Abstract
Pre-harvest autologous blood collection from bone marrow (BM) donors is performed to meet potential post-operative transfusion needs. This study examines the impact of autologous blood transfusion on BM donor’s health and safety. The study included first-time unrelated BM donors from the United States whose BM harvest was facilitated by the National Marrow Donor Program (NMDP) centers between 2006 and 2017. Examination of 7,024 BM donors revealed that 60% received at least 1 unit of autologous blood. The donors who received autologous blood were older, had lower hemoglobin pre-harvest, underwent longer duration of anesthesia and higher volume BM harvest. Only donors who underwent high volume BM harvest, defined as a BM harvest volume > 27% of donor’s blood volume, benefited from autologous transfusion. After a high-volume BM harvest, autologous blood transfusion was shown to decrease grade 2 to 4 collection-associated toxicities within 48 hours of BM donation (p=0.010) and shorten the time to donor-reported “complete” recovery from donation associated symptoms (p<0.001). Therefore, autologous transfusion could be avoided as support of marrow donation in the majority of unrelated BM donors and should be limited to cases where the planned BM harvest volume is expected to exceed 27% of donor’s blood volume.
Keywords: Autologous blood, unrelated donor, bone marrow harvest
INTRODUCTION
Pre-harvest autologous blood collection from healthy bone marrow (BM) donors is performed to meet potential post-operative transfusion needs and minimize the likelihood of allogeneic blood transfusion. In the 1980s, due to increases in the number of transfusion-transmitted diseases, pre-operative collection of 1 to 3 units of autologous blood was recommended by National Marrow Donor Program (NMDP) to minimize the chance that a donor would require transfusion of allogeneic blood 1–3. The number of autologous blood units banked was based on the donor’s hemoglobin and the expected amount of BM to be collected. Despite safer allogeneic transfusions over the past several decades 4,5, collection of autologous blood prior to BM harvest remains a common practice in many US collection centers.
Although transfusion of autologous blood has several advantages over allogeneic blood, including avoidance of transfusion-transmitted diseases 6,7, it is not free from risk 8. Autologous blood collection increases the risk of peri-operative anemia and may increase the need for autologous and/or allogeneic transfusions after BM harvest. Availability of autologous blood may also lead to clinicians over-transfusing at hemoglobin levels above the recommended threshold, which may add unnecessary risks to donors. Bacterial contamination of blood product, misidentification of blood unit at the time of transfusion due to clerical errors and transfusion associated circulatory overload have been seen as commonly with autologous blood as with allogeneic blood transfusions 9–11. The cost of collecting, processing and storage of an autologous blood unit is significantly higher than an allogeneic unit 12,13. In addition, there is a possibility that a large portion of autologous blood remains unused post-procedure and discarded leading to further wasting of resources. These disadvantages have led to a decrease in autologous blood collection in Europe and US 14,15
To date, only a few studies have evaluated the efficacy of autologous blood collection and transfusion in donors undergoing BM harvests 16–19. Those single center studies have been limited by small sample sizes, and there remains an unanswered question whether or not healthy marrow donors benefit from collection and transfusion of autologous blood prior to and following the BM harvest procedure. This study examined variables associated with autologous blood transfusion with an aim to evaluate the impact of autologous blood transfusion on donor health and safety after BM harvest.
METHODS
Study population
The study population included first-time unrelated BM donors from the US whose non-mobilized BM harvest was facilitated by NMDP centers between 2006 and 2017. Due to small numbers (n=25), donors who received an allogeneic blood transfusion post-collection were also excluded from analysis. All donors included in this study provided written informed consent for participation in Center for International Blood and Marrow Transplant Research (CIBMTR) studies approved by the NMDP Institutional Review Board.
Bone Marrow Donation
NMDP has established the acceptable standards for all aspects of unrelated BM donor care including recommendations about the eligibility criteria and evaluation of donor health to ensure donor safety before, during and after the stem cell donation. BM collection from the donor’s posterior iliac crests was performed in an operating room under general or regional anesthesia. Based on the NMDP guidelines, the intended volume of marrow was limited to no more than 20 mL/Kg of donor’s weight. In addition, the duration of anesthesia was limited to less than 150 minutes and the duration of the collection itself less than 120 minutes.
Before 2016 donor centers were advised by NMDP to collect 1 to 3 autologous blood units from the donor prior to the expected marrow volume collection. Since 2016, the practice has been at the discretion of the collection facility. The majority of donors will have the baseline CBC before an autologous blood collection. However, there are rare cases where the autologous blood collection is done before the physical exam to better accommodate donor schedules. Abnormal lab results are addressed by NMDP on a case-by-case basis and donor and harvest centers are queried if results entered on the forms are outside a validated range.
Data Collection
Data collection started at the donor’s medical evaluation to determine donor’s suitability and continued throughout the BM donation process, 2 days, 1 month and 6 months after donation. In addition, donors were contacted by donor centers 2 days after BM donation and weekly thereafter until complete recovery of donation associated symptoms.
Outcomes
The primary objective of this study was to evaluate donor symptoms associated with the BM harvest procedure and to evaluate the impact of autologous blood transfusion on donor health after BM harvest. Additional objectives were to describe the donor and BM collection variables between the two cohorts (those who received autologous blood transfusion versus those who did not receive autologous blood transfusion). Donor toxicities were assessed using the toxicity criteria modeled on National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE V.4). Those symptoms included the incidence of grade 2 to 4 or grade 3 to 4 skeletal pain and the highest toxicity level grade 2 to 4 or grade 3 to 4 across selected body symptoms 2 days, 1 month and 6 months after BM donation. Skeletal pain was defined as pain in at least 1 site including back, bone, headache, hip, limb, joint or neck. The severity of skeletal pain was defined as the maximum grade of pain among these sites. Toxicity was defined as fever in the absence of signs of infection, fatigue, skin rash, local reactions, nausea, vomiting, anorexia, dizziness, syncope and insomnia. The severity of toxicity was defined as the maximum grade of symptoms among these sites. This method of assessing donor toxicity has been extensively validated in previous studies 20.
