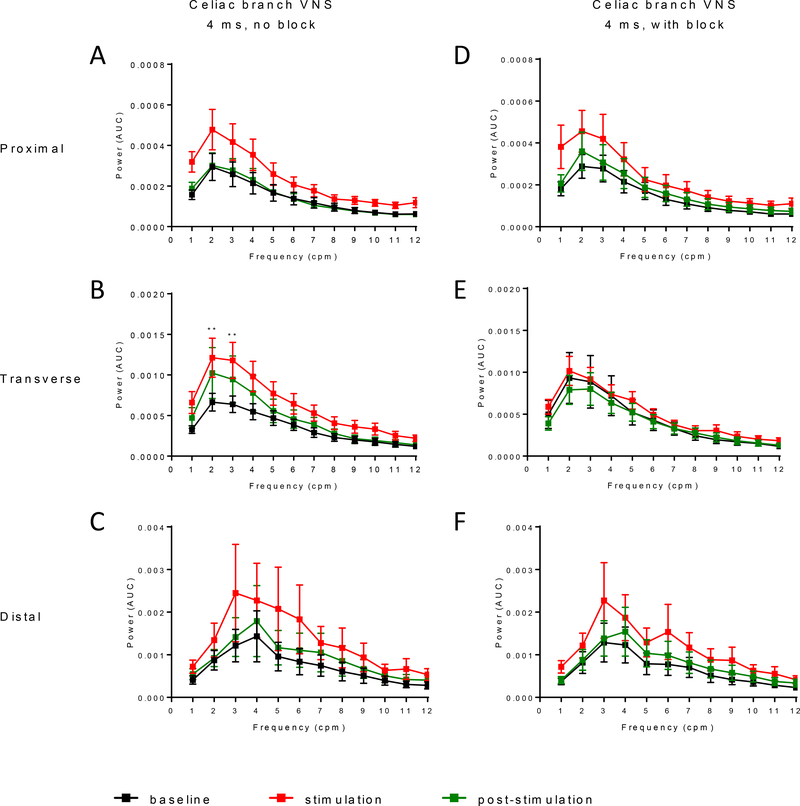
Spectral analysis of the colon response to CBVN ES (4 ms) showed an increase in the power of the dominant frequency (1–6 cpm) across the colon in response to stimulation (Fig. 7A–C), with a statistically significant increase in the 2–3 cpm frequencies (power data; p<0.01 vs baseline) in the tC (Fig. 7B). Following stimulation, the frequency power returned to baseline in the pC and dC but remained elevated in the tC. In the presence of afferent block, the increased frequency power to CBVN ES was attenuated mainly in the tC (Fig. 7D–F), in line with the motility index (AUC) and pressure map response to CBVN ES with or without afferent block.
Figure 7. Influence of celiac branch abdominal vagus nerve stimulation with or without anodal afferent block on regional colonic frequency power spectrum in anesthetized pigs.
Frequency power spectrum analysis of the colonic motility in response to celiac branch vagus nerve stimulation (2 Hz, 5 mA) at pulse width of 4 ms alone or with anodal block at baseline (30 min), during the stimulation (15 min) and post stimulation (30 min) in proximal, transverse and distal (n=7–14) regions. Data are mean ± SEM, Wilcoxon paired or Mann-Whitney unpaired t tests, **p<0.01 vs baseline in respective groups.
Video animation of the pressure changes in response to CBVN ES showed no consistent propagation for longer distances. Instead pressures waves appear to move back and forth in short distance (within 10 cm).
4 |. DISCUSSION
In this study, the influence of different ES modalities applied directly on the colonic tissue or the celiac branch of the abdominal vagus nerve on colonic motility was mapped in an anesthetized porcine model. Direct pC, tC or dC ES primarily caused an immediate local contraction followed with distant colonic regions motility changes. Direct pC and tC stimulations also increased the dominant frequency band contraction (1–6 cpm) power in the pC and tC, respectively. By contrast, CBVN ES (2 Hz, 4 ms) increased colonic motility (monitored as motility index and contraction frequency power) throughout the colonic regions and increased the power of the dominant frequency band in the tC. The colonic motility response was reduced when CBVN ES was done concurrent with afferent nerve anodal block. The study provides, for the first time, the functional (motility) sphere of influence of direct pulse-train ES and CBVN ES on the colonic segments (pC, tC, dC) and spectral analysis of colonic motility in the anesthetized pig.
