Abstract
Purpose
To define the prevalence and risk factors of anxiety and examine rates and predictors of psychotherapy and integrative medicine service use in breast cancer survivors on AIs.
Methods
Observational study of patients with histologically confirmed stage 0 to III hormone receptor-positive breast cancer taking a third generation AI at the time of enrollment. Patients completed self-report measures of anxiety and utilization of psychotherapy and integrative medicine services at a single time-point. We used multivariate logistic regression analyses to identify factors associated with anxiety and receipt of anxiety treatment services.
Results
Among the 1,085 participants, the majority were younger than 65 years of age (n=673, 62.0%) and white (n=899, 82.9%). Approximately one-third (30.8%) reported elevated anxiety (≥8 on the anxiety subscale of the Hospital Anxiety and Depression Scale). Of patients with elevated anxiety, only 24.6% reported receiving psychological counseling, 25.3% used integrative medicine services, and 39.8% received either type of treatment since their diagnosis. Patients with an education level of high school or less were less likely to receive psychological counseling (AOR, .43, 95% CI .19–.95) and integrative medicine services (OR, .30, 95% CI .12–.72) than patients with higher levels of education.
Conclusions
Anxiety is common in breast cancer patients treated with AIs yet the majority of anxious patients do not receive evidence-based treatment, even when these treatments are available. Better systematic anxiety screening and treatment initiation are needed to reduce disparities in care by education level.
Keywords: Breast cancer, anxiety, psychotherapy, integrative medicine
INTRODUCTION
Over 3 million women in the United States have been diagnosed with breast cancer [1] with an estimated 268,600 new diagnoses in 2019 [2]. Survival rates for breast cancer have improved significantly over time with many patients achieving five-year survival and longer [2]. In the past decade, aromatase inhibitors (Als) have been the recommended adjuvant endocrine therapy as an alternative to or in sequence with tamoxifen in postmenopausal women with hormone-receptor-positive breast cancer [3]. Als combined with ovarian suppression are also beneficial for premenopausal women with higher risk breast cancer [4,5], and are associated with improved disease free and overall survival compared to tamoxifen [6]. Long-term use of Als (i.e., ten years) reduces the risk of breast cancer recurrence [7]. Understanding the experience of patients on Als and providing for their needs is vital to maximizing the long-term quality of life and adherence to treatment in a large population of breast cancer survivors.
The prevalence of anxiety in patients with breast cancer varies widely across studies from 10–41% [8–12]. However, elevated anxiety in breast cancer patients is associated with worse treatment adherence [13–15], poor physical quality of life [16], and poor cognitive function over time [17]. In fact, anxiety has been identified as a primary driver of quality of life in breast cancer patients [18,19]. While a causal relationship between anxiety and these variables is unclear, these findings suggest that untreated anxiety is associated with additional problems for patients with breast cancer.
Effective treatments for anxiety have been developed. For example, psychotherapy has been shown to significantly reduce anxiety after cancer diagnosis [20,21]. Informed by these findings, the American Society of Clinical Oncology now recommends cognitive-behavioral psychotherapy as first line treatment for anxiety in cancer patients [22]. In addition, integrative medicine services have been shown to reduce anxiety in breast cancer patients [23,24]. In particular, meditation and yoga are recommended by the Society for Integrative Oncology and American Society for Clinical Oncology for anxiety in breast cancer patients based on accumulated research demonstrating effectiveness [25,26].
Pharmacological interventions are also available to treat anxiety in cancer patients [22]. However, psychotropic medications can have adverse side effects, interact with other medications, and, in some cases, carry risk for dependence [27]. Therefore, this study focused on use of psychotherapy and integrative medicine services as non-pharmacological treatments for anxiety in breast cancer patients on AIs.
The objectives of this study were to: 1) define the prevalence and risk factors of anxiety among postmenopausal breast cancer survivors on AIs and 2) examine rates and predictors of psychotherapy and integrative medicine service use in breast cancer survivors on AIs with elevated anxiety.
METHODS
Study Design and Patient Population
Data for this cross-sectional survey were collected between November 2011 and April 2015 from breast cancer survivors receiving care at the Rowan Breast Cancer Center at the Abramson Cancer Center of the University of the Pennsylvania (Philadelphia, PA). Potential participants were postmenopausal women with histologically confirmed stage 0 to III hormone receptor-positive breast cancer who were currently taking a third generation AI; had completed chemotherapy, radiotherapy, or surgery at least one month prior to enrollment; and had the ability to understand and provide informed consent in English. Research assistants obtained permission from the treating oncologist to contact patients, screened medical records, and approached potential participants for recruitment at their regular follow-up appointments. All participants provided written informed consent. Participants were then given a self-administered survey to complete. Participants who could not complete the survey in time were given a stamped envelope to mail the survey back to the study team. The Institutional Review Board of the University of Pennsylvania approved the study.
Measures
The primary outcome was participants’ self-reported anxiety as measured by the anxiety subscale of the Hospital Anxiety and Depression Scale (HADS). This 7-item instrument was designed to assess anxiety in patient populations on a numerical rating scale ranging from 0 to 3 with higher scores indicating more severe anxiety. Total scores range from 0–21; a total score of ≥8 indicates elevated anxiety [28]. Comorbid depression was assessed with the 7-item depression subscale of the HADS. A total score of ≥8 was used to indicate elevated depression [28].
Patients also indicated whether they used psychological counseling and integrative medicine services since their cancer diagnosis (yes/no). Integrative medicine services included yoga and meditation [25].
