Abstract
Despite the improvement in surgical interventions in the treatment of congenital heart disease, many life-threatening lesions (e.g., hypoplastic left heart syndrome) ultimately require transplantation. However, there is a great limitation in the availability of deceased human cardiac donors of a suitable size. Hearts from genetically-engineered pigs may provide an alternative source. The relatively immature immune system in infants (e.g., absence of anti-carbohydrate antibodies, reduced complement activation, reduced innate immune cell activity) should minimize the risk of early antibody-mediated rejection of a pig graft. Additionally, recipient thymectomy, performed almost routinely as a preliminary to orthotopic heart transplantation in this age group, impairs the T cell response. Because of the increasing availability of genetically-engineered pigs (e.g., triple knockout pigs that do not express any of the three known carbohydrate antigens against which humans have natural antibodies), and the ability to diagnose congenital heart disease during fetal life, cardiac xenotransplantation could be pre-planned to be carried out soon after birth. Because of these several advantages, prolonged graft survival and even the induction of tolerance, e.g., following donor-specific pig thymus transplantation, is more likely to be achieved in infants than in adults. In this review, we summarize the factors in the infant immune system that would be advantageous in the success of cardiac xenotransplantation in this age group.
Keywords: Antibodies; Immune system; Infants; Pigs, genetically-engineered; Tolerance; Xenotransplantation
Introduction
There is a paucity of available organs for cardiac transplantation, particularly in infants and young children. Ironically, the situation is becoming more acute, as an increasing number of infants are being maintained alive by improvements in medical or surgical care. Annually, in the USA, more than 600 children with heart disease are listed for cardiac transplantation 1. Congenital heart abnormalities are common, with an incidence of 1 in 100 live birth 2. Despite improvements in cardiac surgery and therapeutic cardiac catheterization techniques, heart allotransplantation in patients with conditions such as hypoplastic left heart syndrome remains a curative therapy 3–5. Some survivors of palliative surgery in infancy subsequently develop cardiac failure and ultimately require a cardiac transplantation for survival 6,7. Unfortunately, by this time the advantages associated with the immaturity of the immune system in infants have been lost, and sensitization to human leukocyte antigens (HLA) may have developed, further limiting transplantation options.
The immune system in infants is relatively weaker than that of adults (e.g., absence of anti-carbohydrate antibodies, reduced complement activation, reduced innate immune cell activity), and thus antibody-mediated rejection to a pig heart is less likely to occur in this age group 8–10. In addition, the advances made in the genetic engineering of pigs (e.g., triple knockout [TKO] pigs that do not express any of the three known carbohydrate antigens against which humans have natural antibodies). and the ability to prenatally diagnose congenital heart disease, cardiac xenotransplantation could be pre-planned to be carried out soon after birth.
There is considerable evidence that the infant immune system is immunologically less well-developed than in the adult, which therefore lends itself to manipulation 11–13. For example, West et al. demonstrated that ABO-incompatible heart allotransplantation could be readily achieved in infants 14–16. Most infants do not have preformed antibodies against AB carbohydrate antigens, and thus hyperacute rejection of an ABO-incompatible graft does not occur after transplantation. In most patients, donor-specific anti-A/B blood group antibodies remain absent or at low levels throughout the lifetime of the recipient. In some cases, B cell tolerance has been induced (e.g., absence of antibody production to blood group antigens) 17,18, providing encouragement that both B and T cell tolerance (e.g., reprogramming T cells to recognize donor antigens as self, through donor thymus transplantation) might be more likely to be achieved in infants than in adults 11,17,19,20.
The field of transplantation is challenging due to complications associated with organ rejection and chronic immunosuppressive therapy, and tolerance is the ultimate goal 21,22. Strategies for tolerance induction in adults, such as hematopoietic progenitor cell transplantation 23 are unlikely to be feasible in infants due to the toxicity of the pre-transplant nonmyeloablative therapy. Novel therapies are therefore needed for this age group. Because of the unique characteristics of the immature infant immune system, the induction of tolerance may be feasible in this age group 20.
In this review, we summarize the factors in the infant immune system that would be advantageous to the success of cardiac xenotransplantation and the induction of tolerance in this age group.
The immune system in infants
The limited exposure to antigens in the external environment renders the newborn infant’s immune system naïve. After birth, there is an exposure to many antigens of the external world and the immune system responds in an attempt to protect the infant against potential infection 24. At the same time, the infant immune system takes steps to prevent the development of autoimmune disease 25. Below we will discuss the evolution of the infant immune system after birth
Ontogeny of the innate immune system
Innate immune cells (Table 1)
Table 1:
Innate immune cell markers and function in infants, and comparison with adults
| Type of cells | Differences from adults | References |
|---|---|---|
| Neutrophils | Higher numbers (13×109/L) just before birth, and return to adult levels within 72 hours.Lower adhesion, chemotaxis, and phagocytosis. Lower TLR-4 expression. | 26,30 |
| Monocytes/Macrophages | Lower phagocytosis. Lower TLR-4 expression.Lower IL-12 and TNF-α secretion. | 32,33 |
| Dendritic cells(DCs) | Lower numbers.Lower HLA class II and lower CD80/CD86 expression.Lower IL-12 secretion. | 37 |
| Natural killer cells(NK cells) | Higher numbers.Lower IFN-γ secretion. | 40,42 |
HLA = human leukocyte antigen IFN = interferon IL = interleukin TLR = toll like receptor
Neutrophils
Neutrophils are stimulated by granulocyte-colony stimulating factor (G-CSF) just before birth, resulting in infants having higher numbers of neutrophils (1.5–28×109 cells/L) than adults (4.4×109 cells/L) 25. However, the function of these neutrophils is impaired due to lower expression of toll like receptor-4 (TLR-4) 26. Neutrophils in infants demonstrate the following characteristics: (i) 60% lower chemotaxis due to impaired calcium influx 27,28, (ii) lower adhesion due to defective L-selectin, and macrophage-1 antigen (MAC-1) 25,29, and (iii) less phagocytic function due to impaired neutrophil extracellular traps (NETS) 30, all of which make infant neutrophils functionally weaker than in adults 31.
Macrophages
Macrophages are also functionally impaired in infants with lower expression of TLR-4 32, and have an impaired ability to secrete interleukin-12 (IL-12) and tumor necrosis factor-α (TNF-α) (both of which are pro-inflammatory cytokines responsible for inducing a T helper (Th) 1 type immune response and activation of neutrophils). In contrast, they secrete higher levels of IL-10 (an anti-inflammatory cytokine responsible for downregulation of the Th1-type immune response) 33. This deviation implies anti-inflammatory (Th2-polarizing) properties of the infant’s immune system, which have a protective effect against inflammation 32.
Dendritic cells (DCs)
In infants, plasmacytoid dendritic cells (pDCs) have a very limited ability to secrete interferon (IFN) 34,35, and thus infants have an impaired response to viral infection (e.g., respiratory syncytial virus) 36. The number of conventional dendritic cells (cDCs) is fewer in infants, with lower expression of HLA class II and CD80/CD86, and lower IL-12 secretion, which impairs the Th1 response 25. The ratio of pDCs to cDCs in neonates is 3:1 compared to 1:3 in adults. Overall, there is an impairment of the Th1 response, and a more profound Th2 response in infants than in adults 37,38.
Natural killer cells (NK cells)
The cytotoxicity of NK cells in infants is at least three times weaker than in adults 39. Infant NK cells express less leukocyte immunoglobulin-like receptor-1 (LIR-1), which binds to MHC class I, and higher inhibitory NKG2A receptors. Therefore, the cytotoxic capacity of human cord blood NK cells, i.e., their capacity to quickly lyse cognate targets without undergoing differentiation, is at least 3-fold lower than in adults 39,40. They are highly susceptible to inhibition by transforming growth factor-β, and produce limited amounts of IFN-γ 41,42.
Complement (Table 2)
Table 2:
Complement components in infants, and comparison with adults
| Components | Infants (% of adults) | Time to reach adults levels | References |
|---|---|---|---|
| C1q | 65% | 1 year | 44 |
| C2 | 95% | 6 months | 44 |
| C3 | 70% | 1 year | 44 |
| C4 | 73% | 6 months | 44 |
| C5 | 65% | 6 months | 44 |
| C6 | 65% | 6 months | 44 |
| C7 | Same level as adults | 43 | |
| C8 | Low | 6 months | 43 |
| C9 | 10–30% | 1 year | 43 |
| Factor B | 70% | 6 months | 44 |
| Properdin | 25% | 1 year | 46,47 |
The total amount of complement is lower in infants compared to adults (with 10% to 80% of the adult level for most of its components, except for C7 and factor D). C9 remains low throughout the first year of life which is a pore-forming component of the membrane attack complex (MAC) that is responsible for cell lysis. The level of C1 (the major complement component of the classical complement pathway) remains low for 6 months, unlike C2 and C4 which reach normal levels within 1–6 months 43–46. In addition to its direct effect on cell lysis, complement plays a role in phagocytosis and B cell modulation. The classical pathway of complement is also impaired in infants due to the low level of immunoglobulin (see below), and thus its effect depends mainly on the alternative, and lectin pathways 24.