One of the features of the anesthetized pig colon contractile response to local stimulation is that pC and tC stimulation induced ring-like circular contraction (Suppl. Fig. 1A) while dC stimulation response was primarily longitudinal and appeared as a shrinking of the colon wall (Suppl. Fig. 1B) as observed visually. The ring/circular contraction response in the pC and tC is likely to be due to the higher number of gap junctions between circular than longitudinal muscle cells 52, 63 and hence the strong circumferential electrical coupling of intestinal smooth muscle cells 64. Likewise, the dC characteristic longitudinal contraction suggests a stronger electric coupling among the longitudinal than the circular muscle cells and/or the regional heterogeneity of circular vs longitudinal smooth muscles responsiveness to neurohormonal stimuli as reported in humans 65.
Another salient feature of anesthetized pig colon motility is the occurrence of a wide range of contraction frequency band (0->12 cpm) and repetitive phasic pressure events in the 0–12 cpm frequency range, with a dominant frequency band of 1–6 cpm. The colon is generally considered to have three different types of phasic contractions in various species 23, 25, 26, short duration (0–2 s), long duration (10–20 s) and high amplitude propagating contractions/giant contractions. Although the relative proportion of short versus long duration phasic contractions cannot be ascertained in the present study, both short and long duration contractions are observed in the recordings as evidenced in the raw traces. The observed 0–12 cpm frequency band corresponds to the reported irregularly appearing short duration phasic contraction frequency in the human colon 26 and to the short duration spike burst frequency (9–12/min) or colonic slow wave frequency in ambulating pigs 23. On the other hand, the 1–6 cpm motor patterns is likely to be analogous to that of human colon cyclic propagating motor pattern, with a frequency of 2–6 cpm, described in a recent consensus statement on terminology and definitions of colon motility 66. Similarly, in a preliminary chronically prepared study in pigs, a 2–4 cpm frequency band is reported to represent over 50% of all contractile activities 67. These and the currently observed 1–6 cpm dominant band in the colon is likely to be the main feature of colonic motility across species, although the colon unlike the stomach and small intestine, can have multiple frequency components 68, 69.
Direct electrical pulse-train sequential stimulation of two sites (1.5 cm apart) of uncleansed colon at 10 Hz, 2 ms, 15 mA, primarily stimulated contraction on and near the stimulated site (12–15 cm) but also caused delayed responses (inhibition or stimulation) albeit moderately at distant colonic regions (pC, tC and dC) as evidenced by luminal pressure changes. The relatively longer pulse width stimulation used in the present study (2 ms) is in line with the long time-constant of intrinsic myoelectric activity of smooth muscle cells 10. The lack of clear and long-distance propagative contractions in response to direct pC ES may be due to the surgical and anesthesia related ileus. The long luminal distance between the different colonic regions makes it also less likely to capture propagative contractions to such distant regions under the experimental conditions used. On the other hand, the presently observed motility response at distant colonic regions to direct colon electrical stimulation could be due to colo-colic reflex involving the enteric nervous system. In addition, the proximal stimulation site may be devoid of colon pacemakers/hot-spots, described in the human colon as sites responsive to ES with propagative contractions 70. Similar to our observations, however, a recent report in cats indicates that direct proximal colon stimulation is mainly affecting proximal motility 71. It is to note that no histological damage was observed in either colonic region in response to this ES protocol (Suppl. Fig. 4).
The anesthetized pig colon displayed also anterograde and retrograde short duration propagation (see example in Suppl. Fig. 2). Similar back and forth propagation is reported in the human cyclic motor patterns in the colon 72 and the cecum-proximal colon of pigs 73. However, it is possible that random contractions and relaxations occur proximal and distal at different sensors’ positions that give the impression of short-distance propagation. Further studies on the actual distance and direction of propagation is needed. High amplitude propagating contractions were not recorded in the present study, which is likely to be due to the general anesthesia 26 and the surgical manipulation 74, both known to cause ileus. The propulsive contraction observed in prior pig dC studies, could be the result of the combination of multiple sequential stimulation (8 sites at 2 cm interval) and the colon cleansing preparation, itself known to affect colon motility 75–77.
