Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) is an ancestral molecule that was isolated from sheep hypothalamic extracts based on its action to stimulate cAMP production by pituitary cell cultures. PACAP is one of a number of ligands that coordinate with GnRH to control reproduction. While initially viewed as a hypothalamic releasing factor, PACAP and its receptors are widely distributed, and there is growing evidence that PACAP functions as a paracrine/autocrine regulator in the CNS, pituitary, gonads and placenta, among other tissues. This review will summarize current knowledge concerning the expression and function of PACAP in the hypothalamic-pituitary-gonadal axis with special emphasis on its role in pituitary function in the fetus and newborn.
Introduction
Pituitary adenylate cyclase activating polypeptide (PACAP) is one of a number of ligands that coordinate with gonadotropin-releasing hormone (GnRH) to control reproduction. While initially identified as a hypophysiotropic factor, there is accumulating evidence that PACAP is primarily a paracrine/autocrine hormone in the CNS, pituitary and gonad. Overexpression of PACAP in the anterior pituitary in male mice delayed puberty and suppressed serum luteinizing hormone (LH), follicle-stimulating hormone (FSH) and testosterone levels as well as pituitary gonadotropin-releasing hormone receptor (GnRH-R) expression perhaps because pituitary folllistatin expression was markedly increased (1). On the other hand, PACAP knockout male mice are testosterone-deficient with relative LH insufficiency (2), and females are sub-fertile with lower implantation rates (3,4).
1. PACAP and its receptors.
PACAP was isolated in 1989 from sheep hypothalamic extracts based on its action to stimulate cyclic AMP production by rat pituitary cell cultures (5). PACAP is the most highly conserved member of the VIP-secretin-glucagon peptide superfamily (6). There are two PACAP isoforms, a 38 amino acid form and a C-terminally truncated 27 amino acid form, with PACAP-38 accounting for 90% of the protein in most tissues. The human PACAP promoter contains two cAMP-response-like elements, a 12-O-tetradecanoylphorbol 13-acetate-response element, two sequences that are homologous to the consensus sequence for pituitary-specific factor growth hormone transactivator factor-1-binding sites, and six binding domains for the thyroid-specific transcription factor-1 (7). Given PACAP’s effect to increase cAMP production, the CREs and AP1 sites in the PACAP promoter allow for a feed-forward mechanism in which PACAP transcription can be activated by PACAP itself (8) (Moore et al, Molecular and Cellular Endocrinology, in press). This mechanism, shown in Figure 1, likely mediates the change in expression that occurs abruptly between the fetal-newborn pituitary (see section 6).
Figure 1.
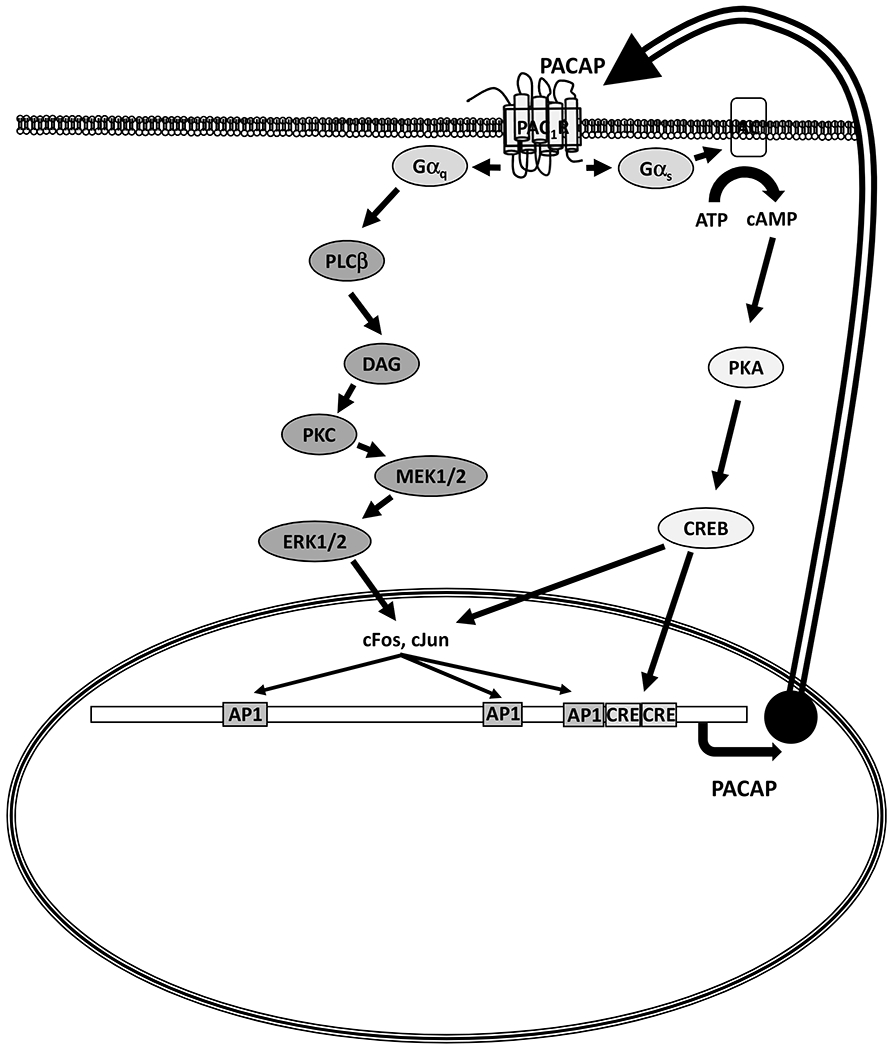
PACAP receptor second messenger signaling in pituitary cells. The schema describes a paracrine/autocrine feed-forward mechanism to maintain a high level of PACAP in the fetal pituitary. PACAP and PAC1-R in gonadotrophs and folliculostellate cells likely contribute to the autocrine/paracrine system. The mouse PACAP promoter is depicted with three AP1 sites (−948, −448, −275) and two CRE sites (−205, −179).
Because it is found in high concentration in the hypothalamus (9), is present in the median eminence (10), and levels in portal blood exceed those of peripheral blood (at least in the rat) (11), PACAP is viewed as a hypophysiotropic factor. However, the portal:peripheral plasma ratio for PACAP was 2:4, compared to 5:120 for GnRH (12). While PACAP is found in peripheral plasma (13), PACAP in the circulation is rapidly degraded with a half-life of 2 to 10 min (14).
PACAP activates three distinct 7- transmembrane receptors: VPAC1 and VPAC2 receptors with relatively similar affinity for VIP and PACAP, and the highly specific PAC1 receptor (PAC1-R; ADCYAP1R1) [3]. There are multiple splice variants of the PAC1-R that result from alternative splicing of two 84 bp exons in the third intracellular loop (designated hip, and hop) and were named null (neither hip nor hop), hip, hop1, hop2 (a shorter version of hop1), and hiphop1 and hiphop2 (15). PAC1-R variants that differ from the null receptor in the amino-terminal extracellular domain have also been identified [5].
VPAC-1 and -2 and all PAC1_R variants bind PACAP38 and PACAP27 with high affinity, and like other members of the group B G-protein coupled receptor family, couple with Gαs to activate adenylate cyclase and increase cAMP signaling. In some cell types, PACAP 38 >>PACAP 27 also couples with Gq/11 to stimulate IP3 production, the MAPK pathway, and increase intracellular calcium (16), as well as nitric-oxide synthase type I and cGMP (17). PAC1-R has also been shown to signal via β-arrestin1 and β-arrestin2 (18). Thus, variable expression and signaling of the PAC1 -R variants would be expected to produce different transcriptomes (7,19,20).
Consequent to the extensive distribution of the ligand and its receptors, PACAP exerts a wide array of functions including protection against neuronal apoptosis and retinal degeneration, neurotransmitter function, vaso- and broncho-dilatation, tear secretion, activation of intestinal motility, bladder pain and micturition, anti-inflammatory and antioxidant effects, immune modulation, thermogenesis, appetite suppression, and sleep and circadian rhythms, as well as effects on endocrine systems (21). There are PACAP effects in the exocrine and endocrine pancreas, hepatocytes, osteoblasts, adrenal medulla and cortex, testis and ovary, thyroid, pineal, hypothalamus, neurohypophysis and pars tuberalis as well as the anterior pituitary.
2. Functions of PACAP as a neuropeptide
Early studies by Arimura et al (9) revealed the highest concentration of immunoreactive PACAP in the hypothalamus. Further research showed that PACAP is widely distributed in the CNS (22) in various brain regions including the cerebral cortex, amygdala, and hippocampus. In situ hybridization methods revealed dense labeling in the supraoptic nucleus (SON) followed by the anterior and posterior hypothalamic regions, the dorso-ventromedial, and arcuate nuclei and in the tubero- and premammillary regions (23), the periventricular region and in the paraventricular nucleus (PVN) (24).
A series of experiment suggest that hypothalamic PACAP functions as a local activator of GnRH secretion. The intracerebroventricular (icv) injection of PACAP into adult male rats produced a small but significant increase in GnRH mRNA levels which was abolished by the PACAP6-38 antagonist which itself suppressed the basal hybridization signal (25). The GnRH neuronal line, GT1-7, was reported to express multiple PACAP receptor splice variants, and to respond to PACAP with an increase in cAMP production (26) and GnRH-R expression (27). The effect on GnRH may be through Kisspeptin neurons since Kisspeptin1 mRNA levels were increased by PACAP38 in mHypoA-50 and mHypoA-55 hypothalamic cell lines although the PACAP 6-38 antagonist produced a similar effect (28).
Using in situ hybridization, we found (29) that Pacap mRNA expression in the PVN and pituitary vary significantly across the estrous cycle in the rat, with the greatest changes occurring on the day of proestrus (Figure 2). Pacap mRNA in the PVN declined significantly on the morning of diestrus. At noon of proestrus, there was a notable peak in PVN Pacap mRNA that occurred three hours before the gonadotropin surge, followed by a decline. Pituitary expression of Pacap mRNA also varied on the afternoon of proestrus with a moderate decline at the time of the gonadotropin surge and a significant increase later in the evening. Expression of the mRNA species encoding the 288 amino acid form of follistatin increased significantly following the rise in pituitary Pacap mRNA, at the termination of the secondary surge in Fshb gene expression.
Figure 2.