The secondary endpoint included time to recovery which is defined as time from BM donation to complete recovery of donation associated symptoms reported by the donor. Donor centers called the donor weekly to determine if donation associated symptoms resolved and they had returned to baseline21.
Statistical Methods
The NMDP data collection donor forms do not capture whether the donor underwent collection of autologous blood, but instead records whether the donor received either an autologous or allogeneic blood transfusion. Therefore, all of the analysis was stratified based on whether or not the donors received an autologous blood transfusion after BM harvest. Using descriptive statistics, the number (percentage) of donors who received autologous blood transfusion was quantified, and the donor and harvest variables among those who received an autologous blood transfusion versus those who did not receive an autologous blood transfusion were described.
The volume of marrow collected was expressed as a percentage of donor’s total blood volume. Total blood volume (mL) was calculated using Nadler’s equation22:
Based on donation volume expressed as a percentage of donor’s total blood volume, donors were grouped into 4 categories according to quartiles of the data: <15%, 15-22%, >22-27%, >27%. For the purpose of this study, high volume donation was defined as BM donation volume more than 27% of donor’s blood volume. Variables were compared between the cohorts using the Pearson χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. To evaluate the impact of autologous blood transfusion on donor outcomes, logistic regression was used to compare the cohorts for skeletal pain and acute toxicities frequently associated with BM harvest. Stepwise variable selection with a significance level of ≤0.01 was used to identify variables to be included in the model. Autologous blood transfusion was forced into the final stepwise logistic regression model as the primary variable of interest. Interactions between autologous blood transfusion and significant variables were tested. Center effect for all outcomes based on the generalized linear mixed model was tested. There was a significant center effect on toxicities within 2 days and pain 2 days, 1 month and 6 months after BM harvest. Generalized estimating equation with logit link function was used to adjust for the center effect on these outcomes.
The Cox proportional hazards model was fitted to model complete recovery from donation. Stepwise selection with a significance level 0.01 was used to select significant variables. Adjusted probabilities of complete recovery from donation were calculated based on the final Cox model23.
Results
Characteristics of bone marrow donors
A total of 7,024 BM donors between 2006 and 2017 were examined. The baseline demographics and collection characteristics are summarized in Table 1. The majority of the donors (60%) received at least 1 unit of autologous blood transfusion. Donors who received autologous blood transfusion were older (median age 31 years) compared to donors who did not (median age 30 years). There were more male donors in the autologous blood transfusion cohort (63% male and 37% female) compared to the cohort without autologous blood transfusion (60% male and 40% female). Donors who received autologous transfusion underwent larger volume BM harvests (25% vs. 15% of donors’ blood volume) and longer duration of anesthesia (98 minutes vs. 80 minutes). In those with available data (n= 3531), the product total nucleated cell dose per kg recipient weight was lower in autologous transfusion cohort compare to the cohort without autologous transfusion (5.6 x108/kg vs. 4.0 x108/kg, respectively). As expected, a decline in transfusion of autologous blood was noted over the past years, especially from 2016 on, when the practice was made optional by NMDP.
Table 1.
Demographic and collection characteristics for first time United States BM donors between 2006 and 2017
| Variable |
No Autologous Blood Transfusion N (%) |
Autologous Blood Transfusion N (%) |
p-value |
|---|---|---|---|
| Number of donors | 2813 | 4211 | 0.001 |
| Autologous blood units transfused | |||
| 1 | 0 | 2957 (70) | |
| 2 | 0 | 1236 (29) | |
| 3 | 0 | 18 (<1) | |
| Donor age at donation | 0.001 | ||
| 18 to 29 | 1408 (50) | 1926 (46) | |
| 30 to 39 | 784 (28) | 1215 (29) | |
| 40 to 49 | 471 (17) | 791 (19) | |
| 50+ | 150 (5) | 279 (7) | |
| Median (Range) | 30 (19-61) | 31 (19-61) | <0.001 |
| Sex | 0.002 | ||
| Female | 1132 (40) | 1539 (37) | |
| Male | 1681 (60) | 2672 (63) | |
| Race | 0.005 | ||
| Caucasian | 1755 (62) | 2735 (65) | |
| Hispanic | 381 (14) | 547 (13) | |