Interestingly, the spectral analysis of colonic contractile response to direct colon tissue stimulation causes a general increase in the power of the 0–12 cpm frequency band bust especially of the 1–6 cpm frequency band. These data point to the possible differential modulation of power by neuromodulation. There is evidence that patients with bowel dysfunction such as slow transit constipation, lack meal-induced cyclic motor pattern (1–6 cpm) in the colon 72. The present data showing the modulation of dominant frequency band motor pattern by ES may have relevance in treating some motility related bowel dysfunctions. However, additional characterization of the differential modulation of frequency band will be critical to understand the complex functions of the colon, including the possible mechanisms behind the ability of the colon to differentially expel gas, liquid and solid luminal contents.
In contrast to the direct colon tissue stimulation, which produced an immediate response restricted locally to the segment being stimulated, stimulation of the CBVN (2 Hz, 0.3 ms or 4 ms and 5 mA, pulse train) caused a pancolonic increase in the motility index across the colonic regions. The motility response to short pulse width stimulation was weaker than the one observed with the longer pulse width. This indicates that the use of the long pulse-width with the current parameter used (2 Hz, 5 mA) produce a stronger stimulation. In addition, vagal nerve stimulation caused an overall increase in the power of the 0–12 cpm frequency band across the colon but especially of the 1–6 cpm band in the tC. In view of the inconsistent report on the extent of colonic innervation by the vagus nerve 78–80, the pancolonic motility response observed in the present study is of significance. This shows that the functional sphere of influence of the vagus on the pig colon is larger (extending to the dC) than the reported structural evidence of vagal innervation in other species mainly limited to cecum and pC 23–26, 52, 81. Interestingly the effect of CBVN ES on motility index as well as the increased power of the 1–6 cpm band is reduced when CBVN afferents are blocked using concurrent afferent nerve block. CBVN stimulation in the current study is done unilaterally (the posterior abdominal vagus). Thus stimulation of the CBVN, activates both afferent and efferent fibers, the former modulating central circuits that feedback to the target organ through both dorsal and ventral vagi. These data suggest that the CBVN ES recruited central vagal network regulating colonic motility 82 and that vagal afferents contribute to the pancolonic response to vagal stimulation. In line with this assumption, vagal stimulation frequencies as low as 1 Hz is reported to activate central vagal network and reduce seizure 59, supporting that our stimulation parameters can indeed recruit central vagal circuits.
The vagal nerve stimulation frequency used in the current study is in the lower frequency range than that used to reduce epileptic seizures in humans (20–30 Hz) 83, 84. However, vagal stimulation at wider ranges (2–300 Hz) is shown to induce electroencephalographic desynchronization 85. Studies show that different electrical vagal stimulation patterns have different effects. For instance, chorda tympani in cats or vagus nerve stimulation in ferrets, using a burst pattern compared to a continuous stimulation pattern, causes higher magnitude of saliva and vasoactive intestinal peptide secretion by the salivary gland in cats and of higher gastric acid secretion and contraction response in ferrets, for the same total number of stimuli 86, 87. In line with this, we show that pulse-train vagal nerve stimulation causes a more robust pancolonic motility than a continuous or square wave stimulation pattern (with same pulse width and intensity). This is likely to be due to the pulse-train stimulation pattern inducing temporal summation at the colonic plexi to release more excitatory neurotransmitters, as shown in cats 88. Thus although the frequency of stimulation used in the current study is low, the combination of pulse-train stimulation pattern with the relatively longer pulse width (0.3–4 ms) and higher intensity (5 mA) stimulation used is likely to account for the recruitment of both afferent and efferent vagal fibers.
