Abstract
Objective –
To develop a method to simultaneously quantify the synthetic contraceptive progestin segesterone acetate (Nestorone®, NES) and the endogenous steroid hormones estradiol (E2), progesterone (P4), and estrone (E1) in human serum samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Study Design –
We analyzed 615 serum samples collected from 67 reproductive-age women actively using a contraceptive vaginal ring (CVR) designed to release NES (200 mcg/d) and E2 (75–200 mcg/d). Samples were taken prior to and up to 30 days after CVR insertion and analyzed for concentrations of NES, E2, P4, and E1 in human serum using a Shimadzu Nexera-LCMS-8050 LC-MS/MS platform. Precision, accuracy, and sensitivity for all analytes were determined across multiple assays.
Results –
The assay ranges for NES, E2, P4, and E1 in this analytical method were 10 pg/mL to 10 ng/mL with a lower limit of quantification of 10 pg/mL for all targets. Assay precisions were less than or equal to 14.5% and accuracies ranged from 87.0% – 110.8%. When applied to the 615 clinical samples, 550 samples had quantifiable concentrations of NES (value range 0.014 ng/mL – 1471 ng/mL). Similarly, 595 samples had quantifiable concentrations of E2 (0.010 ng/mL – 0.312 ng/mL), 596 samples had quantifiable concentrations of P4 (0.010 ng/mL – 5.791 ng/mL), and 609 samples had quantifiable concentrations of E1 (0.010 ng/mL – 0.416 ng/mL).
Conclusions –
The LC-MS/MS platform results in a robust, accurate, and sensitive method for the simultaneous quantification of NES and endogenous steroid hormones in human serum.
Implications –
The analytical method described allows for the simultaneous quantification of NES and endogenous steroids and can be used to monitor NES concentrations during clinical trials and subject adherence to treatment with NES.
Keywords: segesterone acetate, Nestorone®, estradiol, contraception, LC-MS/MS
1. Introduction:
Development of new contraceptive methods for men and women is a goal of the Contraceptive Development Program at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, Bethesda, MD). Access to novel products with decreased side-effects and increased health benefits may improve uptake, adherence, and effectiveness, all essential to addressing the unmet need for contraception. User-controlled reversible contraceptive methods with long-acting duration that do not require daily attention, such as contraceptive vaginal rings (CVR), may improve compliance and have been shown to decrease rates of unplanned pregnancies [1].
Segesterone acetate (Nestorone®; NES) is a 19-norprogesterone derivative that acts as a potent inhibitor of ovulation and demonstrates both high progestational and antiestrogenic effects on the endometrium [2, 3]. NES is inactive when given orally, but is very potent when delivered transdermally, transvaginally, or parenterally [4–7]. Low doses of NES have been found to be highly effective at controlling fertility [8] when administered in combination with an estrogen such as ethinylestradiol (EE) or estradiol (E2) in a CVR [9, 10]. A CVR (Annovera™, TherapeuticsMD, Boca Raton, FL) releasing 0.15 mg EE and 0.013 mg of NES daily and designed for use over one year (13–28 day cycles) was recently approved in the United States [11]. E2-releasing CVRs are currently undergoing evaluation in clinical trials, as use of E2 could improve safety by reducing the thrombosis risk associated with EE [10, 12, 13].
Because NES effectively blocks follicle development and ovarian production of E2, the ability to measure concentrations of both NES and E2 is critical to evaluate the efficacy of this new CVR. There are currently limited methods available to quantify NES in human serum, and these methods require a dedicated aliquot for the measurement of NES [10, 14]. A previous study similar to the study described herein required the measurement of both NES and E2 in study samples; due to limitations at the respective testing laboratories, separate laboratories were required for analysis of NES and E2 [10]. In order to assess the ovulatory-inhibition activity of NES, it is necessary to measure other serum steroids, particularly progesterone (P4), as well as additional estrogen concentrations throughout the treatment period. Herein, we describe a method to simultaneously quantitate NES, E2, P4, and estrone (E1) in human serum samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS). This method can be used to monitor drug levels during clinical trials to evaluate efficacy, as well as adherence in protocols involving treatment with NES.