Complement regulatory proteins (Table 3)
Table 3:
Expression of circulating complement-regulatory proteins in infants, and comparison with adults.
Complement-regulatory proteins are circulating complement components that are responsible for regulation/inhibition of complement. Some components, e.g., C4b-binding protein, are found at lower levels than in adults, or are even absent 47. Other regulatory components are at lower concentrations than in adults, e.g., in term neonates, factor H is 64% of adult concentration, and the factor I level is on average approximately only 50% of adult levels 46. These levels are still lower than adult levels at 6 months after birth 44. McGreal et al. reported that C1 inhibitor (a regulatory protein of the three activation pathways of the complement system) in term neonates is 62% of adult levels. Collectively, the low levels of complement circulatory proteins balance the low levels of complement-regulatory proteins in infants 46.
Natural antibodies
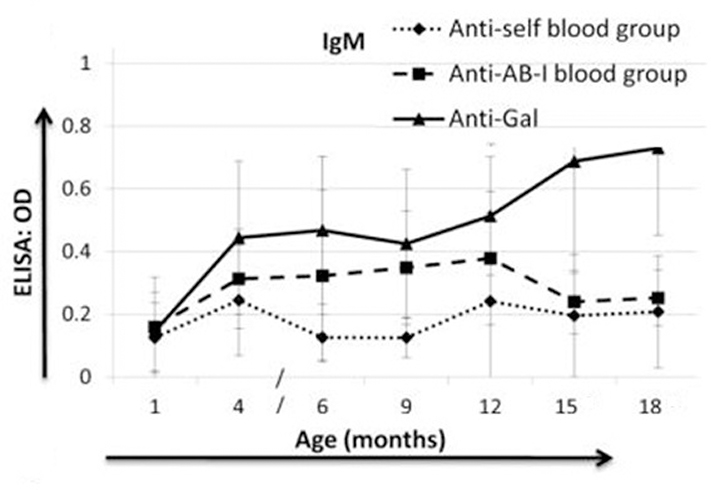
Natural antibodies in infants are mainly immunoglobulin M (IgM) (produced by B1 cells), the pure definition of natural Abs is that it can be created in the absence of exogenous antigens. Yet, it has also been demonstrated that natural Abs are affected by the presence of exogenous/endogenous antigens, and germline composition, all of which can alter natural Abs repertoire 48. Isohemagglutinins, such as anti-A and anti-B blood group antibodies, as well as anti-pig antibodies, are natural IgM antibodies. They generally appear at 3–6 months of age 48–50. Dons et al. showed that there were almost undetectable levels of anti-A/B-incompatible blood group IgM antibodies at one month of age in naïve infant baboons 50 (Figure 1).
Fig. 1:
IgM levels (mean ± SD) of anti-self A/B blood group (auto) antibodies, anti-A/B-Incompatible blood group (anti-AB-I) antibodies, and anti-galactose-α1,3-galactose (Gal) antibodies, in naïve baboons (n=6). There were undetectable IgM levels of anti-AB-I blood group antibodies, and anti-Gal antibodies at one month of age in naïve infant baboons, comparable with anti-self (optical density<0.2). Thereafter, anti-AB-I IgM increased slowly and anti-Gal IgM rose more rapidly with increasing age. (Reprinted with permission from Dons et al. 2012.)
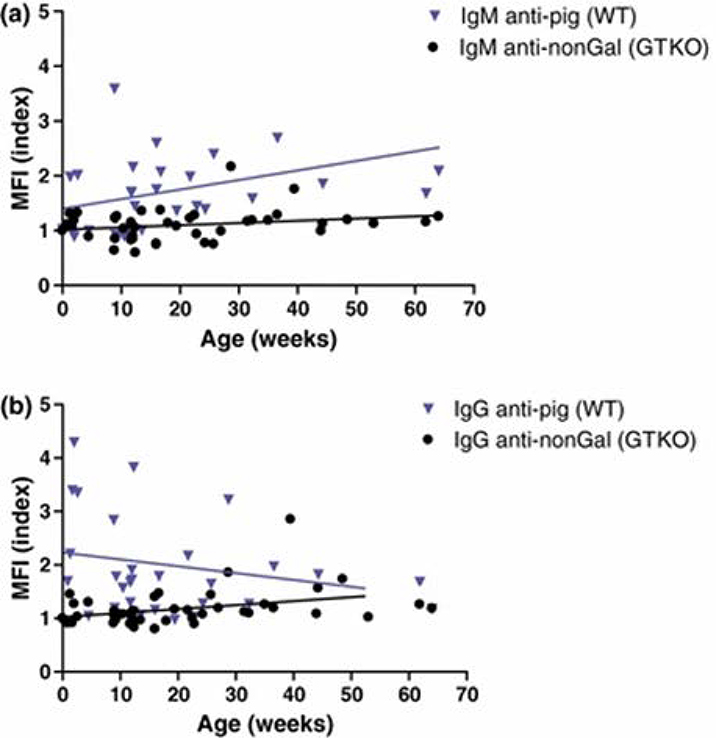
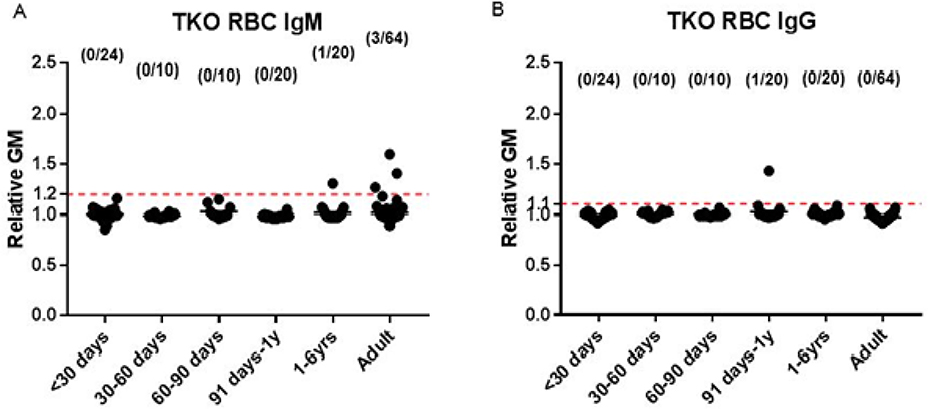
Largely through the development of antibodies to galactose-α1,3-galactose (Gal), the most important xenoantigen, most infants have antibodies to wild-type (i.e., genetically-unmodified) pig carbohydrate antigens within the first few months of life (Figures 1 and 2) 51. Knockout of the gene for α1,3-galactosyltransferase, which is responsible for adding Gal to the underlying carbohydrates on pig cells, results in reduced antibody binding to pig cells (GTKO pigs) 51 (Figure 2). Binding of infant human antibodies to triple-knockout (TKO) pig cells (that do not express any of the three known carbohydrate xenoantigens) is reduced even further (Figure 3). Indeed, almost no infants develop antibodies to TKO pig cells during the first year of life 52 (Figure 3).
Fig. 2:
Binding of infant human (n=42) serum antibodies to WT and GTKO pig PBMCs. (MFI index = mean fluorescence intensity of the serum sample divided by the MFI of the isotype control sample).
(a) Distribution of IgM reactivity against WT and GTKO PBMCs during the first year of life. Correlation of MFI index with age of each group is indicated by a line (versus WT, p=0.073, r=0.316; versus GTKO, p=0.129, r=0.238). There is a slow increase in IgM directed to Gal antigens on cells from wild-type (WT) pigs, but little increase in IgM to nonGal antigens.
(b) Distribution of IgG reactivity against WT and GTKO PBMC. Correlation of MFI index with age of each group is indicated by a line (versus WT, p=0.381, r= −0.158; versus GTKO, p=0.021, r=0.356). The high level of IgG against Gal antigens on wild-type (WT) pig cells at birth is almost certainly related to the presence of maternal IgG (that crosses the placenta) in the neonate. This falls rapidly after birth. IgG directed to nonGal antigens slowly increases throughout the first year of life. (Reprinted with permission from Rood et al. 2007.)
Fig. 3:
Serum IgM (A) and IgG (B) binding to TKO pig red blood cells (RBCs) in human neonates (<30 days of age) (n=24), infants (1–12 months of age, n=40), children (1–6 years of age, n=20), and adults (n=64). (The dotted line represents the lowest limit of detection, indicating no binding.) (A) There is no binding of IgM to TKO pig RBCs during infancy, and only one infant demonstrated any IgG binding. Very few children and adults had any IgM to TKO pig RBCs. (B) Only one infant demonstrated any IgG binding to TKO pig RBCs, and no children or adults demonstrated any binding. (Reprinted with permission from Li et al. 2019.)