In summary, the study provides the first functional/motility-response-map of the colon to electrical neuromodulation by simultaneous monitoring of the pC, tC and dC regions, to direct tissue and celiac branch of the abdominal vagus nerve stimulation in the anesthetized pig. The data show that: 1) direct electrical stimulation primarily causes local contraction but also moderately modulates distant colon regions contraction; 2) celiac branch of the abdominal vagus nerve ES increases contractions across the colon through the recruitment of vagal circuits including afferent mediated central circuitry; and 3) the dominant contraction frequency band in basal states in the anesthetized pig colon is 1–6 cpm in all colonic regions whose power/magnitude is increased during colon or vagal ES. Mapping the motility response of the colon to electroceutical interventions, through simultaneous monitoring of the different regions of the colon, in a model that bears several similarities to humans, provides critical data that can guide translational studies and applications to human patients. The study has limitations in that data are generated in anesthetized pigs that underwent acute abdominal surgery. Thus, animals were under conditions of suppressed colonic motor activity and the actual colonic transit was not assessed. Similarly, the study determined the colonic motility responses to an acute short duration (10–15 min) electrical stimulation of colon tissue or vagus nerve. It is likely that chronic and longer stimulation may exert different responses and, as such, further investigation in chronic conscious models are warranted. Lastly, given that the studies were performed in male castrated pigs, additional studies taking into account the possible effects of sex differences and the influences of sex hormones on colonic motility and on the colonic responses to neuromodulation are needed.
Taken together, the functional mapping data and the characterization of colonic motility analysis in the anesthetized porcine model reported provides a useful frame of reference that will help guide: 1) future mapping studies in the awake and behaving model and 2) neuromodulation interventions while patients are still under anesthesia (for instance to decrease surgery-induced ileus). As such, the study provides a foundational basis on which to develop safe and effective neuromodulation for patients suffering from intractable colonic motility disorders. Selective stimulation of fascicles within a nerve, such as the vagus, could allow targeting specific function and avoid off target effects 89.
Supplementary Material
Funding information and acknowledgement
This work was supported by NIH OT2 OD024899 (PD/PI Y. Taché, Subaward PI: Million Mulugeta), the CURE: Digestive Diseases Research Center P30 DK 41301 (Animal Model Core; MM, YT, ML) and a VA Senior Research Career Scientist Award (YT). Wentai Liu and his Lab was also partially supported by an endowment fund of Chen Soon-Shiong Bionic Engineering Center. We thank Dr. Steve Axelrod for his input in frequency spectrum analysis of colon contractions.
Footnotes
Conflict of interest
Competing Interests: Lo YK and Liu W hold shareholder interest in Niche Biomedical LLC.
All the other authors have no competing interests.
References
- 1.Chang L, Di Lorenzo C, Farrugia G, et al. Functional Bowel Disorders: A Roadmap to Guide the Next Generation of Research. Gastroenterology 2018;154:723–735. [DOI] [PubMed] [Google Scholar]
- 2.Fukudo S, Kanazawa M, Kano M, et al. Exaggerated motility of the descending colon with repetitive distention of the sigmoid colon in patients with irritable bowel syndrome. J Gastroenterol 2002;37 Suppl 14:145–150. [DOI] [PubMed] [Google Scholar]
- 3.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol 2004;99:750–759. [DOI] [PubMed] [Google Scholar]
- 4.Knowles CH, Martin JE. Slow transit constipation: a model of human gut dysmotility. Review of possible aetiologies. Neurogastroenterol Motil 2000;12:181–196. [DOI] [PubMed] [Google Scholar]
- 5.Rabine JC, Barnett JL. Management of the patient with gastroparesis. J Clin Gastroenterol 2001;32:11–18. [DOI] [PubMed] [Google Scholar]