2. Materials and methods:
2.1. Chemicals
NES was purchased from Sigma-Aldrich (St. Louis, MO, USA) for use as a standard. Calibration standards for E2, P4, and E1 were purchased from Cerilliant (Round Rock, TX, USA) as 1.000 mg/mL solutions in acetonitrile (ACN). The stable isotope-labeled internal standards E2-d5, P4-d9, and E1-13C3 were purchased from Cerilliant and arrived as 100 μg/mL stocks in ACN. Ammonium fluoride (NH4F) and dichloromethane (DCM) were purchased from Sigma-Aldrich. Charcoal-stripped human serum was purchased from BioChemed Services (Winchester, VA, USA) and Golden West Biologicals (Temecula, CA, USA). Charcoal-stripped serum (CSS) purchased from BioChemed Services was used in the method validation and assays described herein; CSS from Golden West Biologicals was used only during specificity testing (Sections 2.5 and 3.2.1 below). Normal human serum was purchased from Golden West Biologicals. LC-MS-grade water and methanol were purchased from Honeywell Burdick & Jackson (Muskegon, MI, USA).
2.2. Preparation of calibration curve and quality control samples
Calibration curves were prepared using a working mixture of unlabeled standards for NES, E2, P4, and E1 in methanol at 500 ng/mL each. This mixture was added to CSS for a final concentration of 10 ng/mL for each hormone. Two-fold serial dilutions of the 10 ng/mL mixture were made in CSS to yield 12 calibration standards, which included a blank (0 pg/mL) and ranged from 10 pg/mL to 10 ng/mL for all hormones. Quality control (QC) samples were prepared by spiking the mixture of unlabeled hormone standards described above into normal human serum (Table 1). A high QC was prepared by spiking a mixture of NES, E2, P4, and E1; a low QC was prepared by spiking only NES and E1, as endogenous concentrations of E2 and P4 were used in this QC. Endogenous concentrations of E2 and P4 in normal human serum used for QCs were determined by immunoassay on a Roche cobas e411 automatic clinical analyzer (Roche Diagnostics, Indianapolis, IN). The endogenous E1 concentration in normal human serum was determined via the standard addition method using 12 serial dilutions of serum spiked with 10 ng/mL of E1. These QCs were assayed in triplicate at the beginning, middle, and end of each assay.
Table 1.
Accuracies, precisions (expressed as CVs), matrix effects, and recoveries for the simultaneous analysis of NES and endogenous steroid hormones (n=5).
| Analyte | Spiked Value (ng/mL) | Expected Value (ng/mL) | Assayed Value (ng/mL) | Intra-assay CV | Inter-assay CV | Accuracy | Matrix Effect | Recovery |
|---|---|---|---|---|---|---|---|---|
| Nestorone® QC1Nestorone® QC2 | 0.100 | 0.100 | 0.087 | 10.7% | 14.0% | 87.0% | 93.2% | 83.5% |
| Nestorone® QC2 | 0.500 | 0.500 | 0.480 | 11.1% | 13.5% | 96.0% | ||
| Estradiol QC1 | 0.000 | 0.124 | 0.122 | 5.8% | 6.9% | 98.4% | 105.9% | 92.8% |
| Estradiol QC2 | 0.500 | 0.624 | 0.621 | 6.0% | 6.4% | 99.5% | ||
| Progesterone QC1 | 0.000 | 3.530 | 3.706 | 12.6% | 14.4% | 105.0% | 64.9% | 84.4% |
| Progesterone QC2 | 0.500 | 4.030 | 4.048 | 14.0% | 14.5% | 100.4% | ||
| Estrone QC1 | 0.100 | 0.166 | 0.184 | 5.9% | 6.9% | 110.8% | 102.5% | 93.1% |
| Estrone QC2 | 0.500 | 0.566 | 0.574 | 5.8% | 6.8% | 101.4% | ||
Expected values for QC1 and QC2 for Nestorone were created using spikes of unlabeled standard. Expected values for QC1 for estradiol and progesterone were endogenous values; expected values for QC2 for estradiol, progesterone, and estrone, as well as for QC1 for estrone, are the sum of endogenous and spiked amounts as listed in the table above. QC, quality control; CV, coefficient of variation.