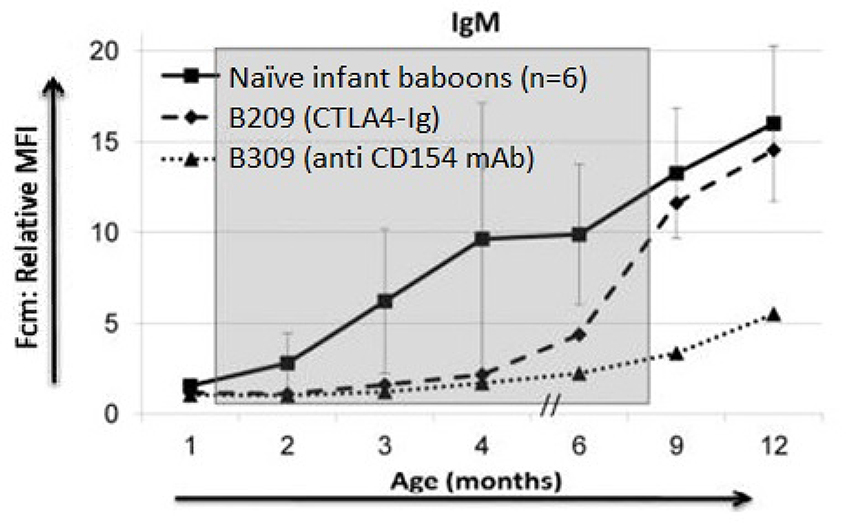
One observation made in our own laboratory questions the assumption that natural antibodies in infants are T cell-independent. Dons et al. provided evidence that anti-pig (mainly anti-Gal) IgM antibodies rose after 3 months of age in naïve infant baboons. In contrast, if T cell costimulation was blocked by the administration of an anti-CD154 monoclonal antibody (mAb), then anti-pig IgM antibodies remained low, but gradually rose after the discontinuation of anti-CD154mAb 50 (Figure 4). When the infant baboon was treated with CTLA4-Ig (instead of an anti-CD154mAb), anti-pig IgM antibodies increased slightly before the discontinuation of CTLA4-Ig, suggesting it was less efficacious in this respect. This observation that natural antibodies are not T cell-independent correlates with observations made by Cretin et al. in a mouse model 53.
Fig. 4:
Anti-pig IgM antibody levels in baboons with or without the administration of anti CD154mAb or CTLA4-Ig. (The shaded area indicates the period of time when immunosuppressive therapy was being administered to B309 and B209.) (Reprinted with permission from Dons et al. 2012.) In naïve, non-immunosuppressed infant baboons (n=6), after 3 months of age, anti-pig IgM antibody levels rose significantly and continued to increase at 6, 9, and 12 months of age. In contrast, anti-pig IgM antibody level in an anti-CD154mAb-treated infant baboon (B309) remained very low while immunosuppressive therapy was being administered, but rose after discontinuation of the anti-CD154 mAb. In a CTLA4-Ig-treated infant baboon (B209), anti-pig IgM antibody began to increase before discontinuation of CTLA4-Ig.
These observations suggest that, with regard to TKO pig xenotransplantation in infants, there is a ‘window of opportunity’ (that lasts virtually throughout the first year of life) in which no natural anti-TKO IgM and IgG pig antibodies are present. Additionally, the activation of complement and innate immune cells is weak, thus negating or minimizing the risk of early antibody-mediated rejection 54.
Ontogeny of the adaptive Immune system (Table 4)
Table 4:
Adaptive immune cells in infants, and comparison with adults
| Type of cells | Difference from adults | References |
|---|---|---|
| T helper 1 (Th1) cells | Lower CD40 ligand (CD40L) expression (lower B cell response). Lower IFN-γ secretion. | 55,60 |
| T helper 2 (Th2) cells | Higher anti-inflammatory cytokine (IL-4, IL-5, and IL-10) secretion. | 38 |
| Cytotoxic T (Tc) cells | Lower cytotoxic function due to lower CD80/CD86 expression and lower IL-12 secretion by antigen-presenting cells (APCs). | 72 |
| T regulatory cells (Tregs) | Higher numbers in human cord blood (∼12% of CD4+ T cells) and neonatal lymph nodes (∼8%). | 65 |
| B cells | Lower B1 cells (50% of B cells at birth), with higher IgM secretion. Lower CD40 expression. | 77 |
The adaptive immune system has two arms – (i) cell-mediated immunity, and (ii) humoral immunity 24,55. The exposure to antigens is very limited during fetal life, and thus, at birth, the adaptive immune system is still naïve, with a more profound response toward T helper 2 (Th2) cells 56.
T cells
T cell precursors can be found in the liver as early as 7 weeks of gestation 27, but they do not express any surface proteins at this time. During the early stages of development, they are divided into two main groups – (i) α/β T cells, and (ii) γ/δ T cells 25. The α/β cells migrate to the thymus, where they complete their maturation 57. The γ/δ cells do not migrate to thymus, but instead secrete cytokines to stimulate adaptive immunity, playing an important role in protection against some microbial infections (e.g., Mycobacterium tuberculosis, Listeria monocytogenes, and Brucella abortus) 25,58. At birth, CD4+T cells and CD8+T cells can be detected in cord blood, contributing approximately 25% of the total lymphocyte count, with CD4+T cells outnumbering CD8+T cells. Most of the T cells in infants are naïve T cells 57,59.
CD4+ T helper cells (Th cells)
The cytokine profile in infants is different from that in adults. Infants have higher levels of anti-inflammatory cytokines (e.g., IL-4, IL-5, and IL-10) and lower levels of pro-inflammatory cytokines (e.g., IL-2, TNF-α, and IFN-γ), which makes the T helper response deviate towards a Th2 (anti-inflammatory) immune response 38,55. Infantile T helper cells also have a limited ability to express CD40 ligand (CD40L, CD154), which plays an important role in activation of the B cell response 60,61.
T regulatory cells (Tregs)
There are numerous subtypes of T regulatory cells (Tregs), including natural Tregs (nTregs) (CD4+CD25+Foxp3+) and induced Tregs (iTregs) 62. They all have suppressive effects on other T cells and play an important role in protection against autoimmune disease 63,64. nTregs are present in relativity high numbers in cord blood and neonatal lymph nodes. They play a major role in the development of central tolerance 65,66. There is inconsistent evidence regarding the presence of iTregs at birth, but it is clear that the peripheral immune system of neonates has a strong tendency to become tolerogenic upon antigenic stimulation. Whether this is related to the presence of nTregs or a mix of both nTregs and iTregs is still debatable 67.
T helper 17 (Th17) cells
T helper 17 (Th17) cells play an important role in protection from infection. However, its high inflammatory potential through IL-17 can also harm the graft and cause rejection 68. de Roock demonstrated that expression of Th17 is nearly absent in neonates 69. There is a reciprocal development pathway between Th17 and Tregs 70. In newborns, there is an impaired production of pro-inflammatory cytokines, which allows for the dominance of Tregs over Th17 cells, leading to the deviation of the Tregs-Th17 axis towards Tregs. This in turn might help in the induction of tolerance due to the suppressive effect of Tregs 20.
CD8+ cytotoxic T cells
Cytotoxic T cells are responsible for defense against viruses and intracellular pathogens 71. In infants, they are not as effective as in adults, due to a defective secretion of IL-12 by antigen-presenting cells and lower expression of CD80/CD86 72,73.
B cells
Early in gestation (7–8 weeks), B cell progenitors are found in the liver. Halfway through gestation, B cell lymphopoiesis takes place in the bone marrow 74,75. During gestation, most of the B cells are immature B1 cells (and are believed to be T cell-independent), and secrete low-affinity, polyreactive IgM antibodies 59. At term, these immature B cells represent 50% of all B cells, compared to 12% in adults. They are responsible for early protection against bacteria (e.g., Borrelia hermsii and streptococcus pneumoniae) 76–78.
The antibody-mediated response in neonates is weaker in comparison to adults due to three main factors – (i) T cell expression of CD40L is insufficient 79; (ii) the lower level of complement, that is in part associated with a weaker antibody response, and (iii) the lymphoid structures in which B cell optimization takes place may not mature during the neonatal period 80–82.
The response to vaccines in infants
The immune response in neonates is deviated towards a Th2 response 83, with a lower Th1 response and a weak humoral response after vaccination. There is, therefore, a defect in the quality and strength of the immune response to vaccination in neonates compared with adults 84,85. However, there are a few vaccines which generate a strong response in neonates, e.g., Bacillus Calmette–Guérin (BCG), hepatitis B, and oral polio 86. These vaccines primarily induce a good Th1 response. The reason behind this remains unclear, but might be explained by the ability of the neonate’s immune system to enhance the expression of costimulatory molecules on antigen-presenting cells (APCs), thus mounting a stronger Th1 response 87,88.
