Introduction
The treatment and prevention of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), collectively known as keratinocyte carcinomas (KC), present a challenge for renal transplant recipients (RTRs) who develop multiple KCs annually. Patients with multiple SCCs have an increased risk of local recurrence and nodal metastasis, in addition to experiencing a significant adverse impact on their quality of life.
Several studies have evaluated the efficacy of retinoids for chemoprevention of skin cancer. Large randomized controlled trials (RCT) evaluating isotretinoin failed to show a reduction in skin cancer formation whereas data on acitretin is inconsistent. Etretinate was utilized up until the late 1990’s before being replaced by acitretin. A few small published studies showed that Etretinate had a prophylactic effect on patients with xeroderma pigmentosum and basal nevoid syndrome. Although there is a qualitative systematic review on acitretin for chemoprevention in renal transplant recipients (RTRs), it did not evaluate the pooled reduction in KCs. The present study sought to review all published literature on acitretin for KC chemoprevention, pool outcome data, and evaluate treatment costs in RTRs.
Methods/Literature Search
The Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines was followed. The review protocol was registered with PROSPERO (registration number: CRD42019129703). MEDLINE (www.nlm.nih.gov), EMBASE (www.embase.com), and CENTRAL (https://www.cochranelibrary.com/central) databases were searched on December 1, 2018 for English language studies published before the search date using the relevant terms. Articles were independently screened for eligibility by two authors (OB and ER). Eligible studies included 5 or more subjects. Studies were excluded if they did not report the number of BCCs and SCCs pre- and post-acitretin therapy, duration of follow up, or any original data. The following data were extracted: number of subjects, duration, dosing, number of BCCs and SCCs, and side-effects. The pre-treatment and post-treatment durations were formatted in years. The number of tumors and follow-up time were pooled. The annual rate of tumor development during the pre- and post-treatment periods were calculated using the period duration, number of tumors, and number of subjects. Though the source data delineated the number of BCCs and SCCs, we included the pool variable KCs to determine acitretin’s efficacy across diagnoses and to simply the cost analysis as both BCCs and SCCs are treated with same modalities.
Cost data was obtained from the National Average Drug Acquisition Cost (NADAC) data and the Massachusetts All-Payer Claims Database (APCD) Council data.4–5 Laboratory monitoring information and Mohs practice patterns were obtained from published resources. The repair costs for MMS and excision were estimated using a weighted average of each type of repair and the relative frequency of each repair in the claims data.