2.3. Sample preparation
Two-hundred μL of standard, QC, or serum samples were pipetted into 350 μL V-bottom 96-well microtiter plates (Shimadzu, Kyoto, Japan), followed by 100 μL of LC-MS-grade water containing 1.8 ng/mL E2-d5, 0.45 ng/mL P4-d9, and 0.75 ng/mL E1-13C3. A double blank of serum with 100 μL of ultrapure water containing no isotope-labeled or unlabeled standards was also prepared in duplicate. Plates were shaken on an orbital shaker for 5 min and the contents were loaded onto 400 μL 96-well Isolute Supported Liquid Extraction (SLE+) plates (Biotage, Uppsala, Sweden). After incubation at room temperature for 5 min, analytes were eluted into 2 mL glass-coated 96-well deep well plates (Thermo Fisher, Waltham, MA) with 2 × 900 μL DCM and dried under forced air at 40°C in a TurboVap 96 automated evaporation system (Biotage). The glass-coated plates were rinsed with 400 μL DCM and again dried under forced air. After careful reconstitution in 50 μL of 25:75 methanol:water (v:v), the contents were transferred to new 350 μL V-bottom 96-well microtiter plates and analyzed by LC-MS/MS.
2.4. LC-MS/MS instrument parameters
Microtiter plates were prepared as described above and loaded onto a SIL-30ACMP autosampler (Shimadzu) set at 10°C. Twenty-five μL of each standard, QC, or sample were injected onto a Raptor Biphenyl column (50 mm × 2.1 mm, 2.7 μm particle size; Restek, Bellefonte, PA) with an in-line Raptor Biphenyl guard column (5 mm × 2.1 mm, 2.7 μm particle size; Restek) at 40°C and eluted using reversed-phase chromatography. Mobile phase A was 0.15 mM NH4F in water; mobile phase B was 100% methanol. The mobile phase gradient was created using two Nexera LC-30AD pumps (Shimadzu) as follows: 0.00–4.00 min, 70%−90% B; 4.01–4.74 min, 90%−100% B; 4.75–4.79 min, 100% B; 4.80 min, return to 70% B and hold for re-equilibration until 7.75 min. The entire gradient was run at a flow rate of 0.25 mL/min. Heated electrospray ionization in both positive (NES, P4) and negative (E2, E1) modes with ultra-fast polarity switching and scheduled multiple reaction monitoring (MRM) on a Shimadzu LCMS-8050 triple-quadrupole mass spectrometer was used for detection of steroids. The interface temperature was 300°C, the desolvation line temperature was 150°C, and the heat block temperature was 500°C. Gas was supplied by a Peak Genius 1051 nitrogen and air generator (Peak Scientific, Inchinnan, UK). Nitrogen gas was used for the nebulizing and drying gases, while air was used for the heating gas. The nebulizing gas flow was 3 L/min, the heating gas flow was 10 L/min, and the drying gas flow was 10 L/min. Ultra-high purity argon (Airgas, Radnor, PA) was used for collision-induced dissociation at 270 kPa. The MS/MS conditions for each hormone were optimized using the flowinjection analysis and automated MRM optimization procedure in LabSolutions software, version 5.72 (Shimadzu). The MRM transitions (m/z) and other MS parameters for all compounds in this method can be found in Table 2.
Table 2.
MS parameters used for simultaneous quantification of NES and endogenous sex steroids.
| Hormone | Type | Precursor m/z | Product m/z | Dwell Time (ms) | Interface voltage (kV) | Q1 pre bias (V) | CE (V) | Q3 pre bias (V) | Retention Time (min) |
|---|---|---|---|---|---|---|---|---|---|
| E2-d5 | ISTD | 276.10 | 147.15 | 38.0 | −2.00 | 19.0 | 42.0 | 28.0 | 1.51 |
| 276.10 | 187.20 | 38.0 | 10.0 | 41.0 | 18.0 | ||||
| E2 | Target | 271.00 | 145.25 | 37.0 | −2.00 | 13.0 | 40.0 | 27.0 | 1.53 |
| 271.00 | 143.10 | 37.0 | 13.0 | 50.0 | 25.0 | ||||
| 271.00 | 239.30 | 37.0 | 13.0 | 39.0 | 25.0 | ||||
| E1-13C3 | ISTD | 272.20 | 148.15 | 29.0 | −2.00 | 18.0 | 39.0 | 13.0 | 2.33 |
| 272.20 | 146.25 | 29.0 | 18.0 | 50.0 | 12.0 | 2.33 | |||
| E1 | Target | 268.90 | 145.30 | 74.0 | −2.00 | 22.0 | 46.0 | 22.0 | 2.33 |
| 268.90 | 143.35 | 74.0 | 22.0 | 54.0 | 23.0 | ||||
| NES | Target | 371.10 | 311.15 | 107.0 | 0.50 | −26.0 | −12.0 | −21.0 | 3.88 |
| 371.10 | 253.30 | 107.0 | −26.0 | −14.0 | −28.0 | ||||
| 371.10 | 269.30 | 107.0 | −26.0 | −16.0 | −28.0 | ||||
| 371.10 | 159.15 | 107.0 | −26.0 | −31.0 | −30.0 | ||||
| P4-d9 | ISTD | 324.15 | 100.20 | 107.0 | 0.50 | −23.0 | −23.0 | −17.0 | 4.42 |
| 324.15 | 113.20 | 107.0 | −24.0 | −25.0 | −22.0 | ||||
| P4 | Target | 315.30 | 109.15 | 107.0 | 0.50 | −12.0 | −24.0 | −21.0 | 4.47 |
| 315.30 | 97.05 | 107.0 | −12.0 | −21.0 | −18.0 | ||||
Bold font indicates the transition used for quantification for each hormone. E2, estradiol; E1, estrone; NES, Nestorone®; P4, progesterone; CE, collision energy.