The role of the thymus in infants
The thymus is responsible for the maturation of T cells 24,89,90 through positive and negative selection. β chain development in T cells gives rise to the T cell receptor (TCR) 91, while the α chain is associated with the expression of CD4+CD8+ double-positive T cells (which are the first cells in the T cell developmental pathway to express α/β TCR complexes on the cell surface). The appropriate TCR signaling is crucial for survival of the double-positive cells during this period, and ultimately determines whether developing T cells are positively or negatively selected. Those cells that could not engage will undergo death by neglect 90,92. The number of naïve T cells remains relatively constant throughout life due to the ability of T cells to maintain peripheral homeostasis 93,94.
The CD31 surface molecule (platelet endothelial cell adhesion molecule or PECAM-1) is present on the T cells exiting the thymus 95,96, enabling CD31 to be used as a marker for recent thymic emigrants (RTEs) 97. Division of T cells in the periphery results in a lower number of CD31+ naïve T cells with aging. This change is more profound in patients who underwent thymectomy in infancy 98.
Effect of thymectomy in infants (Table 5)
Table 5:
Effect of thymectomy in infancy
The exact effects of thymectomy in infants remain unclear due to conflicting evidence in the literature. van den Broek et al. reported that the numbers of CD4+ and CD8+ T cells are lower after total or partial thymectomy during the first year of life 99,100. Other studies claimed that, despite the lower number of CD4+T cells, the number of CD8+T cells remained unchanged 98,101,102. Mancebo et al. found that there were no changes in the numbers of B cells or NK cells, but the number of neutrophils increased 103. In contrast, Brearley et al. reported that there was an increase in the number of B cells and in IgA antibodies, with lower numbers of neutrophils 104.
TCR excision circles are small DNA episomes that are present in specific subtypes of naïve T cells, and are used as a marker for recent thymus emigrants 105,106. As they are not capable of division, they become diluted by the proliferation of T cells, and so (like CD31+ expression) indicate aging of the T cell lineage 100,107,108. The number of TCR excision molecules is reduced and remains low after thymectomy 109. Cao et al suggested that the number of TCR excision molecules may correlate with the thymic volume (mass) that remains after thymectomy 110.
Since the early studies of Billingham and his colleagues 111, many studies suggest a role for thymectomy in the induction of tolerance 112–114. Although the clinical consequences of thymectomy are not certain, some studies suggest there is higher risk for autoimmune disease and a reduced response to vaccines 98,115.
DiGeorge syndrome
DiGeorge syndrome is a primary immunodeficiency disorder resulting from the abnormal development of the third and fourth pharyngeal pouches during embryonic life, resulting in thymic hypoplasia or aplasia, heart defects, and hypoparathyroidism 116. It can be either partial or complete, and the severity of the disease ranges from no circulating T cells to a normal T cell count 117,118. There may be complete athymia, which presents as severe combined immunodeficiency disease (SCID) 119. There is no relation between the T cell count and the size of the thymus 118.
Most infants with DiGeorge syndrome have a low number of CD3+T cells, and most of these cells are exclusively of a memory phenotype (CD4/CD45RO or TCR excision circle-negative) 120. In selected patients, severe combined immunodeficiency disease can be corrected by successful hematopoietic cell transplantation or by thymic transplantation 119,121,122.
The induction of immunological tolerance in neonates and infants
The induction of donor-specific tolerance is the ultimate goal in organ transplantation, as the recipient would no longer require long-term immunosuppressive therapy 114,123,124. Tolerance to maternal antigens develops naturally in the fetus, and is retained throughout life.
As an infant, the thymus plays an important role in maintaining tolerance to self-antigens, while trying to defend the body against the exposure to new antigens that occurs after birth, thus allowing the immune system to respond to infection, but avoiding the development of autoimmune disease. This process depends on the development of (i) central and/or (ii) peripheral tolerance 125,126.
Central tolerance can develop in the thymus as T and B cells undergo positive selection, followed by negative selection 127. However, some T cells escape this process and pass into the periphery where peripheral tolerance may develop through anergy, clonal deletion, and/or the induction of iTregs 128.
The concept of neonatal tolerance and its clinical application is not new 111. As already discussed, West et al. demonstrated that B cell tolerance to an ABO-incompatible heart graft can be readily induced in infants 11. However, it is still unknown whether T cell tolerance can be achieved. In xenotransplantation, even if the transplantation of a heart from a TKO pig negated the need for the induction of B cell tolerance to pig carbohydrate antigens (as the recipient would not be exposed to these antigens on the pig heart), T cell tolerance would be required to prevent rejection induced by the presence of protein antigens on the pig cells, e.g., swine leukocyte class I and II antigens 129,130.
In transplantation, many approaches to the induction of tolerance have been investigated, e.g., donor-specific thymic transplantation, mixed hematopoietic cell chimerism, and anergy 131,132. Almost all studies in large animals, however, have been in adult recipients 133.
In view of the need for intensive pre-transplant immunosuppressive therapy, in our opinion, it is unlikely that hematopoietic cell chimerism will be an approach to the induction of tolerance in infants in the near future. We suggest that tolerance will be more likely achieved by either concomitant donor-specific thymic transplantation or by the induction of T cell anergy (though this latter approach may be associated with loss of tolerance in the event of certain subsequent events, e.g., an infection, in the recipient).
Thymus transplantation
Thymic tissue allotransplantation is already being performed on babies born with DiGeorge syndrome, with good outcomes (around 70% survival), but with some autoimmune complications 134–141. This is a relatively simple and non-invasive technique as the thymic tissue is injected into the quadriceps muscles of the recipient 134.
In a xenotransplantation model, Lee et al. performed thymus transplantation from pig-to-mouse to induce tolerance, and demonstrated that specific T cell tolerance could be achieved 142. However, B cells producing antibodies in a T cell-independent manner can still provide a barrier 143. In large animal models, the transplantation of vascularized thymic tissue increases the success of the induction of tolerance 19,144–146. Fudaba et al. demonstrated that positive selection, and peripheral maturation of T cells are more efficient when combining thymus transplantation with injection of recipient thymic epithelial cells into the donor thymus at the time of recipient thymectomy 147.
We have suggested that, in neonates or infants, the transplantation of donor-specific thymic tissue (using the Markert technique) at the time of pig heart transplantation (following T cell depletion by thymectomy and the administration of anti-thymocyte globulin) may well induce T cell tolerance 7,54.
Anergy
T cell anergy, in which the recipient lymphocytes are functionally inactivated, may result following the absence or suppression of T cell costimulation (the second signal). Tolerance develops through clonal anergy 148, in which T cells encounter donor antigens when the co-stimulatory signal is deficient. In the absence of costimulation, the T cells enter a state of anergy (allowing a transplanted graft to survive), but this state might be reversible, thus threatening the ‘breaking’ of the state of tolerance 149.
Costimulation blockade is key to the induction of anergy, but other factors may play important roles, including suppression of the mTOR pathway, which can be achieved by drugs such as rapamycin 150. Although blockade of the CD28:B7 costimulation pathway was considered to be most important in the context of anergy, the CD40:CD154 pathway is gaining increasing attention 151. This approach has been successful in a mouse allotransplant model 152, but requires further investigation in nonhuman primates and humans 153,154.
Comment
For many neonates and infants with complex congenital heart disease, an immediate heart transplant remains one of the most potent therapies available, and is associated with good survival 155. Given the need for hearts in this age group, we suggest that clinical cardiac xenotransplantation should initially be considered either (i) as an early bridge to allotransplantation in newborns with life-threatening congenital heart disease, or (ii) as an equivalent to allotransplantation. The excellent long-term outcomes of ABO-incompatible heart allotransplantation in infants provide encouragement that, with an effective tolerance-inducing regimen, both B and T cell tolerance to a cardiac allograft may be achieved 24,54.
The lack of availability of hearts from deceased human donors remains a major barrier to allotransplantation. Xenotransplantation using genetically-engineered pigs as sources of organs offers a realistic alternative, at least as a bridge until a suitable cardiac allograft becomes available. The several advantages of xenotransplantation have been summarized previously 156,157.
The advantageous immunological factors that contribute to the success of heart transplantation in infants, and are likely to facilitate the success of xenotransplantation, are summarized in Table 6. Pre-transplant thymectomy is considered an additional beneficial factor.
Table 6:
Factors responsible for the relative weakness of the immune system in infants
| Cells | Function |
|---|---|
| Macrophage | Defective secretion of pro-inflammatory cytokines IL-12 and TNF-α |
| Natural killer (NK) cells | Three times weaker cytotoxic function than in adults |
| Complement | Lower complement levels, and functionally weaker than in adults |
| Natural antibodies | Few present until 3–6 month of age |
| T helper cell response | High anti-inflammatory cytokine profile (IL-4, IL-5 and IL-10), Th2>Th1 |
| Cytotoxic T (Tc) cells | Defective secretion of IL-12 |
| T regulatory cells (Tregs)/Th17 | Absent Th17, and more abundant Tregs, that are responsible for T cell suppression |
| B cells | Defective CD40 expression, and immature B cell response |
We hypothesize that the induction of tolerance to a transplanted pig heart may be possible using a combination of (i) pre-transplant T cell depletion and thymectomy, (ii) a genetically-engineered pig as the source of the heart, (iii) costimulation blockade-based immunosuppressive therapy, and (iv) donor-specific pig thymic transplantation 54. The possibility of achieving a state of tolerance is greater in infants than in adults due to the relatively immature immune system in infants. Even if tolerance cannot be achieved, prolonged survival of a genetically-engineered pig heart in a recipient receiving maintenance immunosuppressive therapy would enable the heart to be employed either as a short-term ‘bridge’ to allotransplantation, or as destination therapy 54.
