Abstract
Background and Aims:
Despite adverse effects like hyperglycemia, New Onset Diabetes After Transplant (NODAT) and infectious complications; corticosteroids remains an important part of liver transplant (LT) immune suppression. Budesonide, a synthetic corticosteroid, undergoes extensive first-pass hepatic metabolism with only 10% systemic bioavailability, providing an opportunity for improved toxicity-therapeutic ratio. Although effective in the treatment of autoimmune hepatitis, effects of budesonide for LT immune suppression are unknown.
Approach and Results:
We conducted a single center phase 2a trial to study the safety and efficacy of budesonide immunosuppressive therapy. From July 2017 to November 2018, twenty subjects undergoing first LT received budesonide tapering doses (9mg-3mg) for 12 weeks. Subjects were compared to matched controls that received prednisone from the same time period. Additionally, both groups received calcineurin inhibitors and mycophenolate. Outcome measures at week 24 included rates of biopsy proven Acute Cellular Rejection (ACR), NODAT (Glycated Hemoglobin > 6.4) and infectious complications.
In the budesonide arm, one subject developed ACR at week 5 and was removed from the study. Another subject stopped the study drug at week 8 due to persistent nausea. Rates of ACR were similar between budesonide and control groups (5% vs 5%, p=1.00). Three patients in the control group developed NODAT vs none in budesonide group (15% vs 0%, p=0.23). There were six infections in the control group as compared to none in the budesonide group (30% vs 0, p=0.02).
Conclusion:
These pilot data suggests that Budesonide has the potential to be a safe and effective alternative to prednisone for LT immune suppression while reducing steroid induced infections and NODAT. Randomized controlled trials are required to validate these findings.
Keywords: New onset diabetes after transplant, infectious complications, hyperglycemia, adrenal suppression, acute cellular rejection
Introduction:
Systemic corticosteroids have been an integral component of the common calcineurin inhibitor (CNI) and mycophenolate mofetil (MMF) backbone of immune suppression post liver transplant (LT), acting upon multiple targets to both prevent and treat acute cellular rejection (ACR). 1,11 However, their use is associated with many well-known adverse effects in LT recipients including elevated blood glucose, new onset diabetes after transplant (NODAT), weight gain, increased rate of infections and metabolic bone disease. These co-morbidities affect patient and graft survival. 2 With recent advancements in the field of immune suppression, multiple studies have tried to evaluate the role of steroid-free regimens for LT recipients.3–10. While steroid-free regimens are associated with lower rates of NODAT and cytomegalovirus (CMV) infections, and reduced serum cholesterol levels, their efficacy, in terms of preventing ACR in allografts is debatable.11–13. The majority of these studies are either retrospective or have a small sample size. 3,10,14–20. Prospective studies have implied different strategies regarding the use of corticosteroids including complete steroid free regimens vs early withdrawal vs late withdrawal.4,6,8 The majority of the studies using steroid-free regimens augmented their immune suppression with antibody induction therapy.3,9,10,20–24 Randomized trials that have used similar methodology and patient selection have provided conflicting evidence in regards to risk of ACR between cohorts using steroid vs steroid-free regimens. 5,7,16,25. Multiple meta-analyses and literature reviews have concluded that steroid free immune suppression regimens lower the risk of NODAT, CMV infections and hypercholesterolemia while having similar efficacy to steroid based regimens in preventing ACR only when steroids were replaced with antibody induction treatment. 11–13. Despite this adverse effect profile, systemic steroids are considered as “necessary evil” and up to 70% of LT centers in United States use systemic corticosteroids for a few months in immediate post-LT period when the risk of ACR is highest.26
Budesonide, a synthetic corticosteroid undergoes complete intestinal absorption after oral intake and extensive first-pass hepatic metabolism with only 10% systemic bioavailability.27 In 2001, Budesonide was approved by Food and Drug Administration for treatment of Crohn’s disease.28 Since then, multiple studies have demonstrated not only its efficacy in the treatment of immune mediated liver conditions such as primary biliary cholangitis and autoimmune hepatitis while also having minimal adverse effects likely due to its limited systemic bioavailability. 29–35 In a phase IIb randomized clinical trial, combination budesonide and azathioprine was found superior to the combination of prednisone and azathioprine to induce biochemical remission in patients with newly diagnosed autoimmune hepatitis while showing significantly lower rates of steroid specific adverse effects.36 Incidence of new onset diabetes was 1% in the budesonide group. In another study of patients with Crohn’s disease, average reduction in bone mineral density was significantly lower (1.04%) for patients who used long term budesonide as compared to patients on prednisone (3.84%) 37. Use of budesonide in immune-mediated liver conditions has been shown to be safe for up to 2 years.38
The role of budesonide as an immunosuppressant post LT is limited to animal models where it has shown to prevent ACR. 39–41 Budesonide has also shown to be effective as a single agent immunosuppressant in a case series of 3 patients with severe sepsis.42 When used in place of prednisone, budesonide has the potential for fewer systemic adverse effects including lower rates of NODAT, infections and metabolic bone disease while providing adequate liver specific immune suppression to prevent ACR. We conducted a pilot phase IIa, prospective, case-control trial to study the safety and efficacy of Budesonide in place of prednisone for liver transplant immune suppression.