We collected self-report information on covariates including age, race, education, and employment and partner status. Clinical variables included stage of cancer, time since cancer diagnosis, and receipt of radiation and chemotherapy and were assessed by self-report and medical chart abstraction.
Statistical Analysis
Data analysis was performed using STATA 12 for Windows (STATA Corporation, College Station, TX). Descriptive statistics were used to report the demographic characteristics of study participants. Logistic regression analyses were used to examine the bivariate relationships between demographic characteristics and depression and anxiety. We then developed a multivariate logistic regression model to identify independent risk factors associated with anxiety. Variables with p-values of <0.10 in the bivariate analyses were included in the multivariate analysis. Bivariate analyses using chi-square tests were then conducted to examine the relationship between anxiety and the use of psychological counseling, integrative medicine services, and either type of treatment (psychological counseling or integrative medicine). Among patients with elevated anxiety, logistic regression analyses were used to examine the bivariate relationships between demographic characteristics and depression and use of psychological counseling, integrative medicine services, or either type of treatment. We developed a multivariate logistic regression model to identify independent risk factors associated with use of these services in patients with elevated anxiety. Variables with p-values of <0.10 in the bivariate analyses were included in the multivariate analysis. Statistical tests were 2-sided, and p values of <0.05 indicated statistical significance.
RESULTS
Participant Characteristics
Of the 1,518 consecutive breast cancer survivors screened, 1,321 (87.0%) agreed to participate and provided consent. Among the 197 (13.0%) who declined, the main reasons were: lack of time to complete the survey (n=62, 31.5%), did not want to participate in research (n=85, 43.1%), and were ineligible (n=50, 25.4%). Additionally, 15 (1.1%) subjects withdrew consent from the study and 26 (2.0%) subjects did not return their survey, resulting in the final sample of 1,280. This population reflects an 87.0% response rate among all initially approached subjects. Additionally, 177 subjects discontinued AIs due to various reasons and n=18 had incomplete data on the Hospital Anxiety and Depression Scale. For this study, we restricted analysis to the 1,085 subjects who were on AIs at the time of enrollment with complete HADS data.
Among these 1,085 participants, the majority were younger than 65 years of age (n=673, 62.0%) and white (n=899, 82.9%). Over three quarters of the sample had a college education or higher (n=863, 79.6%) and approximately half were not currently employed (n=518, 47.7%). Regarding disease characteristics, approximately half of the sample had stage 0 or 1 breast cancer (n=556, 51.8%), were less than two years from diagnosis (n=480, 44.2%), and had not received chemotherapy (n=523, 48.2%). The majority of the sample had been treated with radiation (n=780, 71.9%). Characteristics of the study sample are listed in Table 1.
Table 1.
Bivariate relationships between demographic characteristics and depression and anxiety (n=1,085)
| Total N (%) | None/Normal Anxiety (N, %) | Borderline/Abnormal Anxiety (N, %) | P value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age | ||||
| ≥65 years (Reference) | 412 (38.0%) | 303 (73.5%) | 109 (26.5%) | - |
| <65 years | 673 (62.0%) | 448 (66.6%) | 225 (33.4%) | .02 |
| Race | ||||
| White (Reference) | 899 (82.9%) | 610 (67.9%) | 289 (32.1%) | - |
| None-white | 186 (17.1%) | 141 (75.8%) | 45 (24.2%) | .03 |
| Education | ||||
| College and above (Reference) | 863 (79.6%) | 590 (68.4%) | 273 (31.6%) | - |
| High school or less | 221 (20.4%) | 161 (72.9%) | 60 (27.1%) | .20 |
| Employment status | ||||
| Full-time (Reference) | 418 (38.5%) | 291 (69.6%) | 127 (30.4%) | - |
| Part-time | 149 (13.7%) | 99 (66.4%) | 50 (33.6%) | .47 |
| Not currently employed | 518 (47.7%) | 361 (69.7%) | 157 (30.3%) | .98 |
| Partner status | ||||
| Single (Reference) | 417 (38.7%) | 297 (71.2%) | 120 (28.8%) | |
| Partnered | 661 (61.3%) | 450 (68.1%) | 211 (31.9%) | .28 |
| Disease characteristics | ||||
| Cancer stage | ||||
| Stage 0 & I (Reference) | 556 (51.8%) | 388 (69.8%) | 168 (30.2%) | - |
| Stage II | 379 (35.3%) | 259 (68.3%) | 120 (31.7%) | .64 |
| Stage III | 138 (12.9%) | 97 (70.3%) | 41 (29.7%) | .91 |
| Time since diagnosis | ||||
| >5 years (Reference) | 188 (17.3%) | 127 (67.6%) | 61 (32.5%) | - |
| 2–5 years | 417 (38.4%) | 296 (71.0%) | 121 (29.0%) | .40 |
| <2 years | 480 (44.2%) | 328 (68.3%) | 152 (31.7%) | .85 |
| Received radiation treatment | ||||
| No (Reference) | 305 (28.1%) | 209 (68.5%) | 96 (31.5%) | - |
| Yes | 780 (71.9%) | 542 (69.5%) | 238 (30.5%) | .76 |
| Received chemotherapy | ||||
| No (Reference) | 523 (48.2%) | 371 (70.9%) | 152 (29.1%) | - |
| Chemotherapy without Taxane | 103 (9.5%) | 68 (66.0%) | 35 (34.0%) | .32 |
| Chemotherapy with Taxane | 459 (42.3%) | 312 (68.0%) | 147 (32.0%) | .31 |
| Distress | ||||
| Depressive symptoms | ||||
| None/Normal (Reference) | 958 (88.3%) | 720 (75.2%) | 238 (24.8%) | - |
| Borderline/Abnormal | 127 (11.7%) | 31 (24.4%) | 96 (75.6%) | <.001 |
Note: Anxiety: 0=None/Normal, 1=Borderline/Abnormal
Prevalence and correlates of anxiety
Among the 1,085 participants, 334 (30.8%) reported elevated anxiety (HADS-Anxiety score ≥8). In bivariate analyses, age, race, and depression severity were significantly associated with anxiety (Table 1). Specifically, younger adults (<65 years of age) were more likely to report elevated anxiety than adults 65 years of age and older (33.4% vs. 26.5%; p=0.02) and non-white participants were less likely to report elevated anxiety than white participants (24.2% vs. 32.1%; p=0.03). Finally, participants who reported elevated depression were over nine times more likely to report elevated anxiety than patients who did not report elevated depression (75.6% vs. 24.8%; p<0.001).
