Abstract
Background:
Susceptibility MRI techniques, such as phase and quantitative susceptibility mapping (QSM) reveal lesion heterogeneity in MS, including the presence of lesions with outer rims suggestive of iron accumulation in macrophages and microglia, indicative of chronic-active inflammatory white matter lesions (WMLs).
Objective:
To evaluate the in vivo relationship between chronic-active WMLs (as visualized by rimmed lesions on QSM) and several clinical metrics.
Methods:
39 patients (15 men, 24 women) with MS underwent 7 Tesla brain MRIs and clinical evaluation. Contrast patterns of lesions identified on FLAIR and quantitative susceptibility maps were reviewed and compared to demographic characteristics and disability scores.
Results:
1279 lesions were identified on FLAIR MRI; 846 (66.2%) of these were visible on QSM, 119 (14.1%) of which had visible rims. Lesions visible on QSM were more likely to have rims in men (16.1%, vs 4.9% in women, p=0.009). In a logistic regression model accounting for several factors, male sex conferred a >10-fold risk of having ≥1 rimmed lesion(s) (p=0.026).
Conclusion:
Our findings provide in vivo support for the body of histopathologic literature indicating sex-specific differences in MS WML formation and suggest that QSM can be used to study these sex differences in the future.
Keywords: Multiple sclerosis, magnetic resonance imaging, quantitative susceptibility mapping, rim, disability, sex
Introduction
Multiple Sclerosis (MS) is a chronic demyelinating disorder that affects the central nervous system. Clinicians rely on MRI to diagnose and monitor patients with MS. The inflammatory status of white matter lesions (WMLs) on MRI has classically been assessed with the use of gadolinium-based contrast agents - the leakage of which into a WML is indicative of a new, actively inflamed lesion.1 Histologically, actively demyelinating plaques consist of focal inflammatory infiltrates of lymphocytes, along with activated macrophages/microglia and destruction of myelin and, sometimes, oligodendrocytes.2 After an initial period of active demyelination, WMLs then progress to either inactive astrogliotic scars or chronic-active WMLs, in which lymphocyte infiltration becomes less prominent, but demyelination and oligodendrocyte loss, driven by activated microglia and macrophages, continue.2 Given that chronic-active WMLs do not enhance with contrast, differentiation of this lesion type from inactive WMLs has been previously quite difficult. The ability of susceptibility-based MRI measures, such as quantitative susceptibility mapping (QSM), T2*/R2*, and phase, to visualize changes in both tissue myelin and iron content has led to evaluation of these measures as means to stage the chronicity and activity of WMLs.3–6
In chronic-active WMLs, as demyelination spreads outward radially, an outer rim of active demyelination surrounds an inner, hypocellular core.7 This outer rim is visible on both susceptibility-weighted phase imaging and QSM - likely due to the paramagnetic effects of iron at the outer edge of this lesion subtype. Lesions with susceptibility rims expand more over time than those without rims,4 and persistent phase rims on chronic-active (‘smoldering’) WMLs are correlated histologically with iron-laden immune cells surrounding chronic demyelinated lesions.6 A greater burden of lesions with rims is also associated with progressive MS phenotypes,8 which is consistent with histopathologic findings of a greater chronic-active WML burden in progressive MS.2,9
In addition to progressive phenotype, sex differences may influence the evolution of WMLs. Chronic-active WMLs are found in higher proportions in males,9 which may explain why men with MS have decreased life expectancy,10 faster disability progression,11 are more likely to convert to a secondary progressive MS phenotype12, and represent a greater proportion of those with a primary progressive phenotype than they do in the relapsing-remitting population.13
Multi-echo gradient echo (GRE) acquisitions on 7 Tesla (7T) MRI have been used to produce QSM images for identification of lesion heterogeneity - including lesions with outer susceptibility rims.8,14 Given previously established links between lesions with susceptibility rims on QSM and chronic-active WMLs, we sought to provide in vivo confirmation of histopathologic associations between chronic-active WMLs and clinical and demographic characteristics, including sex.
Materials and Methods
Standard protocol approvals and patient consents
Protocols for this prospective study were approved by the Institutional Review Boards at the University of Maryland School of Medicine, the Johns Hopkins University School of Medicine, and the Kennedy Krieger Institute. Written, informed consent was obtained from all participants.
Participants
Volunteers aged 18 to 65 with diagnoses of relapsing-remitting, secondary progressive, and primary progressive MS according to revised 2010 McDonald Criteria15 were recruited from the Johns Hopkins MS Center and the University of Maryland Center for Multiple Sclerosis Treatment and Research. Participants were excluded for contraindications to MRI (i.e. metallic foreign bodies) or gadolinium contrast (i.e. previous allergy to contrast, renal failure). The participants described in this study have been previously reported.16–18 However, none of the prior reports evaluated the acquired gradient-echo images nor lesion appearances on those images.
MRI protocol
Participants underwent MRI of the brain in a 7T Philips Achieva scanner with a volume transmit/32-channel receive head coil (Novamedical). Dielectric padding was used to improve image homogeneity. All images were acquired as 3-dimensional, whole brain acquisitions at 0.7mm isotropic resolution. A multi-shot turbo spin echo magnetization-prepared FLAIR (MPFLAIR) sequence was obtained with the following parameters: TR 8000ms, TI 2077ms, TE 400ms, SENSE factor 2 × 3, flip angle 90 degrees, 10’48” duration. A multi-echo GRE image was acquired with the following parameters: TR 29ms, 5 echoes with TE1/ΔTE of 5.0/5.0ms, SENSE factor 2.5 × 2, flip angle 10 degrees, 8’14”duration. A multishot magnetization prepared 2 rapid acquisition gradient echoes (MP2RAGE) was acquired with the following parameters: MP2RAGE-TR 8.25s, TI1 1s, TI2 3.3s, TR 6.9ms, TE 1.97ms, SENSE factor 2 × 2, flip angle (both inversion times) = 5 degrees, 9 minutes and 46 seconds duration. MP2RAGE images were repeated after administration of 0.1 mmol/kg of gadoteridol (ProHance).