Patients and Methods
Patient selection
All patients between ages 21 and 75 undergoing primary, liver-only transplant from a deceased donor at University of Cincinnati Medical Center were screened for study enrollment. Patients were excluded if they met any of the following criteria: previous organ transplant recipient, undergoing multiple organ transplants (e.g. simultaneous liver and kidney), inability to take enteral (orally or by tube feed) medications by post-transplant day (POD) 4, history of diabetes mellitus prior to LT (diabetes mellitus defined as use of hypoglycemic agents or glycated hemoglobin (HbA1c) > 6.4), had any severe medical condition requiring acute or chronic treatment that in the investigator’s opinion would interfere with study participation, exposure to an investigational therapy within 30 days prior to enrollment or 5 half-lives of the investigational product, whichever was greater, concomitant use of medications which are inhibitors of CYP3A4 (such as ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, erythromycin) could not be avoided during the study period, pregnant females, and subjects with diminished mental capacity to consent. Potential subjects were given information about the study once being placed on LT wait-list to ensure ample time to review and consider study participation. After candidate underwent LT, study team re-reviewed eligibility criteria between POD 0 and 3, and approached all subjects meeting eligibility criteria to obtain informed consent on POD 4. A total of twenty subjects were enrolled. Study protocol and related documents were approved by University of Cincinnati Institutional Review Board before the start of any study procedures. No donor organs were obtained from executed prisoners or other institutionalized persons.
Control Selection
Each study subject was matched to a control using same inclusion and exclusion criteria described above. In addition, controls were matched to study subjects based on age, gender, autoimmune liver disease as the primary liver disorder and use of anti-thymocyte globulin in peri-operative period. To minimize the selection bias, the matched controls were selected in a manner that he/she would have undergone LT within a 24-week period (8 weeks prior or 16 weeks after) of the LT of the matched study subject. Thus, data from the controls was also collected prospectively. Since outcomes were being measured at week 24 of LT, this protocol ensured that the investigators had no influence on selecting the controls with a known outcome.
Immunosuppressive regimen
Subjects and controls received intravenous corticosteroids in the form of methylprednisolone; 500 mg on POD 0, 250 mg on POD 1, 125 mg on POD 2 and 60 mg POD 3. Subjects were started on oral Budesonide on POD 4 at a dose of 9 mg daily. Controls were started on Prednisone 60 mg daily. Both these drugs were tapered over next 12 weeks (Table 1). Both groups received similar amounts of standard immune suppression which included Tacrolimus twice a day with a goal trough level of 8–12 ng/ml, MMF 500 twice a day with an option to titrate up to 1000 mg twice a day as per discretion of treating physician. For any significant adverse effects or contraindication to Tacrolimus, use of cyclosporine was permitted with a goal trough level 150–200 ng/ml. Selected patients with either pre-transplant renal dysfunction defined as renal replacement therapy or serum creatinine > 2.0mg/dL or post-operative renal dysfunction defined as renal replacement therapy (including intra-operative) or serum creatinine > 2.0 mg/dL within 48 hours of transplant on 2 separate readings received a maximum of 4 doses of anti-thymocyte globulin through POD 7 with a goal of CD3 count suppression to < 25 cells/mm and delayed start of Tacrolimus on POD 7.