| Black / African American | 194 (7) | 301 (7) | |
| Asian / Pacific Islander | 189 (7) | 226 (5) | |
| American Indian / Alaska Native | 34 (1) | 37 (1) | |
| Other / Multiple Race | 250 (9) | 328 (8) | |
| Decline / Unknown | 10 (<1) | 37 (1) | |
| Donor Body Mass Index BMI | 0.001 | ||
| Underweight (<18.5) | 20 (1) | 18 (<1) | |
| Normal (18.5-24.9) | 967 (34) | 1325 (31) | |
| Overweight (25-29.9) | 953 (34) | 1605 (38) | |
| Obese (≥30) | 872 (31) | 1263 (30) | |
| Unknown | 1 (N/A) | 0 (N/A) | |
| Donor Weight, kg | |||
| Median (Range) | 81 (42-155) | 82 (40-164) | 0.026 |
| Hemoglobin at baseline, g/dL | |||
| N Eval | 2813 | 4211 | |
| Median (1st to 99th percentile) | 15 (11.1-17.4) | 15 (11.5-17.3) | 0.631 |
| Hemoglobin pre-BM harvest, g/dL | |||
| N Eval | 2775 | 4154 | |
| Median (1st to 99th percentile) | 14 (10.6-17.2) | 14 (9.9-16.3) | <0.001 |
| Hemoglobin post-BM harvest, pre-transfusion, g/dL | |||
| N Eval | 0 | 1081 | |
| Median (1st to 99th percentile) | 11 (7-15) | ||
| Hemoglobin post-BM harvest, post-transfusion, g/dL | |||
| N Eval | 0 | 3055 | |
| Median (1st to 99th percentile) | 11 (8-15) | ||
| Hemoglobin post-BM harvest, no transfusion, g/dL | |||
| N Eval | 2471 | 0 | |
| Median (1st to 99th percentile) | 12 (8-15) | ||
| Collection volume per kg of donor weight | <0.001 | ||
| <10 mL/kg | 1540 (55) | 550 (13) | |
| 10 to <15 mL/kg | 729 (26) | 1453 (35) | |
| 15 to <20 mL/kg | 391 (14) | 1720 (41) | |
| ≥ 20 mL /kg | 130 (5) | 442 (11) | |
| Unknown | 23 (N/A) | 46 (N/A) | |
| Collection volume per donor volume, % | <0.001 | ||
| N Eval | 2791 | 4165 | |
| Median (Range) | 15 (2-45) | 25 (5-46) | |
| TNC in the product (x108) | <0.001 | ||
| N Eval | 2800 | 4188 | |
| Median (1st to 99th percentile) | 175 (47-474) | 260 (106-525) | |
| TNC in the product per kg recipient weight (x108) | <0.001 | ||
| N Eval | 1394 | 2150 | |
| Median (1St to 99th percentile) | 5.6 (1.4-30.1) | 4.0 (1.4-21.7) | |
| Type of anesthesia | |||
| Epidural | 16 (<1) | 16 (<1) | <0.001 |
| General | 2729 (97) | 4155 (99) | |
| Local | 6 (<1) | 3 (<1) | |
| Spinal | 61 (2) | 35 (<1) | |
| Duration of anesthesia in minutes | <0.001 | ||
| N Eval | 1515 | 3018 | |
| Median (Range) | 80 (25-216) | 98 (25-217) | |
| Duration of collection in minutes | <0.001 | ||
| N Eval | 1524 | 3051 | |
| Median (Range) | 37 (4-210) | 57 (2-221) | |
| Year of collection | <0.001 | ||
| 2006 | 139 (26.6%) | 330 (70.4%) | |
| 2007 | 124 (26.1%) | 352 (73.9%) | |
| 2008 | 157 (30.4%) | 359 (69.6%) | |
| 2009 | 168 (33.5%) | 333 (66.5%) | |
| 2010 | 179 (33.3%) | 358 (66.7%) | |
| 2011 | 204 (34.6%) | 385 (65.4%) | |
| 2012 | 267 (36.8%) | 459 (63.2%) | |
| 2013 | 264 (37.7%) | 436 (62.3%) | |
| 2014 | 262 (39.5%) | 402 (60.5%) | |
| 2015 | 258 (43.7%) | 332 (56.3%) | |
| 2016 | 387 (60.8%) | 250 (39.2%) | |
| 2017 | 404 (65.3%) | 215 (34.7%) |
Hemoglobin concentrations peri-BM collection
Peri-collection hemoglobin (Hb) concentrations based on donor’s gender are summarized in Figure 1, Supplemental table 1. Median Hb concentration at baseline was similar between the two groups (p 0.631). However, immediately before BM harvest, the Hb concentration was lower in donors who later received autologous transfusion (12.0 g/dL in female donors and 14.2 g/dL in male donors) than in those who did not (13.0 g/dL in female donors and 15.0 g/dL in male donors). Among donors who received autologous blood transfusion, the post-marrow collection Hb before and after the transfusion came from 2 mutually exclusive subsets of the donors, depending on whether the post-collection CBC was obtained before or after the transfusion. Among the 4,211 donors who received autologous transfusion, Hb concentration was available for 1,080 donors prior to blood transfusion and 3,055 donors after blood transfusion. After BM harvest and transfusion, the Hb concentration remained slightly lower in donors who received at least 1 unit autologous blood transfusion (10.1g/dL in female donors and 12.1 g/dL in male donors) compared to donors who did not receive autologous blood transfusion (10.7g/dL in female donors and 12.8 g/dL in male donors).
Figure 1:
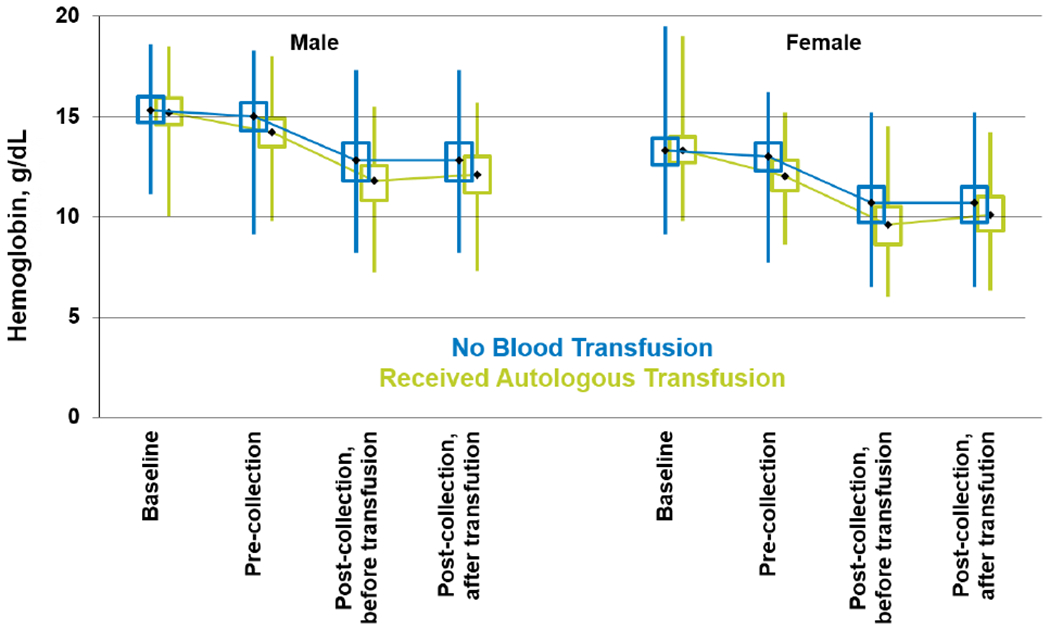
Donors peri-collection hemoglobin (Hb) concentrations based on donor gender (Note: Among donors who received blood transfusion, the post-marrow collection CBC before vs. after the transfusion came from 2 mutually exclusive subsets of the population, depending on whether the post-collection CBC was obtained before or after the transfusion. The before and after transfusion data for the “No Blood Transfusion” group are the same data because these donors did not receive blood transfusion.)