Acknowledgment
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959, and in part by a grant to UAB from United Therapeutics, Silver Spring, MD.
Abbreviations
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- IL
interleukin
- TCR
T cell receptor
- TLR
Toll like receptor
- TNF-α
tumor necrosis factor-α
- TKO
triple gene-knockout
References
- 1.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 Annual Data Report: Heart. Am J Transplant. 2018;18:291–362. doi: 10.1111/ajt.14561 [DOI] [PubMed] [Google Scholar]
- 2.Krasuski RA, Bashore TM. Congenital Heart Disease Epidemiology in the United States: Blindly Feeling for the Charging Elephant. Circulation. 2016;134(2):110–113. doi: 10.1161/CIRCULATIONAHA.116.023370 [DOI] [PubMed] [Google Scholar]
- 3.Aurora P, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirteenth official pediatric lung and heart-lung transplantation report—2010. J Heart Lung Transplant. 2010;29(10):1129–1141. doi: 10.1016/j.healun.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 4.Chinnock R E, Bailey L L. Heart Transplantation for Congenital Heart Disease in the First Year of Life. Curr Cardiol Rev. 2011;7(2):72–84. doi: 10.2174/157340311797484231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gobergs R, Salputra E, Lubaua I. Hypoplastic left heart syndrome: a review. Acta Medica Litu. 2016;23(2). doi: 10.6001/actamedica.v23i2.3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attenhofer Jost CH, Schmidt D, Huebler M, et al. Heart Transplantation in Congenital Heart Disease: In Whom to Consider and When? J Transplant. 2013;2013:1–12. doi: 10.1155/2013/376027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleveland D, Adam Banks C, Hara H, Carlo WF, Mauchley DC, Cooper DKC. The Case for Cardiac Xenotransplantation in Neonates: Is Now the Time to Reconsider Xenotransplantation for Hypoplastic Left Heart Syndrome? Pediatr Cardiol. 2019;40(2):437–444. doi: 10.1007/s00246-018-1998-1 [DOI] [PubMed] [Google Scholar]
- 8.Kaplon RJ, Michler RE, Xu H, Kwiatkowski PA, Edwards NM, Platt JL. Absence of hyperacute rejection in newborn pig-to-baboon cardiac xenografts. Transplantation. 1995;59(1):1–6. doi: 10.1097/00007890-199501150-00001 [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Edwards NM, Chen JM, Dong X, Michler RE. Natural antipig xenoantibody is absent in neonatal human serum. J Heart Lung Transplant. 1995;14(4):749–754. [PubMed] [Google Scholar]
- 10.Minanov OP, Itescu S, Neethling FA, et al. Anti-GaL IgG antibodies in sera of newborn humans and baboons and its significance in pig xenotransplantation. Transplantation. 1997;63(2):182–186. doi: 10.1097/00007890-199701270-00002 [DOI] [PubMed] [Google Scholar]
- 11.Fan X, Ang A, Pollock-BarZiv SM, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004;10(11):1227–1233. doi: 10.1038/nm1126 [DOI] [PubMed] [Google Scholar]
- 12.Lynch RJ, Platt JL. Accommodation in organ transplantation: Curr Opin Organ Transplant. 2008;13(2):165–170. doi: 10.1097/MOT.0b013e3282f6391e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Platt JL, Cascalho M, West L. Lessons from cardiac transplantation in infancy. Pediatr Transplant. 2009;13(7):814–819. doi: 10.1111/j.1399-3046.2009.01143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West LJ, Cardella CJ. ABO-Incompatible Heart Transplantation in Infants. N Engl J Med. 2001:8. [DOI] [PubMed] [Google Scholar]
- 15.West L Targeting Antibody-Mediated Rejection in the Setting of ABO-Incompatible Infant Heart Transplantation: Graft Accommodation vs. B Cell Tolerance. Curr Drug Target -Cardiovasc Hematol Disord. 2005;5(3):223–232. doi: 10.2174/1568006054064762 [DOI] [PubMed] [Google Scholar]
- 16.West LJ. ABO-incompatible hearts for infant transplantation: Curr Opin Organ Transplant. 2011;16(5):548–554. doi: 10.1097/MOT.0b013e32834a97a5 [DOI] [PubMed] [Google Scholar]
- 17.Conway J, Manlhiot C, Allain-Rooney T, McCrindle BW, Lau W, Dipchand AI. Development of Donor-Specific Isohemagglutinins Following Pediatric ABO-Incompatible Heart Transplantation: Isohemagglutinins and Heart Transplant. Am J Transplant. 2012;12(4):888–895. doi: 10.1111/j.1600-6143.2011.03910.x [DOI] [PubMed] [Google Scholar]
- 18.Urschel S, West LJ. ABO-incompatible heart transplantation: Curr Opin Pediatr. 2016;28(5):613–619. doi: 10.1097/MOP.0000000000000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griesemer AD, Hirakata A, Shimizu A, et al. Results of Gal-Knockout Porcine Thymokidney Xenografts. Am J Transplant. 2009;9(12):2669–2678. doi: 10.1111/j.1600-6143.2009.02849.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan H, Gazarian A, Dubernard J-M, Belot A, Michallet M-C, Michallet M. Transplant Tolerance Induction in Newborn Infants: Mechanisms, Advantages, and Potential Strategies. Front Immunol. 2016;7. doi: 10.3389/fimmu.2016.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder RA, Marroquin CE, Kuo PC. Tolerance and the “Holy Grail” of transplantation. J Surg Res. 2003;111(1):109–119. doi: 10.1016/S0022-4804(03)00081-7 [DOI] [PubMed] [Google Scholar]
- 22.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and Withdrawal of Immunosuppressive Drugs in Patients Given Kidney and Hematopoietic Cell Transplants: Human Tolerance and Chimerism. Am J Transplant. 2012;12(5):1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai T, Tolkoff-Rubin N, Shaffer J, et al. HLA-Mismatched Renal Transplantation without Maintenance Immunosuppression. N Engl J Med. 2008:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282(1821):20143085. doi: 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. 2014;10(9):1171–1184. doi: 10.1586/1744666X.2014.942288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal Sepsis and Neutrophil Insufficiencies. Int Rev Immunol. 2010;29(3):315–348. doi: 10.3109/08830181003792803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55(8):688–697. doi: 10.1034/j.1398-9995.2000.00118.x [DOI] [PubMed] [Google Scholar]
- 28.Weinberger B, Laskin DL, Mariano TM, et al. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. J Leukoc Biol. 2001;70(6):969–976. [PMC free article] [PubMed] [Google Scholar]
- 29.Ygberg S, Nilsson A. The developing immune system - from foetus to toddler. Acta Paediatr Oslo Nor 1992. 2012;101(2):120–127. doi: 10.1111/j.1651-2227.2011.02494.x [DOI] [PubMed] [Google Scholar]
- 30.Yost CC, Cody MJ, Harris ES, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–6427. doi: 10.1182/blood-2008-07-171629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi Z, Mou W, He J, Gui J. General Characteristics of Human Neonate Immunity. Am J Biomed Sci. October 2014:265–277. doi: 10.5099/aj140400265 [DOI] [Google Scholar]
- 32.Chelvarajan RL, Collins SM, Doubinskaia IE, et al. Defective macrophage function in neonates and its impact on unresponsiveness of neonates to polysaccharide antigens. J Leukoc Biol. 2004;75(6):982–994. doi: 10.1189/jlb.0403179 [DOI] [PubMed] [Google Scholar]
- 33.Kraft JD, Horzempa J, Davis C, Jung J-Y, Peña MMO, Robinson CM. Neonatal macrophages express elevated levels of interleukin-27 that oppose immune responses. Immunology. 2013;139(4):484–493. doi: 10.1111/imm.12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Danis B, George TC, Goriely S, et al. Interferon regulatory factor 7-mediated responses are defective in cord blood plasmacytoid dendritic cells. Eur J Immunol. 2008;38(2):507–517. doi: 10.1002/eji.200737760 [DOI] [PubMed] [Google Scholar]
- 35.Yu JC, Khodadadi H, Malik A, et al. Innate Immunity of Neonates and Infants. Front Immunol. 2018;9:1759. doi: 10.3389/fimmu.2018.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cormier SA, Shrestha B, Saravia J, et al. Limited Type I Interferons and Plasmacytoid Dendritic Cells during Neonatal Respiratory Syncytial Virus Infection Permit Immunopathogenesis upon Reinfection. J Virol. 2014;88(16):9350–9360. doi: 10.1128/JVI.00818-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128(1):118–123. doi: 10.1046/j.1365-2249.2002.01817.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debock I, Flamand V. Unbalanced Neonatal CD4+ T-Cell Immunity. Front Immunol. 2014;5. doi: 10.3389/fimmu.2014.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalle J-H, Menezes J, Wagner É, et al. Characterization of Cord Blood Natural Killer Cells: Implications for Transplantation and Neonatal Infections. Pediatr Res. 2005;57(5 Part 1):649–655. doi: 10.1203/01.PDR.0000156501.55431.20 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Xu H, Zheng X, Wei H, Sun R, Tian Z. High Expression of NKG2A/CD94 and Low Expression of Granzyme B Are Associated with Reduced Cord Blood NK Cell Activity. Mol Immunol. 2007;4(5):6. [PubMed] [Google Scholar]