2.5. Specificity and stability
The specificity of each assay was confirmed by ensuring that no interference peaks for signals at the expected retention time for each analyte were detected in blank matrix. We did not formally assess analyte stability. However, we demonstrated that hormones were stable after extraction for the duration of at least one analytical run (approximately 25 hours) as QC concentrations and CV values were statistically indistinguishable at the front, middle, and end of each assay. In addition, we found no discoloration or increase in sample viscosity after extraction suggesting that no interferences from hemoglobin or other blood products were present.
2.6. Precision, accuracy, recovery, and matrix effects
Precision and accuracy were calculated using QCs prepared by spiking normal human serum with unlabeled standards and analyzed across five assays (n=9 replicates per assay). Extraction efficiency (recovery) was calculated by comparing CSS spiked before extraction and CSS spiked after extraction. Matrix effects were assessed by analysis of unlabeled standards spiked into CSS after extraction compared to unlabeled standards spiked into 25:75 methanol:water without extraction.
2.7. Limits of quantification and detection
Because of the low noise found in most modern MS/MS instruments, we did not use the traditional approach of signal/noise (S/N) ratio to evaluate each assay lower limit of quantification (LLOQ) or limit of detection (LOD). Instead, the limits of quantification were determined to be the lowest and highest concentration on the calibration curve with accuracy 80%−120% and precision <20% as measured by the coefficient of variation (CV). The method detection limit (MDL) is defined as the minimum concentration of an analyte that can be measured and reported with 99% confidence that the analyte concentration is greater than zero. The MDL was determined from analysis of replicate standard injections in matrix at concentrations near the LLOQ to evaluate the uncertainty in the analysis system. For determination of MDLs for this method, nine replicate injections of the lowest-concentration calibrator were analyzed for each analyte and MDLs were calculated using an established formula [15].
2.8. Clinical samples
Clinical samples came from a multi-centered, double-blind, randomized Phase IIa dose-finding study sponsored and developed jointly by the Population Council (New York, NY) and the Contraceptive Discovery and Development Branch, NICHD. The respective sites’ Institutional Review Boards approved the study protocol and all participants underwent written informed consent. The trial was registered with clinicaltrials.gov (NCT0158600). Subjects were randomized 1:1:1 to receive CVRs containing one of three doses of E2 (75, 100 or 200 mcg/day) in combination with a single dose of NES (200 mcg/day) to determine if a dose-response for E2 was observed in CVR users [16]. Visits occurred twice weekly for one month, at which time site staff collected blood samples and used a refrigerated centrifuge to separate serum, which was stored at −80°C prior to shipment on dry ice to the Oregon National Primate Research Center for analysis of NES, E2, P4, and E1. A total of 615 samples were collected from 67 participants and analyzed using the LC-MS/MS method described herein.
2.9. Data analysis
Data were analyzed using LabSolutions software (Shimadzu). Target reference ion ratios for the qualifying ions were set based on the area ratio between the quantifying ion and qualifying ion observed in the highest standard. For calibration curves, ratios of the peak area for each standard to that of its respective internal standard were linearly regressed using 1/C weighting. As no stable isotope-labeled internal standard for NES was commercially available without custom synthesis at the time of method development and sample analysis, E1-13C3 was used as the internal standard for NES.