Peri-collection Hb concentrations stratified based on volume of BM harvest are shown in Figure 2. The volume of BM harvested is expressed as a percentage of donor’s blood volume. Based on the percentage of the donor’s blood volume collected during BM harvest, donors were divided into 4 categories based on quartiles; <15%, 15%-22%, 23%-27% and >27%. Immediately before BM harvest, the median Hb concentration was lower in donors who received autologous blood transfusion than those who did not. As expected, the larger volume BM harvest led to a greater decline in the Hb level. Immediately after BM harvest (prior to autologous blood transfusion), there was no significant difference in Hb concentrations in donors who had autologous transfusion and the ones who did not in each of the categories.
Figure 2:
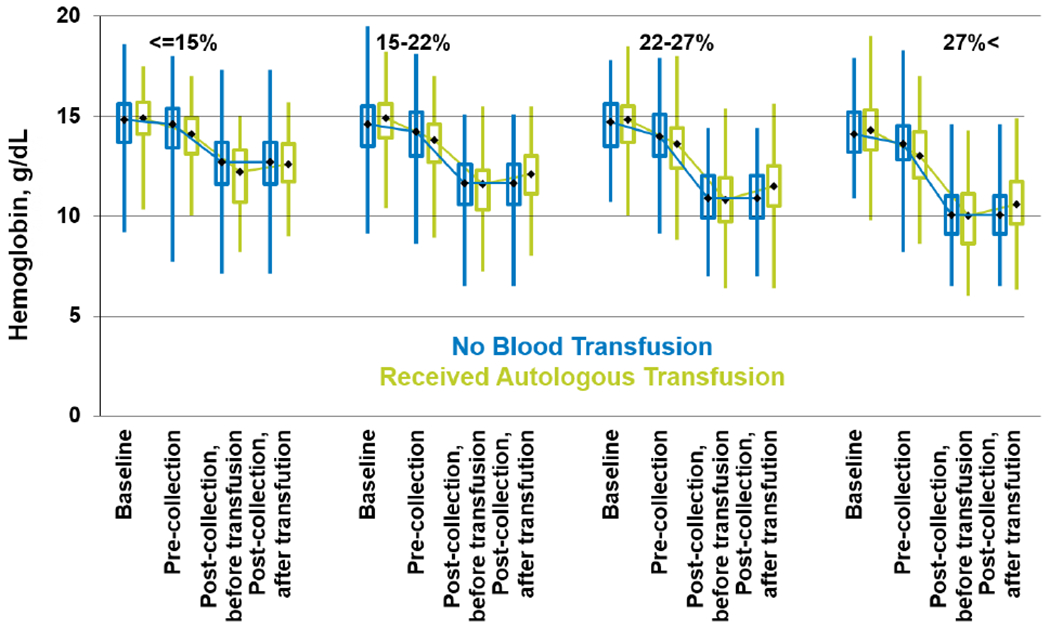
Peri-collection donors Hb concentrations stratified based on volume of BM harvest. Volume of bone marrow harvested is expressed as percentage of donor’s blood volume. Note: Among donors who received blood transfusion, the post-marrow collection CBC before vs. after the transfusion came from 2 mutually exclusive subsets of the population, depending on whether the post-collection CBC was obtained before or after the transfusion. The before and after transfusion data for the “No Blood Transfusion” group are the same data because these donors did not receive blood transfusion.)
Pain and toxicity experiences in BM donors
Table 2 shows the time course and extent of toxicities experienced by BM donors based on whether or not the donors received autologous blood transfusions. At baseline, skeletal pain and other donation associated toxicities were comparable among the two groups. In multivariate analysis, there were no significant differences in grade 2 to 4 toxicities within 48 hours after BM donation between cohorts who did or did not receive autologous blood transfusion. Female gender (p < 0.0001), larger collection volume (p< 0.0001) and longer duration of anesthesia (p < 0.0001) were associated with an increased risk of grade 2 to 4 toxicities.
Table 2.