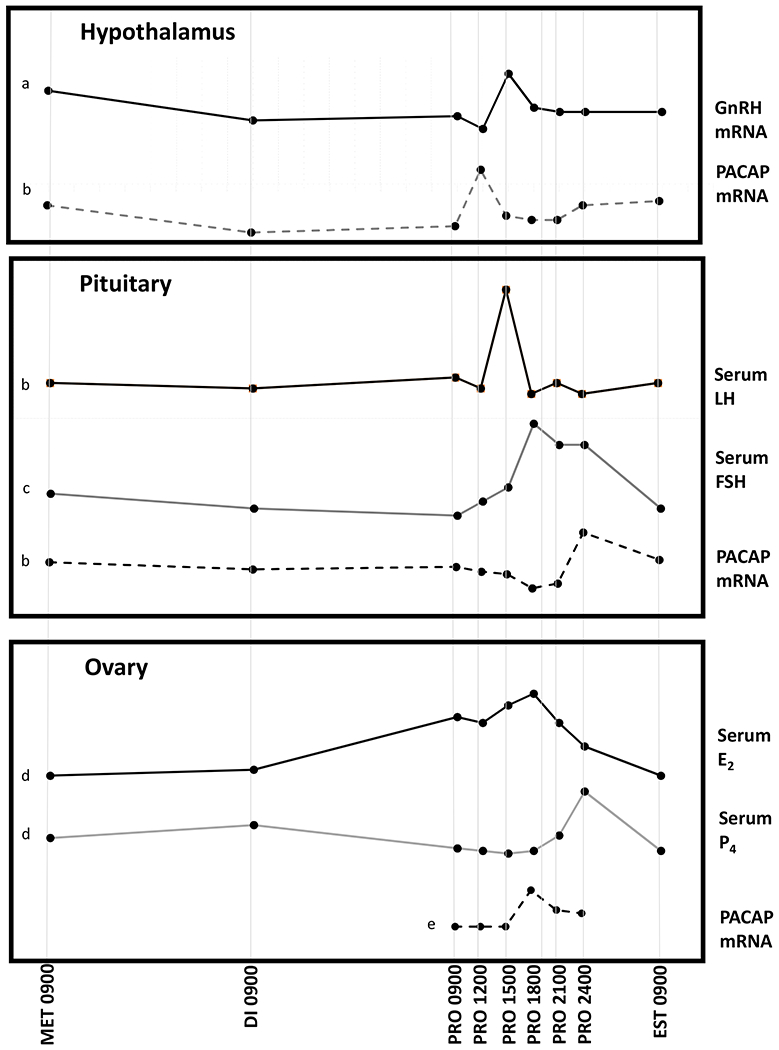
Pattern of PACAP expression in the hypothalamus (PVN), anterior pituitary, and ovaries during the rat estrus cycle. PACAP expression increases in the PVN just prior to the rise in GnRH in the preoptic area which initiates the surge in serum LH and FSH. PACAP expression in the pituitary rises prior to a decline in FSH secretion. PACAP expression in the ovary rises in response to the proestrus surge in circulating gonadotropins, and soon thereafter serum progesterone increases. Abbreviations: MET Metestrus; DI Diestrus; PRO Proestrus; EST Estrus; E2 estradiol; P4 progesterone. a: Schirman-Hildesheim, 2005 (150), b: Moore, 2003 (151) c: Ozawa, 2005 (152), d: Smith, 1975 (153), e: Ko, 1999 (154).
While these observations suggest a faciliatory role for PACAP in the rat estrus cycle, in vivo experiments to examine the effects of exogenous PACAP or the PACAP 6-38 antagonist have produced variable results depending on dose and route of administration, sex and species (30). For example, intra-atrial or icv administration of PACAP38 stimulated LH release in adult male rats (31) but icv PACAP38 on the day of proestrus inhibited the release of LH and ovulation in rats (32) while in a second study, icv PACAP38 suppressed but PACAP27 enhanced the LH surge (33). Choi et al (34) reported that PAC1-R antisense oligodeoxynucleotide administered icv to immature female rats suppressed the prepubertal increases in GnRH and GnRH-R mRNA levels and delayed sexual maturation. On the other hand, Szabo et al found that the subcutaneous (sc) administration of PACAP to neonatal rats delayed vaginal opening and reduced immunoreactive GnRH in the pre-optic area in 30-day old rats (35).
The importance of PACAP to adult reproductive function has been demonstrated by studies of PACAP- and PAC-1-knockout mice which have revealed many instances of endocrine dysfunction as summarized in Table 1. PACAP knockout mice have substantial newborn mortality. Surviving females have normal sexual maturation and estrous cycles but have disrupted mating behavior, a lower implantation rate with reduced fertility, and lower prolactin and progesterone levels (4,36). PACAP knockout males developed testosterone deficiency apparently because of LH insufficiency insofar as LH levels were normal or reduced in the setting of low testosterone. The LH activated steroidogenesis enzymes, testicular steroidogenic acute regulatory protein (StAR) and P450c17 were reduced. As these animals age (15 mo), knockout mice were protected from the germ cell depletion and vacuolization that was observed in w/t testes (37). Mice rendered deficient in PAC-R1 had increased mortality at weaning but no fertility disturbance has been noted (38,39).
Table 1.
Endocrine changes with PACAC genetic manipulation
| Reproductive Function | ||
|---|---|---|
| PAC1R-KO | Disrupted estrus cycle | Jamen, 2000 (38) |
| PACAP-KO | Testosterone deficient with LH insufficiency | Lacombe, 2006 (37) |
| Delayed testicular aging | Lacombe, 2006 (37) | |
| Abnormal sperm shape and size | Brubel, 2012 (96) | |
| Sub-fertile females | Isaac, 2008 (36) | |
| Decreased progesterone and prolactin levels during pregnancy | Isaac, 2008 (36) | |
| Reduced uterine implantation rates | Shintani, 2002 (4) | |
| Lpr+ PMV neurons | Delayed female sexual maturation | Ross, 2018 (43) |
| Disruption of LH surge | Ross, 2018 (43) | |
| Low pup number per litter | Ross, 2018 (43) | |
| αGSU-PACAP | Delayed male sexual maturation | Moore, 2012 (1) |
| Lifelong suppressed gonadotropins | Moore, 2012 (1) | |
| glucose homeostasis | ||
| PAC1R-KO | Glucose intolerance | Winzell, 2007 (137) Persson, 2002(138) |
| impaired insulin secretion | Winzell, 2007 (137) | |
| Decreased glucagon secretion | Winzell, 2007 (137) Persson, 2002(138) |
|
| Less body wt & white adipose tissue Hypoinsulinemia | Tomimoto, 2008 (139) | |
| Increased insulin sensitivity | Tomimoto, 2008 (139) | |
| PACAP-KO | Microvesicular fat accumulation in | Gray, 2001 (140) |
| liver, skeletal muscle and heart increased triglycerides and cholesterol | Gray, 2001 (140) | |
| increased insulin and hypoglycemia | Gray, 2001 (140) | |
| impaired thermoregulation | Gray, 2002 (140) | |
| Susceptibility to insulin-induced hypoglycemia, and related death | Hamelink, 2002 (141) | |
| β-cell overexpression | Increased insulin secretion | Yamamoto, 2003 (142) |
| Improved insulin sensitivity in high fat diet-induced diabetes | Tomimoto, 2004 (143) Sakuri, 2012 (144) |
|
| Stress Response | ||
| PAC1R-KO | Reduced contextual fear conditioning | Otto, 2001 (145) |
| Impaired hippocampal long-term potentiation | Otto 2001 (146) | |
| PACAP-KO | Lack of stress-induced CRH upregulation | Agarwal, 2005 (112) |
| Disrupted stress-induced catecholamine release from splanchnic nerves. | Stroth, 2010 (147) | |
| Impaired stress-induced ACTH secretion | Stroth, 2010 (147) | |
| Reduced stress-induced corticosterone secretion | Stroth, 2010 (147) | |
| Impaired stress-induced adrenal steroidogenesis | Stroth, 2010 (147) | |
| Feeding Behavior | ||
| PAC1R-KO | Reduced POMC expression in the arcuate nucleus | Mounien, 2009 (148) |
| Increased postprandial ghrelin | Vu, 2015 (40) | |
| Decreased postprandial GLP-1, insulin and leptin | Vu, 2015 (40) | |
| Loss of PACAP-induced reduction in food intake | Vu, 2015 (40) | |
| PACAP-KO | Reduced carbohydrate intake | Nakata, 2004 (149) |
| Impaired leptin-stimulated adipose tissue sympathetic nerve activity | Tanida. 2013 (41) | |
| reduced food intake and body weight | Nakata, 2004 (149) | |
| reduced body weight | Gray, 2001 (140) | |
PACAP is a potent suppressor of feeding behavior, by decreasing ghrelin which suppresses appetite, and by increasing leptin and GLP1 which increase satiety (40). PACAP also appears to mediate in part the effects of leptin on food intake (41) and perhaps its effect to enhance pubertal development and reproductive function (42). Ross et al (43) developed a conditional knockout model of PACAP from ventral premammillary nucleus (PMV) neurons that express the leptin receptor. They found delayed sexual maturation in females, disruption of the LH surge, and fewer pups per litter. Kisspeptin induction of LH secretion was unaffected. They also deleted PACAP from the PMV of adult female Adcyap1fl/fl mice with bilateral stereotaxic injections of an adenovirus carrying cre-recombinase, and again found dysregulation of the estrus cycle. They propose that PACAP plays a role in conveying the signal between nutrition and GnRH release.
PACAP immunoreactivity in and around magnocellular neurons and colocalization of PAC-R by in situ hybridization in arginine vasopressin (AVP) neurons in the SON suggest a role for PACAP in the control of vasopressin secretion (44).
3. PACAP and its receptors in the pituitary
Although initially identified as a hypothalamic peptide, PACAP is also produced in the pituitary. An early immunoassay detected PACAP in the adult rat pituitary, although at much lower levels than in the hypothalamus, with higher levels in the posterior than in the anterior lobe (9). No PACAP mRNA was found in the adult rat pituitary by Northern blotting (45). Subsequently, Koves et al (46) used dual immunohistochemistry to localize PACAP to gonadotrophs, and Jin et al (47) identified PACAP mRNA using RT/PCR in pituitary folliculostellate (FS) cells obtained by laser-capture microdissection from adult female rats. FS cells are agranular and star-shaped with long cytoplasmic processes (48) that intermingle with and are joined to the endocrine cells by a variety of intercellular junctions (49) allowing for intercellular communication. Thus, PACAP in gonadotrophs could have an autocrine effect, and PACAP from FS cells might be a paracrine regulator of gonadotrophs.
Each of the pituitary secretory cells, as well as FS cells, express at least one form of the PACAP receptor (19,50,51). Studies using rat (52–54) or ovine (55) pituitary cell monolayer cultures revealed, however, only small effects of added PACAP on the release of PRL, GH, and ACTH. There are some species-specific differences, as PRL synthesis and secretion are substantially stimulated by PACAP in pituitary cultures from fish (56). PACAP also effectively stimulates the release of GH and PRL from rat GH3 cells, and ACTH from the mouse pituitary tumor cell line AtT-20 (57,58).
Gonadotrophs, on the other hand, are clearly regulated by PACAP, with direct effects on gonadotropin secretion and subunit gene expression, and indirect effects by modulating the actions of GnRH. PACAP directly stimulates the release of LH and uncombined glycoprotein α-subunit from primary pituitary cell cultures (52,54) although the effect is less than that of GnRH, and desensitizes rapidly (59). Most notably, PACAP enhances LH and FSH secretion by pituitary cells that are stimulated with GnRH (52,60), an effect that is especially pronounced when pituitary cells are perifused and stimulated with pulses of GnRH as a model of the hypothalamic-pituitary unit. In this model, PACAP also increased α-subunit mRNA levels, lengthened LHb mRNA but suppressed Fshb mRNA levels (60).