Chi-squared tests were used to compare the percent reduction in KC formation pre- and post-acitretin treatment. All analyses were conducted using Stata, version 12.0 (StataCorp, College Station, TX).
Results
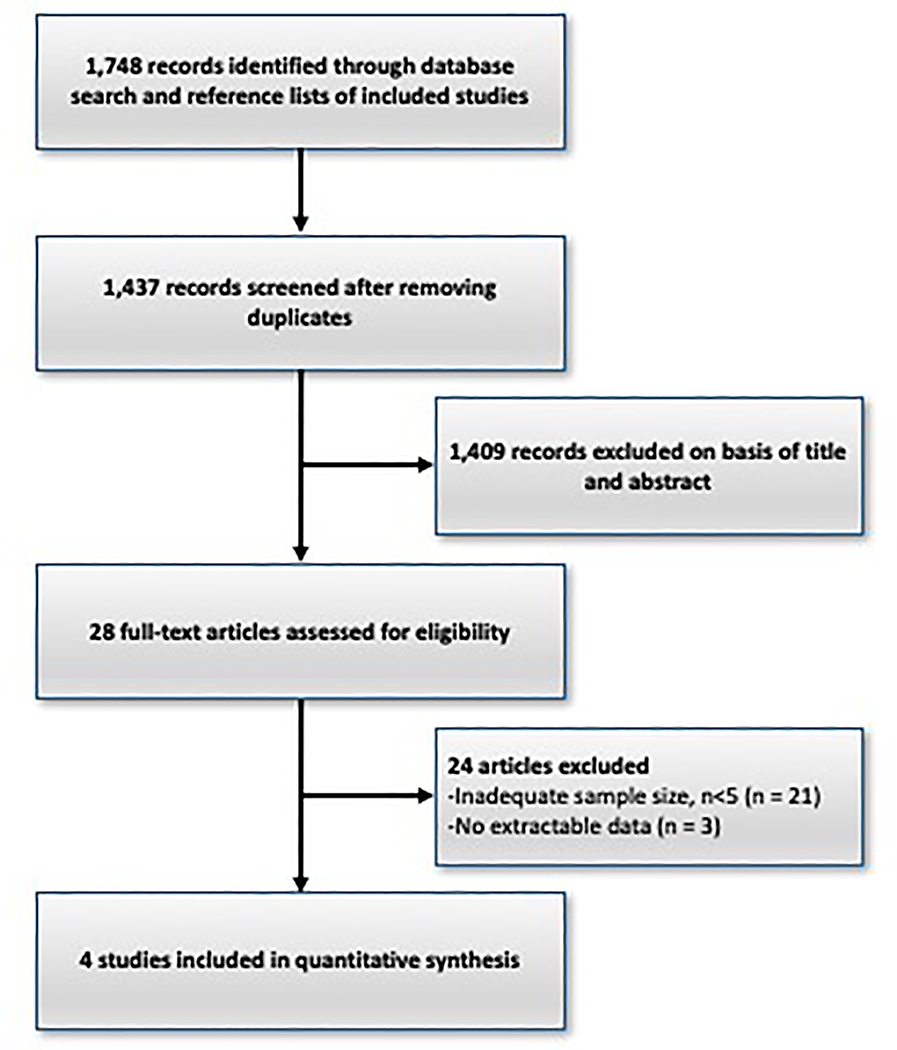
The database search identified 1,748 articles. There were 1,720 articles excluded as they did not relate to the study topic or were duplicates. The remaining 28 articles were reviewed in detail. Additional articles were excluded for the following reason: lack of information (3) and case series with fewer than 5 subjects (21).
During the mean 2.05-year pre-treatment period, 103 patients developed 37 BCCs and 232 SCCs. During the mean 1.38-year post-treatment period (range 0.5 to 3.17 years), there were 8 BCCs and 71 SCCs. This corresponded to a 73% reduction in BCC (mean: 0.10 per patient per year), 54% reduction in SCC (mean: 0.57 per patient per year), and 56% reduction in KC (mean: 0.68 per patient per year) (Table 2). There was no statistical difference in the reduction by tumor subtype (p>0.05). Nearly all patients experienced some mucocutaneous xerosis, 14 (14%) discontinued therapy, and 1 (1%) took a drug holiday.
Table 2.
Summary of number of pre- and post-acitretin SCC, BCC, and KC.
| Author (Year) | Patient (n) | Average Pre-treatment duration (years) | Average Post-treatment duration (years) | SCC | BCC | KC | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Reduction (annual) | Pre-Treatment | Post-Treatment | Reduction (annual) | Pre-Treatment | Post-Treatment | Reduction (annual) | ||||||||||||||||
| Total reported | Annually | Total reported | Annually | Abs. | Mean (per patient) | % | Total reported | Annually | Total reported | Annually | Abs. | Mean (per patient) | % | Total reported | Annually | Total reported | Annually | Abs. | Mean (per patient) | % | ||||
| Transplant | ||||||||||||||||||||||||
| Bavinck et al (1995) | 38 | 0.5 | 0.5 | 16 | 32 | 2 | 4 | 28 | 0.74 | 88 | 2 | 4 | 0 | 0 | 4 | 0.1 | 100 | 18 | 36 | 2 | 4 | 32 | 0.84 | 89 |
| McKenna et al (1999) | 16 | 3.17 | 3.17 | 100 | 31.54 | 18 | 5.68 | 25.87 | 1.62 | 82 | 11 | 3.5 | 3 | 0.95 | 2.52 | 0.16 | 73 | 111 | 35 | 21 | 6.62 | 28.39 | 1.78 | 81 |
| George et al (2002) | 23 | 5 | 2 | 88 | 17.6 | 21 | 10.5 | 7.1 | 0.31 | 40 | 21 | 4.2 | 4 | 2 | 2.2 | 0.1 | 52 | 109 | 21.8 | 25 | 12.5 | 9.3 | 0.4 | 42 |
| de Sevaux et al (2003) | 26 | 1 | 1 | 28 | 28 | 30 | 30 | −2 | −.08 | −7 | 3 | 3 | 1 | 1 | 2 | 0.08 | 67 | 31 | 31 | 31 | 31 | 0 | 0 | 0 |
| Total (weighted) | 103 | 2.05 | 1.38 | 232 | 109.14 | 71 | 50.18 | 58.97 | 0.57 | 54 | 37 | 14.7 | 8 | 3.95 | 10.72 | 0.10 | 73 | 269 | 123.8 | 79 | 54.12 | 69.69 | 0.68 | 56 |
Abbreviations: Abs, absolute; SCC, squamous cell carcinoma; BCC, basal cell carcinoma; KC, keratinocyte carcinoma