Multivariate analysis: Pain and toxicities experiences by donors after bone marrow harvest
| N | OR | OR Lower CI | OR Upper CI | P-value | |
|---|---|---|---|---|---|
| Highest toxicity level of key symptoms grade 2 to 4, 2 days after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 2813 | 1 | 0.2708 | ||
| Yes | 4209 | 0.8663 | 0.671 | 1.1184 | 0.2708 |
| Donor Sex | |||||
| Female | 2670 | 1 | < 0.0001 | ||
| Male | 4352 | 0.4441 | 0.3857 | 0.5114 | < 0.0001 |
| Collection volume per donor blood volume as a % (Nadler’s) | |||||
| <= 15 | 1706 | 1 | < 0.0001 | ||
| 15 - 22 | 1782 | 1.3637 | 1.1682 | 1.5919 | < 0.0001 |
| 22 - 27 | 1581 | 1.6021 | 1.2417 | 2.0671 | 0.0003 |
| 27 < | 1883 | 1.8289 | 1.4685 | 2.2777 | < 0.0001 |
| Missing | 70 | 1.5262 | 0.8675 | 2.6848 | 0.1424 |
| Duration of anesthesia in minutes | |||||
| <= 74 | 1142 | 1 | < 0.0001 | ||
| 74 - 91 | 1178 | 1.0999 | 0.8626 | 1.4024 | 0.4426 |
| 91 - 115 | 1113 | 1.347 | 1.0579 | 1.7152 | 0.0157 |
| 115 < | 1099 | 1.901 | 1.4821 | 2.4384 | < 0.0001 |
| Missing | 2490 | 1.389 | 1.0596 | 1.8209 | 0.0173 |
| Bone pain grade 2 to 4, 2 days after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 2813 | 1 | 0.596 | ||
| Yes | 4211 | 1.1522 | 0.9943 | 1.3352 | 0.596 |
| Donor sex | |||||
| Female | 2671 | 1 | <0.0001 | ||
| Male | 4353 | 0.645 | 0.5798 | 0.7176 | <0.0001 |
| Duration of collection in minutes | |||||
| ≤36 | 1171 | 1 | <0.0001 | ||
| 36-50 | 1132 | 1.4904 | 1.2848 | 1.729 | <0.0001 |
| 50-70 | 1162 | 1.8447 | 1.515 | 2.2461 | <0.0001 |
| >70 | 1108 | 2.4433 | 1.8881 | 3.1618 | <0.0001 |
| Missing | 2451 | 1.5126 | 1.2163 | 1.8811 | 0.0002 |
| Number of marrow harvests/year at collection centers | |||||
| ≤ 7 | 1643 | 1 | 0.0011 | ||
| 7-15 | 1677 | 0.8562 | 0.6882 | 1.0654 | 0.1639 |
| 15-45 | 1510 | 0.6363 | 0.4842 | 0.8363 | 0.0012 |
| > 45 | 1575 | 0.753 | 0.6415 | 0.8840 | 0.0005 |
| Missing | 619 | 0.9402 | 0.7082 | 1.2482 | 0.6697 |
| Bone pain grade 2 to 4, 1 month after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 2590 | 1 | 0.459 | ||
| Yes | 3953 | 1.1733 | 0.7686 | 1.7911 | 0.459 |
| Bone pain grade 2 to 4, 6 months after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 2402 | 1 | 0.4574 | ||
| Yes | 3681 | 0.8415 | 0.5338 | 1.338 | 0.4574 |
| Donor age at bone marrow collection | |||||
| 18 to 29 | 2838 | 1 | <0.0001 | ||
| 30 to 39 | 1730 | 1.3264 | 0.8901 | 1.9765 | 0.1652 |
| 40 to 49 | 1130 | 2.5104 | 1.7105 | 3.6844 | <0.0001 |
| 50+ | 385 | 2.3874 | 1.2908 | 4.4157 | 0.0055 |
Grade 2 to 4 skeletal pain within 48 hours of donation, 1 and 6 months after donation were also comparable between the two cohorts. Women were more likely to experience pain compared with men in the early post-donation period (p< 0.001). Longer duration of BM harvest was also independently associated with grade 2 to 4 pain within 48 hours of BM harvest (p<0.0001). In addition, older donors were at higher risk for persistent grade 2 to 4 pain at 6 months after BM donation (p < 0.0001).
Pain and toxicity experiences after high volume donation
For the purpose of this study, high volume donation was defined as BM donation volume more than 27% of donor’s blood volume. A collected BM volume equal or greater than 20 mL/kg of donor body weight was found to translate into at least 27% of total blood volume in 99% of cases. BM volume of 15-20 mL/kg was equal to 27% of donor’s blood volume in 60% of the donors (supplemental table 2).
The majority of donors (1528 of 1853, 81.5%) who underwent high volume BM harvest received at least one unit of autologous blood transfusion. Fatigue and insomnia were the most common complaints, with more fatigue (59.0% vs. 56%) and insomnia (11.1% vs. 8.7%) noted in BM donors who did not receive transfusion (Supplemental table 3). Multivariate analysis of the impact of autologous blood transfusion on donation-associated pain and toxicities after high volume BM harvest are shown in Table 3. Donors who received autologous blood transfusion were less likely to experience grade 2 to 4 donation-associated toxicities within 48 hours of BM donation (p= 0.010). However, there were no differences in grade 2 to 4 donation-associated pain within 48 hours, 1 month and 6 months after BM harvest based on whether or not the donor received autologous blood transfusion. Autologous blood transfusion did not impact donation-associated toxicities and pain in donors who underwent donation of volumes less than 27% of their blood volume (Supplemental table 4).
Table 3.
Outcomes of donors who underwent high volume bone marrow harvest (volume greater than 27% of donor blood volume). Nadler’s equation was used to estimate the donor blood volume
| N | OR | OR Lower CI | OR Upper CI | p_value | |
|---|---|---|---|---|---|
| MTC grade 2 to 4, 2 days after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 355 | 1 | 0.01 | ||
| Yes | 1528 | 0.7267 | 0.5701 | 0.9265 | 0.01 |
| Bone pain grade 2 to 4, 2 days after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 355 | 1 | 0.8273 | ||
| Yes | 1528 | 1.0282 | 0.8006 | 1.3205 | 0.8273 |
| Bone pain grade 3 to 4, 2 days after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 355 | 1 | 0.3048 | ||
| Yes | 1528 | 0.56 | 0.185 | 1.6949 | 0.3048 |
| Bone pain grade 2 to 4, 1-month after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 332 | 1 | 0.0411 | ||
| Yes | 1430 | 0.5132 | 0.2706 | 0.9733 | 0.0411 |
| Bone pain grade 2 to 4, 6-months after BM harvest | |||||
| Autologous blood transfusion | |||||
| No | 298 | 1 | 0.9819 | ||
| Yes | 1348 | 0.989 | 0.3811 | 2.5665 | 0.9819 |
Probability of complete recovery after BM donation
Multivariate analysis of probability of donor-reported “complete” recovery after BM harvest based on BM collection volume is shown on supplemental table 5. Autologous blood transfusion did not have an impact on the time to complete recovery of donation associated symptoms after low volume harvest (BM collection less than 15% of donors’ blood volume, p 0.308) and intermediate volume harvests (BM collection volume 15%-22% and 22%-27% of donors’ blood volume, p 0.561 and p 0.059 respectively). However, after high volume BM harvests (> 27% of donors’ blood volume), donors who received autologous transfusion were more likely to recover within the first 2 weeks of BM harvest compared to the donors who did not receive autologous blood transfusion (p < 0.0001) (Figure 3). The median time to complete recovery was 21 days and 26 days in the cohort with and without autologous blood transfusion after high volume harvest, respectively.