- 6.Wiley JW, Chang L. Functional Bowel Disorders. Gastroenterology 2018;155:1–4. [DOI] [PubMed] [Google Scholar]
- 7.Liu CW, Huang CC, Yang YH, Chen SC, Weng MC, Huang MH. Relationship between neurogenic bowel dysfunction and health-related quality of life in persons with spinal cord injury. J Rehabil Med 2009;41:35–40. [DOI] [PubMed] [Google Scholar]
- 8.Qi Z, Middleton JW, Malcolm A. Bowel Dysfunction in Spinal Cord Injury. Curr Gastroenterol Rep 2018;20:47. [DOI] [PubMed] [Google Scholar]
- 9.Scolapio JS. Current update of short-bowel syndrome. Curr Opin Gastroenterol 2004;20:143–145. [DOI] [PubMed] [Google Scholar]
- 10.Chen JD, Yin J, Wei W. Electrical therapies for gastrointestinal motility disorders. Expert Rev Gastroenterol Hepatol 2017;11:407–418. [DOI] [PubMed] [Google Scholar]
- 11.Horn CC, Ardell JL, Fisher LE. Electroceutical Targeting of the Autonomic Nervous System. Physiology (Bethesda) 2019;34:150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevcencu C A review of electrical stimulation to treat motility dysfunctions in the digestive tract: effects and stimulation patterns. Neuromodulation 2007;10:85–99. [DOI] [PubMed] [Google Scholar]
- 13.Furness JB. Integrated Neural and Endocrine Control of Gastrointestinal Function. Adv Exp Med Biol 2016;891:159–173. [DOI] [PubMed] [Google Scholar]
- 14.Southwell BR. Electro-Neuromodulation for Colonic Disorders-Review of Meta-Analyses, Systematic Reviews, and RCTs. Neuromodulation 2020. 10.1111/ner.13099 [DOI] [PubMed] [Google Scholar]
- 15.Payne SC, Furness JB, Stebbing MJ. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat Rev Gastroenterol Hepatol 2019;16:89–105. [DOI] [PubMed] [Google Scholar]
- 16.Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery 2008;143:723–731. [DOI] [PubMed] [Google Scholar]
- 17.McCallum RW, Sarosiek I, Parkman HP, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil 2013;25:815–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ducrotte P, Coffin B, Bonaz B, et al. Gastric Electrical Stimulation Reduces Refractory Vomiting in a Randomized Crossover Trial. Gastroenterology 2020;158:506–514.e502. [DOI] [PubMed] [Google Scholar]
- 19.Martellucci J, Valeri A. Colonic electrical stimulation for the treatment of slow-transit constipation: a preliminary pilot study. Surg Endosc 2014;28:691–697. [DOI] [PubMed] [Google Scholar]
- 20.Shafik A, Shafik AA, El-Sibai O, Ahmed I. Colonic pacing: a therapeutic option for the treatment of constipation due to total colonic inertia. Arch Surg 2004;139:775–779. [DOI] [PubMed] [Google Scholar]
- 21.Leroi AM, Parc Y, Lehur PA, et al. Efficacy of sacral nerve stimulation for fecal incontinence: results of a multicenter double-blind crossover study. Ann Surg 2005;242:662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinning PG, Fuentealba SE, Kennedy ML, Lubowski DZ, Cook IJ. Sacral nerve stimulation induces pan-colonic propagating pressure waves and increases defecation frequency in patients with slow-transit constipation. Colorectal Dis 2007;9:123–132. [DOI] [PubMed] [Google Scholar]
- 23.Fioramonti J, Bueno L. Motor activity in the large intestine of the pig related to dietary fibre and retention time. Br J Nutr 1980;43:155–162. [DOI] [PubMed] [Google Scholar]
- 24.Gourcerol G, Wang L, Adelson DW, Larauche M, Tache Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol 2009;296:G992–g1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol 2002;283:G544–552. [DOI] [PubMed] [Google Scholar]
- 26.Sarna SK. Integrated Systems Physiology: From Molecule to Function to Disease Colonic Motility: From Bench Side to Bedside. San Rafael (CA):Morgan & Claypool Life Sciences; Copyright (c) 2010 by Morgan & Claypool Life Sciences., 2010. [PubMed] [Google Scholar]
- 27.Shafik A, Shafik AA, El-Sibai O, Ahmed I. Electrophysiologic identification of the location of the colonic pacemakers in humans: further study. J Invest Surg 2003;16:289–297. [PubMed] [Google Scholar]
- 28.Vodicka P, Smetana K Jr., Dvorankova B, et al. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci 2005;1049:161–171. [DOI] [PubMed] [Google Scholar]