Table 1:
Immunosuppressive Therapy per Study Group
| Time Post LT* (post-operative day (POD)) | Budesonide Group N=20 | Control Group N=20 |
|---|---|---|
| POD 0–3 | SISǂ + Intravenous corticosteroids | SISǂ + Intravenous corticosteroids |
| POD 4–30 | SISǂ + Budesonide 9 mg | SISǂ + Prednisone 60 mg tapered to 15 mg |
| POD 31–45 | SISǂ + Budesonide 6 mg | SISǂ + Prednisone 10 mg |
| POD 46–90 | SISǂ + Budesonide 3 mg | SISǂ + Prednisone 10 mg tapered to discontinue |
| POD ≥ 91 | SISǂ,¥ | SISǂ,¥ |
LT: Liver Transplant
SIS: standard immune suppression which consisted of the following: tacrolimus twice daily dosed to achieve goal trough level 8–12 ng/ml + mycophenolate mofetil 500 mg twice daily, +/− Thymoglobulin with goal CD3 suppression < 25 with delayed initiation of tacrolimus
If autoimmune hepatitis prior to LT continue prednisone 5 mg daily indefinitely
Antimicrobial prophylaxis:
Antimicrobial prophylaxis was administered to both groups as per our center’s standard protocol (Table 2).
Table 2:
Antimicrobial prophylaxis for LTǂ recipients
| Patient Population | Medication | Dosing and Length of Treatment | ||
|---|---|---|---|---|
| Peri-Operative | Non-Penicillin Allergic | Ceftriaxone AND Ampicillin | Pre-op: ceftriaxone 2g IV AND ampicillin 2g IV Post-op: ceftriaxone 2g IV x 1 dose 24h after pre-op dose AND ampicillin 1g IV q6h (or per renal function) x 48 hours |
|
| Penicillin Allergic | Vancomycin AND Ciprofloxacin | Pre-op vancomycin: 20mg/kg Pre-op ciprofloxacin: 400mg – 600mg IV Post-op: vancomycin 15mg/kg IV x 1 dose and ciprofloxacin 400mg IV x 1 dose 12 hours after pre-operative dose and then as per renal function for 48 hours total |
||
| Fungal | High Risk Defined as > 1 of the following:
|
Fluconazole | 200 mg orally daily for 1 month | |
| All others | No therapy | |||
| Viral | High Risk | CMV IgG Donor + / Recipient − | Valganciclovir | 900 mg orally daily for 6 months |
| Intermediate Risk | CMV IgG Donor + / Recipient + CMV IgG Donor − / Recipient + | Valganciclovir | 450 mg orally daily for 3 months | |
| Low Risk | CMV IgG Donor − / Recipient − | Acyclovir | 800 mg orally twice daily for 1 month | |
LT: liver transplant IV, intravenous; PRBC, packed red blood cells; IgG, immunoglobulin G
Study procedures
Study enrollment was 24 weeks. Study visits were performed during the standard of care ambulatory follow up visits at post-LT weeks 2, 4, 6, 8, 12, 18 and 24. To study the effect of budesonide on hypothalamus-pituitary-adrenal axis, early morning (between 6 AM and 8 AM) serum cortisol levels were measured at weeks 4 and 8. A low dose cosyntropin stimulation was performed at week 12
Outcome measures
Primary Outcome:
Primary outcome was rate of biopsy proven ACR during the first 24 weeks of LT amongst study and control group. This outcome was used to assess efficacy of budesonide as compared to prednisone. The definition of ACR was based on widely accepted Banff criteria of liver histology.43 A Banff score of 3 or more on liver histology as interpreted by a blinded, study assigned liver pathologist was considered as ACR. Decision to obtain a liver biopsy was as per treating physician based on liver chemistries and patient’s clinical course. Protocol biopsies were not performed since yield of liver biopsy to show ACR with normal liver chemistries is expected to be very low and would render unnecessary risk to study participants. Rates of ACR during first 24 weeks after LT were calculated for the study subjects and compared to controls.