Gonadotroph-derived cell lines developed by Dr. Pamela Mellon and colleagues (61) have been instrumental in understanding the intracellular signaling pathways through which PACAP regulates the gonadotropin subunit genes. αT3-1 cells, viewed as immature because they express gonadotropin-α but not β-subunits, also express PAC1-R at a high level with the hop and short variants predominating although other forms (PAC1-R hiphop and hip) were also observed (51). In these cells, PACAP stimulates cAMP production and activates inositol phosphate to increase cytosolic Ca2+(62) with the former pathway leading to an increase in Cga mRNA levels (62–64).
In LβT2 cells, with characteristics of differentiated gonadotrophs (65), PAC1-R expression is very low although PCR products are consistent with the short and the hop1, hop2 or hip forms (66). In this cell line, PAC1-R overexpression has been used to study PACAP signaling to Lhb and Fshb transcription (67) including experiments designed to understand differences among the various splice variants. In these cells, PACAP stimulates the Lhb promoter in part through cAMP-PKA (68) and increased EGR-1 (69). Stimulation of the Lhb promoter was more evident when cells were stimulated with intermittent PACAP pulses (67) perhaps because of desensitization with continuous PACAP. PACAP likewise activated the Lhb promoter in pituitary cultures from mice expressing a rat Lhb-luciferase transgene (68).
In contrast to its activation of Cga, and Lhb, PACAP stimulation of Fshb is transient in primary rat pituitary cell cultures, and is followed by suppression (70,71), although not in LβT-2 cells (66,67,71). A likely explanation for this difference involves robust stimulation of follistatin expression by PACAP in the normal pituitary (70) which is essentially undetectable in LbT2 cells (66). According to this paradigm, ongoing PACAP stimulation of Fshb transcription is blocked in the normal pituitary when follistatin increases, binds activin, and prevents the multiplicity of activin effects including up-regulation of the Fshb and Gnrhr genes (72). How PACAP may differentially regulate the gonadotropin subunit genes is shown in Figure 3, and evidence that this mechanism is important to the ontogeny of the gonadotropins in the fetus and newborn is summarized in Section 6.
Figure 3.
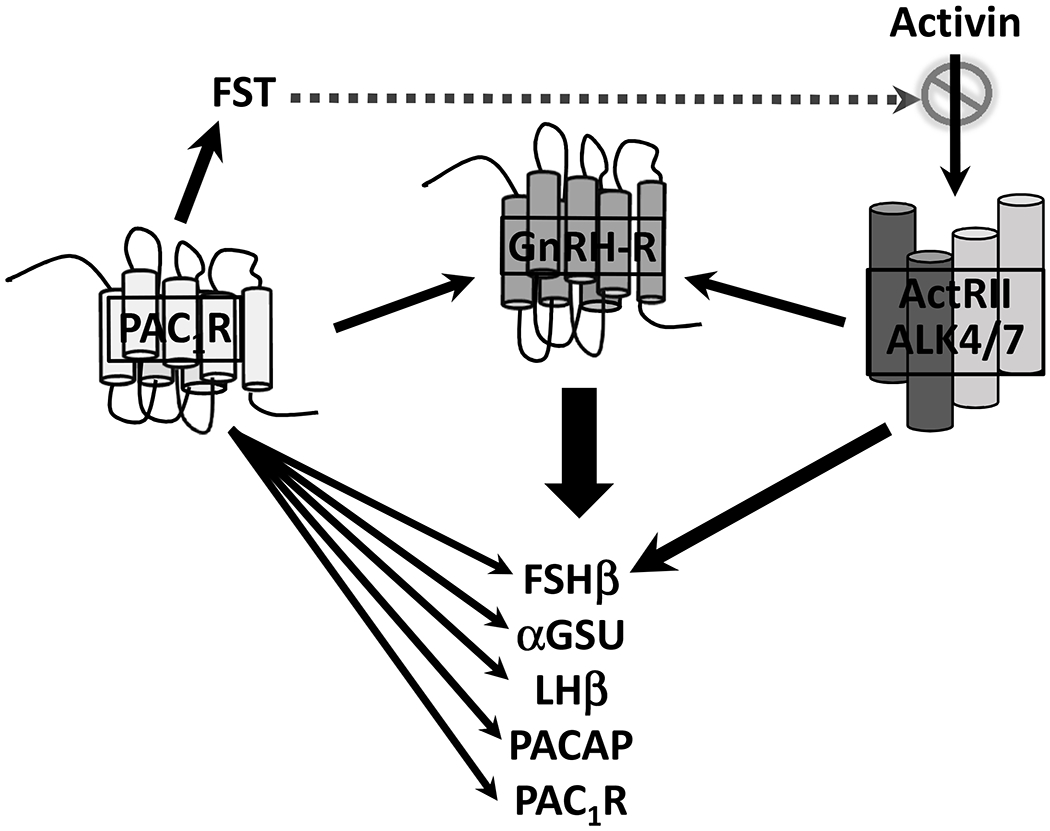
A model for the mechanism by which PACAP differentially regulates gonadotropin expression. Experiments using primary pituitary cell cultures and/or immortalized gonadotroph cell lines have shown that PACAP directly stimulates transcription of each of the gonadotropin subunits, enhances GnRH-stimulated expression of α-subunit and LHb as well as expression of PACAP and the PAC1 receptor. PACAP also stimulates expression of GnRH-R and enhances GnRH signaling. On the other hand, PACAP suppresses Fshb and GnRH-R expression indirectly by stimulating follistatin (FST) expression in gonadotrophs and folliculo-stellate cells, which neutralizes stimulation of Fshb and GnRH-R by activin.
In addition to direct effects on gonadotropin subunit genes, there is substantial cross-talk between PACAP and GnRH signaling pathways in gonadotrophs. PACAP has been found to increase GnRH-R expression (73) and to enhance GnRH-R signaling (74). Gnrhr transcription is increased by PACAP in LβT2 cells through CREB and SF-1 [28], and Gnrhr mRNA levels in αT3-1 cells are increased by PKA activation in which GnRH-stimulated IP production is increased synergistically by PACAP (62). The nitric oxide pathway also appears to contribute to the interaction between PACAP and GnRH as both ligands increase nitric oxide synthase type I protein levels in rat gonadotrophs via cAMP leading to an increase in cyclic GMP (75).
Furthermore, effects may be amplified because GnRH increases PACAP and PAC1-R expression in primary pituitary cell cultures (76) as well as in LβT2 gonadotroph cells (77). On the other hand, GnRH blunts PACAP-induced cAMP accumulation by up to 70% in both αT3-1 (78) and LβT2 cells (79) in which GnRH phosphorylates PAC1-R (79).
Pituitary PACAP may play an important role in gonadotroph function in the transition from the fetus to newborn (Section 6) as well as in the timing of pubertal development. We created a transgenic model of pituitary PACAP overexpression using the gonadotropin-α subunit promoter (1). Male transgenic mice had delayed sexual maturation based on testis weight and balano-preputial separation, with delayed spermiogenesis. LH and FSH levels were suppressed, and GnRH-receptor expression was decreased. The effect of PACAP to stimulate follistatin transcription (80) may have mediated these changes. Pituitary follistatin expression in w/t mice declines from day 10 to day 30, and then remains stable, and there is a reciprocal rise in GnRH-R expression. In the transgenic mice, however, pituitary follistatin was substantially higher while Gnrhr mRNA was suppressed through day 40. It is likely follistatin binding to activin blocked activin paracrine upregulation of the GnRH-R to produce gonadotropin deficiency.
4. PACAP in the testes and ovaries
In addition to regulating reproduction via hypothalamic-pituitary functioning, PACAP has direct effects on the testis and ovaries. PACAP is present in high concentration in testis (9) including the human testis (81), and is primarily found in germ cells (82,83). Accordingly, levels increase from age 20 to 60 days in the rat, and are low when spermatogenesis is disrupted, e.g. by cryptorchidism (84). A shorter PACAP mRNA is expressed in the testis from rats, mice, bovine and humans, and reflects a novel testis-specific first exon upstream from the transcriptional start-site. The gene encodes a PACAP precursor with no signal peptide suggesting a paracrine function. The level of expression across the rat seminiferous tubule varies during the spermatogenic cycle, and presumably reflects a role for PACAP in spermatogenesis (85,86).
PACAP receptors are widely distributed in testis although localization has varied by species. VPAC2 binding sites in the rat predominate in seminiferous tubules by in situ hybridization and are implied by ligand specificity (87,88) but localized to Leydig cells in immature mice by immunohistochemistry (89). VPAC2 knock-out mice (both males and females) were healthy and fertile young adults, and produced normal sized litters, but in older males (31 wks), VPAC2 deficiency caused diffuse seminiferous tubular degeneration with hypospermia and reduced fertility (90) supporting a role for this receptor in the effect of PACAP on spermatogenesis. Rat Sertoli cells (SC) predominantly express a unique PAC1-R splice variant (91). PAC1-R mRNA is found in rat Leydig cells (92) and in the clonal TM3 Leydig cell tumor cell line (93).
PACAP may play a role in the function of the fetal and immature testis. PACAP simulates cAMP production by SC cultures from immature rats, although less effectively than FSH, but like FSH, PACAP stimulation becomes less potent in cultures as animals mature (94). Testosterone production by fetal Leydig cells is evident by E15.5 which is thought to precede the presence of LH in the circulation. The implication is that some factor, either endocrine, autocrine or paracrine activates steroidogenesis in early fetal life. To assess this idea, El-Gehani et al (95) examined the effects of hCG and PACAP on testosterone synthesis by LC from E15.5 to E21.5 pups as well as adult rats. They found that PACAP stimulates testosterone production far more potently in fetal than in adult LC whereas the effect of hCG is sustained. The presence of PACAP mRNA, albeit at a low level, in the fetal testis supports the idea that PACAP is an autocrine stimulator of fetal LC function. Finally, LH-receptor deficient males have a normal male phenotype leading to speculation that PACAP was the sexual differentiation factor in rats; however, PACAP knock-out males likewise have a male phenotype.
As noted above, PACAP knock-out males are testosterone deficient probably because of gonadotropin deficiency, but have normal testicular morphology (37) while PAC1-R knock-out mice of both sexes had increased mortality at weaning but no fertility disturbance was noted (38,39).
Effects of PACAP on sperm have also been reported. In one study, sperm heads from PACAP-deficient mice were smaller, with more abnormal-shape, than in w/t littermates. In the same study, adding PACAP to semen samples from fertile and infertile men increased the motility of low -motility sperm (96). A study of PACAP and sperm quality in obesity was recently published (97). A high fat diet is known to reduce sperm motility, capacitation and oocyte membrane binding, and to increase intracellular reactive oxygen species in mice. PACAP i.p. daily for 4 wks partially blocked high fat diet -induced obesity in adult mice, and improved testis morphology and sperm function which was thought to be mediated through the p53 deacetylase Sirt 1 (silent information regulator 1) leading to suppression of apoptosis. Sirtl protein was found to be lower in sperm of those obese infertile men who also had decreased semen quality (97).
There is also evidence that PACAP is a paracrine regulator of ovarian function (30,98). Female gametogenesis begins with the differentiation of primordial germ cells (PGC) into oogonia and then oocytes. Akin to its role in stem cell proliferation and survival, PACAP immunoreactivity was identified on the gonadal ridge adjacent to the PGC surface in the e11-12 mouse, and PAC1-R is expressed in PGC cells in which PACAP increases cAMP production and promotes survival in culture. (99). When immature rats (100) or mice (101) are stimulated with PMSG/hCG, PACAP mRNA and protein are found in the majority of granulosa and cumulus cells from large preovulatory follicles, suggesting a role in ovulation. PACAP also inhibited the growth of preantral follicles suggesting an additional role in follicular recruitment (102).