In the multivariate logistic regression model, age (AOR, 1.41,95% CI 1.05–1.89; p=0.02), race (AOR, .58, 95% CI .39–.87; p=0.008), and depression (AOR, 9.81, 95% CI 6.34–15.19; p<0.001) remained significantly associated with anxiety (Table 2).
Table 2.
Multivariate logistic regression predicting anxiety (n=1,085)
| Unadjusted Odds ratio | Adjusted Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| Age | ||||
| ≥65 years (Reference) | - | - | - | - |
| <65 years | 1.40 | 1.41 | 1.05–1.89 | .02 |
| Race | ||||
| White (Reference) | - | - | - | - |
| None-white | .67 | .58 | .39–.87 | .008 |
| Depression | ||||
| None/Normal (Reference) | - | - | - | - |
| Borderline/Abnormal | 9.37 | 9.81 | 6.34–15.19 | <.001 |
Note: Anxiety: 0=None/Normal, 1=Borderline/Abnormal
Relationship between anxiety and psychological counseling and integrative medicine service use
Across the entire sample, less than one-fifth of participants had received psychological counseling services (n=166, 15.3%), 18.9% (n=205) had utilized integrative medicine services and 28.6% (n=310) received psychological counseling or integrative medicine services since their cancer diagnosis. Regarding specific integrative medicine services, 12.4% (n=134) of the sample had used meditation and 14.5% (n=157) had used yoga.
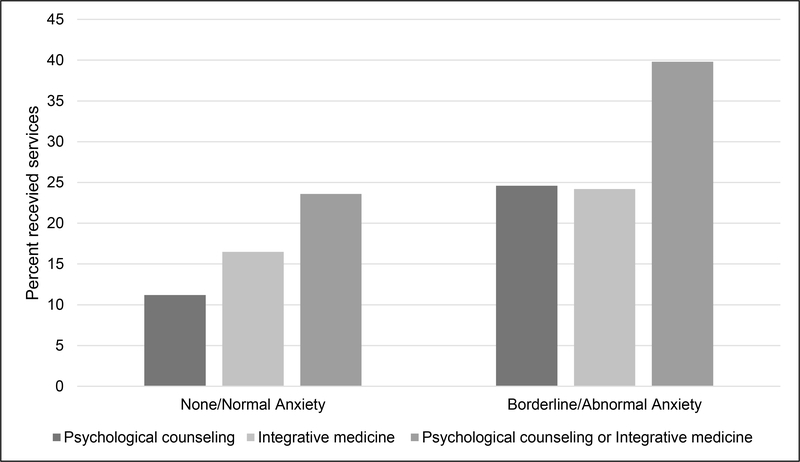
Participants with elevated anxiety were more likely to receive psychological counseling (n=82, 24.6%) than patients with normal anxiety levels (n=84, 11.2%; X2(1, N=1,085)=31.87, p<.001; Figure 1). Similarly, patients with elevated anxiety were more likely to use integrative medicine services (n=81,24.2%) than patients with normal anxiety levels (n=124, 16.5%; X2(1, N=1,084)=8.98, p<.003). Finally, patients with elevated anxiety were more likely to use psychological counseling or integrative medicine services (n=133, 39.8%) than patients with normal anxiety levels (n=177, 23.6%; X2(1, N=1,085)=29.92, p<.001).
Figure 1.
Relationship between anxiety and psychological counseling and integrative medicine service use
Predictors of psychological counseling and integrative medicine service use in patients with elevated anxiety
In univariate logistic regression models predicting receipt of psychological counseling in patients with elevated anxiety, education and depression were significantly associated with psychological counseling use (Table 3). Specifically, patients with an education level of high school or less were less likely to receive psychological counseling than patients with a college education or higher (13.3% vs. 26.7%; p=.03). Patients with elevated depression were more likely to receive psychological counseling than patients with normal levels of depressive symptoms (31.3% vs. 21.9%; p=.07).
Table 3.