Image processing and analysis
MP2RAGE images were processed as per Marques et al19 to create a T1-weighted image and T1 map. Images were subsequently manipulated in MIPAV (version 7.2, http://mipav.cit.nih.gov). As part of a larger analysis pipeline, which was created using the Java Image Science Toolkit (version 3.0, https://www.nitrc.org/projects/jist),20 skull stripping was performed on the MP2RAGE T1 map image and MPFLAIR images were linearly registered to the T1 map. GRE magnitude images also underwent linear registration to MP2RAGE space. QSM generation was performed by processes previously described8,14,21.
MS lesions were selected based on their hyperintensity on MPFLAIR images. This was performed by two independent raters (B.T. and H.C.), with a consensus review performed by a third rater (D.H.) with extensive experience in clinical MS practice and imaging interpretation, including susceptibility MRI. All analyses made were based on the consensus review. Similar to processes described elsewhere,8,14 two independent raters (B.T. and W.Z.) subsequently reviewed co-registered MPFLAIR and QSM images with overlaid voxels of interest for visibility of these lesions on QSM, and, if visualized, noted whether this lesion has an outer rim. Identification of a rim was based on having at least a ~50% circum-lesion presence, as well as a generally thin (in relation to lesion size) and curved appearance that is distinct both from the intensity of the lesion core and surrounding white matter (Figure 1). If a lesion was noted to have a rim, the intensity of both the lesion’s rim and core were described compared to surrounding white matter. A consensus review with arbitration was performed by D.H. for any lesion in which the independent raters did not concur. Examples of the potential lesion patterns noted are shown in Figure 2. Post-contrast T1-weighted MP2RAGE images were also reviewed for lesion contrast enhancement status.
Figure 1: Lesion identification.
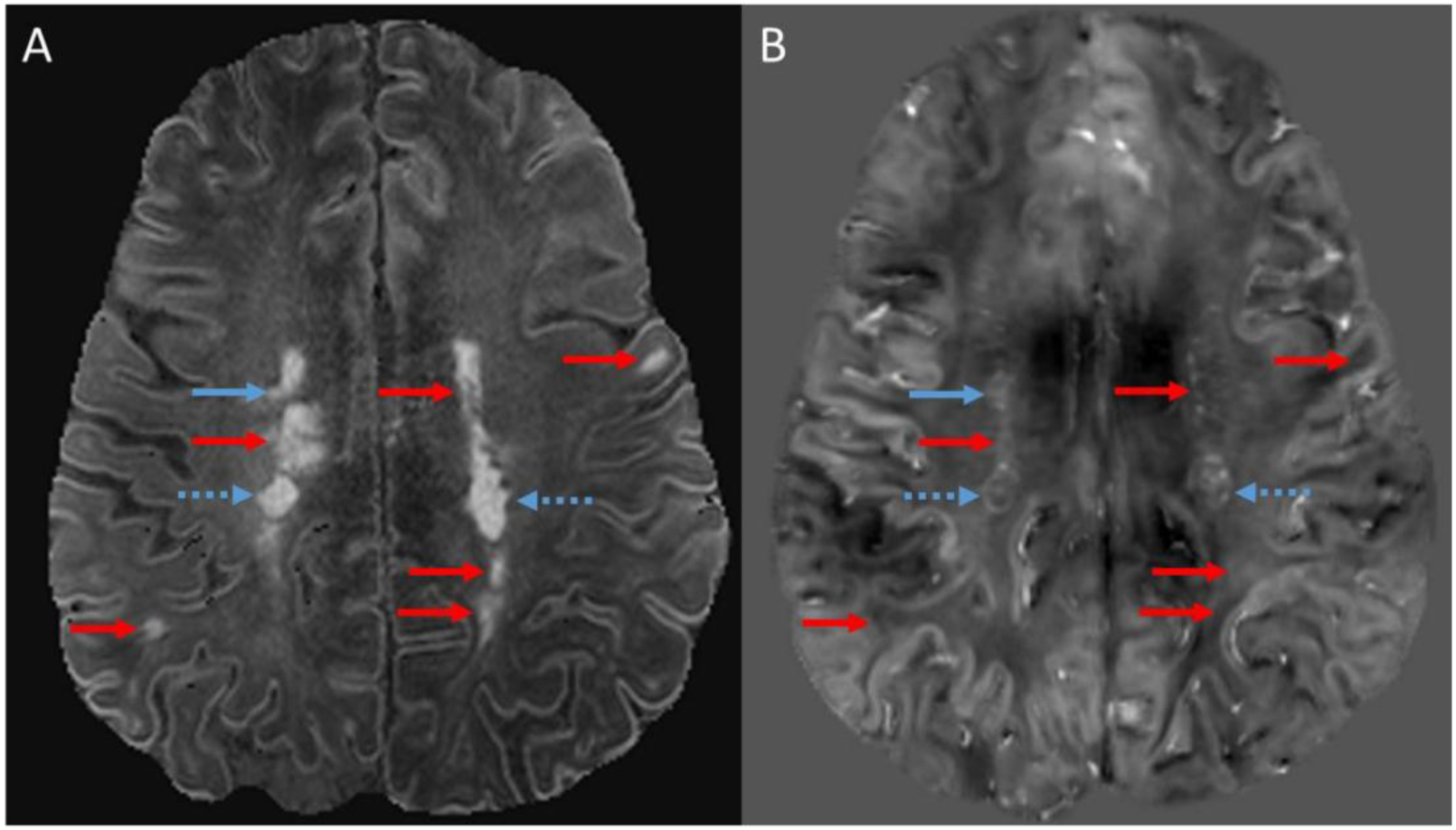
Lesions identified on magnetization-prepared FLAIR (MPFLAIR). B) Corresponding quantitative susceptibility map (QSM). Blue arrows denote lesions visible on both MPFLAIR and QSM, with dashed blue arrows indicating lesions with outer rims. Red arrows indicate lesions visible on MPFLAIR, but not visible on QSM (thus only their corresponding coordinates indicated by red arrow on QSM).
Figure 2: Example of lesion patterns on quantitative susceptibility maps (QSM).