RT-PCR revealed PAC1-R and VPAC2-R in rat granulosa cells whereas only VPAC1-R and VPAC2-R were found in thecae-interstitial cells (103). PACAP is a potent activator of cAMP production and estradiol and progesterone secretion by immature rat granulosa cells (104) in which PACAP inhibited cell apoptosis (103). Human granulosa-luteal cells obtained at the time of IVF also express PACAP and VPAC1-R and VPAC2-R mRNAs that are increased by LH/FSH (105)
PACAP and PACAP type I receptor mRNAs are also expressed in the rat corpus luteum (106) in which PACAP mRNA gradually increases in pregnancy. Finally, PACAP and its receptors are present in human placenta (107) where it may play a role in cell proliferation and angiogenesis.
5. PACAP, stress and reproduction.
Stress is a major cause of reproductive dysfunction (108), and there is considerable evidence linking PACAP to the stress response (109). PACAP and PAC1-R are highly expressed in the amygdala and the bed nucleus of the stria terminalis (BNST), together with corticotropin-releasing hormone (CRH) and other stress-related peptides (110). When adult male rats were exposed to a 7-day variate stress paradigm, PACAP mRNA was markedly increased in the BNST (111). PACAP produces stresslike effects in rats when injected icv or specifically into the paraventricular nucleus (PVN) or the central amygdala, and potentiates acoustic startle when injected into the BNST (111–114). In a comprehensive series of experiments, Eiden et al have shown that PACAP functions in the response to stress as a neurotransmitter to CRF-ACTH (115), and is an activator of catecholamine synthesis and secretion (116). Restraint stress increased hypothalamic CRH mRNA as well as fos and Egr1 in hypothalamic sections from wild-type mice but not in mice deficient in PACAP (117) in which the corticosterone response was also partially attenuated [31]. Many lines of evidence indicate that stress suppresses GnRH secretion by activating the CRF system as well as sympathoadrenal pathways (118,119) which may be linked to effects of PACAP. For example, the combination of a CRF antagonist and naloxone partially restored LH secretion in female rats in which LH was suppressed by PACAP on proestrus (120).
PACAP may also play a role in stress-associated reproductive dysfunction through it effect to suppress food intake (121,122), which may indicate a role for PACAP in eating disorders, anxiety and depression, as well as the risk for drug and alcohol abuse (123). Furthermore, a SNP in a putative estrogen response element in PAC1-R has been associated with posttraumatic stress disorder in heavily traumatized humans (124).
6. Pituitary PACAP and the control of gonadotrophs in the fetus and newborn
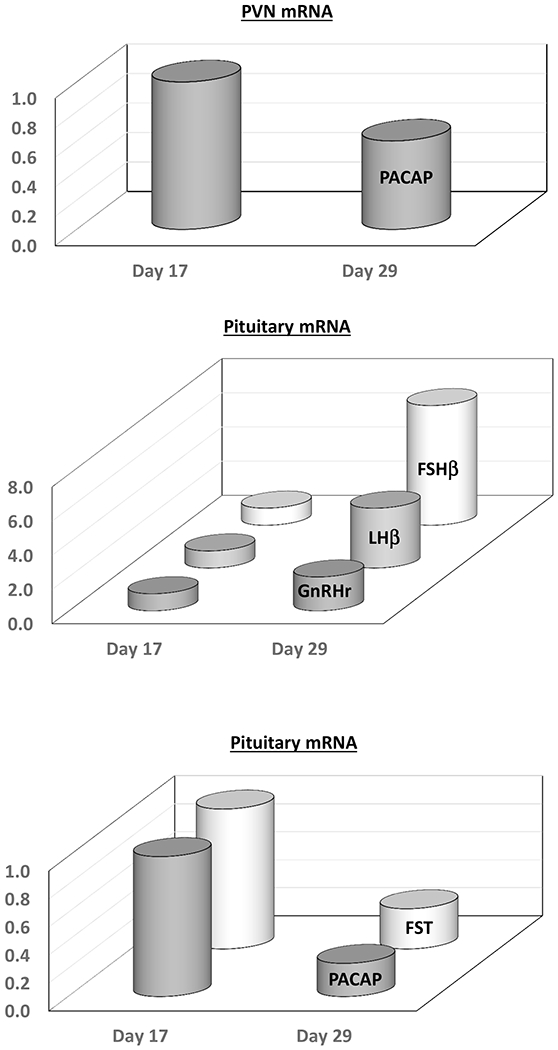
Targeted pituitary PACAP overexpression in male mice (1) delayed sexual maturation associated with a suppression of gonadotropin subunit and Gnrhr mRNAs, and a large increase in follistatin expression. In male rats, there is a significant decline in pituitary PACAP mRNA expression between postnatal days 17 and 21 (125). This decline is paralleled by a significant decline in pituitary follistatin expression and a reciprocal and preferential rise in circulating FSH and pituitary Fshb mRNA levels. These data, summarized in Figure 4, support the notion that alterations in pituitary PACAP expression are involved in developmental changes in gonadotropin synthesis during key periods of sexual maturation.
Figure 4.
Hypothalamic (PVN) and pituitary PACAP expression in the male rat during early maturation. PACAP expression in the PVN and pituitary decrease significantly between postnatal days 17 and 29. There is parallel decrease in pituitary follistatin (FST) expression and a reciprocal rise for the gonadotropin subunits (LHb and Fshb) and GnRH-R.
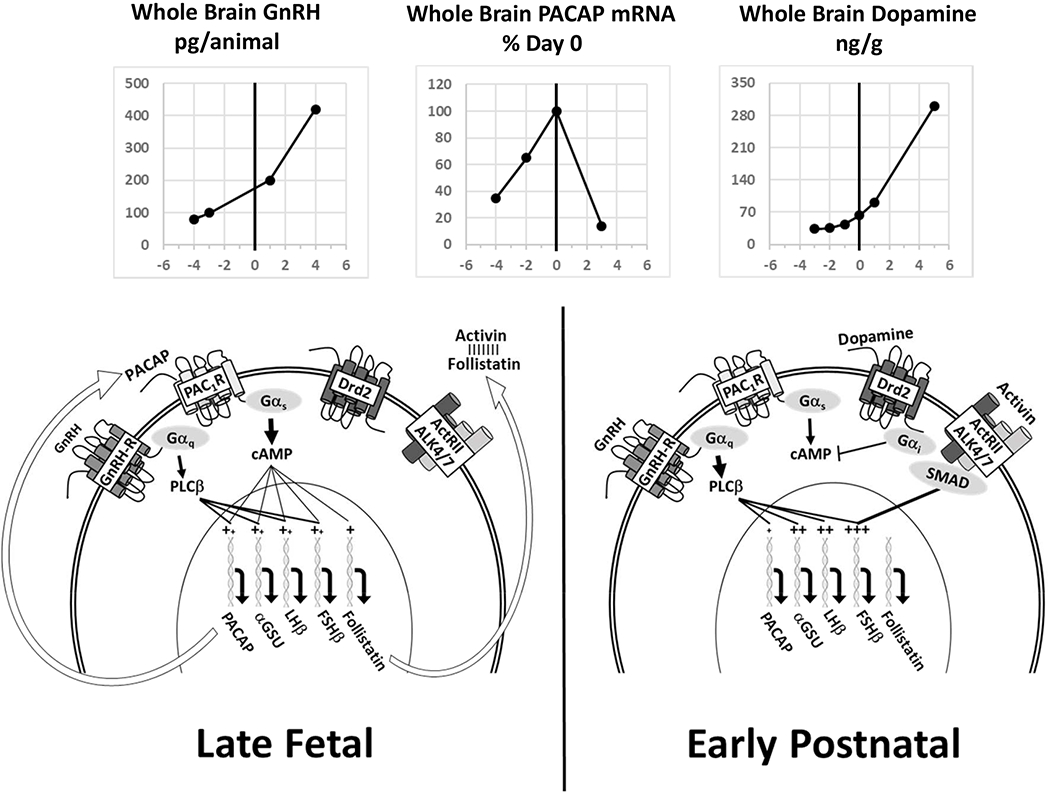
A more substantial change in pituitary PACAP expression is observed during the perinatal period (Figure 5). During rodent embryonic development, pituitary LH immunoreactivity is detectable as early as embryonic day 12 (E12) while FSH is not found until E19 or E21 (126,127). We identified a significant and abrupt decline in pituitary PACAP expression at or around the time of birth (128). A coincident decrease in pituitary follistatin expression and reciprocal increase in FSHb and GnRH-R expression were also observed. Furthermore, in rat pituitary cell cultures, exposure to a potent PACAP antagonist decreased basal LH secretion in both pre- and postnatal cultures, decreased aGSU expression, but selectively increased Fshb mRNA levels only in cultures from prenatal animals. These results suggest that high levels of PACAP expression in the fetal pituitary preferentially suppress FSH synthesis through stimulation of follistatin expression. This hypothesis is supported further by the observation that pituitary PACAP expression is significantly higher in postnatal day 1 females when compared to males, and their post-natal rise in FSH secretion (129) and FSH expression is delayed.
Figure 5.
Schematic representation of a model elucidating the possible role of pituitary PACAP expression in the ontogeny of sexual development in the perinatal period. In late fetal life, pituitary PACAP expression is very high and stimulates expression of αGSU and LHb. PACAP stimulation also produces high levels of follistatin that effectively block activin signaling to GnRH-R and fshb. As the hypothalamic dopaminergic system develops, increased dopamine exposure activates type 2 dopamine receptors (Drd2) and thereby Gαi which suppresses cAMP production and block the PACAP-stimulated cAMP feed-forward mechanism. As PACAP signaling declines, follistatin production is reduced, and activin is freed to stimulate GnRH-R and FSH synthesis, among other genes. The top graphs depict the rep orted changes in brain levels of GnRH (155), PACAP (156) and dopamine (132).
In subsequent experiments designed to identify potential factors that could inhibit pituitary PACAP expression during the perinatal period, we identified activation of dopamine-2 receptor (Drd2) signaling as a mechanism that may explain the pronounced and abrupt decline in PACAP expression in the perinatal pituitary (130). Drd2 is present on the membranes of rat gonadotrophs, and Drd2 mRNA was detected in individual gonadotrophs from postnatal day 1 rats (130,131). In cultures of E19 rat pituitaries, adding the Drd2 agonist, bromocriptine, significantly reduced PACAP mRNA expression in a dose-dependent manner. Conversely, daily subcutaneous injections of the dopamine antagonist, haloperidol from postnatal day 1 to 3 in rats, partially reversed the early postnatal decline in pituitary PACAP expression. These data suggest that the perinatal decline in pituitary PACAP may be mediated by the dramatic increases in brain dopamine levels which occur just prior to the time of birth (132). This sequence of events is summarized in Figure 5.