The average cost of a 25mg pill of acitretin in May 2018 was $15.02.10 Based on a daily dosing regimen, the annual prescription cost for acitretin is $5482.20 and when including $503.52 in monitoring fees the total annual cost per year was $5985.72.4–5 The average number of Mohs stages was 1.74, based on data from the APCD data. By comparison, the cost of Mohs micrographic surgery (MMS) with repair, excision of malignant lesion with repair, and ED&C is $949.36, $359.02, and $102.77, respectively (Table 3).4–5 The financial breakeven point for use of acitretin occurs when patients develop 11, 30 and 103 KC’s per year compared to only treating the tumors with MMS, excision and ED&C, respectively. This analysis does not factor other important factors such as the morbidity associated with surgery, patient preferences, and indirect costs (e.g. opportunity costs).
Table 3.
Summary of Acitretin and surgical costs.
| Reference | Description | Cost |
|---|---|---|
| Acitretin Related Costs | ||
| APCD 2 | E/M | $58.20 |
| APCD 2 | CBC | $10.03 |
| APCD 2 | LFT | $26.97 |
| APCD 2 | Lipid panel | $20.14 |
| APCD 2 | BMP | $9.09 |
| NADAC 1 | Acitretin 25mg pill (U.S. as of May 2018) | $15.02 |
| Canadarxconnection.com | Acitretin 25mg pill (International as of May 2018) | $1.50–$4.00 |
| Surgical Costs | ||
| APCD 2 | Average Mohs micrographic surgery (MMS) first stage | $392.33 |
| APCD 2 | Average Mohs micrographic surgery (MMS) additional stage | $338.32 |
| APCD 2 | Average excision of malignant lesion | $156.16 |
| APCD 2 | Average intermediate/complex repair | $202.86 |
| APCD 2 | Average flap/graft repair | $634.75 |
| APCD 2 | Electrodessication and curettage (ED&C) | $102.77 |
Abbreviations: APCD, All-Payer Claims Database; E/M, evaluation and management; CBC, complete blood count; LFT, liver function test; BMP, basic metabolic panel
Discussion
The analysis presented found a reduction in BCCs (73%) in addition to SCCs (54%). These findings support most of the smaller published studies regarding SCC and also demonstrate efficacy for BCC reduction.
Despite the efficacy demonstrated herein of acitretin for KC chemoprevention, the medication is likely underutilized for a few reasons. The first is the direct cost of the medication and associated monitoring. Based on a 25mg daily dosing regimen, the annual cost is $5985.72 in the United States (U.S.), which increased 157.5% from 2000–2008 and continued to increase until 2013.3 Based on the same 25mg daily dosing regimen, the annual prescription cost in Canada is $547.50 and the total annual cost with monitoring is $1051.02, which is almost 600% lower than in the U.S. There are other factors that impede acitretin utilization such as the need for frequent lab studies, bothersome side effects, and rebound after cessation of efficacious therapy.