3. Results:
3.1. LC-MS/MS analysis
Linear response was observed for each hormone within the calibration range (0.010 – 10 ng/mL; R2>0.999). Simple chromatographic conditions were selected to provide optimal peak heights and run time. These requirements were met by using a biphenyl column with a mobile phase combination of 0.15 mM NH4F in water (mobile phase A) and methanol with no additive (mobile phase B). Using the gradient program described above (section 2.4), the combination of the selected column and mobile phases provided favorable separation of the target hormones (Figure 1). Ammonium fluoride was selected as an additive as it provides up to 11 times more sensitivity in negative mode and over two times more sensitivity in positive mode than other commonly used additives [17]. This enhanced sensitivity yielded acceptable LLOQs for all targets in the method without further manipulation such as derivatization during sample preparation.
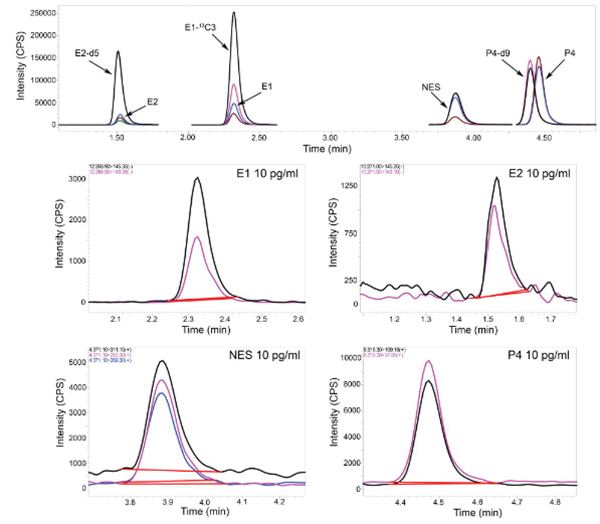
Figure 1.
Representative multiple reaction monitoring (MRM) chromatograms of unlabeled and stable isotope-labeled endogenous steroid hormones and Nestorone. The chromatogram in the top panel is representative off the simultaneous analysis of E2, E1, NES, and P4 (0.150 ng/mL each). Below are representative chromatograms for each individual analyte from a single injection of a mixed standard at the lower limit of quantification (LLOQ) for each hormone, with the LLOQ indicated in each respective panel. Peaks are colored to represent the different ions used in analysis of a particular target. MRMs used for quantification and other MS parameters can be found in Table 2. E2, estradiol; E1, estrone; NES, Nestorone; P4, progesterone; d, deuterium; CPS, counts per second; min, minutes.
3.2. Method validation
Validation of the analytical method was performed largely according to FDA guidelines [18], by assessing specificity, precision, accuracy, recovery, and sensitivity.
3.2.1. Specificity
The specificity of each assay in the method was confirmed by ensuring that no interfering peaks or signals at the expected retention times for each analyte were detectable in blank matrix for all MRMs used within the method. The blank matrices tested were eight different lots of CSS from two different vendors (Golden West Biologicals and BioChemed Services). In addition, the specificity of the NES assay was confirmed by utilizing several in-house prepared pools of nonhuman primate serum from animals that were not exposed to NES and that were not spiked with unlabeled NES standard.
3.2.2. Precision, accuracy, and recovery
Precision, accuracy, and recovery for all analytes were determined as averages across multiple assays (n=5). Intra- and inter-assay precisions for each compound are reported as CVs. Intra-assay CVs were less than 14.0% and inter-assay CV were less than 14.5% for all targets in the method (Table 1). Accuracies for all targets ranged from 87.0–110.8% (Table 1). Recoveries (extraction efficiencies) were 83.5% for NES, 92.8% for E2, 84.4% for P4, and 93.1% for E1 (Table 1) and were compensated for by using appropriate internal standards for each target. Matrix effects ranged from 64.9%−105.9% (Table 1) and did not vary with analyte concentration and were stable across replicates in each analysis. Isotopically labeled NES was not commercially available at the time of method development; it was determined that E1-13C3 was the most appropriate substitute based on the accuracy and precision of the NES assay when E1-13C3 was used for NES normalization compared to E2-d5 and P4-d9 (data not shown).
3.2.3. Sensitivity
The LLOQ was 10 pg/mL and the upper limit of quantification (ULOQ) was 10 ng/mL for each assay in the method, with accuracies between 80–120% at these levels. The double blank injected following the highest calibration standard confirmed absence of carryover in this method. The MDL for NES was 4 pg/mL and the MDLs for E2, P4, and E1 were each 3 pg/mL (Table 3).
Table 3.