Additional studies using αT3-1 and LβT2 gonadotroph cells were performed to address the mechanism by which Drd2 signaling may suppress PACAP expression. Drd2 mRNA and peptide are present in αT3-1 and LβT2 cells (133,134). Furthermore, αT3-1 cells express high levels of PACAP and PAC1-R mRNA whereas LβT-2 cells express low levels of PAC1-R and PACAP is nearly undetectable (51,66,135,136) mimicking the differences in PACAP expression observed in late fetal and early postnatal pituitaries (128). In αT3–1 cell cultures, the Drd2 agonist, bromocriptine, produced a dose- and time-dependent decrease in PACAP mRNA levels and suppressed the activity of a transiently transfected PACAP promoter-reporter construct (130). Arimura and colleagues discovered PACAP based on its ability to stimulate cAMP production in the pituitary, and PACAP is known to stimulate its own production through a cAMP response element in the PACAP promoter (Moore et al., in press). Bromocriptine also decreased PACAP-stimulated cAMP production in αT3–1 cells suggesting that Drd2 coupling to Gαl, and the resultant inhibition of adenyl cyclase activity, may be the signal that interrupts a feed-forward mechanism in which PACAP will self-regulate and maintain high levels of pituitary PACAP expression until interrupted by Drd2 signaling at the time of birth. More studies are needed to confirm this hypothesis.
7. Summary and future considerations
PACAP is an ancestral protein which functions as a paracrine regulator in the CNS, pituitary and gonads, and may be a hypophysiotropic factor. Many intriguing findings have been summarized in this chapter, yet there are substantial knowledge gaps in understanding PACAP’s role in reproduction. While many studies demonstrate in vitro effects, some observations are inconsistent or contradictory, and there are few in vivo studies in which there are sex- and species-differences. The diversity in PACAP actions is partly due to its multiple receptors and signaling pathways which extend its therapeutic potential but raise suspicion about the impact of lack of specificity. Moreover, the wide distribution and actions of the peptide and its receptors can limit the interpretation of results. Specifically, neonatal mortality and sensitivity to stress in global PACAP knock-out mice may have mediated many of the reported negative effects on reproduction, and tissue-specific knock-out models are needed. The role of PACAP in the estrus cycle remains to be defined. Our results suggest that PACAP may be especially important in the fetus. Perhaps the strongest evidence identifies a PACAP feed-forward mechanism that controls gonadotropin subunit and Gnrhr gene expression through follistatin and activin signaling in the fetal-newborn transition, at least in rodents. While PACAP may be involved in a wide array of human diseases from neurodegenerative disorders to cancer, there are presently no human disease conditions that are clearly linked to PACAP or its receptors. Research on this important molecule continues in a wide variety of biological systems.
Highlights.
While originally identified as a potential hypophysiotropic factor, PACAP and its receptors are co-expressed in the pituitary and gonads, implying a paracrine role in reproductive functioning.
PACAP stimulates expression of each of the gonadotropin subunit genes while pronounced stimulation of follistatin blocks activin signaling to suppress FSHb-and GnRH-R expression.
PACAP stimulates its own promoter through a cAMP mechanism establishing a feed forward mechanism allowing for rapid changes in expression levels.
Pituitary PACAP and follistatin expression decline rapidly at or around the time of birth in rodents allowing GnRH-R and FSH-b expression to increase and initiate sexual maturation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore JP Jr., Yang RQ, and Winters SJ (2012) Targeted pituitary overexpression of pituitary adenylate-cyclase activating polypeptide alters postnatal sexual maturation in male mice. Endocrinology 153, 1421–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, and Vilain E (2006) [A neuropeptide at the origin of testicular aging?]. Med Sci (Paris) 22, 809–811 [DOI] [PubMed] [Google Scholar]
- 3.Adams BA, Gray SL, Isaac ER, Bianco AC, Vidal-Puig AJ, and Sherwood NM (2008) Feeding and metabolism in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology 149, 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shintani N, Mori W, Hashimoto H, Imai M, Tanaka K, Tomimoto S, Hirose M, Kawaguchi C, and Baba A (2002) Defects in reproductive functions in PACAP-deficient female mice. Regul Pept 109, 45–48 [DOI] [PubMed] [Google Scholar]
- 5.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, and Coy DH (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164, 567–574 [DOI] [PubMed] [Google Scholar]
- 6.Sherwood NM, Krueckl SL, and McRory JE (2000) The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 21, 619–670 [DOI] [PubMed] [Google Scholar]
- 7.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, and Vaudry H (2009) Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61, 283–357 [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto H, Hagihara N, Koga K, Yamamoto K, Shintani N, Tomimoto S, Mori W, Koyama Y, Matsuda T, and Baba A (2000) Synergistic induction of pituitary adenylate cyclase-activating polypeptide (PACAP) gene expression by nerve growth factor and PACAP in PC12 cells. J Neurochem 74, 501–507 [DOI] [PubMed] [Google Scholar]
- 9.Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, and Kitada C (1991) Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129, 2787–2789 [DOI] [PubMed] [Google Scholar]
- 10.Mikkelsen JD, Hannibal J, Fahrenkrug J, Larsen PJ, Olcese J, and McArdle C (1995) Pituitary adenylate cyclase activating peptide-38 (PACAP-38), PACAP-27, and PACAP related peptide (PRP) in the rat median eminence and pituitary. J Neuroendocrinol 7, 47–55 [DOI] [PubMed] [Google Scholar]
- 11.Dow RC, Bennie J, and Fink G (1994) Pituitary adenylate cyclase-activating peptide-38 (PACAP)-38 is released into hypophysial portal blood in the normal male and female rat. J Endocrinol 142, R1–4 [DOI] [PubMed] [Google Scholar]
- 12.Sarkar DK, and Minami S (1995) Diurnal variation in luteinizing hormone-releasing hormone and beta-endorphin release in pituitary portal plasma during the rat estrous cycle. Biol Reprod 53, 38–45 [DOI] [PubMed] [Google Scholar]
- 13.Borzsei R, Mark L, Tamas A, Bagoly T, Bay C, Csanaky K, Banki E, Kiss P, Vaczy A, Horvath G, Nemeth J, Szauer E, Helyes Z, and Reglodi D (2009) Presence of pituitary adenylate cyclase activating polypeptide-38 in human plasma and milk. Eur J Endocrinol 160, 561–565 [DOI] [PubMed] [Google Scholar]
- 14.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, Schmidt K, Bagchi A, Griffin PR, Thornberry NA, and Sinha Roy R (2003) The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38). J Biol Chem 278, 22418–22423 [DOI] [PubMed] [Google Scholar]
- 15.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, and Journot L (1993) Differential signal transduction by five splice variants of the PACAP receptor. Nature 365, 170–175 [DOI] [PubMed] [Google Scholar]
- 16.Rawlings SR, and Hezareh M (1996) Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland. Endocr Rev 17, 4–29 [DOI] [PubMed] [Google Scholar]
- 17.Garrel G, Lozach A, Bachir LK, Laverriere JN, and Counis R (2002) Pituitary adenylate cyclase-activating polypeptide stimulates nitric-oxide synthase type I expression and potentiates the cGMP response to gonadotropin-releasing hormone of rat pituitary gonadotrophs. J Biol Chem 277, 46391–46401 [DOI] [PubMed] [Google Scholar]
- 18.Shintani Y, Hayata-Takano A, Moriguchi K, Nakazawa T, Ago Y, Kasai A, Seiriki K, Shintani N, and Hashimoto H (2018) beta-Arrestin1 and 2 differentially regulate PACAP-induced PAC1 receptor signaling and trafficking. PLoS One 13, e0196946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirabayashi T, Nakamachi T, and Shioda S (2018) Discovery of PACAP and its receptors in the brain. J Headache Pain 19, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao C, de Molliens MP, Schneebeli ST, Brewer M, Song G, Chatenet D, Braas KM, May V, and Li J (2019) Targeting the PAC1 Receptor for Neurological and Metabolic Disorders. Curr Top Med Chem 19, 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denes V, Geck P, Mester A, and Gabriel R (2019) Pituitary Adenylate Cyclase-Activating Polypeptide: 30 Years in Research Spotlight and 600 Million Years in Service. J Clin Med 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannibal J (2002) Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. The Journal of comparative neurology 453, 389–417 [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, and Baba A (1996) Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol 371, 567–577 [DOI] [PubMed] [Google Scholar]
- 24.Piggins HD, Stamp JA, Burns J, Rusak B, and Semba K (1996) Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J Comp Neurol 376, 278–294 [DOI] [PubMed] [Google Scholar]
- 25.Li S, Grinevich V, Fournier A, and Pelletier G (1996) Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on gonadotropin-releasing hormone and somatostatin gene expression in the rat brain. Brain Res Mol Brain Res 41, 157–162 [DOI] [PubMed] [Google Scholar]
- 26.Olcese J, McArdle CA, Middendorff R, and Greenland K (1997) Pituitary adenylate cyclase-activating peptide and vasoactive intestinal peptide receptor expression in immortalized LHRH neurons. J Neuroendocrinol 9, 937–943 [DOI] [PubMed] [Google Scholar]
- 27.Kanasaki H, Mijiddorj T, Sukhbaatar U, Oride A, and Miyazaki K (2013) Pituitary adenylate cyclase-activating polypeptide (PACAP) increases expression of the gonadotropin-releasing hormone (GnRH) receptor in GnRH-producing GT1–7 cells overexpressing PACAP type I receptor. Gen Comp Endocrinol 193, 95–102 [DOI] [PubMed] [Google Scholar]
- 28.Tumurbaatar T, Kanasaki H, Oride A, Okada H, Hara T, Tumurgan Z, and Kyo S (2019) Effect of pituitary adenylate cyclase-activating polypeptide (PACAP) in the regulation of hypothalamic kisspeptin expression. Gen Comp Endocrinol 270, 60–66 [DOI] [PubMed] [Google Scholar]
- 29.Moore JP Jr., Burger LL, Dalkin AC, and Winters SJ (2005) Pituitary adenylate cyclase activating polypeptide messenger RNA in the paraventricular nucleus and anterior pituitary during the rat estrous cycle. Biol Reprod 73, 491–499 [DOI] [PubMed] [Google Scholar]