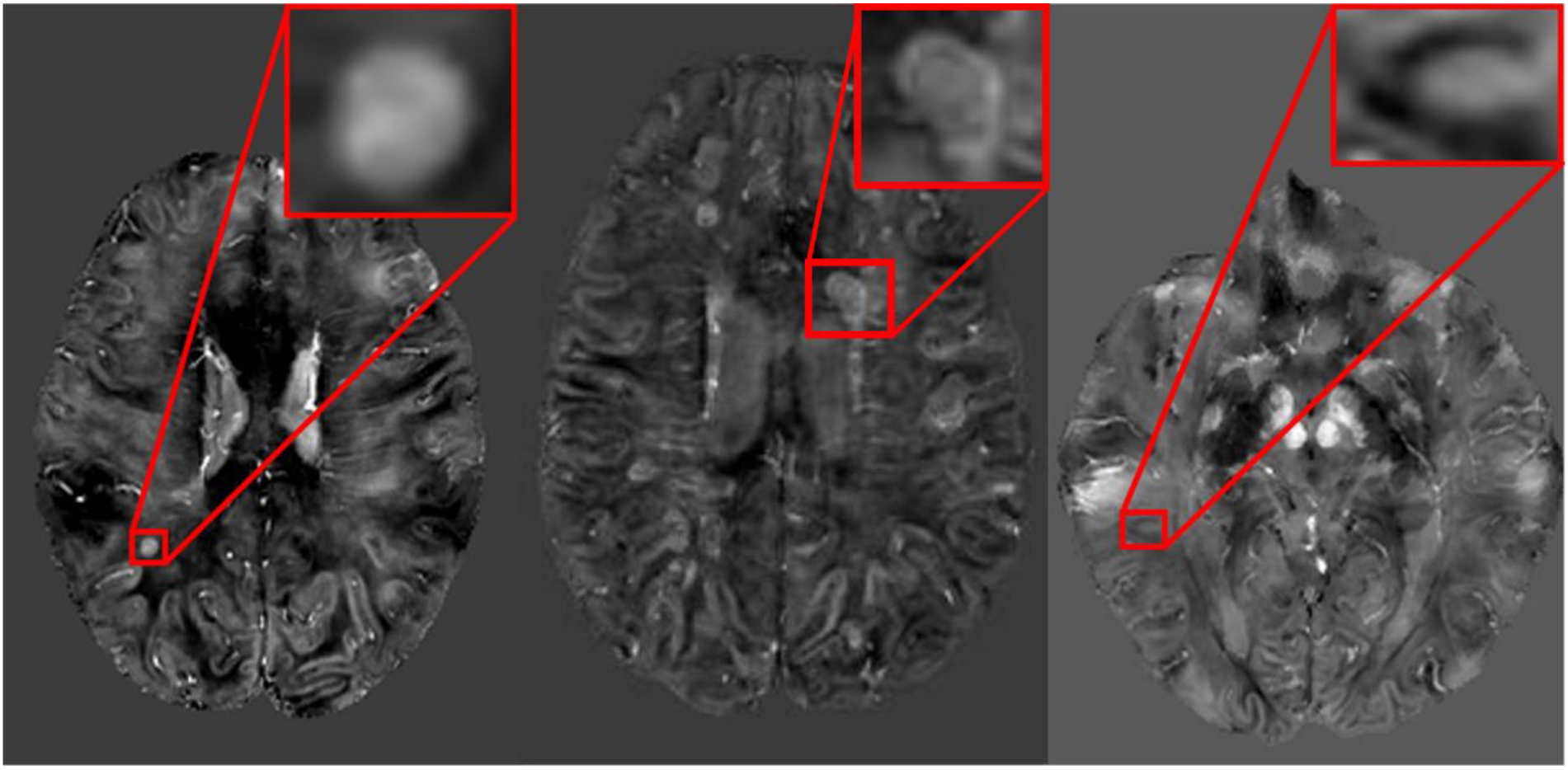
A) Hyperintense core without a rim. B) Isointense core with a hyperintense rim. C) Isointense core with a hypointense rim.
Disability measures
Disability examinations were performed within one week of the MRI. Disability was characterized by the Kurtzke Expanded Disability Status Scale (EDSS) score and the component tests of the multiple sclerosis functional composite (MSFC).22,23 To allow for statistical analysis using dichotomous variables, EDSS scores were placed into groups of <5 or ≥5. The Symbol Digits Modalities Test and Modified Fatigue Impact Scale were also acquired.
Statistical analysis
Statistical analysis was performed in Stata (StataCorp, College Station, Texas) version 10.1 IC. Comparisons between groups were performed using t-test, except for those involving EDSS, for which a non-parametric equivalent (Wilcoxon rank sum) was used. Variables showing significant association with the presence or absence of lesion rims were further explored using logistic regression, with odds ratios reported.
Because of the exploratory nature of the study, adjustment for multiple comparisons was not performed, as to not inhibit hypothesis-generating research by overcorrection for false discovery.24 When possible, actual p-values are provided to allow the reader to draw conclusions as to the significance of the findings.
Results
39 patients with MS were included; 27 (69.2%) with relapsing-remitting, 4 (10.3%) with secondary progressive, and 8 (20.5%) with primary progressive MS. The cohort was 38.5% men with an average age of 46.51 ± 11.11 years. At the time of the baseline study visit, the cohort had a mean 12.33 ± 8.60 year duration since first MS symptom onset, and most patients (n=27, 69.2%) were on disease-modifying therapy. Primary progressive patients in this cohort were more often men, whereas women in the cohort were more likely relapsing-remitting or secondary progressive (p=0.014). Basic demographic information including disability scores are shown in Table 1.
Table 1.