LLOQ and MDL for simultaneous analysis of NES and endogenous steroid hormones (n=5).
| Analyte | LLOQ | Measured Concentration at LLOQ | MDL | |||
|---|---|---|---|---|---|---|
| pg/mL | pg/col | pg/mL | CV | pg/mL | pg/col | |
| Estrone | 10 | 0.9 | 9 | 12.5% | 3 | 0.27 |
| Estradiol | 10 | 0.9 | 11 | 9.4% | 3 | 0.27 |
| Nestorone® | 10 | 0.9 | 9 | 14.6% | 4 | 0.36 |
| Progesterone | 10 | 0.9 | 9 | 16.3% | 3 | 0.27 |
The amount injected is indicated as pg/col. LLOQ, lower limit of quantification; col, column; MDL, method detection limit; CV, coefficient of variation
3.3. Application of the method to human serum samples
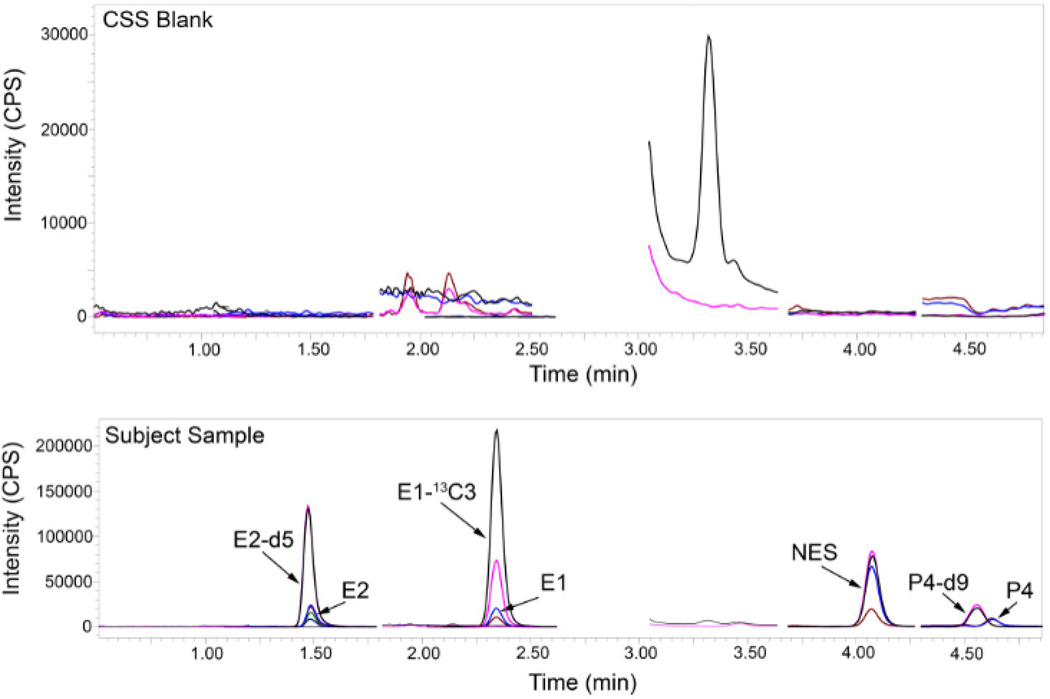
The validated analytical method was applied to 615 samples collected from 67 women administered a combination of NES and E2 via CVR (Table 4). For NES, 550 samples had quantifiable levels with a sample concentration range of 0.014 ng/mL – 1.471 ng/mL; the 65 samples that were below the LLOQ were baseline samples taken before the administration of NES. Similarly, 595 samples had quantifiable concentrations of E2 (concentration range 0.010 ng/mL – 0.312 ng/mL), 596 samples had quantifiable concentrations of P4 (concentration range 0.010 ng/mL – 5.791 ng/mL), and 609 samples had quantifiable concentrations of E1 (concentration range 0.010 ng/mL – 0.416 ng/mL). Seventeen samples, 18 samples, and 6 samples had concentrations that were detectable (>MDL) but below the LLOQ for E2, P4, and E1, respectively. Three samples, one sample, and no samples had concentrations that were below the MDL for E2, P4, and E1, respectively. Figure 2 contains a representative MRM chromatogram from a subject sample. A representative chromatogram of charcoal-stripped serum (blank matrix) is included in the figure to show the baseline.
Table 4.