- 30.Koves K, Kantor O, Lakatos A, Szabo E, Kirilly E, Heinzlmann A, and Szabo F (2014) Advent and recent advances in research on the role of pituitary adenylate cyclase-activating polypeptide (PACAP) in the regulation of gonadotropic hormone secretion of female rats. J Mol Neurosci 54, 494–511 [DOI] [PubMed] [Google Scholar]
- 31.Osuga Y, Mitsuhashi N, and Mizuno M (1992) In vivo effect of pituitary adenylate cyclase activating polypeptide 38 (PACAP 38) on the secretion of luteinizing hormone (LH) in male rats. Endocrinol Jpn 39, 153–156 [DOI] [PubMed] [Google Scholar]
- 32.Koves K, Molnar J, Kantor O, Lakatos A, Gorcs TJ, Somogyvari-Vigh A, Furst Z, and Arimura A (1996) PACAP participates in the regulation of the hormonal events preceeding the ovulation. Acta Biol Hung 47, 239–249 [PubMed] [Google Scholar]
- 33.Kantora O, Molnar J, Arimura A, and Koves K (2000) PACAP38 and PACAP27 administered intracerebroventricularly have an opposite effect on LH secretion. Peptides 21, 817–820 [DOI] [PubMed] [Google Scholar]
- 34.Choi EJ, Ha CM, Kim MS, Kang JH, Park SK, Choi WS, Kang SG, and Lee BJ (2000) Central administration of an antisense oligodeoxynucleotide against type I pituitary adenylate cyclase-activating polypeptide receptor suppresses synthetic activities of LHRH-LH axis during the pubertal process. Brain Res Mol Brain Res 80, 35–45 [DOI] [PubMed] [Google Scholar]
- 35.Szabo F, Horvath J, Heinzlmann A, Arimura A, and Koves K (2002) Neonatal PACAP administration in rats delays puberty through the influence of the LHRH neuronal system. Regul Pept 109, 49–55 [DOI] [PubMed] [Google Scholar]
- 36.Isaac ER, and Sherwood NM (2008) Pituitary adenylate cyclase-activating polypeptide (PACAP) is important for embryo implantation in mice. Mol Cell Endocrinol 280, 13–19 [DOI] [PubMed] [Google Scholar]
- 37.Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, and Vilain E (2006) Delayed testicular aging in pituitary adenylate cyclase-activating peptide (PACAP) null mice. Proc Natl Acad Sci U S A 103, 3793–3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamen F, Rodriguez-Henche N, Pralong F, Jegou B, Gaillard R, Bockaert J, and Brabet P (2000) PAC1 null females display decreased fertility. Ann N Y Acad Sci 921, 400–404 [DOI] [PubMed] [Google Scholar]
- 39.Hannibal J, Jamen F, Nielsen HS, Journot L, Brabet P, and Fahrenkrug J (2001) Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J Neurosci 21, 4883–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vu JP, Goyal D, Luong L, Oh S, Sandhu R, Norris J, Parsons W, Pisegna JR, and Germano PM (2015) PACAP intraperitoneal treatment suppresses appetite and food intake via PAC1 receptor in mice by inhibiting ghrelin and increasing GLP-1 and leptin. Am J Physiol Gastrointest Liver Physiol 309, G816–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanida M, Hayata A, Shintani N, Yamamoto N, Kurata Y, Shibamoto T, Morgan DA, Rahmouni K, and Hashimoto H (2013) Central PACAP mediates the sympathetic effects of leptin in a tissue-specific manner. Neuroscience 238, 297–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahima RS, Saper CB, Flier JS, and Elmquist JK (2000) Leptin regulation of neuroendocrine systems. Frontiers in neuroendocrinology 21, 263–307. [DOI] [PubMed] [Google Scholar]
- 43.Ross RA, Leon S, Madara JC, Schafer D, Fergani C, Maguire CA, Verstegen AM, Brengle E, Kong D, Herbison AE, Kaiser UB, Lowell BB, and Navarro VM (2018) PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. Elife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shioda S, Yada T, Nakajo S, Nakaya K, Nakai Y, and Arimura A (1997) Pituitary adenylate cyclase-activating polypeptide (PACAP): a novel regulator of vasopressin-containing neurons. Brain Res 765, 81–90 [DOI] [PubMed] [Google Scholar]
- 45.Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, and Bloom SR (1993) Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol 136, 159–166 [DOI] [PubMed] [Google Scholar]
- 46.Koves K, Kantor O, Scammell JG, and Arimura A (1998) PACAP colocalizes with luteinizing and follicle-stimulating hormone immunoreactivities in the anterior lobe of the pituitary gland. Peptides 19, 1069–1072 [DOI] [PubMed] [Google Scholar]
- 47.Jin L, Tsumanuma I, Ruebel KH, Bayliss JM, and Lloyd RV (2001) Analysis of homogeneous populations of anterior pituitary folliculostellate cells by laser capture microdissection and reverse transcription-polymerase chain reaction. Endocrinology 142, 1703–1709 [DOI] [PubMed] [Google Scholar]
- 48.Devnath S, and Inoue K (2008) An insight to pituitary folliculo-stellate cells. J Neuroendocrinol 20, 687–691 [DOI] [PubMed] [Google Scholar]
- 49.Chauvet N, El-Yandouzi T, Mathieu MN, Schlernitzauer A, Galibert E, Lafont C, Le Tissier P, Robinson IC, Mollard P, and Coutry N (2009) Characterization of adherens junction protein expression and localization in pituitary cell networks. J Endocrinol 202, 375–387 [DOI] [PubMed] [Google Scholar]
- 50.Vigh S, Arimura A, Gottschall PE, Kitada C, Somogyvari-Vigh A, and Childs GV (1993) Cytochemical characterization of anterior pituitary target cells for the neuropeptide, pituitary adenylate cyclase activating polypeptide (PACAP), using biotinylated ligands. Peptides 14, 59–65 [DOI] [PubMed] [Google Scholar]
- 51.Rawlings SR, Piuz I, Schlegel W, Bockaert J, and Journot L (1995) Differential expression of pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptor subtypes in clonal pituitary somatotrophs and gonadotrophs. Endocrinology 136, 2088–2098 [DOI] [PubMed] [Google Scholar]
- 52.Culler MD, and Paschall CS (1991) Pituitary adenylate cyclase-activating polypeptide (PACAP) potentiates the gonadotropin-releasing activity of luteinizing hormone-releasing hormone. Endocrinology 129, 2260–2262 [DOI] [PubMed] [Google Scholar]
- 53.Jarry H, Leonhardt S, Schmidt WE, Creutzfeldt W, and Wuttke W (1992) Contrasting effects of pituitary adenylate cyclase activating polypeptide (PACAP) on in vivo and in vitro prolactin and growth hormone release in male rats. Life Sci 51, 823–830 [DOI] [PubMed] [Google Scholar]
- 54.Hart GR, Gowing H, and Burrin JM (1992) Effects of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, on pituitary hormone release in rats. J Endocrinol 134, 33–41 [DOI] [PubMed] [Google Scholar]
- 55.Sawangjaroen K, Anderson ST, and Curlewis JD (1997) Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal polypeptide (VIP) on hormone secretion from sheep pituitary cells in vitro. J Neuroendocrinol 9, 279–286 [DOI] [PubMed] [Google Scholar]
- 56.Lin C, Jiang X, He M, Zhao L, Huang T, Bian Z, and Wong AO (2017) Mechanisms for PACAP-induced prolactin gene expression in grass carp pituitary cells. J Endocrinol 233, 37–51 [DOI] [PubMed] [Google Scholar]
- 57.Propato-Mussafiri R, Kanse SM, Ghatei MA, and Bloom SR (1992) Pituitary adenylate cyclase-activating polypeptide releases 7B2, adrenocorticotrophin, growth hormone and prolactin from the mouse and rat clonal pituitary cell lines AtT-20 and GH3. J Endocrinol 132, 107–113 [DOI] [PubMed] [Google Scholar]
- 58.Koch B, and Lutz-Bucher B (1992) Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates cyclic AMP formation as well as peptide output of cultured pituitary melanotrophs and AtT-20 corticotrophs. Regul Pept 38, 45–53 [DOI] [PubMed] [Google Scholar]
- 59.Tsujii T, and Winters SJ (1995) Effects of pulsatile pituitary adenylate cyclase activating polypeptide (PACAP) on gonadotropin secretion and subunit mRNA levels in perifused rat pituitary cells. Life Sci 56, 1103–1111 [DOI] [PubMed] [Google Scholar]
- 60.Tsujii T, Ishizaka K, and Winters SJ (1994) Effects of pituitary adenylate cyclase-activating polypeptide on gonadotropin secretion and subunit messenger ribonucleic acids in perifused rat pituitary cells. Endocrinology 135, 826–833 [DOI] [PubMed] [Google Scholar]
- 61.Windle JJ, Weiner RI, and Mellon PL (1990) Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4, 597–603 [DOI] [PubMed] [Google Scholar]
- 62.Schomerus E, Poch A, Bunting R, Mason WT, and McArdle CA (1994) Effects of pituitary adenylate cyclase-activating polypeptide in the pituitary: activation of two signal transduction pathways in the gonadotrope-derived alpha T3-1 cell line. Endocrinology 134, 315–323 [DOI] [PubMed] [Google Scholar]
- 63.Tsujii T, Attardi B, and Winters SJ (1995) Regulation of alpha-subunit mRNA transcripts by pituitary adenylate cyclase-activating polypeptide (PACAP) in pituitary cell cultures and alpha T3-1 cells. Mol Cell Endocrinol 113, 123–130 [DOI] [PubMed] [Google Scholar]
- 64.Attardi B, and Winters SJ (1998) Transcriptional regulation of the glycoprotein hormone alpha-subunit gene by pituitary adenylate cyclase-activating polypeptide (PACAP) in alphaT3-1 cells. Mol Cell Endocrinol 137, 97–107 [DOI] [PubMed] [Google Scholar]
- 65.Thomas P, Mellon PL, Turgeon J, and Waring DW (1996) The L beta T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137, 2979–2989 [DOI] [PubMed] [Google Scholar]
- 66.Fujii Y, Okada Y, Moore JP Jr., Dalkin AC, and Winters SJ (2002) Evidence that PACAP and GnRH down-regulate follicle-stimulating hormone-beta mRNA levels by stimulating follistatin gene expression: effects on folliculostellate cells, gonadotrophs and LbetaT2 gonadotroph cells. Mol Cell Endocrinol 192, 55–64 [DOI] [PubMed] [Google Scholar]
- 67.Kanasaki H, Mutiara S, Oride A, Purwana IN, and Miyazaki K (2009) Pulse frequency-dependent gonadotropin gene expression by adenylate cyclase-activating polypeptide 1 in perifused mouse pituitary gonadotroph LbetaT2 cells. Biol Reprod 81, 465–472 [DOI] [PubMed] [Google Scholar]
- 68.Ferris HA, Walsh HE, Stevens J, Fallest PC, and Shupnik MA (2007) Luteinizing hormone beta promoter stimulation by adenylyl cyclase and cooperation with gonadotropin-releasing hormone 1 in transgenic mice and LBetaT2 Cells. Biol Reprod 77, 1073–1080 [DOI] [PubMed] [Google Scholar]
- 69.Horton CD, and Halvorson LM (2004) The cAMP signaling system regulates LHbeta gene expression: roles of early growth response protein-1, SP1 and steroidogenic factor-1. J Mol Endocrinol 32, 291–306 [DOI] [PubMed] [Google Scholar]
- 70.Winters SJ, Dalkin AC, and Tsujii T (1997) Evidence that pituitary adenylate cyclase activating polypeptide suppresses follicle-stimulating hormone-beta messenger ribonucleic acid levels by stimulating follistatin gene transcription. Endocrinology 138, 4324–4329 [DOI] [PubMed] [Google Scholar]