Bivariate relationships between demographic characteristics and depression and psychological counseling and integrative medicine use in patients with elevated anxiety (n=334)
| Psychological Counseling | Integrative Medicine | Psychological Counseling or Integrative Medicine | ||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | Odds ratio | 95% CI | |
| Demographic characteristics | ||||||
| Age | ||||||
| ≥65 years (Reference) | - | - | - | - | - | - |
| <65 years | 1.23 | .71–2.12 | 1.20 | .70–2.07 | 1.45 | .90–2.33 |
| Race | ||||||
| White (Reference) | - | - | - | - | - | - |
| None-white | .53 | .23–1.23 | .75 | .35–1.64 | .65 | .33–1.26 |
| Education | ||||||
| College and above (Reference) | - | - | - | - | - | - |
| High school or less | .42 | .19–.93 | .30 | .12–.72 | .36 | .19–.69 |
| Employment status | ||||||
| Full-time (Reference) | - | - | - | - | - | - |
| Part-time | 1.45 | .70–3.01 | 1.40 | .68–2.86 | 1.56 | .81–3.02 |
| Not currently employed | 1.08 | .62–1.87 | .79 | .45–1.38 | .78 | .48–1.26 |
| Partner status | ||||||
| Single (Reference) | - | - | - | |||
| Partnered | .85 | .51–1.43 | 1.16 | .68–1.96 | 1.11 | .70–1.75 |
| Distress | ||||||
| Depressive symptoms | ||||||
| None/Normal (Reference) | - | - | - | - | - | - |
| Borderline/Abnormal | 1.63 | .96–2.76 | 1.14 | .66–1.97 | 1.42 | .88–2.29 |
Note: Psychological counseling use: 0=No, 1=Yes
In multivariate analyses, only education was associated with use of psychological counseling (AOR, .43, 95% CI .19–.95; p=.04). Patients with a lower level of education were less likely to use psychological counseling services. Depression did not significantly predict service use.
In univariate analyses predicting integrative medicine use in patients with elevated anxiety, only education was associated with use of integrative medicine services. Patients with an education level of high school or lower were less likely to receive integrative medicine services than patients with a level of education of college or greater (10.0% vs. 27.1%; p=.007; Table 3).
In univariate analyses predicting use of psychological counseling or integrative medicine service use, patients with an education of high school or less were less likely to receive these services than patients with higher education levels (21.7% vs. 43.6%; p=0.002; Table 3).
DISCUSSION
In this cross-sectional study of over 1,000 women with breast cancer on AI therapy, nearly one in three reported elevated anxiety; however, 60% of those with anxiety had not received evidence-based treatments such as psychological counseling or integrative medicine care since their diagnosis despite institutional availability of those services. In addition, among patients with elevated anxiety, patients educated at a high school level or lower were more than 60% less likely to receive psychotherapy or integrative medicine service. This finding points to a concerning disparity in the provision of these services with the potential for lower quality care in patients with lower education levels.
Rates of anxiety in this study are consistent with prior research in breast cancer patients [8–11] and other cancer populations [29,30,11]. However, this study sample is unique in that the majority of patients (52–72%) had received chemotherapy and/or radiation, treatments that often require extensive appointments with more severe side effects than AIs. Yet, the prevalence of anxiety was high even after patients transitioned from more burdensome treatments to AIs. Similarly, prior research suggests that distress remains high after patients transition from active treatment to survivorship, despite the benefits of completing active treatment [31,8,32]. Therefore, repeat anxiety screening and provision of anxiety treatments remain important components of patient care, even after patients transition to AIs. Given that patients taking AIs likely have fewer oncology appointments, remote screening using the telephone [33–35] or an electronic assessment may increase the feasibility of repeat anxiety screening [36].
Predictors of anxiety in this study are consistent with research in other cancer populations. Younger age has been associated with elevated anxiety across cancer types and stage [11,37–40]. However, among patients with elevated anxiety in this study, age was not associated with receipt of psychotherapy or integrative medicine service use. White patients were also more likely to report elevated anxiety than racial/ethnic minority patients. This finding is inconsistent with prior studies demonstrating higher distress in racial/ethnic minority patients [41,42]. While many anxiety screening measures have not been normed in older adults and racial and ethnic minority patients [43], measures validated in these populations are available [44]. The current study did not use population-specific measures of anxiety. Such measures may more accurately capture anxiety in patients who would benefit from treatment, thereby reducing disparities in the provision of these services.
Elevated depression was associated with greater likelihood of elevated anxiety, consistent with other studies of cancer patients [45,46]. Fortunately, many treatments for anxiety also improve depressive symptoms. For example, cognitive-behavioral therapy [47,20,21], mindfulness-based interventions [48–50], and meditation [25] have been shown to reduce symptoms of anxiety and depression. Integrating evidence-based interventions like cognitive-behavioral psychotherapy and meditation into patient care may be an effective and cost-efficient strategy for reducing anxiety and depression in this patient population.
Rates of anxiety in this population were high. Yet, only 40% of patients with elevated anxiety received psychotherapy or integrative medicine services. To our knowledge, this study is the first to assess psychotherapy and integrative medicine service use in anxious breast cancer patients treated with AIs. However, the results of this study are consistent with findings in other populations. In a study of patients with metastatic breast cancer, only 29% of patients with elevated anxiety or depression reported use of mental health services [34]. In a large observational study of distress and psychosocial service use in 652 breast cancer patients across the care trajectory (i.e., diagnosis, treatment, follow-up), 45.1% of patients endorsed past, present, or intended future use of psychosocial services [51]. These rates are likely higher than actual use due to the inclusion of intended future use. However, these findings indicate low rates of psychological service use in breast cancer patients and those with elevated distress, including breast cancer patients with anxiety treated with AIs.