Secondary Outcomes:
Secondary outcomes included
Rate of NODAT at week-24 post LT amongst study group and control group. Variable definitions of NODAT have been used in liver and kidney transplant literature. Expert recommendations from an international consensus meeting suggest that NODAT should be differentiated from transient hyperglycemia during corticosteroids use since in majority of cases transient hyperglycemia resolves once corticosteroids are stopped.44 For our study, subjects were assessed for NODAT at week 24 of LT which was 12 weeks after discontinuation of budesonide or prednisone while subjects were on stable doses of standard immune suppression. NODAT was defined as HbA1c of > 6.4 % or requirement of insulin or any hypoglycemic agent.
Study the effect of budesonide on hypothalamus-pituitary-adrenal axis and estimate the magnitude of adrenal suppression. Early morning serum cortisol levels during first 12 weeks post-LT were used as a screening test for adrenal suppression due to exogenous use of corticosteroids. The blood samples were collected at weeks 4 and 8 between 6AM and 8AM. A serum cortisol value of 3 μg/dl or below was used as a cutoff for adrenal suppression. A one-time, low dose, cosyntropin stimulation test at week 12 (when study drug was being tapered off) was performed by administering cosyntropin at a dose of 0.5 μg per 1.73 m2 of body surface area and checking the serum cortisol levels at baseline and 30 minutes after cosyntropin injection. This test was used to assess adrenal insufficiency with a high sensitivity. A serum cortisol value of less than 11 μg/dl after cosyntropin stimulation was used to define adrenal suppression.45 Hypothalamus-pituitary-adrenal assessment was only performed in budesonide group. The Roche cobas® Cortisol II assay was used to measure cortisol levels.
-
Infectious Complications: Rates of overall infections were measured and compared between groups.
Bacterial infections: Positive blood, wound or fluid culture, or positive Clostridium difficile DNA amplification assay.
CMV: Serum nucleic acid amplification assay value of 2000 copies/ml or more or tissue histology with positive CMV stain
Fungal infection: Positive blood, wound or fluid culture or positive serologic test for aspergillus, cryptococcus or histoplasma.
Rate of adverse events and severe adverse events during the first 24 weeks post LT amongst recipients receiving budesonide (study group only). Adverse events and severe adverse events were recorded at each study visit and at any time these were being reported by the subject to the study team or treating physician. Interim data safety review was performed by an independent Data Safety Monitoring Committee after enrollment of first 10 subjects.
All the secondary outcomes were designed to help with safety assessment of budesonide.
Statistical Analyses
Sample size was based on primary outcome measure of efficacy (rate of ACR). Review of data from our center revealed rate of ACR ~ 10% in the first 24 weeks of LT. We hypothesized that similar rates of ACR will be observed using budesonide. Since this was designed to be a pilot, proof of concept study, a large sample size was not feasible. We planned to estimate the rates of ACR along with 95% confidence intervals in both groups. As such with the proposed sample size of 20 subjects in each group, a two-sided 95% confidence interval would be able to estimate this rate within 0.13 range.
Data was analyzed using Statistical Package for the Social Sciences (SPSS) version 26. Categorical data is portrayed in proportions and was analyzed using chi-square test. Continuous data is portrayed as medians and interquartile ranges and was analyzed using t-test. Early morning serum cortisol levels were analyzed using paired t-test.
Results
From July 2017 to November 2018 a total of 139 potential subjects were screened for study enrollment (Figure 1). Of these, 59 met the inclusion and exclusion criteria, with 19 (32%) declining participation, 20 who underwent primary deceased donor LT enrolled in the study, and another 20 patients consented as matched controls. All participants were followed for 24 weeks post LT. Baseline characteristics are summarized in Table 3. Both groups of LT recipients were well matched with respect to gender, age, race, cause of liver failure, Model of End-stage Liver Disease-Sodium score and tacrolimus trough levels. There were no deaths or graft losses in either group for the duration of the study. Results of our primary and secondary outcomes are summarized in Table 4.