- 71.Yeh DM, and Coss D (2019) PACAP induces FSHbeta gene expression via EPAC. Mol Cell Endocrinol 492, 110438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fortin J, Ongaro L, Li Y, Tran S, Lamba P, Wang Y, Zhou X, and Bernard DJ (2015) Minireview: Activin Signaling in Gonadotropes: What Does the FOX say... to the SMAD? Mol Endocrinol 29, 963–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pincas H, Laverriere JN, and Counis R (2001) Pituitary adenylate cyclase-activating polypeptide and cyclic adenosine 3’,5’-monophosphate stimulate the promoter activity of the rat gonadotropin-releasing hormone receptor gene via a bipartite response element in gonadotrope-derived cells. J Biol Chem 276, 23562–23571 [DOI] [PubMed] [Google Scholar]
- 74.Halvorson LM (2014) PACAP modulates GnRH signaling in gonadotropes. Mol Cell Endocrinol 385, 45–55 [DOI] [PubMed] [Google Scholar]
- 75.Garrel G, Lerrant Y, Siriostis C, Berault A, Magre S, Bouchaud C, and Counis R (1998) Evidence that gonadotropin-releasing hormone stimulates gene expression and levels of active nitric oxide synthase type I in pituitary gonadotrophs, a process altered by desensitization and, indirectly, by gonadal steroids. Endocrinology 139, 2163–2170 [DOI] [PubMed] [Google Scholar]
- 76.Zheng W, Grafer CM, and Halvorson LM (2014) Interaction of gonadal steroids and gonadotropin-releasing hormone on pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP receptor expression in cultured rat anterior pituitary cells. ReprodSci 21, 41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Purwana IN, Kanasaki H, Oride A, Mijiddorj T, Shintani N, Hashimoto H, Baba A, and Miyazaki K GnRH-induced PACAP and PAC1 receptor expression in pituitary gonadotrophs: A possible role in the regulation of gonadotropin subunit gene expression. Peptides [DOI] [PubMed] [Google Scholar]
- 78.McArdle CA, Poch A, Schomerus E, and Kratzmeier M (1994) Pituitary adenylate cyclase-activating polypeptide effects in pituitary cells: modulation by gonadotropin-releasing hormone in alpha T3-1 cells. Endocrinology 134, 2599–2605 [DOI] [PubMed] [Google Scholar]
- 79.Lariviere S, Garrel-Lazayres G, Simon V, Shintani N, Baba A, Counis R, and Cohen-Tannoudji J (2008) Gonadotropin-releasing hormone inhibits pituitary adenylyl cyclase-activating polypeptide coupling to 3’,5’-cyclic adenosine-5’-monophosphate pathway in LbetaT2 gonadotrope cells through novel protein kinase C isoforms and phosphorylation of pituitary adenylyl cyclase-activating polypeptide type I receptor. Endocrinology 149, 6389–6398 [DOI] [PubMed] [Google Scholar]
- 80.Winters SJ, Ghooray D, Fujii Y, Moore JP Jr., Nevitt JR, and Kakar SS (2007) Transcriptional regulation of follistatin expression by GnRH in mouse gonadotroph cell lines: evidence for a role for cAMP signaling. Mol Cell Endocrinol 271, 45–54 [DOI] [PubMed] [Google Scholar]
- 81.Nakamura K, Nakamachi T, Endo K, Ito K, Machida T, Oka T, Hori M, Ishizaka K, and Shioda S (2014) Distribution of pituitary adenylate cyclase-activating polypeptide (PACAP) in the human testis and in testicular germ cell tumors. Andrologia 46, 465–471 [DOI] [PubMed] [Google Scholar]
- 82.Hannibal J, and Fahrenkrug J (1995) Expression of pituitary adenylate cyclase activating polypeptide (PACAP) gene by rat spermatogenic cells. Regul Pept 55, 111–115 [DOI] [PubMed] [Google Scholar]
- 83.Shioda S, Legradi G, Leung WC, Nakajo S, Nakaya K, and Arimura A (1994) Localization of pituitary adenylate cyclase-activating polypeptide and its messenger ribonucleic acid in the rat testis by light and electron microscopic immunocytochemistry and in situ hybridization. Endocrinology 135, 818–825 [DOI] [PubMed] [Google Scholar]
- 84.Lv CM, Cheng DL, Zhao W, and Zhu H (2011) Pituitary adenylate cyclase-activating polypeptide mRNA expression in rat testis and epididymis during postnatal development and experimental cryptorchidism. Mol Med Rep 4, 793–798 [DOI] [PubMed] [Google Scholar]
- 85.Daniel PB, and Habener JF (2000) Pituitary adenylate cyclase-activating polypeptide gene expression regulated by a testis-specific promoter in germ cells during spermatogenesis. Endocrinology 141, 1218–1227 [DOI] [PubMed] [Google Scholar]
- 86.Tominaga A, Sugawara H, Futagawa T, Inoue K, Sasaki K, Minamino N, Hatakeyama M, Handa H, and Miyata A Characterization of the testis-specific promoter region in the human pituitary adenylate cyclase-activating polypeptide (PACAP) gene. Genes Cells 15, 595–606 [DOI] [PubMed] [Google Scholar]
- 87.Krempels K, Usdin TB, Harta G, and Mezey E (1995) PACAP acts through VIP type 2 receptors in the rat testis. Neuropeptides 29, 315–320 [DOI] [PubMed] [Google Scholar]
- 88.Li M, Funahashi H, Mbikay M, Shioda S, and Arimura A (2004) Pituitary adenylate cyclase activating polypeptide-mediated intracrine signaling in the testicular germ cells. Endocrine 23, 59–75 [DOI] [PubMed] [Google Scholar]
- 89.Prisco M, Rosati L, Morgillo E, Mollica MP, Agnese M, Andreuccetti P, and Valiante S (2020) Pituitary adenylate cyclase-activating peptide (PACAP) and its receptors in Mus musculus testis. Gen Comp Endocrinol 286, 113297. [DOI] [PubMed] [Google Scholar]
- 90.Asnicar MA, Koster A, Heiman ML, Tinsley F, Smith DP, Galbreath E, Fox N, Ma YL, Blum WF, and Hsiung HM (2002) Vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptor 2 deficiency in mice results in growth retardation and increased basal metabolic rate. Endocrinology 143, 3994–4006 [DOI] [PubMed] [Google Scholar]
- 91.Daniel PB, Kieffer TJ, Leech CA, and Habener JF (2001) Novel alternatively spliced exon in the extracellular ligand-binding domain of the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) selectively increases ligand affinity and alters signal transduction coupling during spermatogenesis. J Biol Chem 276, 12938–12944 [DOI] [PubMed] [Google Scholar]
- 92.Monts BS, Breyer PR, Rothrock JK, and Pescovitz OH (1996) Peptides of the growth hormone-releasing hormone family : Differential expression in rat testis. Endocrine 4, 73–78 [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto S, Arakawa Y, Ohishi M, Yanaihara H, Iwanaga T, and Kurokawa N (2008) Suppressive action of pituitary adenylate cyclase activating polypeptide (PACAP) on proliferation of immature mouse Leydig cell line TM3 cells. Biomed Res 29, 321–330 [DOI] [PubMed] [Google Scholar]
- 94.Heindel JJ, Powell CJ, Paschall CS, Arimura A, and Culler MD (1992) A novel hypothalamic peptide, pituitary adenylate cyclase activating peptide, modulates Sertoli cell function in vitro. Biol Reprod 47, 800–806 [DOI] [PubMed] [Google Scholar]
- 95.El-Gehani F, Tena-Sempere M, and Huhtaniemi I (2000) Evidence that pituitary adenylate cyclase-activating polypeptide is a potent regulator of fetal rat testicular steroidogenesis. Biol Reprod 63, 1482–1489 [DOI] [PubMed] [Google Scholar]
- 96.Brubel R, Kiss P, Vincze A, Varga A, Varnagy A, Bodis J, Mark L, Jambor E, Maasz G, Hashimoto H, Helyes Z, Toth G, Tamas A, Koppan M, and Reglodi D (2012) Effects of pituitary adenylate cyclase activating polypeptide on human sperm motility. J Mol Neurosci 48, 623–630 [DOI] [PubMed] [Google Scholar]
- 97.Yan Q, Huang H, Lu S, Ou B, Feng J, Shan W, Li H, Wang Z, Hong A, and Ma Y (2020) PACAP ameliorates fertility in obese male mice via PKA/CREB pathway-dependent Sirt1 activation and p53 deacetylation. J Cell Physiol [DOI] [PubMed] [Google Scholar]
- 98.Reglodi D, Tamas A, Koppan M, Szogyi D, and Welke L (2012) Role of PACAP in Female Fertility and Reproduction at Gonadal Level - Recent Advances. Front Endocrinol (Lausanne) 3, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pesce M, Canipari R, Ferri GL, Siracusa G, and De Felici M (1996) Pituitary adenylate cyclase-activating polypeptide (PACAP) stimulates adenylate cyclase and promotes proliferation of mouse primordial germ cells. Development 122, 215–221 [DOI] [PubMed] [Google Scholar]
- 100.Gräs S, Hannibal J, Georg B, and Fahrenkrug J (1996) Transient periovulatory expression of pituitary adenylate cyclase activating peptide in rat ovarian cells. Endocrinology 137, 4779–4785 [DOI] [PubMed] [Google Scholar]
- 101.Barberi M, Muciaccia B, Morelli MB, Stefanini M, Cecconi S, and Canipari R (2007) Expression localisation and functional activity of pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal polypeptide and their receptors in mouse ovary. Reproduction 134, 281–292 [DOI] [PubMed] [Google Scholar]
- 102.Latini S, Chiarpotto M, Muciaccia B, Vaccari S, Barberi M, Guglielmo MC, Stefanini M, Cecconi S, and Canipari R (2010) Inhibitory effect of pituitary adenylate cyclase activating polypeptide on the initial stages of rat follicle development. Mol Cell Endocrinol 320, 34–44 [DOI] [PubMed] [Google Scholar]
- 103.Vaccari S, Latini S, Barberi M, Teti A, Stefanini M, and Canipari R (2006) Characterization and expression of different pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptors in rat ovarian follicles. J Endocrinol 191, 287–299 [DOI] [PubMed] [Google Scholar]
- 104.Heindel JJ, Sneeden J, Powell CJ, Davis B, and Culler MD (1996) A novel hypothalamic peptide, pituitary adenylate cyclase-activating peptide, regulates the function of rat granulosa cells in vitro. Biol Reprod 54, 523–530 [DOI] [PubMed] [Google Scholar]
- 105.Morelli MB, Barberi M, Gambardella A, Borini A, Cecconi S, Coticchio G, and Canipari R (2008) Characterization, expression, and functional activity of pituitary adenylate cyclase-activating polypeptide and its receptors in human granulosa-luteal cells. J Clin Endocrinol Metab 93, 4924–4932 [DOI] [PubMed] [Google Scholar]
- 106.Kotani E, Usuki S, and Kubo T (1997) Rat corpus luteum expresses both PACAP and PACAP type IA receptor mRNAs. Peptides 18, 1453–1455 [DOI] [PubMed] [Google Scholar]
- 107.Horvath G, Reglodi D, Brubel R, Halasz M, Barakonyi A, Tamas A, Fabian E, Opper B, Toth G, Cohen M, and Szereday L (2014) Investigation of the possible functions of PACAP in human trophoblast cells. J Mol Neurosci 54, 320–330 [DOI] [PubMed] [Google Scholar]