Conclusion
Although this study is subject to limitations due to variability in study characteristics (i.e. follow-up time, and variable dosing), generalizability (i.e. non-RTRs), and cost data (i.e. indirect costs and cost saving due to a reduction in metastasis and actinic keratoses), the data shows that acitretin is efficacious for chemoprevention of both BCC and SCC. Although the cost of acitretin has been decreasing since 2013, it is still 4 to 10 times higher than in other countries and is a barrier to providing appropriate care to patients most in need of skin cancer chemoprevention.
Figure 1.
Flow chart of studies included in the systematic review.
This figure summarizes the literature search methodology based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria.
Table 1.
Summary of studies included in the systematic review.
| Reference | Publication Year | Study Design | Quality of Evidence‡ | Inclusion criteria | Medium FU (Range), Months | No. of Cases | Dose | Patient Population |
|---|---|---|---|---|---|---|---|---|
| Bavinck et al.4 | 1995 | RCT | 1 | 10+ keratotic lesions | 6 | 38 (n=19 in each arm) | 30mg QD | Renal Transplant |
| McKenna et al.5 | 1999 | Prospective | 4 | 2+ KC | 38 | 16 | 0.3mg/kg/day | Renal Transplant |
| George el al.6 | 2002 | Randomized cross over trial | 1 | 3+ KC or 10 actinic keratoses | 24 | 23 | 25mg QD or QOD | Renal Transplant |
| de Sevaux et al.7 | 2003 | RCT using 2 different doses | 1 | 1+ KC with 10+ actinic keratoses | 12 | 26 | 0.4mg/kg/d (n=14) or 0.4 × 3 months -> 0.2mg/kg/d × 9 months (n=12) | Renal Transplant |
Abbreviations: FU: follow up; RCT: randomized controlled trial; KC: keratinocyte carcinoma; No.: number; d: day; QD: one a day; QOD: one every other day; Wk: week
Quality of evidence assessed using the Quality Rating Scheme for Studies and Other Evidence14: 1) Properly powered and conducted randomized clinical trial or systematic review with meta-analysis; 2) Well-designed controlled trial without randomization or prospective comparative cohort trial; 3) Case-control studies or retrospective cohort study; 4) Case series with or without intervention or cross-sectional study; and 5) Opinion of respected authorities or case reports.
Acknowledgments
Funding sources: Mr. Karia is supported by the Cancer Epidemiology, Prevention, and Control Training Grant (NCI T32 CA009314).
Footnotes
Conflicts of Interest: None declared.
IRB approval status: This study was exempted from IRB review.
Reprint requests: Emily Stamell Ruiz
Attachments: None
References
- 1.Centers for Medicare and Medicaid National Average Drug Acquisition Cost (NADAC) Files. https://data.medicaid.gov/Drug-Pricing-and-Payment/NADAC-National-Average-Drug-Acquisition-Cost-/a4y5-998d/data. Accessed December 29, 2018.
- 2.All-Payer Claims Database (APCD) Council Files. https://www.apcdcouncil.org/. Accessed December 29, 2018.
- 3.Beyer V, Wolverton SE. Recent Trends in Systemic Psoriasis Treatment Costs. JAMA Derm. 2010; 146(1):46–45. [DOI] [PubMed] [Google Scholar]
- 4.Bavinck JN, Tieben LM, Van der Woude FJ, Tegzess AM, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol 1995; 13(8):1933–1938. [DOI] [PubMed] [Google Scholar]
- 5.McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol 1999; 140(4):656–660. [DOI] [PubMed] [Google Scholar]
- 6.George R, Weightman W, Russ GR, Bannister KM, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol 2002; 43(4):269–273. [DOI] [PubMed] [Google Scholar]
- 7.de Sévaux RG, Smit JV, de Jong EM, van de Kerkhof PC, et al. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol 2003; 49(3):407–412. [DOI] [PubMed] [Google Scholar]