Similar findings have been reported for integrative medicine services. A meta-analysis of 148 articles on use of integrative therapies found that 50% of patients in the United States had used these treatments [52]. More specifically, in a sample of breast cancer patients (n=764), approximately 30% of patients had used a mind-body technique during their cancer experience with 15% using meditation or and 10% using yoga [53]. In a study of newly diagnosed breast cancer patients, 24.2% of the sample reported using meditation and 22.2% engaged in yoga [54]. This study found similar rates of integrative medicine use in breast cancer patients treated with AIs. However, direct comparison of studies is limited by varying definitions of integrative medicine services across studies.
These low treatment rates are problematic given the association between anxiety and poor cancer treatment adherence [13–15], physical quality of life [16], and cognitive function [17]. Treating anxiety in breast cancer patients taking AIs has the potential to reduce this distress and improve other associated patient outcomes. Reducing anxiety in this population has the potential for long-term positive impact on patients’ emotional and physical quality of life due to recommended long-term use of AIs. Identifying the barriers to uptake of psychotherapy and integrative medicine services is the first step in the development of strategies to improve access to these services.
Patients with at least a college education were more likely to receive psychotherapy and integrative medicine services than patients with education levels of high school or less. This finding is concerning given that breast cancer patients with lower education levels may be at greater risk for psychological comorbidities in long-term survivorship [11]. Patients with lower education levels are less likely to have sufficient information about available support services than patients with higher education levels [11]. Further, insurance coverage of integrative medicine services is often limited [55,56]. Patients with lower education levels may have fewer financial resources available for integrative medicine services. Direct indicators of financial resources such as insurance status, socioeconomic status, and financial burden due to cancer were not evaluated in this study although employment was not associated with receipt of psychotherapy and integrative medicine services. Assessment of these variables in future studies will identify specific financial characteristics that may impact patients’ access to these services. Further, reducing the cost of integrative medicine services through insurance coverage or institutional resources and ensuring that patients across education levels are provided with information on all available support services may increase use of these services in patients with elevated anxiety and reduce disparities in care associated with education.
Limitations and future directions
The findings of this study should be interpreted in the context of study limitations. This study was conducted at a single center in an urban setting with a largely white and highly educated sample, limiting the generalizability of these findings to other populations and settings. The cross-sectional nature of the data also limits our conclusions. Causal relationships cannot be determined from these data. Further, the HADS was used to assess current distress while the assessment of service utilization referred to services received since the breast cancer diagnosis. Therefore, the experience of distress and receipt of psychological counseling and integrative medicine services may not have co-occurred.
Anxiety levels were assessed at a single point in time. Over half of the sample had received their cancer diagnosis over two years prior to study participation. Data on the trajectory of anxiety from diagnosis through AI treatment would provide greater understanding of patients’ experience and inform the timing of psychotherapy and integrative medicine services. In addition, approximately half of the sample had stage 0 or 1 breast cancer and did not receive chemotherapy. Cancer stage and receipt of chemotherapy and radiation were not associated with anxiety in this study. However, examination of anxiety and psychotherapy and integrative medicine service use in patients with advanced disease receiving more aggressive treatment would provide information on the needs of a potentially more vulnerable patient population.
This study is also limited by the nature of available data on psychotherapy and integrative medicine services. Patients indicated which services they received since their cancer diagnosis; data on the duration and intensity of services are not available. In addition, we are unable to determine whether patients received psychotherapy and integrative medicine services for their anxiety or a different presenting problem and whether these services were effective. Future studies that collect data on the timing and dose of psychotherapy and integrative medicine services received and their impact on anxiety will inform the tailoring of interventions to meet the needs of breast cancer patients treated with AIs.
Conclusions
The results of this study indicate that a large proportion of breast cancer patients receiving AI treatment experience elevated anxiety. However, few receive psychotherapy or integrative medicine services. Further, patients with higher education levels are more likely to receive these services, pointing to a potential disparity in care. Our findings highlight the need for improved dissemination and implementation of interventions to improve the availability and accessibility of evidence-based psychological and integrative medicine services.
Acknowledgments