Simultaneous analysis of NES and endogenous steroid hormones in samples collected from women using a NES- and E2-releasing CVR (n=5).
| Parameter | NES | E2 | P4 | E1 |
|---|---|---|---|---|
| Assay Range (ng/mL) | 0.010 – 10 | 0.010 – 10 | 0.010 – 10 | 0.010 – 10 |
| Sample Concentration Range (median) (ng/mL) | 0.014 – 1.471 (0.178) | 0.010 – 0.312 (0.034) | 0.010 – 5.791 (0.049) | 0.010 – 0.416 (0.033) |
| Number of Sampes Quantified | 550/615* | 595/615 | 596/615 | 609/615 |
| Number of Samples <LLOQ and >MDL | 0/615** | 17/615 | 18/615 | 6/615 |
| Number of Samples <MDL | 0/615** | 3/615 | 1/615 | 0/615 |
the 65 baseline samples for each subject before administration of NES were not detected (<MDL).
does not include baseline samples
NES, Nestorone®; E2, estradiol; P4, progesterone; E1, estrone; LLOQ, lower limit of quantification; MDL, method detection limit.
Figure 2.
Representative multiple reaction monitoring (MRM) chromatograms of unlabeled and stable isotope-labeled endogenous steroid hormones and Nestorone in a charcoal-stripped serum (CSS) blank and a subject sample. The chromatogram in the top panel is a representative CSS blank, demonstrating absence of appreciable endogenous steroids. The chromatogram in the bottom panel is a representative simultaneous analysis of E2 (180 pg/mL), E1 (102 pg/mL), NES (178 pg/mL), and P4 (45 pg/mL) in a subject sample. E2, estradiol; E2, estrone; NES, Nestorone; P4, progesterone; d, deuterium; CPS, counts per second; min, minutes; CSS, charcoal-stripped serum.
4. Discussion:
In this report, we describe a method for the simultaneous assay of the synthetic progestin NES and three endogenous steroid hormones, E2, P4, and E1, by LC-MS/MS. While reports of hormonal contraceptive and endogenous steroid analysis by LC-MS/MS are abundant, there are limited reports of an assay for NES [10, 14]. The method described here is the first to include simultaneous analysis of endogenous steroid hormones and NES. One report [10] utilized a radioimmunoassay to measure NES while another [14] reported an LC-MS/MS method for analysis of NES concentrations in women exposed to men using a topical NES/testosterone contraceptive gel. The latter method had identical sensitivity (LLOQ of 10 pg/mL for both methods) and similar accuracy (88% versus 87%, QC1, Table 1) to the method described herein, though it was more precise as expressed by CV (<10% vs. 14.0%, QC1, Table 1). This superior precision is likely due to their use of a custom-made NES-13C3 internal standard for normalization of NES data. However, the intra-day precision of <11.1% and inter-day precisions of <14.0% reported here are acceptable according to the guidelines we followed while developing the method [18].
The method described by Yuen et al. [14] was for the assay of NES alone and requires a separate sample aliquot and analysis for the determination of additional steroid concentrations. Concurrent monitoring of endogenous steroid hormone concentrations with NES concentrations is advantageous because it can provide additional information about the mechanism of NES action and give insight into contraceptive efficacy. Simultaneous analysis also requires less sample volume and may result in collecting less blood from subjects during contraceptive hormone monitoring in clinical studies. Analysis can also be completed more quickly and more economically with simultaneous hormone determinations.
Advantages of the LC-MS/MS method for NES analysis we describe here include requirement of a relatively small sample size (200 μL) and a sample preparation method that does not require derivatization for the simultaneous measurement of endogenous estrogens. While derivatization is common to reach an appropriate LLOQ for E2 measurement [19], we were able to create a method with an adequate LLOQ for E2 without the use of chemical derivatization during sample preparation. Of the 615 samples to which the method was applied, three samples were undetectable or below the 3 pg/mL MDL for E2; one was less than the MDL for P4, and no samples measured below the MDL for E1. Two samples had undetectable concentrations of NES (below 4 pg/mL) when baseline samples (before administration of NES) are discounted (Table 4). These results underscore the utility of this method for analyzing these hormones in women actively using NES (Table 4).
In order to improve the robustness of the assay, a QC could be added to reflect a concentration in the upper portion of the assay range. During development we anticipated sample analyte concentrations would exist in the lower portion of the assay range, and our QCs were created accordingly. Because the assays were linear throughout the calibration range (R2>0.999) we do not expect any accuracy issues with sample analyte values in the upper portion of the calibration range despite the lack of monitoring by QCs. A further limitation of our paper is the focus on assay characteristics, and not on the clinical performance of the CVR. However, the pharmacokinetics of investigational NES and E2 CVRs, including the CVR used in this paper, have been previously reported [10, 16].