- 29.Yin L, Yang H, Li J, et al. Pig models on intestinal development and therapeutics. Amino Acids 2017;49:2099–2106. [DOI] [PubMed] [Google Scholar]
- 30.Ziegler A, Gonzalez L, Blikslager A. Large Animal Models: The Key to Translational Discovery in Digestive Disease Research. Cell Mol Gastroenterol Hepatol 2016;2:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 1995;16:351–380. [DOI] [PubMed] [Google Scholar]
- 32.Miller ER, Ullrey DE. The pig as a model for human nutrition. Annu Rev Nutr 1987;7:361–382. [DOI] [PubMed] [Google Scholar]
- 33.Pang X, Hua X, Yang Q, et al. Inter-species transplantation of gut microbiota from human to pigs. Isme j 2007;1:156–162. [DOI] [PubMed] [Google Scholar]
- 34.de Camp NV, Heimann A, Kempski O, Bergeler J. Accelerometer-Based Assessment of Intestinal Peristalsis: Toward Miniaturized Low-Power Solutions for Intestinal Implants. IEEE J Transl Eng Health Med 2018;6:2700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiemer JF, Heimann A, Somerlik-Fuchs KH, et al. Five-fold Gastrointestinal Electrical Stimulation With Electromyography-based Activity Analysis: Towards Multilocular Theranostic Intestinal Implants. J Neurogastroenterol Motil 2019;25:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sevcencu C, Rijkhoff NJ, Gregersen H, Sinkjaer T. Propulsive activity induced by sequential electrical stimulation in the descending colon of the pig. Neurogastroenterol Motil 2005;17:376–387. [DOI] [PubMed] [Google Scholar]
- 37.Sevcencu C, Rijkhoff NJ, Gregersen H, Sinkjaer T. Electrical stimulation to induce propulsive contractions in the porcine descending colon. Artif Organs 2005;29:246–249. [DOI] [PubMed] [Google Scholar]
- 38.Aellen S, Wiesel PH, Gardaz JP, et al. Electrical stimulation induces propagated colonic contractions in an experimental model. Br J Surg 2009;96:214–220. [DOI] [PubMed] [Google Scholar]
- 39.Bertschi M, Schlageter V, Vesin JM, et al. Direct electrical stimulation using a battery-operated device for induction and modulation of colonic contractions in pigs. Ann Biomed Eng 2010;38:2398–2405. [DOI] [PubMed] [Google Scholar]
- 40.Vaucher J, Cerantola Y, Gie O, et al. Electrical colonic stimulation reduces mean transit time in a porcine model. Neurogastroenterol Motil 2010;22:88–92, e31. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Chen JD. Colonic electrical stimulation regulates colonic transit via the nitrergic pathway in rats. Dig Dis Sci 2006;51:502–505. [DOI] [PubMed] [Google Scholar]
- 42.Sevcencu C, Rijkhoff NJ, Sinkjaer T. Muscular vs. Neural Activation in Propulsion Induced by Electrical Stimulation in the Descending Colon of Rats. Neuromodulation 2005;8:131–140. [DOI] [PubMed] [Google Scholar]
- 43.Chen S, Liu L, Guo X, et al. Effects of colonic electrical stimulation using different individual parameter patterns and stimulation sites on gastrointestinal transit time, defecation, and food intake. Int J Colorectal Dis 2016;31:429–437. [DOI] [PubMed] [Google Scholar]
- 44.Amaris MA, Rashev PZ, Mintchev MP, Bowes KL. Microprocessor controlled movement of solid colonic content using sequential neural electrical stimulation. Gut 2002;50:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanmiguel CP, Casillas S, Senagore A, Mintchev MP, Soffer EE. Neural gastrointestinal electrical stimulation enhances colonic motility in a chronic canine model of delayed colonic transit. Neurogastroenterol Motil 2006;18:647–653. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Li Y, Yao S, et al. Implantable Colonic Electrical Stimulation Improves Gastrointestinal Transit and Defecation in a Canine Constipation Model. Neuromodulation 2016;19:108–115. [DOI] [PubMed] [Google Scholar]
- 47.Andersen IS, Buntzen S, Rijkhoff NJ, Dalmose AL, Djurhuus JC, Laurberg S. Ano-rectal motility responses to pelvic, hypogastric and pudendal nerve stimulation in the Gottingen minipig. Neurogastroenterol Motil 2006;18:153–161. [DOI] [PubMed] [Google Scholar]