Baseline characteristics of study participants.
| Variables | n = 39 | Men (n=15) | Women (n=24) | p4 | |||
|---|---|---|---|---|---|---|---|
| Age (mean (SD)) | 46.51 (11.11) years | 46.7 (9.1) years | 46.4 (12.4) years | 0.923 | |||
| Phenotype | Relapsing-Remitting | 27 (69.2%) | 9 (60%) | 15 (75%) | 0.014 | ||
| Secondary Progressive | 4 (10.3%) | 0 | 4 (17%) | ||||
| Primary Progressive | 8 (20.5%) | 6 (40%) | 2 (8%) | ||||
| EDSS* score (median (range)) | 3 (1 – 7) | 3.9 (1.7) | 3.4 (1.7) | 0.328 | |||
| SDMT* score (median (range)) | 52 (29 – 83) | 47.4 (7.3) | 54.7 (14.0) | 0.040 | |||
| MFIS* score (median (range)) | 41 (0 – 84) | 38.2 (15.9) | 43.0 (24.8) | 0.511 | |||
| PASAT*1 (median (range)) | 43 (1 – 60) | 47.2 (9.3) | 41.0 (14.3) | 0.144 | |||
| 9HPT2 | 38 (16 – 403) | 60.7 (97.1) | 24.3 (10.2) | 0.170 | |||
| T25FW3 | 7 (4 – 28) | 5.7 (1.8) | 7.3 (5.9) | 0.228 | |||
| On Disease-Modifying Therapy | 27 (69.2%) | 12 (80%) | 15 (63%) | 0.426 | |||
| Interferon Beta-1a | 4 | 2 (13%) | 2 (8%) | ||||
| Glatiramer acetate | 5 | 2 (13%) | 3 (13%) | ||||
| Natalizumab | 3 | 1 (7% | 2 (8%) | ||||
| Teriflunomide | 1 | 0 | 1 (4%) | ||||
| Fingolimod | 4 | 2 (13%) | 2 (8%) | ||||
| Dimethyl fumarate | 10 | 5 (33%) | 5 (21%) | ||||
| Duration of Symptoms (mean (SD)) | 12.33 (8.60) years | 8.9 (5.8) years | 14.5 (9.5) years | 0.028 | |||
| Follow-up (mean (SD)) | 1.48 (0.98) years | 1.42 (0.95) years | 1.58 (1.05) years | 0.628 | |||
9HPT = 9-Hole Peg Test; T25FW = Timed 25-Foot Walk; EDSS = Expanded Disability Status Scale; PASAT = Paced Auditory Serial Addition Test; SD = standard deviation; SDMT = Symbol Digit Modalities Test; MFIS = Modified Fatigue Impact Scale.
at baseline visit
2 values missing
average of dominant and non-dominant hands
average of two trials
difference between men and women. Chi-square test was used for categorical variables (phenotype, use of disease-modifying therapy); independent samples t-test was used for the remaining continuous variables
In total, 1279 MS lesions were identified by two reviewers on MPFLAIR sequencing. Only 6 lesions (0.5%) enhanced with contrast administration (4 cases with 1 enhancing lesion each, 1 case with 2 lesions). 846 (66.1%) lesions were visible on QSM, 119 (14.1%) of which were characterized as having an outer rim. Almost all (n=115, 96.6%) rims were hyperintense on QSM compared to surrounding white matter, with the remaining (n=4, 3.4%) rims appearing hypointense. All rims were found on scans from 18 participants (46.2%), with 21 participants (53.8%) having no rimmed lesions. Concordance rates among the two initial reviewers for lesion identification were as follows: 83.9% for visibility of FLAIR-identified lesion on QSM, 89.1% for presence/absence of rim, 88% for intensity of the rim, and 73% for concordance on all 3 former characteristics. All analyses below are based on consensus review and arbitration of all discrepant lesions by the third reviewer.
The profile of MS lesions, including their appearance on QSM, was compared among four characteristics: MS phenotype, EDSS score (< 5.0 versus ≥ 5.0), treatment status at time of MRI, and sex (Table 2, Supplemental Table 1). There were no significant differences among lesion appearance or burden when comparing among MS phenotypes nor treatment status. Patients with an EDSS of ≥ 5.0 had, on average, more visible lesions on QSM (33.30 ± 31.22 vs 17.69 ± 12.06, p=0.028), more lesions with rims (7.30 ± 13.93 vs 1.59 ± 2.72, p=0.039), and more lesions with hyperintense rims (5.40 ± 10.51 vs 0.93 ± 1.81, p=0.031) compared to patients with EDSS < 5.0, respectively. Men had a greater proportion of lesions with rims compared to women (11.8% vs 3.9% of all lesions, p=0.021, and 16.1% vs 4.9% of lesions visible on QSM, p=0.009), as well as a greater proportion of lesions with hyperintense rims (10.5% vs 3.6% of all lesions, p=0.026, and 14.5% vs 4.6% of lesions visible on QSM, p=0.011). Though both the male and EDSS ≥5 cohorts tended to have more lesions with rims, there was no correlation between EDSS score and sex (p=0.328).
Table 2.
Analysis of lesion and rim characteristics among multiple sclerosis phenotype, disability score, baseline treatment, and sex.