- 41.Slavica L, Nordström I, Karlsson MN, et al. TLR3 impairment in human newborns. J Leukoc Biol. 2013;94(5):1003–1011. doi: 10.1189/jlb.1212617 [DOI] [PubMed] [Google Scholar]
- 42.Ivarsson MA, Loh L, Marquardt N, et al. Differentiation and functional regulation of human fetal NK cells. J Clin Invest. 2013;123(9):3889–3901. doi: 10.1172/JCI68989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adinolfi M, Beck SE. Human complement C7 and C9 in fetal and newborn sera. Arch Dis Child. 1975;50(7):562–564. doi: 10.1136/adc.50.7.562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis CA, Vallota EH, Forristal J. Serum Complement Levels in Infancy: Age Related Changes. Pediatr Res. 1979;13(9):1043–1046. doi: 10.1203/00006450-197909000-00019 [DOI] [PubMed] [Google Scholar]
- 45.Ueda H, Nakanishi A, Ichijo M. Immunochemical quantitation of serum complement components in SFD and AFD infants. Tohoku J Exp Med. 1980;132(1):111–116. doi: 10.1620/tjem.132.111 [DOI] [PubMed] [Google Scholar]
- 46.McGreal EP, Hearne K, Spiller OB. Off to a slow start: Under-development of the complement system in term newborns is more substantial following premature birth. Immunobiology. 2012;217(2):176–186. doi: 10.1016/j.imbio.2011.07.027 [DOI] [PubMed] [Google Scholar]
- 47.de Paula PF, Barbosa JE, Junior PR, et al. Ontogeny of Complement Regulatory Proteins - Concentrations of Factor H, Factor I, C4b-Binding Protein, Properdin and Vitronectin in Healthy Children of Different Ages and in Adults. Scand J Immunol. 2003;58(5):572–577. doi: 10.1046/j.1365-3083.2003.01326.x [DOI] [PubMed] [Google Scholar]
- 48.Holodick NE, Rodríguez-Zhurbenko N, Hernández AM. Defining Natural Antibodies. Front Immunol. 2017;8:872. doi: 10.3389/fimmu.2017.00872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between Human Natural Anti-o-Galactosyl Immunoglobulin G and Bacteria of the Human Florat. Infect Immun. 1988;56:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dons EM, Montoya C, Long CE, et al. T-Cell-Based Immunosuppressive Therapy Inhibits the Development of Natural Antibodies in Infant Baboons: Transplantation. 2012;93(8):769–776. doi: 10.1097/TP.0b013e3182481168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rood PPM, Tai H-C, Hara H, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int. 2007;20(12):1050–1058. doi: 10.1111/j.1432-2277.2007.00546.x [DOI] [PubMed] [Google Scholar]
- 52.Li Q, Hara H, Banks CA, et al. ANTI-PIG ANTIBODY IN INFANTS: CAN A GENETICALLY-ENGINEERED PIG HEART BRIDGE TO ALLOTRANSPLANTATION? Ann Thorac Surg. September 2019:S0003497519314523. doi: 10.1016/j.athoracsur.2019.08.061 [DOI] [PubMed] [Google Scholar]
- 53.Cretin N, Bracy J, Hanson K, Iacomini J. The Role of T Cell Help in the Production of Antibodies Specific for Galα1–3Gal. J Immunol. 2002;168(3):1479–1483. doi: 10.4049/jimmunol.168.3.1479 [DOI] [PubMed] [Google Scholar]
- 54.Cooper DKC, Hara H, Iwase H, Banks CA, Cleveland DC. An approach to induction of tolerance to pig cardiac xenografts in neonates. Xenotransplantation. 2018;25(6):e12454. doi: 10.1111/xen.12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30(12):585–591. doi: 10.1016/j.it.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35(7):299–310. doi: 10.1016/j.it.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fink PJ. The Biology of Recent Thymic Emigrants. Annu Rev Immunol. 2013;31(1):31–50. doi: 10.1146/annurev-immunol-032712-100010 [DOI] [PubMed] [Google Scholar]
- 58.Engelmann I, Moeller U, Santamaria A, Kremsner PG, Luty AJF. Differing activation status and immune effector molecule expression profiles of neonatal and maternal lymphocytes in an African population. Immunology. 2006;119(4):515–521. doi: 10.1111/j.1365-2567.2006.02466.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Randolph DA. The Neonatal Adaptive Immune System. NeoReviews. 2005;6(10):e454–e462. doi: 10.1542/neo.6-10-e454 [DOI] [Google Scholar]
- 60.Kaminski BA, Kadereit S, Miller RE, et al. Reduced expression of NFAT-associated genes in UCB versus adult CD4+ T lymphocytes during primary stimulation. Blood. 2003;102(13):4608–4617. doi: 10.1182/blood-2003-05-1732 [DOI] [PubMed] [Google Scholar]
- 61.Mastelic B, Kamath AT, Fontannaz P, et al. Environmental and T Cell–Intrinsic Factors Limit the Expansion of Neonatal Follicular T Helper Cells but May Be Circumvented by Specific Adjuvants. J Immunol. 2012;189(12):5764–5772. doi: 10.4049/jimmunol.1201143 [DOI] [PubMed] [Google Scholar]
- 62.Gol-Ara M, Jadidi-Niaragh F, Sadria R, Azizi G, Mirshafiey A. The Role of Different Subsets of Regulatory T Cells in Immunopathogenesis of Rheumatoid Arthritis. Arthritis. 2012;2012:1–16. doi: 10.1155/2012/805875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mills KHG. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4(11):841–855. doi: 10.1038/nri1485 [DOI] [PubMed] [Google Scholar]
- 64.Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19(7):665–673. doi: 10.1038/s41590-018-0120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michaëlsson J, Mold JE, McCune JM, Nixon DF. Regulation of T Cell Responses in the Developing Human Fetus. J Immunol. 2006;176(10):5741–5748. doi: 10.4049/jimmunol.176.10.5741 [DOI] [PubMed] [Google Scholar]
- 66.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 2012;26(6):2253–2276. doi: 10.1096/fj.11-193672 [DOI] [PubMed] [Google Scholar]
- 67.Burt TD. Fetal Regulatory T Cells and Peripheral Immune Tolerance In Utero : Implications for Development and Disease. Am J Reprod Immunol. 2013;69(4):346–358. doi: 10.1111/aji.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: Effector T cells with inflammatory properties. Semin Immunol. 2007;19(6):362–371. doi: 10.1016/j.smim.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Roock S, Stoppelenburg AJ, Scholman R, et al. Defective T H 17 development in human neonatal T cells involves reduced RORC2 mRNA content. J Allergy Clin Immunol. 2013;132(3):754–756.e3. doi: 10.1016/j.jaci.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 70.Miyara M, Yoshioka Y, Kitoh A, et al. Functional Delineation and Differentiation Dynamics of Human CD4+ T Cells Expressing the FoxP3 Transcription Factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 71.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol. 2002;2(6):401–409. doi: 10.1038/nri819 [DOI] [PubMed] [Google Scholar]
- 72.Lee H-H, Hoeman CM, Hardaway JC, et al. Delayed maturation of an IL-12–producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205(10):2269–2280. doi: 10.1084/jem.20071371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruckwardt TJ, Malloy AMW, Morabito KM, Graham BS. Quantitative and Qualitative Deficits in Neonatal Lung-Migratory Dendritic Cells Impact the Generation of the CD8+ T Cell Response. Garcia-Sastre A, ed. PLoS Pathog. 2014;10(2):e1003934. doi: 10.1371/journal.ppat.1003934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hofman FM, Danilovs J, Husmann L, Taylor CR. Ontogeny of B cell markers in the human fetal liver. J Immunol Baltim Md 1950. 1984;133(3):1197–1201. [PubMed] [Google Scholar]
- 75.LeBien TW. Fates of human B-cell precursors. Blood 2000;96(1):15. [PubMed] [Google Scholar]
- 76.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b Cells Exhibit Distinct Developmental Requirements and Have Unique Functional Roles in Innate and Adaptive Immunity to S. pneumoniae. Immunity. 2005;23(1):7–18. doi: 10.1016/j.immuni.2005.04.011 [DOI] [PubMed] [Google Scholar]
- 77.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b Lymphocytes Confer T Cell-Independent Long-Lasting Immunity. Immunity. 2004;21(3):379–390. doi: 10.1016/j.immuni.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 79.Melville JM, Moss TJM. The immune consequences of preterm birth. Front Neurosci. 2013;7. doi: 10.3389/fnins.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pihlgren M, Friedli M, Tougne C, Rochat A-F, Lambert P-H, Siegrist C-A. Reduced Ability of Neonatal and Early-Life Bone Marrow Stromal Cells to Support Plasmablast Survival. J Immunol. 2006;176(1):165–172. doi: 10.4049/jimmunol.176.1.165 [DOI] [PubMed] [Google Scholar]