- 108.Whirledge S, and Cidlowski JA (2017) Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends in endocrinology and metabolism: TEM 28, 399–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.King SB, Toufexis DJ, and Hammack SE (2017) Pituitary adenylate cyclase activating polypeptide (PACAP), stress, and sex hormones. Stress (Amsterdam, Netherlands) 20, 465–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, and May V (2010) Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J Mol Neurosci 42, 327–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, and May V (2009) Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Agarwal A, Halvorson LM, and Legradi G (2005) Pituitary adenylate cyclase-activating polypeptide (PACAP) mimics neuroendocrine and behavioral manifestations of stress: Evidence for PKA-mediated expression of the corticotropin-releasing hormone (CRH) gene. Brain Res Mol Brain Res 138, 45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Norrholm SD, Das M, and Legradi G (2005) Behavioral effects of local microinfusion of pituitary adenylate cyclase activating polypeptide (PACAP) into the paraventricular nucleus of the hypothalamus (PVN). Regul Pept 128, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, and Diamond DM (2007) Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast 2007, 79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stroth N, Holighaus Y, Ait-Ali D, and Eiden LE (2011) PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Annals of the New York Academy of Sciences 1220, 49–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eiden LE, Emery AC, Zhang L, and Smith CB (2018) PACAP signaling in stress: insights from the chromaffin cell. Pflugers Archiv : European journal of physiology 470, 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stroth N, and Eiden LE (2010) Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience 165, 1025–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li XF, Knox AM, and O’Byrne KT (2010) Corticotrophin-releasing factor and stress-induced inhibition of the gonadotrophin-releasing hormone pulse generator in the female. Brain Res 1364, 153–163 [DOI] [PubMed] [Google Scholar]
- 119.Kageyama K (2013) Regulation of gonadotropins by corticotropin-releasing factor and urocortin. Front Endocrinol (Lausanne) 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kantor O, Molnar J, Heinzlmann A, Arimura A, Furst Z, and Koves K (2001) Study on the hypothalamic factors mediating the inhibitory effect of PACAP38 on ovulation. Peptides 22, 2163–2168 [DOI] [PubMed] [Google Scholar]
- 121.Matsuda K, Azuma M, Maruyama K, and Shioda S (2013) Neuroendocrine control of feeding behavior and psychomotor activity by pituitary adenylate cyclase-activating polypeptide (PACAP) in vertebrates. Obes Res Clin Pract 7, e1–e7 [DOI] [PubMed] [Google Scholar]
- 122.Kocho-Schellenberg M, Lezak KR, Harris OM, Roelke E, Gick N, Choi I, Edwards S, Wasserman E, Toufexis DJ, Braas KM, May V, and Hammack SE (2014) PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology 39, 1614–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gargiulo AT, Curtis GR, and Barson JR (2020) Pleiotropic pituitary adenylate cyclase-activating polypeptide (PACAP): Novel insights into the role of PACAP in eating and drug intake. Brain Res 1729, 146626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, and May V (2011) Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470, 492–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore JP Jr., and Winters SJ (2008) Weaning and the developmental changes in follicle-stimulating hormone, pituitary adenylate cyclase-activating polypeptide, and inhibin B in the male rat. Biol Reprod 78, 752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nemeskeri A, Kurcz M, and Halasz B (1984) Changes in hypophyseal luteinizing hormone (LH) content during fetal and early postnatal life, and capacity of fetal and early postnatal pituitaries to synthesize and release LH in vitro. Neuroendocrinology 38, 393–396 [DOI] [PubMed] [Google Scholar]
- 127.Aubert ML, Begeot M, Winiger BP, Morel G, Sizonenko PC, and Dubois PM (1985) Ontogeny of hypothalamic luteinizing hormone-releasing hormone (GnRH) and pituitary GnRH receptors in fetal and neonatal rats. Endocrinology 116, 1565–1576 [DOI] [PubMed] [Google Scholar]
- 128.Moore JP Jr., Villafuerte BC, Unick CA, and Winters SJ (2009) Developmental Changes in Pituitary PACAP Expression during the Perinatal Period: Possible Role in Fetal Gonadotroph Regulation. Endocrinology 150, 4802–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chowdhury M, and Steinberger E (1976) Pituitary and plasma levels of gonadotrophins in foetal and newborn male and female rats. J Endocrinol 69, 381–384 [DOI] [PubMed] [Google Scholar]
- 130.Winters SJ, Ghooray DT, Yang RQ, Holmes JB, O’Brien AR, Morgan J, and Moore JP Jr. (2014) Dopamine-2 receptor activation suppresses PACAP expression in gonadotrophs. Endocrinology 155, 2647–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goldsmith PC, Cronin MJ, and Weiner RI (1979) Dopamine receptor sites in the anterior pituitary. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society 27, 1205–1207 [DOI] [PubMed] [Google Scholar]
- 132.Hooghe-Peters EL, Belayew A, Herregodts P, Velkeniers B, Smets G, Martial JA, and Vanhaelst L (1988) Discrepancy between prolactin (PRL) messenger ribonucleic acid and PRL content in rat fetal pituitary cells: possible role of dopamine. Mol Endocrinol 2, 1163–1168 [DOI] [PubMed] [Google Scholar]
- 133.Kanasaki H, Yonehara T, Yamada Y, Takahashi K, Hata K, Fujiwaki R, Yamamoto H, Takeuchi Y, Fukunaga K, Miyamoto E, and Miyazaki K (2002) Regulation of gonadotropin alpha subunit gene expression by dopamine D(2) receptor agonist in clonal mouse gonadotroph alphaT3-1 cells. Biol Reprod 67, 1218–1224 [DOI] [PubMed] [Google Scholar]
- 134.Mutiara S, Kanasaki H, Harada T, and Miyazaki K (2006) Dopamine D(2) receptor expression and regulation of gonadotropin alpha-subunit gene in clonal gonadotroph LbetaT2 cells. Mol Cell Endocrinol 259, 22–29 [DOI] [PubMed] [Google Scholar]
- 135.Radleff-Schlimme A, Leonhardt S, Wuttke W, and Jarry H (1998) Evidence for PACAP to be an autocrine factor on gonadotrope cells. Ann N Y Acad Sci 865, 486–491 [DOI] [PubMed] [Google Scholar]
- 136.Purwana IN, Kanasaki H, Oride A, Mijiddorj T, and Miyazaki K (2011) Expression of the pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1R) potentiates the effects of GnRH on gonadotropin subunit gene expression. Biochem Biophys Res Commun 410, 295–300 [DOI] [PubMed] [Google Scholar]
- 137.Winzell MS, and Ahren B (2007) Role of VIP and PACAP in islet function. Peptides 28, 1805–1813 [DOI] [PubMed] [Google Scholar]
- 138.Persson K, and Ahren B (2002) The neuropeptide PACAP contributes to the glucagon response to insulin-induced hypoglycaemia in mice. Acta Physiol Scand 175, 25–28 [DOI] [PubMed] [Google Scholar]
- 139.Tomimoto S, Ojika T, Shintani N, Hashimoto H, Hamagami K, Ikeda K, Nakata M, Yada T, Sakurai Y, Shimada T, Morita Y, Ishida C, and Baba A (2008) Markedly reduced white adipose tissue and increased insulin sensitivity in adcyap1-deficient mice. J Pharmacol Sci 107, 41–48 [DOI] [PubMed] [Google Scholar]
- 140.Gray SL, Cummings KJ, Jirik FR, and Sherwood NM (2001) Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Mol Endocrinol 15, 1739–1747 [DOI] [PubMed] [Google Scholar]
- 141.Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, and Eiden LE (2002) Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A 99, 461–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamamoto K, Hashimoto H, Tomimoto S, Shintani N, Miyazaki J, Tashiro F, Aihara H, Nammo T, Li M, Yamagata K, Miyagawa J, Matsuzawa Y, Kawabata Y, Fukuyama Y, Koga K, Mori W, Tanaka K, Matsuda T, and Baba A (2003) Overexpression of PACAP in transgenic mouse pancreatic beta-cells enhances insulin secretion and ameliorates streptozotocin-induced diabetes. Diabetes 52, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 143.Tomimoto S, Hashimoto H, Shintani N, Yamamoto K, Kawabata Y, Hamagami K, Yamagata K, Miyagawa J, and Baba A (2004) Overexpression of pituitary adenylate cyclase-activating polypeptide in islets inhibits hyperinsulinemia and islet hyperplasia in agouti yellow mice. J Pharmacol Exp Ther 309, 796–803 [DOI] [PubMed] [Google Scholar]
- 144.Sakurai Y, Inoue H, Shintani N, Arimori A, Hamagami K, Hayata-Takano A, Baba A, and Hashimoto H (2012) Compensatory recovery of blood glucose levels in KKA(y) mice fed a high-fat diet: insulin-sparing effects of PACAP overexpression in β cells. J Mol Neurosci 48, 647–653 [DOI] [PubMed] [Google Scholar]
- 145.Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, and Schutz G (2001) Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res 92, 78–84 [DOI] [PubMed] [Google Scholar]
- 146.Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Gröne HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, and Schmtz G (2001) Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci 21, 5520–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stroth N, Liu Y, Aguilera G, and Eiden LE (2011) Pituitary adenylate cyclase-activating polypeptide controls stimulus-transcription coupling in the hypothalamic-pituitary-adrenal axis to mediate sustained hormone secretion during stress. Journal of neuroendocrinology 23, 944–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, and Jegou S (2009) Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology 34, 424–435 [DOI] [PubMed] [Google Scholar]
- 149.Nakata M, Kohno D, Shintani N, Nemoto Y, Hashimoto H, Baba A, and Yada T (2004) PACAP deficient mice display reduced carbohydrate intake and PACAP activates NPY-containing neurons in the rat hypothalamic arcuate nucleus. Neurosci Lett 370, 252–256 [DOI] [PubMed] [Google Scholar]
- 150.Schirman-Hildesheim TD, Bar T, Ben-Aroya N, and Koch Y (2005) Differential gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acid expression patterns in different tissues of the female rat across the estrous cycle. Endocrinology 146, 3401–3408 [DOI] [PubMed] [Google Scholar]
- 151.Moore JP Jr., Wilson L, Dalkin AC, and Winters SJ (2003) Differential expression of the pituitary gonadotropin subunit genes during male rat sexual maturation: reciprocal relationship between hypothalamic pituitary adenylate cyclase-activating polypeptide and follicle-stimulating hormone beta expression. Biol Reprod 69, 234–241 [DOI] [PubMed] [Google Scholar]
- 152.Ozawa K, and Wakabayashi K (1988) Dynamic change in charge heterogeneity of pituitary FSH throughout the estrous cycle in female rats. Endocrinol Jpn 35, 321–332 [DOI] [PubMed] [Google Scholar]
- 153.Smith MS, Freeman ME, and Neill JD (1975) The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96, 219–226 [DOI] [PubMed] [Google Scholar]
- 154.Ko C, In YH, and Park-Sarge OK (1999) Role of progesterone receptor activation in pituitary adenylate cyclase activating polypeptide gene expression in rat ovary. Endocrinology 140, 5185–5194 [DOI] [PubMed] [Google Scholar]
- 155.Moore JP Jr., and Wray S (2000) Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology 141, 4486–4495 [DOI] [PubMed] [Google Scholar]
- 156.Shuto Y, Uchida D, Onda H, and Arimura A (1996) Ontogeny of pituitary adenylate cyclase activating polypeptide and its receptor mRNA in the mouse brain. Regul Pept 67, 79–83 [DOI] [PubMed] [Google Scholar]