| Lesion Characteristic | Phenotype | EDSS | Treatment Status | Sex | ||||
|---|---|---|---|---|---|---|---|---|
| Relapsing-Remitting (n = 27) | Progressive (n = 12) | EDSS < 5.0 (n = 29) | EDSS > 5.0 (n = 10) | Not Treated (n = 12) | Treated (n = 27) | Women (n = 24) | Men (n = 15) | |
| Mean # of FLAIR-visible lesions (SD) | 30.52 (21.12) | 37.92 (34.16) | 29.14 (20.73) | 43.4 (35.50) | 41.83 (34.81) | 28.78 (19.75) | 27.63 (20.78) | 41.07 (30.81) |
| Mean # of FLAIR-visible lesions visible on QSM (SD) | 19.22 (12.54) | 27.25 (30.20) | 17.69 (12.06) | 33.30 (31.22)* | 29.75 (29.77) | 18.11 (12.06) | 17.96 (14.30) | 27.67 (25.47) |
| Mean % of FLAIR-visible lesions visible on | 65.9 (15.2) | 62.6 (20.1) | 63.2 (15.6) | 69.9 (19.5) | 67.2 (17.1) | 63.9 (16.7) | 62.9 (17.0) | 68.1 (16.2) |
| QSM (SD) | ||||||||
| Mean # of FLAIR-visible lesions with rims on QSM (SD) | 1.96 (2.99) | 5.5 (13) | 1.59 (2.72) | 7.30 (13.93)* | 6.25 (12.91) | 1.63 (2.66) | 1.46 (3.37) | 5.60 (11.26) |
| Mean % of FLAIR-visible lesions with rim on QSM (SD) | 7.0 (10.3) | 6.7 (11.8) | 6.0 (10.0) | 9.5 (12.5) | 9.4 (14.2) | 5.8 (8.7) | 3.9 (8.7) | 11.8 (11.9)* |
| Mean # of lesions with hyper rims (SD) | 1.89 (2.91) | 5.33 (12.98) | 1.52 (2.63) | 7.10 (7.56)* | 6.08 (12.86) | 1.56 (2.67) | 1.38 (3.15) | 5.47 (11.31) |
| Mean # of lesions with hypo rims (SD) | 0.07 (2.67) | 0.17 (0.38) | 0.07 (0.26) | 0.20 (0.42) | 0.17 (0.39) | 0.07 (0.27) | 0.08 (0.28) | 0.13 (0.35) |
| Mean # of lesions with iso core, hyperintense rim (SD) | 1.22 (2.15) | 4 (9.77) | 0.93 (1.81) | 5.40 (10.51)* | 4.5 (9.76) | 1.00 (1.82) | 1.00 (2.47) | 3.80 (8.55) |
| Mean # of lesions with hyper core, hyperintense rim (SD) | 0.41 (0.89) | 1.00 (3.16) | 0.31 (0.85) | 1.40 (3.41) | 1.25 (3.14) | 0.30 (0.82) | 0.21 (0.51) | 1.20 (2.91) |
| Mean # of lesions with hypo core, hyperintense rim (SD) | 0.26 (0.59) | 0.25 (0.87) | 0.24 (0.58) | 0.30 (0.95) | 0.25 (0.87) | 0.26 (0.59) | 0.17 (0.64) | 0.40 (0.74) |
| Mean # of lesions with iso core, hypointense rim (SD) | 0 | 0.08 (0.29) | 0 | 0.10 (0.32) | 0.08 (0.29) | 0 | 0.04 (0.20) | 0 |
| Mean # of lesions with hyper core, hypointense rim (SD) | 0.07 (0.27) | 0.08 (0.29) | 0.07 (0.26) | 0.10 (0.32) | 0.08 (0.29) | 0.07 (0.27) | 0.04 (0.20) | 0.13 (0.35) |
| Mean # of lesions with hypo core, hypointense rim (SD) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean % of lesions with rims (SD) | 9.6 (13.4) | 8.3 (14.1) | 8.5 (13.2) | 11.4 (14.5) | 11.8 (17.4) | 8.0 (11.4) | 4.9 (11.0) | 16.1 (14.4)** |
| Mean % of lesions with hypo rim (SD) | 0.9 (3.9) | 0.5 (1.2) | 0.9 (3.8) | 0.6 (1.4) | 0.6 (1.5) | 0.9 (3.9) | 0.3 (1.1) | 1.6 (5.2) |
| Mean % of lesions with hyper rim (SD) | 8.7 (11.6) | 7.8 (13.9) | 7.6 (11.4) | 10.8 (14.5) | 11.3 (16.4) | 7.2 (9.8) | 4.6 (10.0) | 14.5 (13.1)* |
| Mean % of lesions with iso core, hyper rim (SD) | 6.3 (9.5) | 5.9 (10.6) | 5.4 (9.2) | 8.5 (11.2) | 8.5 (13.5) | 5.2 (7.5) | 3.5 (8.6) | 10.5 (10.0)* |
| Mean % of lesions with hyper core, hyper rim (SD) | 1.3 (2.6) | 1.0 (2.9) | 1.0 (2.5) | 1.8 (3.2) | 1.9 (3.3) | 0.9 (2.4) | 0.7 (1.7) | 2.0 (3.7) |
| Mean % of lesions with hypo core, hyper rim (SD) | 1.3 (3.5) | 0.5 (1.7) | 1.2 (3.4) | 0.6 (1.9) | 0.5 (1.7) | 1.3 (3.5) | 0.5 (1.6) | 1.9 (4.5) |
| Mean % of lesions with iso core, hypo rim (SD) | 0 | 0.2 (0.6) | 0 | 0.2 (0.6) | 0.2 (0.6) | 0 | 0.1 (0.4) | 0 |
| Mean % of lesions with hyper core, hypo rim (SD) | 0.9 (3.9) | 0.3 (1.2) | 0.9 (3.8) | 0.4 (1.3) | 0.4 (1.4) | 0.9 (3.9) | 0.2 (1.0) | 1.6 (5.2) |
| Mean % of lesions with hypo core, hypo rim (SD) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
p < 0.05
p <0.01
All proportions in the table are derived from the number of lesions in that category divided by the number of QSM-visible lesions, unless otherwise stated.
EDSS = Expanded Disability Status Scale; “hypo” = hypointense; “hyper” = hyperintense; “iso” = isointense; QSM = Quantitative Susceptibility Mapping; SD = Standard Deviation
In further dividing lesions with rims based on signal intensity of the cores, men had a significantly greater percentage of QSM-visible lesions that had a hyperintense rim with an isointense core (10.5% vs 3.5% in women, p=0.027). The proportion of other core/rim lesion patterns were not significantly different among the sexes, MS phenotype, EDSS score, or treatment status.
Patients with rimmed lesions were then compared to patients without based on several factors including radiologic profile (i.e. lesion burden, % visible on QSM), age, symptom duration, and disability scores (Table 3). Again, patients with rims were more likely to be men (61.1% vs 19.0% of patients without rims, p=0.007). In addition, patients with lesions with rims had more visible lesions on QSM (mean 29.22 vs 15.24 compared to those without rimmed lesions, p=0.025). PASAT scores decreased (thus, worsened) by an average 4.00 ± 8.20 points in the year following MRI acquisition in patients with lesions with rims, compared to an average increase (thus, improved) by 5.06 ± 11.55 points in patients without rimmed lesions (p=0.023). However, changes in PASAT scores between the 2nd and 3rd years, and between the 1st and 3rd years after imaging were not significantly different among these groups, nor were changes in other disability scores over time. Logistic regression analysis (see Table 4) showed that the risk of having lesions with hyperintense rims was significantly higher in men compared to women with an adjusted odds ratio (OR (CI)) of 6.68 (1.68 – 31.51). The significantly higher risk sustained with covariate adjustment for the total number of lesions, age, symptom duration, EDSS, and progressive phenotype (OR, CI: 10.58, 1.33 – 84.18)
Table 3.