Figure 3.
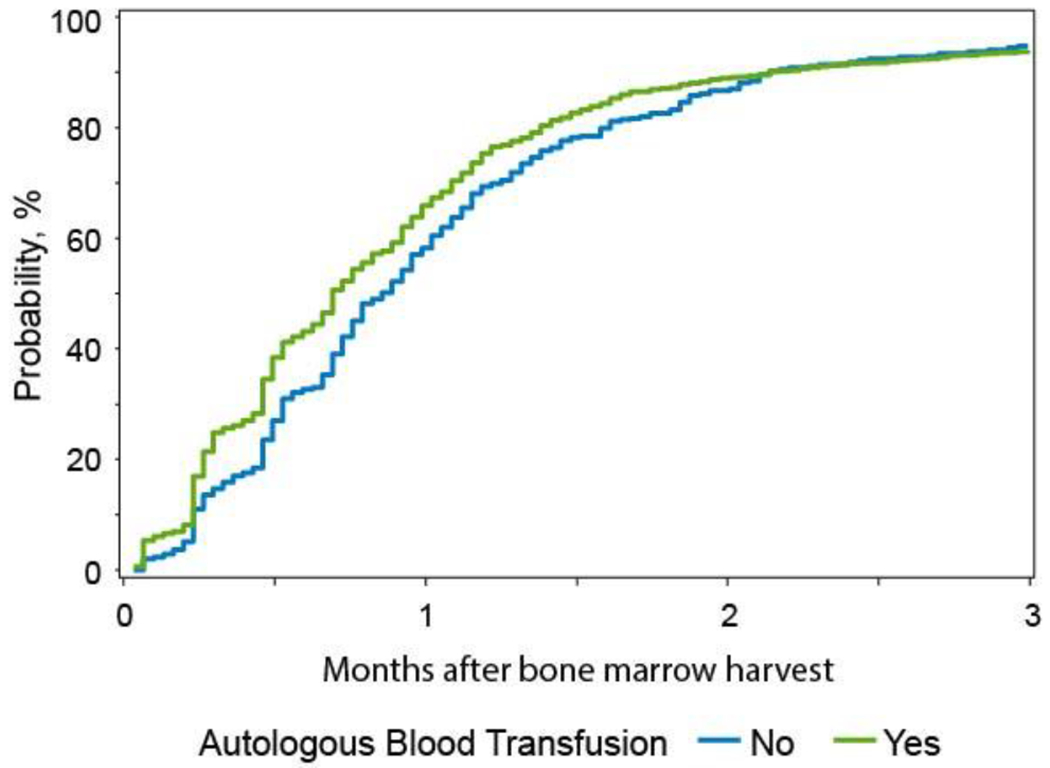
Multivariate cumulative incidence of donors’ reported complete recovery from BM donation after high volume bone marrow (BM) harvest (BM collection volume > 27% of donors’ blood volume)
Other characteristics that significantly impacted the time to complete recovery were donor gender, donor age and duration of BM harvest. More specifically, male gender, younger age and shorter duration of BM collection procedure were associated with faster time to donor recovery.
Discussion
Over the past decade, there have been increasing data calling into question the rationale, safety and cost-effectiveness of routine preoperative autologous blood collection before BM harvest 16–18,24,25. Most of those studies were relatively small single center reports with methodology used to assess donor health and safety varying widely, with no use of CTCAE elements or standardized pain scales. The World Marrow Donor Association (WMDA) and NMDP also raised questions about this practice and currently do not make specific recommendations regarding autologous blood collection prior to BM harvest26. Therefore, the collection and transfusion of autologous blood in the US is at discretion of the collection center physician and/or the collection center preferences. This has resulted in a wide variation in collection center practices.
This study is the first large multicenter study to compare unrelated donor experiences and health outcomes based on whether or not the donor received an autologous blood transfusion after BM harvest. Our study shows that more than half of BM donors received autologous blood transfusion, although there was a steady decline over time, which could represent a growing skepticism in the value of the practice. We observed a lower median Hb concentration prior to BM harvest in donors who received autologous blood transfusion compared to those who did not. Although, the time interval between autologous blood collection and BM harvest is not available in this study, lower Hb concentrations in donors prior to BM harvest who had autologous blood transfusion may be reflective of insufficient time between the autologous blood collection and the BM harvest for Hb levels to recover. If pre-procedure autologous blood collection is performed, ideally, an adequate time-interval between autologous blood collection and BM harvest to allow for a resolution of anemia should be planned, but due to the expediency of the needs of the recipient, this may not always occur. Based on prior studies, it takes at least 20 to 30 days from the first appearance of erythroid progenitors in the BM to the appearance of mature red blood cells in the peripheral blood 27. In a randomized clinical trial of 215 healthy blood donors, the median time to 80% Hb recovery after donation of one unit of blood for participants taking iron supplements was 32 days and for those not taking iron was longer than 78 days (p <0 .001)28. In a recent survey evaluating transfusion practices for BM harvest, among the centers that routinely collect autologous blood, 42% indicated collection of blood within 3–7 days of the BM harvest, which is a clearly inadequate time for sufficient red cell recovery29. Although longer intervals would allow for better Hb recovery between blood donations and BM harvest, this may also pose a problem when BM harvest is postponed, leading to expiration of the stored blood units.