Figure 1:
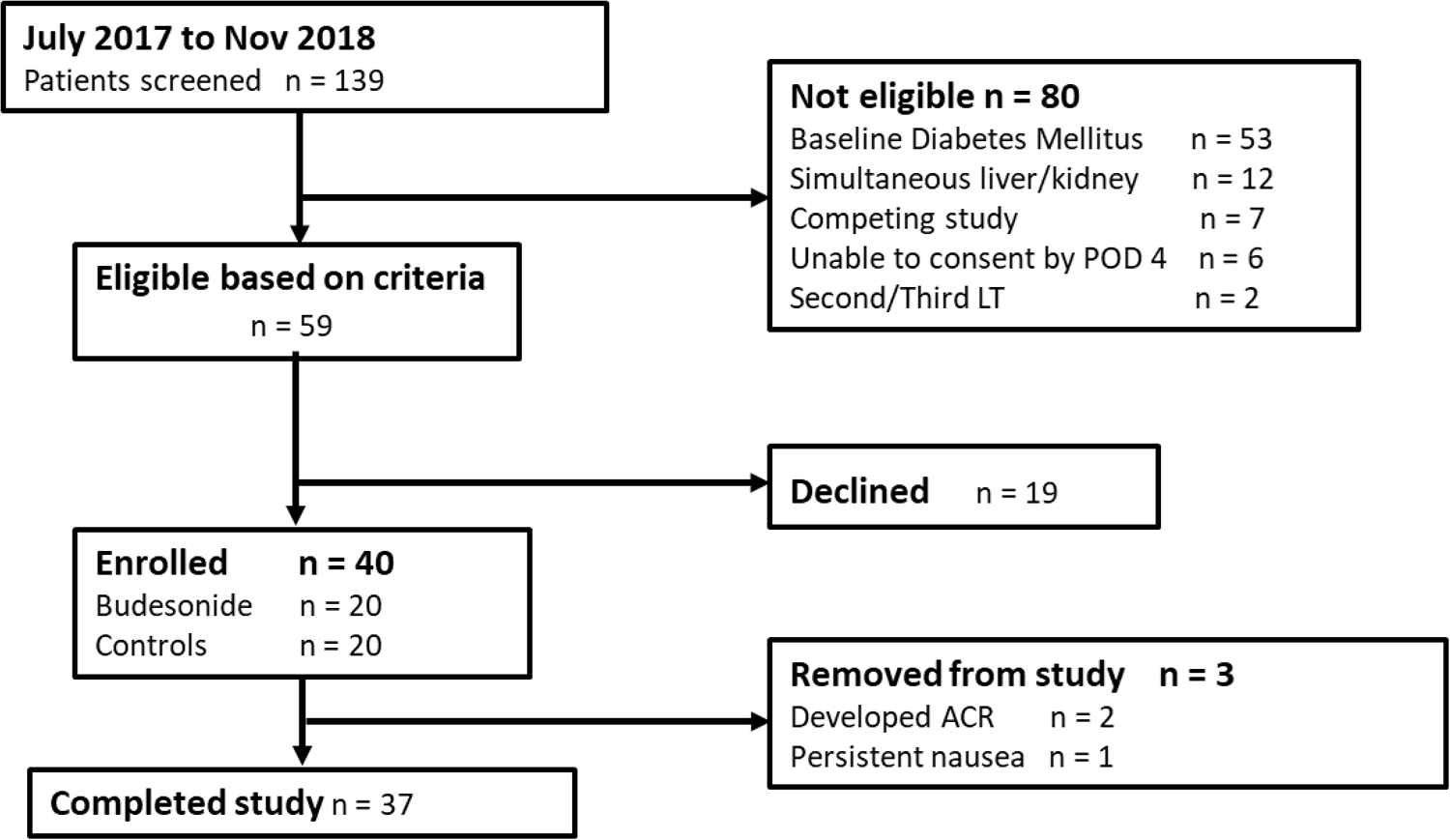
Flow of study subjects
Table 3:
Baseline characteristics per study group illustrated as proportions and interquartile ranges
| Characteristic | Budesonide Group N=20 | Control Group N=20 |
|---|---|---|
| Age | 57 (42–64) | 56 (44–63) |
| Gender (Male) | 13 (65%) | 13 (65%) |
| Hispanic | 1 (5%) | 2 (10%) |
| Other | 0 | 4 (20%) |
| Median MELD-Naǂ at liver transplant | 23 (17–28) | 22 (19–27) |
| Acute on chronic liver failure | 4 (20%) | 3 (15%) |
| History of portal venous thrombosis prior to Liver Transplantation | 4 (20%) | 4 (20%) |
| Hepatocellular carcinoma | 4 (20%) | 1 (5%) |
| Portal vein thrombectomy performed at the time of transplantation | 3 (15%) | 2 (10%) |
| Median Tacrolimus trough level over 24 weeks (ng/mL) | 7.8 (6.5–9.4) | 8 (7–9) |
| Hepatic artery thrombosis | 0 | 1 (5%) |
MELD-Na, Model for End Stage Liver Disease with Sodium
Table 4:
Outcome Measures per Study Group
| Outcome | Budesonide Group N=20 | Control Group N=20 | P |
|---|---|---|---|
| Acute Cellular Rejection | 1 (5%) | 1 (5%) | 1.00 |
| NODATǂ | 0 | 3 (15%) | 0.23 |
| Cytomegalovirus infection | 0 | 2 (10%) |
NODAT, New Onset Diabetes After Transplant
Acute Cellular Rejection
During the 24-week study period, a total of nine liver biopsies were performed; 6 in budesonide group and 3 in prednisone group. One patient in each group (5% vs 5%, p = 1.00) was confirmed to have biopsy proven ACR. The subject in budesonide group that developed ACR was a 35-year-old female who underwent LT for liver failure secondary to autoimmune hepatitis and primary biliary cholangitis overlap syndrome. On POD 7, patient developed significant anemia with a hemoglobin level 6.5 g/dl without any evidence of blood loss. After an extensive work up, she was diagnosed with autoimmune