Analysis of lesion burden and several clinical characteristics based on absence/presence of rimmed lesions on baseline quantitative susceptibility mapping (QSM).
| Characteristic | Presence of Rims on QSM | ||
|---|---|---|---|
| No Rims (n = 21) | Rims (n = 18) | p | |
| On MS treatment (%) | 66.7 | 72.2 | 0.708 |
| Male sex (%) | 19.0 | 61.1 | 0.007 |
| Relapsing-Remitting (%) | 1.9 | 77.8 | 0.284 |
| Mean number of lesions on FLAIR (SD) | 25.71 (20.37) | 41.06 (29.00) | 0.061 |
| Mean number of lesions visible on QSM (SD) | 15.24 (12.39) | 29.22 (23.87) | 0.025 |
| Mean % of total lesions that are visible on QSM (SD) | 58.71 (15.92) | 72.11 (14.82) | 0.010 |
| Mean age (SD) | 48.86 (10.55) | 43.78 (11.41) | 0.157 |
| Mean symptom duration in years (SD) | 13.42 (9.58) | 11.07 (7.38) | 0.404 |
| Baseline Mean MFIS Score (SD) | 44.38 (23.34) | 37.06 (19.16) | 0.305 |
| Mean change in MFIS between years 1 and 2 (SD) | −7.47 (12.19) | −4.08 (14.12) | 0.486 |
| Mean change in MFIS between years 1 and 3 (SD) | −2.86 (10.60) | −8.45 (16.72) | 0.318 |
| Baseline Mean SDMT Score (SD) | 53.05 (12.54) | 50.56 (12.25) | 0.536 |
| Mean change in SDMT between years 1 and 2 (SD) | 4.12 (15.62) | 1.38 (7.49) | 0.566 |
| Mean change in SDMT between years 1 and 3 (SD) | 4.57 (13.26) | 2.18 (6.43) | 0.590 |
| Baseline mean T25FW, average of 2 trials (SD) | 6.14 (2.52) | 7.22 (6.53) | 0.488 |
| Mean change in T25FW between years 1 and 2 (SD) | 0.00 (1.12) | 3.62 (11.35) | 0.200 |
| Mean change in T25FW between years 1 and 3 (SD) | 0.50 (1.61) | 3.18 (13.67) | 0.472 |
| Baseline 9HPT dominant, average of 2 trials (SD) | 23.29 (8.02) | 73.78 (177.12) | 0.199 |
| Baseline 9HPT non-dominant, average of 2 trials (SD) | 29.70 (22.33) | 30.17 (14.45) | 0.940 |
| Baseline 9HPT average of dominant/non-dominant (SD) | 26.43 (13.58) | 52.11 (89.64) | 0.202 |
| Mean change in average of dominant/non-dominant 9HPT between years 1 and 2 (SD) | −1.53 (2.76) | −5.31 (10.86) | 0.177 |
| Mean change in average of dominant/non-dominant 9HPT between years 1 and 3 (SD) | 22.4 (89.5) | −5.1 (10.0) | 0.324 |
| Baseline Mean PASAT (SD) | 42.1 (14.5) | 45.0 (10.5) | 0.501 |
| Mean change in PASAT between years 1 and 2 (SD) | 5.1 (11.6) | −4.0 (8.2)* | 0.023 |
| Mean change in PASAT between years 1 and 3 (SD) | 0.6 (10.2) | −2.3 (9.9) | 0.480 |
| Baseline Mean EDSS Score (SD) | 3.6 (1.6) | 3.6 (1.9) | 0.910 |
| Mean change in EDSS between years 1 and 2 (SD) | −0.2 (1.0) | −0.1 (0.8) | 0.770 |
| Mean change in EDSS between years 1 and 3 (SD) | −0.1 (1.0) | 0.2 (0.9) | 0.389 |
9HPT = 9-hole Peg Test; EDSS = Expanded Disability Status Scale; FLAIR = Fluid Attenuated Inversion Recovery; MFIS = Modified Fatigue Impact Scale; PASAT = Paced Auditory Serial Addition Test; QSM = Quantitative Susceptibility Mapping; SD = Standard Deviation; T25FW = Timed 25-foot walk
Table 4.
Logistic regression analysis for predictors of rimmed QSM lesions in patients with MS
| Predictor Variable | Co-variates | Odds Ratio of Rim: No Rim | 95% Confidence Interval | p-value |
|---|---|---|---|---|
| Male sex | None | 6.68 | 1.68 – 31.51 | 0.010 |
| Male sex | Total # of lesions Age Symptom duration EDSS Progressive phenotype | 10.58 | 1.33 – 84.18 | 0.026 |
Discussion
The findings of this study provide in vivo substantiation of histopathologic data demonstrating higher proportions of chronic-active WMLs in men with MS.9,14,25 Lesions with susceptibility rims on QSM from 7T GRE images were significantly more likely to be present in men and the burden of lesions with rims and presence of rims was associated with disability. This data further supports the notion that WMLs with susceptibility rims represent chronic-active WMLs and that visualization of these lesions by susceptibility techniques is an effective means to investigate the heterogeneity of WMLs in MS and its clinical impact.