Among the 1,081 donors with available pre- transfusion Hb levels, the median Hb concentration prior to autologous blood transfusion was 10.9 g/dL, which is significantly higher than recommended restrictive transfusion threshold of 7 to 8 g/dL in healthy asymptomatic adults30,31. The decision to transfuse may not be solely based on Hb level. Transfusion above the specified Hb threshold may be dictated by the clinical context including pre-existing coronary artery disease and presence of symptoms of anemia. However, in case of “healthy unrelated donors”, this may reflect over-transfusion due to availability of autologous blood unit. Unfortunately, the information on the criteria used to transfuse the BM donors is not available in this study.
This study also revealed that most donors experience approximately the same levels of peri-collection pain and toxicities and probability of recovery after completion of the BM harvest procedure regardless of autologous blood transfusion. Based on the multivariate analysis, the only subgroup that marginally benefited from autologous blood transfusion was donors who underwent high volume BM harvest. More specifically, in donors with BM harvest volume equal or greater than 27% of donor’s blood volume, transfusion of autologous blood was shown to be associated with slightly decreased early collection-associated toxicities, the most prominent of which was fatigue. In addition, autologous blood transfusion after high volume BM harvest was shown to increase the speed of donors’ reporting full recovery by 5 days. Of note, a collected BM volume of greater than 20 mL/kg of donor body weight, the limit set by NMDP for a safe BM harvest, was found to translate into at least 27% of total blood volume. A limit of 20 ml/kg has been shown in 2 large studies of pediatric donation to be associated with avoidance of the need for allogeneic blood transfusions, and considered standard in that setting not to exceed this limit 32,33. Therefore, collection of autologous blood prior to BM harvest, with subsequent transfusion may be justified if the planned total volume of BM harvest calculated based on the total nucleated cells per kg of recipient body weight is equal to, or more than, 27% of the donor’s blood volume. However, one would question this particular practice as it violates NMDP safe BM harvest policy and established practices that avoid the need for allogeneic blood transfusions in near 20% of donors.
There are several limitations to the current study, including lack of data regarding whether the donors underwent autologous blood collection among the donors who did not receive transfusions, lack of data on the time interval between autologous blood collection and BM harvest, and whether or not iron supplementation was given before the BM harvest. Many of these limitations have been addressed by the clear findings of little or no effect of autologous blood transfusion at all levels of collection except for the most extreme. Another limitation is the lack of the Hb level post-harvest but prior to the autologous blood transfusion in more than half of the donors, which makes it difficult to ascertain the criteria that were used to transfuse or not transfuse the BM donors. For those donors where a Hb level was obtained prior to transfusion, practice varied widely, with transfusions given at many levels of Hb, indicating that some centers have a low threshold for transfusing patients when an autologous unit is collected.
In conclusion, the results of this study do not support the routine use of autologous blood transfusion for all unrelated BM donors. Our data suggest that autologous transfusion may be beneficial in cases where the planned BM harvest volume exceeds 27% of donor’s blood volume, and there is sufficient time between the autologous collection and the planned BM harvest for hematopoiesis to replace a substantial portion of the donor’s lost blood. Even this practice may be questionable, as such high-volume harvests may not be in the best interest of the donor.
Supplementary Material
ACKNOWLEDGEMENTS:
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 1U24HL138660 from NHLBI and NCI; a contract HHSH250201700006C with Health Resources and Services Administration (HRSA/DHHS); three Grants N00014-17-1-2388, N00014-17-1-2850 and N00014-18-1-2045 from the Office of Naval Research; and grants from Adaptive Biotechnologies; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; *CytoSen Therapeutics, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite Pharma, Inc.; Medac, GmbH; *Mediware; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Pharmaceuticals Corporation; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
CONFLICTS OF INTEREST:
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wallace EL, Surgenor DM, Hao HS, An J, Chapman RH, Churchill WH. Collection and transfusion of blood and blood components in the United States, 1989. Transfusion 1993; 33: 139–144. [DOI] [PubMed] [Google Scholar]
- 2.Wallace EL, Churchill WH, Surgenor DM, Cho GS, McGurk S. Collection and transfusion of blood and blood components in the United States, 1994. Transfusion 1998; 38: 625–636. [DOI] [PubMed] [Google Scholar]
- 3.Busch MP, Young MJ, Samson SM, Mosley JW, Ward JW, Perkins HA. Risk of human immunodeficiency virus (HIV) transmission by blood transfusions before the implementation of HIV-1 antibody screening. The Transfusion Safety Study Group. Transfusion 1991; 31: 4–11. [DOI] [PubMed] [Google Scholar]
- 4.Zou S, Stramer SL, Dodd RY. Donor testing and risk: current prevalence, incidence, and residual risk of transfusion-transmissible agents in US allogeneic donations. Transfus Med Rev 2012; 26: 119–128. [DOI] [PubMed] [Google Scholar]