Funding. This research is funded in part by grants from the National Cancer Institute / National Institutes of Health (R01 CA158243, P30-CA008748), and the Translational Research and Integrative Medicine Fund at the Memorial Sloan Kettering Cancer Center, and the National Institute on Aging/National Institutes of Health and American Federation for Aging Research (Trevino, K23AG048632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Conflict of interest. Dr. Iyengar reported receiving personal fees from Novartis outside the submitted work. All other authors have no conflicts of interest to disclose.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Ethical approval. All procedures performed in this study were in accordance with the ethical standards of the institutional review board of participating institutions and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Data archive. Dr. Jun J. Mao has full control of all primary data and agrees to allow the journal to review the data if requested.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al. , (eds). SEER Cancer Statistics Review, 1975–2016, National Cancer Institute; Based on November 2017 SEER data submission, Posted to the SEER web site, April 2018. [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1975–2016), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. [Google Scholar]
- 3.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, Giordano SH, Hudis CA, Solky AJ, Stearns V, Winer EP, Griggs JJ (2019) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol 37 (5):423–438. doi: 10.1200/jco.18.01160 [DOI] [PubMed] [Google Scholar]
- 4.Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Láng I, Gomez HL, Tondini C, Burstein HJ, Perez EA, Ciruelos E, Stearns V, Bonnefoi HR, Martino S, Geyer CE, Pinotti G, Puglisi F, Crivellari D, Ruhstaller T, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Bernhard J, Luo W, Ribi K, Viale G, Coates AS, Gelber RD, Goldhirsch A, Francis PA (2014) Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. J Clin Oncol 371 (2): 107–118. doi: 10.1056/NEJMoa1404037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saha P, Regan MM, Pagani O, Francis PA, Walley BA, Ribi K, Bernhard J, Luo W, Gomez HL, Burstein HJ, Parmar V, Torres R, Stewart J, Bellet M, Perello A, Dane F, Moreira A, Vorobiof D, Nottage M, Price KN, Coates AS, Goldhirsch A, Gelber RD, Colleoni M, Fleming GF (2017) Treatment efficacy, adherence, and quality of lLife among women younger than 35 years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J Clin Oncol 35 (27):3113–3122. doi: 10.1200/jco.2016.72.0946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryden L, Heibert Arnlind M, Vitols S, Hoistad M, Ahlgren J (2016) Aromatase inhibitors alone or sequentially combined with tamoxifen in postmenopausal early breast cancer compared with tamoxifen or placebo - Meta-analyses on efficacy and adverse events based on randomized clinical trials. Breast 26:106–114. doi: 10.1016/j.breast.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 7.Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, Gelmon K, Whelan T, Strasser-Weippl K, Rubin S, Sturtz K, Wolff AC, Winer E, Hudis C, Stopeck A, Beck JT, Kaur JS, Whelan K, Tu D, Parulekar WR (2016) Extending aromatase-inhibitor adjuvant therapy to 10 years. NEJM 375 (3):209–219. doi: 10.1056/NEJMoa1604700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandubert C, Carriere I, Escot C, Soulier M, Hermes A, Boulet P, Ritchie K, Chaudieu I (2009) Onset and relapse of psychiatric disorders following early breast cancer: A case-control study. Psychooncology 18 (10):1029–1037. doi: 10.1002/pon.1469 [DOI] [PubMed] [Google Scholar]
- 9.Mayer M (2010) Lessons learned from the metastatic breast cancer community. Seminars in Oncology Nursing 26 (3):195–202. doi: 10.1016/j.soncn.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Tsaras K, Papathanasiou IV, Mitsi D, Veneti A, Kelesi M, Zyga S, Fradelos EC (2018) Assessment of depression and anxiety in breast cancer patients: Prevalence and associated factors. Asian Pacific Journal of Cancer Prevention 19 (6):1661–1669. doi: 10.22034/apjcp.2018.19.6.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehnert A, Koch U (2008) Psychological comorbidity and health-related quality of life and its association with awareness, utilization, and need for psychosocial support in a cancer register-based sample of long-term breast cancer survivors. J Psychosom Res 64 (4):383–391. doi: 10.1016/j.jpsychores.2007.12.005 [DOI] [PubMed] [Google Scholar]
- 12.Mehnert A, Brahler E, Faller H, Harter M, Keller M, Schulz H, Wegscheider K, Weis J, Boehncke A, Hund B, Reuter K, Richard M, Sehner S, Sommerfeldt S, Szalai C, Wittchen HU, Koch U (2014) Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol 32 (31):3540–3546. doi: 10.1200/jco.2014.56.0086 [DOI] [PubMed] [Google Scholar]
- 13.Haskins CB, McDowell BD, Carnahan RM, Fiedorowicz JG, Wallace RB, Smith BJ, Chrischilles EA (2019) Impact of preexisting mental illness on breast cancer endocrine therapy adherence. Breast Cancer Res Treat 174 (1):197–208. doi: 10.1007/s10549-018-5050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith KL, Yeruva SLH, Blackford A, Huang CY, Westbrook KE, Harding BA, Smith A, Fetting J, Wolff AC, Jelovac D, Miller RS, Connolly R, Armstrong D, Nunes R, Visvanathan K, Stearns V (2018) Predictors of adherence to adjuvant endocrine therapy (ET) for early breast cancer (BC) in a prospective clinic-based cohort. Cancer Research 78 (4). doi: 10.1158/15387445.SABCS17-P3-12-02 [DOI] [Google Scholar]