In summary, we have developed a method for the simultaneous quantification of NES, E2, P4, and E1 in human serum. This method uses a small amount of sample and is rapid, sensitive, accurate, and precise. This method can be used for monitoring adherence to contraceptive methods using NES and also for development of new contraceptive methods using NES.
Acknowledgements:
The authors would like to acknowledge the staff of the Endocrine Technologies Core at Oregon National Primate Research Center for technical assistance.
Funding:
This work was supported by the NIH grant P51 OD011092 and NICHD Contraceptive Clinical Trial Network HHSN275200403378I.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. [DOI] [PubMed] [Google Scholar]
- [2].Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update. 2006;12:169–78. [DOI] [PubMed] [Google Scholar]
- [3].Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2008;61:151–7. [DOI] [PubMed] [Google Scholar]
- [4].Heikinheimo O, Ranta S, Moo-Young A, Lahteenmaki P, Gordon K. Parenteral administration of progestin Nestorone to lactating cynomolgus monkeys: an ideal hormonal contraceptive at lactation? Hum Reprod. 1999;14:1993–7. [DOI] [PubMed] [Google Scholar]
- [5].Heikinheimo O, Noe G, Haukkamaa M, Lahteenmaki P. The progestin ST 1435--rapid metabolism in man. Contraception. 1994;50:275–89. [DOI] [PubMed] [Google Scholar]
- [6].Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000;65:629–36. [DOI] [PubMed] [Google Scholar]
- [7].Noe G, Salvatierra A, Heikinheimo O, Maturana X, Croxatto HB. Pharmacokinetics and bioavailability of ST 1435 administered by different routes. Contraception. 1993;48:548–56. [DOI] [PubMed] [Google Scholar]
- [8].Haukkamaa M, Laurikka-Routti M, Heikinheimo O, Moo-Young A. Contraception with subdermal implants releasing the progestin ST-1435: a dose-finding study. Contraception. 1992;45:49–55. [DOI] [PubMed] [Google Scholar]
- [9].Prasad PV, Shrivastav TG. Nestorone: A new hope for gynecologists, andrologists, and neurologists. Women’s Health & Gynecology. 2015;1. [Google Scholar]
- [10].Jensen JT, Edelman AB, Chen BA, et al. Continuous dosing of a novel contraceptive vaginal ring releasing Nestorone(R) and estradiol: pharmacokinetics from a dose-finding study. Contraception. 2018;97:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nelson AL. Comprehensive overview of the recently FDA-approved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: A new year-long, patient controlled, reversible birth control method. Expert Review of Clinical Pharmacology. 2019;12:953–63. [DOI] [PubMed] [Google Scholar]
- [12].Cheek MG, Mashchak AC, Lau BH. Toxic shock syndrome in a postpartum patient. Am J Obstet Gynecol. 1982;142:927–8. [DOI] [PubMed] [Google Scholar]
- [13].Sitruk-Ware R, Plu-Bureau G, Menard J, et al. Effects of oral and transvaginal ethinyl estradiol on hemostatic factors and hepatic proteins in a randomized, crossover study. J Clin Endocrinol Metab. 2007;92:2074–9. [DOI] [PubMed] [Google Scholar]
- [14].Yuen F, Wu S, Thirumalai A, et al. Preventing secondary exposure to women from men applying a novel nestorone/testosterone contraceptive gel. Andrology. 2019;7:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].U.S. EPA - Title 40: Protection of the environment; Part 136 - Guidelines establishing test procedures for the analysis of pollutants; Appendix B to Part 136 - Definition and procedure for the determination of the method detection limit - revision 1.11. 2011. [Google Scholar]
- [16].Chen MJ, Creinin MD, Turok DK, et al. Dose-finding study of a 90-day contraceptive vaginal ring releasing estradiol and segesterone acetate. Contraception. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hindle R. Improved analysis of trace hormones in drinking water by LC/MS/MS (EPA 539) using the Agilent 6460 triple quadrupole LC/MS [5991–2473EN]. Agilent Technologies tech note. 2013. [Google Scholar]
- [18].Food and Drug Administration guidance for industry: Bioanalytical method validation. 2013. [Google Scholar]
- [19].Zhang J, Tang C, Oberly PJ, et al. A sensitive and robust UPLC-MS/MS method for quantitation of estrogens and progestogens in human serum. Contraception. 2019;99:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]