Sex differences in clinical outcomes in MS are well known, with men tending towards a more aggressive, progressive clinical course.26 Correspondingly, multiple imaging studies report MRI markers of more severe pathology in WMLs in men, including higher proportions of T1 ‘black holes’ and greater longitudinal changes in diffusivity measures of demyelination and axonal integrity on diffusion tensor imaging.27,28 Detailed immunohistochemistry of WMLs have recently shed light on the underlying biologic reason for gender differences in WML formation in MS. Tissue in lesions and perilesional white matter in men are more likely to favor production of estrogen and express estrogen and ER-β signaling pathways, whereas lesions and perilesional white matter in women show an environment favoring production of progesterone and progesterone signaling.9,25 This hormonal difference within lesions is quite consequential, as estrogens are known to influence the expression of pro-inflammatory cytokines (i.e. TNF-α) and to increase T-cell trafficking and cellular adhesion molecule expression.25,29 On the other hand, progesterone and dihydroprogesterone are known to ameliorate the rodent model of MS, reduce reactive gliosis, increase remyelination, and reduce microglial activation.30–32
These hormonal differences likely influence the eventual fate of a new WML, resulting in development of a chronic-active (or ‘smoldering’) WML rather than a chronic-inactive WML. Chronic-active WMLs, which are defined as those with a demyelinated, gliotic, and hypocellular core and accumulation of HLA+ microglia/macrophages at their outer rim,33 make up about 15% of lesions seen at autopsy. This lesion subtype is found at higher rates in men with MS - a finding that has been replicated in multiple autopsy series. This gender difference is consequential, as chronic-active WMLs, compared to chronic-inactive WMLs, are more likely to show signs of severe axonal injury, poor remyelination, and neurodegenerative changes.34 Chronic-active WMLs also undergo chronic expansion in size and are found in higher proportion in those with progressive MS phenotypes.4,9,35
Given the clear clinical importance of chronic-active WMLs in MS, a well-validated imaging biomarker is necessary, especially considering the lack of gadolinium enhancement in chronic-active WMLs. Histopathologic analyses confirm that WMLs with paramagnetic rims on MRI do indeed represent chronic-active WMLs, with paramagnetic rims corresponding to regions of iron-laden macrophages and active demyelination.36 Visualization of lesions with paramagnetic rims on unwrapped phase images or QSM are increasingly recognized as means by which to quantify chronic-active MS lesions and their impact on clinical outcomes.37 With this knowledge, it is clear that our data provides in vivo confirmation of autopsy data suggesting a higher proportion of chronic-active WMLs in men with MS.9 Of the possible lesion core and rim combinations, those with isointense cores and hyperintense rims yielded the greatest difference in distribution among men and women (Supplemental Table 1), though not statistically significant in our study. This subset of lesions likely corresponds most directly to chronic-active WMLs, with the hyperintense core reflecting iron-rich macrophage activity and demyelination (both of which would increase susceptibility) and a relatively hypocellular core with iron loss due to oligodendrocyte loss (leading to counterbalance of the effect of demyelination on susceptibility, and resultant isointensity).8,9,36 The ability to measure this pathology and associated sex-based differences in living patients provides an opportunity to further explore the underlying source of variance in clinical outcomes in men versus women with MS. These sex-based differences in chronic-active WMLs may, for example, explain the increased proportion of secondary progressive phenotype MS seen in males,12 including those in our cohort. Further, the findings of this study provide more support for the notion that advanced MRI techniques, especially those at 7T, present opportunities for in vivo investigations of pathology previously only possible at autopsy. Consideration should be given towards the integration of chronic-active WML quantification by MRI into clinical trials, evaluating the effects of disease modifying therapy on chronic-active lesion development and persistence. Knowledge of the masculine predominance for WMLs with susceptibility rims on MRI, as evidenced in our study, will be informative towards the development and analysis of such clinical trials.
Although this study was performed at 7T, ultra-high field images are not required to perform analyses of magnetic susceptibility in MS. GRE techniques at 3 Tesla (3T) also demonstrate visualization of paramagnetic lesions with rims with only minimal discrepancy from 7T and allow for QSM calculation.3,5 Analyses of lesions with paramagnetic rims at 3T and 7T have confirmed a clear link between chronic-active WML burden and worsening disability scores37 - supporting the notion that measurement of chronic-active lesions, and targeting of treatments towards these lesions, may be necessary in MS care.
Symptom duration, phenotype, and lesion burden varied among the sexes in our cohort, which could be sources of confounding. However, despite the small sample size of the study, the relationship between lesions with rims and male sex was independent of the number of lesions, age, and symptom duration in our regression model. This suggests that the predisposition for men to form chronic-active WMLs in our cohort was independent of other factors. Some differences (symptom duration, phenotype distribution) in men and women present in our cohort are expected based on the known epidemiologic differences between men and women with MS.9,26 Since we hypothesize that a higher proportion of chronic-active WMLs may actually be causative of the more aggressive clinical phenotype seen in men, analyses requiring full matching of disease severity measures may filter out any such relationship. Given further weakness of a small sample size and lack of longitudinal follow up, we hope to confirm our findings in future, larger, longitudinal studies.
In an era in which precision, personalized medicine approaches are actively being sought out for most conditions, visualization of lesion rims on QSM may have promise to that end. The presence of this lesion subtype in only 46.2% of participants in this study, the overwhelming majority of which were men, suggests the possibility that this imaging methodology may aide in screening for a subset of patients with differing pathophysiology. Knowledge of the underlying biology of chronic-active lesions and WML formation in MS in men may suggest that patients with lesions with rims on QSM may respond better to medications that can target the innate immune system and/or alter immune-hormonal interactions. It is hoped that the imaging data described in this study will inspire further exploration of this hypothesis, leading to utilization of the techniques described as screening tools for further clinical studies and outcome measures for clinical trials.
Supplementary Material
Highlights.
Quantitative Susceptibility Mapping (QSM) is a susceptibility MRI technique used in MS
An outer rim on a QSM lesion may reflect chronic-active inflammation
Males are more likely to have rimmed QSM lesions than females
These radiologic findings may reflect sex-specific differences in MS pathophysiology
Acknowledgements:
We would like to thank the MRI technicians at the Kirby Center (Terri Brawner, Kathleen Kahl, and Ivana Kusevic) for their work to safely and effectively perform the MRI scans used in this analysis. We would also like to thank the study coordinators and study nurses, Julie Fiol and Kerry Naunton, without whom this work would not be possible.
Disclosures: Data acquisition was funded by grants from NIH (1K23NS072366-01A1, PI: Harrison) and EMD-Serono (PI: Harrison). Dr. Harrison is also supported by 1R01NS104403–01. Dr. Harrison has received consulting fees from EMD-Serono, Genzyme, Biogen, and Genentech.
Conflict of Interest/Role of Funding Sources:
Data acquisition was funded by grants from NIH (1K23NS072366-01A1, PI: Harrison) and EMD-Serono (PI: Harrison). Dr. Harrison is also supported by 1R01NS104403–01. Dr. Harrison has received consulting fees from EMD-Serono, Genzyme, Biogen, and Genentech.