- 15.Bender CM, Gentry AL, Brufsky AM, Casillo FE, Cohen SM, Dailey MM, Donovan HS, Dunbar-Jacob J, Jankowitz RC, Rosenzweig MQ, Sherwood PR, Sereika SM (2014) Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum 41 (3):274–285. doi: 10.1188/14.onf.274-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faller H, Strahl A, Richard M, Niehues C, Meng K (2017) Symptoms of depression and anxiety as predictors of physical functioning in breast cancer patients. A prospective study using path analysis. Acta Oncologica 56 (12):1677–1681. doi: 10.1080/0284186x.2017.1333630 [DOI] [PubMed] [Google Scholar]
- 17.Merriman JD, Sereika SM, Brufsky AM, McAuliffe PF, McGuire KP, Myers JS, Phillips ML, Ryan CM, Gentry AL, Jones LD, Bender CM (2017) Trajectories of self-reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psycho- Oncol 26 (1):44–52. doi: 10.1002/pon.4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lidgren M, Wilking N, Jönsson B, Rehnberg C (2007) Health related quality of life in different states of breast cancer. Qual Life Res 16 (6):1073–1081. doi: 10.1007/s11136-007-9202-8 [DOI] [PubMed] [Google Scholar]
- 19.Chow S, Wan BA, Pidduck W, Zhang L, DeAngelis C, Chan S, Yee C, Drost L, Leung E, Sousa P, Lewis D, Lam H, Chow R, Lock M, Chow E (2019) Symptoms predictive of overall quality of life using the Edmonton Symptom Assessment Scale in breast cancer patients receiving radiotherapy. Clinical Breast Cancer 19 (6):405–410. doi: 10.1016/j.clbc.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 20.Sun H, Huang H, Ji S, Chen X, Xu Y, Zhu F, Wu J (2019) The efficacy of cognitive behavioral therapy to treat depression and anxiety and improve quality of life among early-stage breast cancer patients. Integrative Cancer Therapies 18. doi: 10.1177/1534735419829573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye M, Du K, Zhou J, Zhou Q, Shou M, Hu B, Jiang P, Dong N, He L, Liang S, Yu C, Zhang J, Ding Z, Liu Z (2018) A meta-analysis of the efficacy of cognitive behavior therapy on quality of life and psychological health of breast cancer survivors and patients. Psycho-Oncol 27 (7): 1695–1703. doi: 10.1002/pon.4687 [DOI] [PubMed] [Google Scholar]
- 22.Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, Rowland JH (2014) Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. J Clin Oncol 32 (15):1605–1619. doi: 10.1200/jco.2013.52.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lengacher CA, Johnson-Mallard V, Post-White J, Moscoso MS, Jacobsen PB, Klein TW, Widen RH, Fitzgerald SG, Shelton MM, Barta M, Goodman M, Cox CE, Kip KE (2009) Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psycho-Oncol 18 (12):1261–1272. doi: 10.1002/pon.1529 [DOI] [PubMed] [Google Scholar]
- 24.Wurtzen H, Dalton SO, Elsass P, Sumbundu AD, Steding-Jensen M, Karlsen RV, Andersen KK, Flyger HL, Pedersen AE, Johansen C (2013) Mindfulness significantly reduces self-reported levels of anxiety and depression: results of a randomised controlled trial among 336 Danish women treated for stage I-III breast cancer. Eur J Cancer 49 (6): 1365–1373. doi: 10.1016/j.ejca.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 25.Greenlee H, DuPont-Reyes MJ, Balneaves LG, Carlson LE, Cohen MR, Deng G, Johnson JA, Mumber M, Seely D, Zick SM, Boyce LM, Tripathy D (2017) Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 67 (3):194–232. doi: 10.3322/caac.21397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyman GH, Greenlee H, Bohlke K, Bao T, DeMichele AM, Deng GE, Fouladbakhsh JM, Gil B, Hershman DL, Mansfield S, Mussallem DM, Mustian KM, Price E, Rafte S, Cohen L (2018) Integrative yherapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol 36 (25):2647–2655. doi: 10.1200/jco.2018.79.2721 [DOI] [PubMed] [Google Scholar]
- 27.Thekdi SM, Trinidad A, Roth A (2014) Psychopharmacology in cancer. Current Psychiatry Reports 17 (1):529. doi: 10.1007/s11920-014-0529-x [DOI] [PubMed] [Google Scholar]
- 28.Hopwood P, Howell A, Maguire P (1991) Screening for psychiatric morbidity in patients with advanced breast cancer: Validation of two self-report questionnaires. British Journal of Cancer 64 (2):353–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polanski J, Chabowski M, Chudiak A, Uchmanowicz B, Janczak D, Rosinczuk J, Mazur G (2018) Intensity of anxiety and depression in patients with lung cancer in relation to quality of life. Advances in Experimental Medicine and Biology 1023:29–36. doi: 10.1007/5584_2017_50 [DOI] [PubMed] [Google Scholar]
- 30.Jung JY, Lee JM, Kim MS, Shim YM, Zo JI, Yun YH (2017) Comparison of fatigue, depression, and anxiety as factors affecting posttreatment health-related quality of life in lung cancer survivors. Psychooncology. doi: 10.1002/pon.4513 [DOI] [PubMed] [Google Scholar]
- 31.Deimling GT, Kahana B, Bowman KF, Schaeffer ML (2002) Cancer survivorship and psychological distress in later life. Psycho-Oncol 11 (6):479–494. doi: 10.1002/pon.614 [DOI] [PubMed] [Google Scholar]
- 32.Greer JA, Solis JM, Temel JS, Lennes IT, Prigerson HG, Maciejewski PK, Pirl WF (2011) Anxiety disorders in long-term survivors of adult cancers. Psychosomatics 52 (5):417–423. doi: 10.1016/j.psym.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moadel A, Kolidas E, Ghavamian R (2016) Psychosocial needs assessment of underserved prostate cancer patients: Survivorship program planning. Journal of Clinical Oncology 34 [Google Scholar]
- 34.Mosher CE, DuHamel KN (2012) An examination of distress, sleep, and fatigue in metastatic breast cancer patients. Psycho-Oncol 21 (1):100–107. doi: 10.1002/pon.1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes KL, Sargeant H, Hawkes AL (2011) Acceptability of the Distress Thermometer and Problem List to community-based telephone cancer helpline operators, and to cancer patients and carers. BMC Cancer 11 (46). doi: 10.1186/1471-2407-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdonck-De Leeuw I, Krebber AM, Cuijpers P, De Bree R, Leemans CR (2013) Benefits and costs of screening in clinical practice to identify head and neck cancer patients with untreated psychological distress after treatment. Psycho-Oncol 22:49. doi: 10.1111/j.1099-1611.2013.3393 [DOI] [Google Scholar]