- 81.Klein Klouwenberg P, Bont L. Neonatal and Infantile Immune Responses to Encapsulated Bacteria and Conjugate Vaccines. Clin Dev Immunol. 2008;2008:1–10. doi: 10.1155/2008/628963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsay GJ, Zouali M. The Interplay Between Innate-Like B Cells and Other Cell Types in Autoimmunity. Front Immunol. 2018;9:1064. doi: 10.3389/fimmu.2018.01064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saso A, Kampmann B. Vaccine responses in newborns. Semin Immunopathol. 2017;39(6):627–642. doi: 10.1007/s00281-017-0654-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchant A, Goldman M. T cell-mediated immune responses in human newborns: ready to learn? Clin Exp Immunol. 2005;141(1):10–18. doi: 10.1111/j.1365-2249.2005.02799.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.PrabhuDas M, Adkins B, Gans H, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12(3):189–194. doi: 10.1038/ni0311-189 [DOI] [PubMed] [Google Scholar]
- 86.Siegrist C-A. The Challenges of Vaccine Responses in Early Life: Selected Examples. J Comp Pathol. 2007;137:S4–S9. doi: 10.1016/j.jcpa.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 87.Marchant A, Goetghebuer T, Ota MO, et al. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guérin vaccination. J Immunol. 1999;163(4):2249–2255. [PubMed] [Google Scholar]
- 88.Pichichero ME. Challenges in vaccination of neonates, infants and young children. Vaccine. 2014;32(31):3886–3894. doi: 10.1016/j.vaccine.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller JFAP. Role of the Thymus in Immunity. Br Med J. 1963;2(5355):459–468.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stosio M, Ruszkowski J, Mikosik-Roczyńska A, Haponiuk I, Witkowski JM. The significance of neonatal thymectomy for shaping the immune system in children with congenital heart defects. Pol J Cardio-Thorac Surg. 2017;4:258–262. doi: 10.5114/kitp.2017.72231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hedrick SM. Thymus Lineage Commitment: A Single Switch. Immunity. 2008;28(3):297–299. doi: 10.1016/j.immuni.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 92.Singer A, Adoro S, Park J-H. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8(10):788–801. doi: 10.1038/nri2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walker LSK. Maintaining a Competitive Edge: New Rules for Peripheral T Cell Homeostasis. Immunity. 2012;37(4):598–600. doi: 10.1016/j.immuni.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 94.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol. 2014;14(6):377–391. doi: 10.1038/nri3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood. 2009;113(4):769–774. doi: 10.1182/blood-2008-02-139154 [DOI] [PubMed] [Google Scholar]
- 96.Gui J, Mustachio LM, Su D-M, Craig RW. Thymus Size and Age-related Thymic Involution: Early Programming, Sexual Dimorphism, Progenitors and Stroma. Aging Dis. 2012;3(3):11. [PMC free article] [PubMed] [Google Scholar]
- 97.Tanaskovic S, Fernandez S, Price P, Lee S, French MA. CD31 (PECAM-1) is a marker of recent thymic emigrants among CD4 + T-cells, but not CD8 + T-cells or γδ T-cells, in HIV patients responding to ART. Immunol Cell Biol. 2010;88(3):321–327. doi: 10.1038/icb.2009.108 [DOI] [PubMed] [Google Scholar]
- 98.Zlamy M, Almanzar G, Parson W, et al. Efforts of the human immune system to maintain the peripheral CD8+ T cell compartment after childhood thymectomy. Immun Ageing. 2016;13(1):3. doi: 10.1186/s12979-016-0058-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sauce D, Larsen M, Fastenackels S, et al. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest. 2009;119(10):3070–3078. doi: 10.1172/JCI39269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van den Broek T, Delemarre EM, Janssen WJM, et al. Neonatal thymectomy reveals differentiation and plasticity within human naive T cells. J Clin Invest. 2016;126(3):1126–1136. doi: 10.1172/JCI84997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eysteinsdottir JH, Freysdottir J, Haraldsson A, et al. The influence of partial or total thymectomy during open heart surgery in infants on the immune function later in life. Clin Exp Immunol. 2004;136(2):349–355. doi: 10.1111/j.1365-2249.2004.02437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arron S, Ribeiro R, Gettie A, et al. Impact of thymectomy on the peripheral T cell pool in rhesus macaques before and after infection with simian immunodeficiency virus. Eur J Immunol. 2005;35(1):46–55. doi: 10.1002/eji.200424996 [DOI] [PubMed] [Google Scholar]
- 103.Mancebo E, Clemente J, Sanchez J, et al. Longitudinal analysis of immune function in the first 3 years of life in thymectomized neonates during cardiac surgery. Clin Exp Immunol. 2008;154(3):375–383. doi: 10.1111/j.1365-2249.2008.03771.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brearley S, Gentle TA, Baynham MI, Roberts KD, Abrams LD, Thompson RA. Immunodeficiency following neonatal thymectomy in man. Clin Exp Immunol. 1987;70(2):322–327. [PMC free article] [PubMed] [Google Scholar]
- 105.Ye P, Kirschner DE. Reevaluation of T Cell Receptor Excision Circles as a Measure of Human Recent Thymic Emigrants. J Immunol. 2002;168(10):4968–4979. doi: 10.4049/jimmunol.168.10.4968 [DOI] [PubMed] [Google Scholar]
- 106.Lynch HE, Sempowski GD. Molecular Measurement of T Cell Receptor Excision Circles. Methods Mol Biol. 2013:147–159. doi: 10.1007/978-1-62703-290-2_12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ogle BM, West LJ, Driscoll DJ, et al. Effacing of the T Cell Compartment by Cardiac Transplantation in Infancy. J Immunol. 2006;176(3):1962–1967. doi: 10.4049/jimmunol.176.3.1962 [DOI] [PubMed] [Google Scholar]
- 108.Collier FM, Tang MLK, Martino D, et al. The ontogeny of naïve and regulatory CD4+ T-cell subsets during the first postnatal year: a cohort study. Clin Transl Immunol. 2015;4(3):e34. doi: 10.1038/cti.2015.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Madhok AB, Chandrasekran A, Parnell V, Gandhi M, Chowdhury D, Pahwa S. Levels of Recent Thymic Emigrant Cells Decrease in Children Undergoing Partial Thymectomy during Cardiac Surgery. Clin Vaccine Immunol. 2005;12(5):563–565. doi: 10.1128/CDLI.12.5.563-565.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao Q, Yin M, Zhou Y, Liu J, Sun K, Li B. Effect of thymectomy on cellular immune function. Front Biosci (Landmark Ed). 2011;16:3036–3042. doi: 10.2741/3896 [DOI] [PubMed] [Google Scholar]
- 111.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172(4379):603–606. doi: 10.1038/172603a0 [DOI] [PubMed] [Google Scholar]
- 112.Habiro K, Sykes M, Yang Y-G. Induction of Human T-Cell Tolerance to Pig Xenoantigens via Thymus Transplantation in Mice with an Established Human Immune System. Am J Transplant. 2009;9(6):1324–1329. doi: 10.1111/j.1600-6143.2009.02646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Griesemer AD, Sorenson EC, Hardy MA. The Role of the Thymus in Tolerance: Transplantation. 2010;90(5):465–474. doi: 10.1097/TP.0b013e3181e7e54f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yamada K, Sykes M, Sachs DH. Tolerance in xenotransplantation: Curr Opin Organ Transplant. 2017;22(6):522–528. doi: 10.1097/MOT.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prelog M, Keller M, Geiger R, et al. Thymectomy in early childhood: Significant alterations of the CD4+CD45RA+CD62L+ T cell compartment in later life. Clin Immunol. 2009;130(2):123–132. doi: 10.1016/j.clim.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 116.McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 Deletion Syndrome (DiGeorge Syndrome/Velocardiofacial Syndrome): Medicine (Baltimore). 2011;90(1):1–18. doi: 10.1097/MD.0b013e3182060469 [DOI] [PubMed] [Google Scholar]
- 117.Shah SS, Lai SY, Ruchelli E, Kazahaya K, Mahboubi S. Retropharyngeal Aberrant Thymus. Pediatrics. 2001;108(5):e94–e94. doi: 10.1542/peds.108.5.e94 [DOI] [PubMed] [Google Scholar]
- 118.Bale PM, Sotelo-Avila C. Maldescent of the Thymus: 34 Necropsy and 10 Surgical Cases, Including 7 Thymuses Medial to the Mandible. Pediatr Pathol. 1993;13(2):181–190. doi: 10.3109/15513819309048205 [DOI] [PubMed] [Google Scholar]