Abbreviations:
- EDSS
Expanded Disability Status Score
- GRE
Gradient Echo
- MFIS
Modified Fatigue Impact Scale
- MP2RAGE
Magnetization Prepared Rapid Acquisition of 2 Gradient Echoes
- MPFLAIR
Magnetization-prepared Fluid Attenuated Inversion Recovery
- MRI
Magnetic Resonance Imaging
- MS
Multiple Sclerosis
- PASAT
Paced Auditory Serial Addition Test
- QSM
Quantitative Susceptibility Mapping
- SDMT
Symbol Digit Modalities Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller DH, Rudge P, Johnson G, et al. Serial gadolinium enhanced magnetic resonance imaging in multiple sclerosis. Brain. 1988;111 (Pt 4):927–939. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707–717. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Gauthier SA, Gupta A, et al. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology. 2014;271(1):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2017;133(1):25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Absinta M, Sati P, Fechner A, Schindler MK, Nair G, Reich DS. Identification of Chronic Active Multiple Sclerosis Lesions on 3T MRI. AJNR Am J Neuroradiol. 2018;39(7):1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest. 2016;126(7):2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaunzner UW, Kang Y, Zhang S, et al. Quantitative susceptibility mapping identifies inflammation in a subset of chronic multiple sclerosis lesions. Brain. 2019;142(1):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison DM, Li X, Liu H, et al. Lesion Heterogeneity on High-Field Susceptibility MRI Is Associated with Multiple Sclerosis Severity. AJNR Am J Neuroradiol. 2016;37(8):1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 2018;135(4):511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunde HMB, Assmus J, Myhr KM, Bo L, Grytten N. Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 2017;88(8):621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribbons KA, McElduff P, Boz C, et al. Male Sex Is Independently Associated with Faster Disability Accumulation in Relapse-Onset MS but Not in Primary Progressive MS. PLoS One. 2015;10(6):e0122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch M, Kingwell E, Rieckmann P, Tremlett H, Neurologists UMC. The natural history of secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81(9):1039–1043. [DOI] [PubMed] [Google Scholar]
- 13.Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain. 2013;136(Pt 12):3609–3617. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Harrison DM, Liu H, et al. Magnetic susceptibility contrast variations in multiple sclerosis lesions. J Magn Reson Imaging. 2016;43(2):463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison DM, Wang KY, Fiol J, et al. Leptomeningeal Enhancement at 7T in Multiple Sclerosis: Frequency, Morphology, and Relationship to Cortical Volume. J Neuroimaging. 2017;27(5):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas SN, Izbudak I, Frazier AA, Harrison DM. Longitudinal Persistence of Meningeal Enhancement on Postcontrast 7T 3D-FLAIR MRI in Multiple Sclerosis. AJNR Am J Neuroradiol. 2018;39(10):1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ighani M, Jonas S, Izbudak I, et al. No association between cortical lesions and leptomeningeal enhancement on 7-Tesla MRI in multiple sclerosis. Mult Scler. 2019:1352458519876037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271–1281. [DOI] [PubMed] [Google Scholar]
- 20.Lucas BC, Bogovic JA, Carass A, et al. The Java Image Science Toolkit (JIST) for rapid prototyping and publishing of neuroimaging software. Neuroinformatics. 2010;8(1):5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Hua J, Ross CA, Cai S, van Zijl PCM, Li X. Altered brain iron content and deposition rate in Huntington’s disease as indicated by quantitative susceptibility MRI. J Neurosci Res. 2019;97(4):467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444–1452. [DOI] [PubMed] [Google Scholar]
- 23.Fischer JS, Rudick RA, Cutter GR, Reingold SC. The Multiple Sclerosis Functional Composite Measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5(4):244–250. [DOI] [PubMed] [Google Scholar]
- 24.Perneger TV. What’s wrong with Bonferroni adjustments. Bmj. 1998;316(7139):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luchetti S, van Eden CG, Schuurman K, van Strien ME, Swaab DF, Huitinga I. Gender differences in multiple sclerosis: induction of estrogen signaling in male and progesterone signaling in female lesions. J Neuropathol Exp Neurol. 2014;73(2):123–135. [DOI] [PubMed] [Google Scholar]
- 26.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–532. [DOI] [PubMed] [Google Scholar]
- 27.Pozzilli C, Tomassini V, Marinelli F, Paolillo A, Gasperini C, Bastianello S. ‘Gender gap’ in multiple sclerosis: magnetic resonance imaging evidence. Eur J Neurol. 2003;10(1):95–97. [DOI] [PubMed] [Google Scholar]
- 28.Klistorner A, Wang C, Yiannikas C, Graham SL, Parratt J, Barnett MH. Progressive Injury in Chronic Multiple Sclerosis Lesions Is Gender-Specific: A DTI Study. PLoS One. 2016;11(2):e0149245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greer JM, McCombe PA. Role of gender in multiple sclerosis: clinical effects and potential molecular mechanisms. J Neuroimmunol. 2011;234(1–2):7–18. [DOI] [PubMed] [Google Scholar]
- 30.Giatti S, Caruso D, Boraso M, et al. Neuroprotective effects of progesterone in chronic experimental autoimmune encephalomyelitis. J Neuroendocrinol. 2012;24(6):851–861. [DOI] [PubMed] [Google Scholar]
- 31.Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135(1):47–58. [DOI] [PubMed] [Google Scholar]
- 32.Muller E, Kerschbaum HH. Progesterone and its metabolites 5-dihydroprogesterone and 5–3-tetrahydroprogesterone decrease LPS-induced NO release in the murine microglial cell line, BV-2. Neuro Endocrinol Lett. 2006;27(5):675–678. [PubMed] [Google Scholar]
- 33.van der Valk P, De Groot CJ. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol. 2000;26(1):2–10. [DOI] [PubMed] [Google Scholar]
- 34.Frischer JM, Weigand SD, Guo Y, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78(5):710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134(Pt 12):3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Absinta M, Sati P, Masuzzo F, et al. Association of Chronic Active Multiple Sclerosis Lesions With Disability In Vivo. JAMA Neurol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