- 37.Burgoyne MJ, Bingen K, Leuck J, Dasgupta M, Ryan P, Hoffmann RG (2015) Cancer-related distress in young adults compared to middle-aged and senior adults. J Adolesc Young Adult Oncol 4 (2):56–63. doi: 10.1089/jayao.2014.0005 [DOI] [PubMed] [Google Scholar]
- 38.Rose JH, O’Toole EE, Einstadter D, Love TE, Shenko CA, Dawson NV (2008) Patient age, well-being, perspectives, and care practices in the early treatment phase for late-stage cancer. J Gerontol A Biol Sci Med Sci 63 (9):960–968 [DOI] [PubMed] [Google Scholar]
- 39.Linden W, Vodermaier A, Mackenzie R, Greig D (2012) Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J Affect Disord 141 (2–3):343–351. doi: 10.1016/j.jad.2012.03.025 [DOI] [PubMed] [Google Scholar]
- 40.Jensen RE, Potosky AL, Moinpour CM, Lobo T, Cella D, Hahn EA, Thissen D, Smith AW, Ahn J, Luta G, Reeve BB (2017) United States population-based estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol 35 (17):1913–1920. doi: 10.1200/jco.2016.71.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alcala HE (2014) Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health 14:930. doi: 10.1186/1471-2458-14-930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apenteng BA, Hansen AR, Opoku ST, Mase WA (2016) Racial disparities in emotional distress among cancer survivors: Insights from the Health Information National Trends Survey (HINTS). Journal of Cancer Education 32 (3): 556–565. doi: 10.1007/s13187-016-0984-7 [DOI] [PubMed] [Google Scholar]
- 43.Grassi L, Caruso R, Sabato S, Massarenti S, Nanni MG, the UniFe Psychiatry Working Group Coauthors (2015) Psychosocial screening and assessment in oncology and palliative care settings. Front Psychol 5 (1485). doi: 10.3389/fpsyg.2014.01485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pachana NA, Byrne GJ, Siddle H, Koloski N, Harley E, Arnold E (2007) Development and validation of the Geriatric Anxiety Inventory. International Psychogeriatrics 19 (1):103–114. doi: 10.1017/s1041610206003504 [DOI] [PubMed] [Google Scholar]
- 45.Brown LF, Kroenke K, Theobald DE, Wu J, Tu W (2010) The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology 19 (7):734–741. doi: 10.1002/pon.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson KG, Chochinov HM, Skirko MG, Allard P, Chary S, Gagnon PR, Macmillan K, De Luca M, O’Shea F, Kuhl D, Fainsinger RL, Clinch JJ (2007) Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage 33 (2): 118–129. doi: 10.1016/j.jpainsymman.2006.07.016 [DOI] [PubMed] [Google Scholar]
- 47.Xiao F, Song X, Chen Q, Dai Y, Xu R, Qiu C, Guo Q (2017) Effectiveness of psychological interventions on depression in patients after breast cancer surgery: A meta-analysis of randomized controlled trials. Clinical Breast Cancer 17 (3): 171–179. doi: 10.1016/j.clbc.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 48.Schellekens MPJ, van den Hurk DGM, Prins JB, Donders ART, Molema J, Dekhuijzen R, van der Drift MA, Speckens AEM (2017) Mindfulness-based stress reduction added to care as usual for lung cancer patients and/or their partners: A multicentre randomized controlled trial. Psycho-Oncol 26 (12):2118–2126. doi: 10.1002/pon.4430 [DOI] [PubMed] [Google Scholar]
- 49.Bisseling EM, Schellekens MPJ, Jansen ETM, van Laarhoven HWM, Prins JB, Speckens AEM (2017) Mindfulness-based stress reduction for breast cancer patients: a mixed method study on what patients experience as a suitable stage to participate. Supportive Care in Cancer 25 (10):3067–3074. doi: 10.1007/s00520-017-3714-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenne Sarenmalm E, Mårtensson LB, Andersson BA, Karlsson P, Bergh I (2017) Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Medicine 6 (5): 1108–1122. doi: 10.1002/cam4.1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carlson LE, Angen M, Cullum J, Goodey E, Koopmans J, Lamont L, MacRae JH, Martin M, Pelletier G, Robinson J, Simpson JS, Speca M, Tillotson L, Bultz BD (2004) High levels of untreated distress and fatigue in cancer patients. British Journal of Cancer 90 (12):2297–2304. doi: 10.1038/sj.bjc.6601887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M (2012) How many cancer patients use complementary and alternative medicine: A systematic review and meta-analysis. Integr Cancer Ther 11 (3):187–203. doi: 10.1177/1534735411423920 [DOI] [PubMed] [Google Scholar]
- 53.Link AR, Gammon MD, Jacobson JS, Abrahamson P, Bradshaw PT, Terry MB, Teitelbaum S, Neugut A, Greenlee H (2013) Use of self-care and practitioner-based forms of complementary and alternative medicine before and after a diagnosis of breast cancer. Evidence-Based Complementary and Alternative Medicine 2013. doi: 10.1155/2013/301549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenlee H, Kwan ML, Ergas IJ, Sherman KJ, Krathwohl SE, Bonnell C, Lee MM, Kushi LH (2009) Complementary and alternative therapy use before and after breast cancer diagnosis: The Pathways Study. Breast Cancer Res Treat 117 (3):653–665. doi: 10.1007/s10549-009-0315-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winter RW, Korzenik JR (2017) The practical pros and cons of complementary and alternative medicine in practice: Integrating complementary and alternative medicine into clinical care. Gastroenterology Clinics of North America 46 (4):907–916. doi: 10.1016/j.gtc.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 56.Nahin RL, Barnes PM, Stussman BJ (2016) Insurance coverage for complementary health approaches among adult users: United States, 2002 and 2012. NCHS data brief (235):1–8 [PubMed] [Google Scholar]