- 48.Moller FV, Buntzen S, Rijkhoff NJ, Laurberg S. Pelvic nerve stimulation evokes nitric oxide mediated distal rectal relaxation in pigs. Dis Colon Rectum 2008;51:1261–1267. [DOI] [PubMed] [Google Scholar]
- 49.Moller FV, Buntzen S, Rijkhoff NJ, Laurberg S. Rectal evacuation and antegrade colonic luminal transport by sacral anterior root stimulation in pigs. Dis Colon Rectum 2009;52:1650–1656. [DOI] [PubMed] [Google Scholar]
- 50.Sobocki J, Nowakowski M, Herman RM, et al. Laparoscopically implanted system for stimulation of the hypogastric plexus induces colonic motility, defecation, and micturition: experimental study. Surg Innov 2015;22:70–76. [DOI] [PubMed] [Google Scholar]
- 51.Wattchow D, Brookes S, Murphy E, Carbone S, de Fontgalland D, Costa M. Regional variation in the neurochemical coding of the myenteric plexus of the human colon and changes in patients with slow transit constipation. Neurogastroenterol Motil 2008;20:1298–1305. [DOI] [PubMed] [Google Scholar]
- 52.Gonella J, Bouvier M, Blanquet F. Extrinsic nervous control of motility of small and large intestines and related sphincters. Physiol Rev 1987;67:902–961. [DOI] [PubMed] [Google Scholar]
- 53.Delmas JL G Anatomie médico-chirurgicale du système nerveux végétatif: (sympathique & parasympathique). Paris: 1933. [Google Scholar]
- 54.Collman PI, Grundy D, Scratcherd T. Vagal control of colonic motility in the anaesthetized ferret: evidence for a non-cholinergic excitatory innervation. J Physiol 1984;348:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pahlin PE, Kewenter J. The vagal control of the ileo-cecal sphincter in the cat. Acta Physiol Scand 1976;96:433–442. [DOI] [PubMed] [Google Scholar]
- 56.Tong WD, Ridolfi TJ, Kosinski L, Ludwig K, Takahashi T. Effects of autonomic nerve stimulation on colorectal motility in rats. Neurogastroenterol Motil 2010;22:688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larauche MW P-M, Dubrovsky G; Wang Y; Lo Y-K; Hsiang I; Dunn J; Liu W; Taché Y; Million M Electroceuticals: Porcine model development to study the effect of neuromodulation on colonic motility. Neurogastroenterol Motil 2018;30:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dubrovsky G, Lo YK, Wang PM, et al. Intestinal Electrical Stimulation to Increase the Rate of Peristalsis. J Surg Res 2019;236:153–158. [DOI] [PubMed] [Google Scholar]
- 59.Lo YK, Chang CW, Liu W. Bio-impedance characterization technique with implantable neural stimulator using biphasic current stimulus. Conf Proc IEEE Eng Med Biol Soc 2014;2014:474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lo YK, Wang PM, Dubrovsky G, et al. A Wireless Implant for Gastrointestinal Motility Disorders. Micromachines (Basel) 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stakenborg N, Wolthuis AM, Gomez-Pinilla PJ, et al. Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 62.Larauche MW P-M, Dubrovsky G; Wang Y; Lo Y-K; Hsiang I; Dunn J; Liu W; Taché Y; Million M Influence of direct colon tissue electrical stimulation on colonic motility in anesthetized male Yucatan minipig.: Blackfynn, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 2012;9:633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarna SK. Myoelectrical and contractile activities of the gastrointestinal tract In: Schuster MMC, M.D.; K.L. Koch, ed. Atlas of Gastrointestinal Motility in Health and Disease., Second ed. edn. Hamilton. London: BC Decker Inc; 2002: 1–18. [Google Scholar]
- 65.Maselli MA, Piepoli AL, Riezzo G, Pezzolla F. Motor responsiveness of proximal and distal human colonic muscle layers to carbachol and neurotensin. Dig Dis Sci 1998;43:1685–1689. [DOI] [PubMed] [Google Scholar]
- 66.Corsetti M, Costa M, Bassotti G, et al. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat Rev Gastroenterol Hepatol 2019;16:559–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crowell MD, Musial F, French W, Kittur D, Anderson D, Whitehead WE. Prolonged ambulatory monitoring of colonic motor activity in the pig. Physiol Behav 1992;52:471–474. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Song GQ, Yin J, Koothan T, Chen JD. Electroacupuncture improves impaired gastric motility and slow waves induced by rectal distension in dogs. Am J Physiol Gastrointest Liver Physiol 2008;295:G614–620. [DOI] [PubMed] [Google Scholar]