- 5.Dorsey KA, Moritz ED, Steele WR, Eder AF, Stramer SL. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion 2013; 53: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 6.Kattermann R, Gille H. [Total protein determination in lipemic or icteric specimens]. Med Lab (Stuttg) 1975; 28: 130–131. [PubMed] [Google Scholar]
- 7.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev 2007; 21: 327–348. [DOI] [PubMed] [Google Scholar]
- 8.Goel R, Tobian AAR, Shaz BH. Non-infectious transfusion associated adverse events and their mitigation strategies. Blood 2019; 133: 1831–1839. [DOI] [PubMed] [Google Scholar]
- 9.Domen RE. Adverse reactions associated with autologous blood transfusion: evaluation and incidence at a large academic hospital. Transfusion 1998; 38: 296–300. [DOI] [PubMed] [Google Scholar]
- 10.Vamvakas EC. Meta-analysis of randomized controlled trials comparing the risk of postoperative infection between recipients of allogeneic and autologous blood transfusion. Vox Sang 2002; 83: 339–346. [DOI] [PubMed] [Google Scholar]
- 11.Goldman M, Rémy-Prince S, Trépanier A, Décary F. Autologous donation error rates in Canada. Transfusion 1997; 37: 523–527. [DOI] [PubMed] [Google Scholar]
- 12.Etchason J, Petz L, Keeler E, Calhoun L, Kleinman S, Snider C et al. The cost effectiveness of preoperative autologous blood donations. N Engl J Med 1995; 332: 719–724. [DOI] [PubMed] [Google Scholar]
- 13.Tretiak R, Laupacis A, Rivière M, McKerracher K, Souêtre E. Cost of allogeneic and autologous blood transfusion in Canada. Canadian Cost of Transfusion Study Group. Can Med Assoc J 1996; 154: 1501–1508. [PMC free article] [PubMed] [Google Scholar]
- 14.Whitaker BI, Hinkins S. The 2011 national blood collection and utilization survey report. US Department of Health and Human Services; 2011. [Google Scholar]
- 15.Janssen MP, van Hoeven LR, Rautmann G. Trends and Observations on the Collection, Testing and Use of Blood and Blood Components in Europe. 2001-2011 report. Strasbourg: Council of Europe; 2015. [Google Scholar]
- 16.Arora K, Kelley J, Martinez F, Tholpady A. Preoperative autologous blood collection before bone marrow harvests in haploidentical related donors: is it justified? Transfusion 2018; 58: 1618–1625. [DOI] [PubMed] [Google Scholar]
- 17.Parkkali T, Juvonen E, Volin L, Partanen J, Ruutu T. Collection of autologous blood for bone marrow donation: how useful is it? Bone Marrow Transplant 2005; 35: 1035–1039. [DOI] [PubMed] [Google Scholar]
- 18.Mijovic A, Britten C, Regan F, Harrison J. Preoperative autologous blood donation for bone marrow harvests: are we wasting donors’ time and blood? Transfus Med 2006; 16: 57–62. [DOI] [PubMed] [Google Scholar]
- 19.Manuel SP, Spitzer TR, Ishizawa Y. Preoperative autologous blood donation in healthy bone marrow donors contributes to pre-procedure anemia. Bone Marrow Transplant 2017; 52: 1191–1193. [DOI] [PubMed] [Google Scholar]
- 20.Pulsipher MA, Chitphakdithai P, Logan BR, Shaw BE, Wingard JR, Lazarus HM et al. Acute toxicities of unrelated bone marrow versus peripheral blood stem cell donation: results of a prospective trial from the National Marrow Donor Program. Blood 2013; 121: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JP, Perry EH, Price TH, Bolan CD, Karanes C, Boyd TM et al. Recovery and safety profiles of marrow and PBSC donors: experience of the National Marrow Donor Program. Biol Blood Marrow Transplant 2008; 14: 29–36. [DOI] [PubMed] [Google Scholar]
- 22.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery 1962; 51: 224–232. [PubMed] [Google Scholar]
- 23.Zhang X, Loberiza FR, Klein JP, Zhang M-J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed 2007; 88: 95–101. [DOI] [PubMed] [Google Scholar]
- 24.Brecher ME, Goodnough LT. The rise and fall of preoperative autologous blood donation. Transfusion 2001; 41: 1459–1462. [DOI] [PubMed] [Google Scholar]
- 25.Gouëzec H, Ferré N, Hervé F, Lapart C, Leberre C, Bernard M et al. [Suitability of autologous blood donation before bone marrow donation]. Transfus Clin Biol 2015; 22: 71–75. [DOI] [PubMed] [Google Scholar]
- 26.Worel N, Buser A, Greinix HT, Hägglund H, Navarro W, Pulsipher MA et al. Suitability Criteria for Adult Related Donors: A Consensus Statement from the Worldwide Network for Blood and Marrow Transplantation Standing Committee on Donor Issues. Biol Blood Marrow Transplant 2015; 21: 2052–2060. [DOI] [PubMed] [Google Scholar]
- 27.Bunn HF. Pathophysiology of the anemias. Harrison’s Principle of Internal Medicine New York… 1991.
- 28.Kiss JE, Brambilla D, Glynn SA, Mast AE, Spencer BR, Stone M et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA 2015; 313: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer TR, Sugrue MW, Gonzalez C, O’Donnell P, Confer D, Fuchs E et al. Transfusion practices for bone marrow harvests: a survey analysis from the AABB Bone Marrow Quality Improvement Initiative Working Group. Bone Marrow Transplant 2017; 52: 1199–1200. [DOI] [PubMed] [Google Scholar]
- 30.Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016; 316: 2025–2035. [DOI] [PubMed] [Google Scholar]
- 31.Carson JL, Stanworth SJ, Roubinian N, Fergusson DA, Triulzi D, Doree C et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev 2016; 10: CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Styczynski J, Balduzzi A, Gil L, Labopin M, Hamladji R-M, Marktel S et al. Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective European Group for Blood and Marrow Transplantation Pediatric Diseases Working Party study. Blood 2012; 119: 2935–2942. [DOI] [PubMed] [Google Scholar]
- 33.Pulsipher MA, Logan BR, Kiefer DM, Chitphakdithai P, Riches ML, Rizzo JD et al. Higher Risks of Toxicity and Incomplete Recovery in 13- to 17-Year-Old Females after Marrow Donation: RDSafe Peds Results. Biol Blood Marrow Transplant 2019; 25: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