- 119.Markert ML, Devlin BH, Alexieff MJ, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007;109(10):4539–4547. doi: 10.1182/blood-2006-10-048652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Markert ML, Alexieff MJ, Li J, et al. Complete DiGeorge syndrome: development of rash, lymphadenopathy, and oligoclonal T cells in 5 cases. J Allergy Clin Immunol. 2004;113(4):734–741. doi: 10.1016/j.jaci.2004.01.766 [DOI] [PubMed] [Google Scholar]
- 121.Gennery AR, Slatter MA, Grandin L, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602-610.e1–11. doi: 10.1016/j.jaci.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 122.Davies EG, Cheung M, Gilmour K, et al. Thymus transplantation for complete DiGeorge syndrome: European experience. J Allergy Clin Immunol. 2017;140(6):1660–1670.e16. doi: 10.1016/j.jaci.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Esquivel CO, Benden C. Clinical models of tolerance induction in pediatric transplantation. Pediatr Transplant. 2009;13(4):397–399. doi: 10.1111/j.1399-3046.2008.01082.x [DOI] [PubMed] [Google Scholar]
- 124.Touraine J-L, Sanhadji K. Transplantation Tolerance Induced in Humans at the Fetal or the Neonatal Stage. J Transplant. 2011;2011:1–4. doi: 10.1155/2011/760319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dutta P, Burlingham WJ. Tolerance to noninherited maternal antigens in mice and humans: Curr Opin Organ Transplant. 2009;14(4):439–447. doi: 10.1097/MOT.0b013e32832d6683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salisbury EM, Game DS, Lechler RI. Transplantation tolerance. Pediatr Nephrol. 2014;29(12):2263–2272. doi: 10.1007/s00467-013-2659-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sprent J, Kishimoto H. The thymus and central tolerance. Morris PJ, Wood KJ, eds. Philos Trans R Soc Lond B Biol Sci. 2001;356(1409):609–616. doi: 10.1098/rstb.2001.0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2010;11(1):21–27. doi: 10.1038/ni.1817 [DOI] [PubMed] [Google Scholar]
- 129.Martens GR, Reyes LM, Butler JR, et al. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs: Transplantation. 2017;101(4):e86–e92. doi: 10.1097/TP.0000000000001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ladowski JM, Reyes LM, Martens GR, et al. Swine Leukocyte Antigen Class II Is a Xenoantigen: Transplantation. 2018;102(2):249–254. doi: 10.1097/TP.0000000000001924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miyake T, Hosaka N, Cui W, et al. Adult thymus transplantation with allogeneic intra-bone marrow-bone marrow transplantation from same donor induces high thymopoiesis, mild graft-versus-host reaction and strong graft-versus-tumour effects. Immunology. 2009;126(4):552–564. doi: 10.1111/j.1365-2567.2008.02920.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kinnear G, Jones ND, Wood KJ. Costimulation Blockade: Current Perspectives and Implications for Therapy. Transplant J. 2013;95(4):527–535. doi: 10.1097/TP.0b013e31826d4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fuchimoto Y, Huang CA, Yamada K, et al. Mixed chimerism and tolerance without whole body irradiation in a large animal model. J Clin Invest. 2000;105(12):1779–1789. doi: 10.1172/JCI8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Markert ML, Sarzotti M, Ozaki DA, et al. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. Blood. 2003;102(3):1121–1130. doi: 10.1182/blood-2002-08-2545 [DOI] [PubMed] [Google Scholar]
- 135.Markert ML, Devlin BH, Chinn IK, McCarthy EA, Li YJ. Factors Affecting Success of Thymus Transplantation for Complete DiGeorge Anomaly. Am J Transplant. 2008;8(8):1729–1736. doi: 10.1111/j.1600-6143.2008.02301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Markert ML, Devlin BH, McCarthy EA. Thymus transplantation. Clin Immunol. 2010;135(2):236–246. doi: 10.1016/j.clim.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rice HE, Skinner MA, Mahaffey SM, et al. Thymic transplantation for complete DiGeorge syndrome: Medical and surgical considerations. J Pediatr Surg. 2004;39(11):1607–1615. doi: 10.1016/j.jpedsurg.2004.07.020 [DOI] [PubMed] [Google Scholar]
- 138.Chinn IK, Olson JA, Skinner MA, et al. Mechanisms of tolerance to parental parathyroid tissue when combined with human allogeneic thymus transplantation. J Allergy Clin Immunol. 2010;126(4):814–820.e8. doi: 10.1016/j.jaci.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chinn IK, Milner JD, Scheinberg P, Douek DC, Markert ML. Thymus transplantation restores the repertoires of forkhead box protein 3 (FoxP3) + and FoxP3 − T cells in complete DiGeorge anomaly: FoxP3 + T cells in complete DiGeorge anomaly. Clin Exp Immunol. 2013;173(1):140–149. doi: 10.1111/cei.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li B, Li J, Devlin BH, Markert ML. Thymic microenvironment reconstitution after postnatal human thymus transplantation. Clin Immunol. 2011;140(3):244–259. doi: 10.1016/j.clim.2011.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee JH, Markert ML, Hornik CP, et al. Clinical Course and Outcome Predictors of Critically Ill Infants With Complete DiGeorge Anomaly Following Thymus Transplantation: Pediatr Crit Care Med. 2014;15(7):e321–e326. doi: 10.1097/PCC.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee LA, Gritsch HA, Sergio JJ, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci U S A. 1994;91(23):10864–10867. doi: 10.1073/pnas.91.23.10864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodriguez-Barbosa J-I, Zhao Y, Houser S, Zhao G, Sykes M. Fetal porcine thymus engraftment, survival and CD4 reconstitution in αGal-KO mice is impaired in the presence of high levels of antibodies against αGal. Xenotransplantation. 2003;10(1):24–40. doi: 10.1034/j.1399-3089.2003.01104.x [DOI] [PubMed] [Google Scholar]
- 144.Barth RN, Yamamoto S, LaMattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. evidence for pig-specific T-cell unresponsiveness1: Transplantation. 2003;75(10):1615–1624. doi: 10.1097/01.TP.0000064335.50622.20 [DOI] [PubMed] [Google Scholar]
- 145.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. doi: 10.1038/nm1172 [DOI] [PubMed] [Google Scholar]
- 146.Yamada K, Scalea J. Current progress in xenogeneic tolerance: Curr Opin Organ Transplant. 2012;17(2):168–173. doi: 10.1097/MOT.0b013e32835090f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fudaba Y, Onoe T, Chittenden M, et al. Abnormal Regulatory and Effector T Cell Function Predispose to Autoimmunity following Xenogeneic Thymic Transplantation. J Immunol. 2008;181(11):7649–7659. doi: 10.4049/jimmunol.181.11.7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lechler R, Chai J-G, Marelli-Berg F, Lombardi G. The contributions of T-cell anergy to peripheral T-cell tolerance. Immunology. 2001;103(3):262–269. doi: 10.1046/j.1365-2567.2001.01250.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110 [DOI] [PubMed] [Google Scholar]
- 150.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr Opin Immunol. 2010;22(5):552–559. doi: 10.1016/j.coi.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Uehara M, McGrath MM. The Role of Costimulatory Pathways in Transplant Tolerance. Clin Lab Med. 2019;39(1):87–106. doi: 10.1016/j.cll.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 152.Meng L, Wu Z, Wang Y, et al. Differential Impact of CD154 Costimulation Blockade on Alloreactive Effector and Regulatory T Cells in Murine Renal Transplant Recipients: Transplantation. 2008;85(9):1332–1338. doi: 10.1097/TP.0b013e31816c4f2b [DOI] [PubMed] [Google Scholar]
- 153.Cardona K, Korbutt GS, Milas Z, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12(3):304–306. doi: 10.1038/nm1375 [DOI] [PubMed] [Google Scholar]
- 154.Kim SC, Wakwe W, Higginbotham LB, et al. Fc-Silent Anti-CD154 Domain Antibody Effectively Prevents Nonhuman Primate Renal Allograft Rejection. Am J Transplant. 2017;17(5):1182–1192. doi: 10.1111/ajt.14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.John M, Bailey LL. Neonatal heart transplantation. Ann Cardiothorac Surg. 2018;7(1):118–125. doi: 10.21037/acs.2018.01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research: Genetically engineered pigs in xenotransplantation. J Pathol. 2016;238(2):288–299. doi: 10.1002/path.4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Groth CG. The potential advantages of transplanting organs from pig to man: A transplant Surgeon’s view. Indian J Urol. 2007;23(3):305–309. doi: 10.4103/0970-1591.33729 [DOI] [PMC free article] [PubMed] [Google Scholar]