- 69.Sarna SK, Bardakjian BL, Waterfall WE, Lind JF. Human colonic electrical control activity (ECA). Gastroenterology 1980;78:1526–1536. [PubMed] [Google Scholar]
- 70.Shafik A, El-Sibai O, Shafik AA, Ahmed I. The motor efficacy of the artificial colonic pacemaker in colonic inertia patients. Front Biosci 2002;7:b6–13. [DOI] [PubMed] [Google Scholar]
- 71.Bourbeau D, Aamoth K, Brose S, Gustafson K. Electrical Colon Stimulation Reflexively Increases Colonic Activity. Neuromodulation 2019. doi: 10.1111/ner.13035. [DOI] [PubMed] [Google Scholar]
- 72.Dinning PG, Sia TC, Kumar R, et al. High-resolution colonic motility recordings in vivo compared with ex vivo recordings after colectomy, in patients with slow transit constipation. Neurogastroenterol Motil 2016;28:1824–1835. [DOI] [PubMed] [Google Scholar]
- 73.Hipper K, Ehrlein HJ. Motility of the large intestine and flow of digesta in pigs. Res Vet Sci 2001;71:93–100. [DOI] [PubMed] [Google Scholar]
- 74.Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg 2003;138:206–214. [DOI] [PubMed] [Google Scholar]
- 75.Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. Prolonged multi-point recording of colonic manometry in the unprepared human colon: providing insight into potentially relevant pressure wave parameters. Am J Gastroenterol 2001;96:1838–1848. [DOI] [PubMed] [Google Scholar]
- 76.Sarna SK. Effect of fluid perfusion and cleansing on canine colonic motor activity. Am J Physiol 1992;262:G62–68. [DOI] [PubMed] [Google Scholar]
- 77.Sloots CE, Felt-Bersma RJ. Effect of bowel cleansing on colonic transit in constipation due to slow transit or evacuation disorder. Neurogastroenterol Motil 2002;14:55–61. [DOI] [PubMed] [Google Scholar]
- 78.Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol 1991;260:R200–207. [DOI] [PubMed] [Google Scholar]
- 79.Bessant AR, Robertson-Rintoul J. Origin of the parasympathetic preganglionic fibers to the distal colon of the rabbit as demonstrated by the horseradish peroxidase method. Neurosci Lett 1986;63:17–22. [DOI] [PubMed] [Google Scholar]
- 80.Devroede G, Lamarche J. Functional importance of extrinsic parasympathetic innervation to the distal colon and rectum in man. Gastroenterology 1974;66:273–280. [PubMed] [Google Scholar]
- 81.Maruyama S, Okabe S, Endo M, Sato K, Iwai T. The role of the rectal branches of pelvic plexus in defecation and colonic motility in a canine model. J Med Dent Sci 2003;50:275–284. [PubMed] [Google Scholar]
- 82.Tache Y, Garrick T, Raybould H. Central nervous system action of peptides to influence gastrointestinal motor function. Gastroenterology 1990;98:517–528. [DOI] [PubMed] [Google Scholar]
- 83.Hachem LD, Yan H, Ibrahim GM. Invasive Neuromodulation for the Treatment of Pediatric Epilepsy. Neurotherapeutics 2019;16:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res 2018;11:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zanchetti A, Wang SC, Moruzzi G. The effect of vagal afferent stimulation on the EEG pattern of the cat. Electroencephalogr Clin Neurophysiol 1952;4:357–361. [DOI] [PubMed] [Google Scholar]
- 86.Andersson PO, Bloom SR, Edwards AV, Jarhult J. Effects of stimulation of the chorda tympani in bursts on submaxillary responses in the cat. J Physiol 1982;322:469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grundy D, Scratcherd T. Effect of stimulation of the vagus nerve in bursts on gastric acid secretion and motility in the anaesthetized ferret. J Physiol 1982;333:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Birks RI. A long-lasting potentiation of transmitter release related to an increase in transmitter stores in a sympathetic ganglion. J Physiol 1977;271:847–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson N, Mastitskaya S, Holder D.Avoiding off-target effects in electrical stimulation of the cervical vagus nerve: Neuroanatomical tracingtechniques to study fascicular anatomy of the vagus nerve. J Neurosci Methods. 2019;325:108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.