hemolytic anemia secondary to Tacrolimus. Her immune suppression was switched to Cyclosporine on POD 21. Four days after starting cyclosporine, her trough level was 45 ng/ml with mild elevation of aminotransferases (Aspartate aminotransferase (AST) 67 U/L and Alanine aminotransferase (ALT) 52 U/L) and bilirubin (total 1.7 mg/dl and direct 0.78 mg/dl). Eight days after starting cyclosporine, her trough level was 59 ng/ml with worsening elevation of aminotransferases (AST 170 U/L and ALT 148 U/L) and bilirubin (total 1.9 mg/dl and direct 0.95 mg/dl). A biopsy of the allograft revealed severe ACR with plasma rich infiltrates. The rejection activity index score was eight. C4d stain was negative. Patient was removed from the study protocol and started on intravenous methylprednisolone for 3 days followed by oral prednisone taper along with an increase in her standard immune suppression (CNI and MMF). Her aminotransferase levels slowly trended down to normal range within 4 weeks. The patient in control group that developed ACR was a 46 years old female who underwent LT for liver failure secondary to primary sclerosing cholangitis. On POD 5, her total bilirubin showed a marked incline from 3.2 mg/dl to 10.8 mg/dl within a 48-hour period while aminotransferases were slow to recover from ischemia-reperfusion injury (AST 346 U/L and ALT 536 U/L). Ten-hour tacrolimus trough level was 9 ng/ml. Her bilirubin continued to rise despite undergoing endoscopic retrograde cholangiopancreatography that revealed a mild anastomosis stricture which was treated with ductoplasty and placement of biliary stents. She underwent a liver biopsy. Histology was consistent with moderate ACR with a rejection activity index score of 5. The patient was treated with intravenous methylprednisolone for 3 days followed by oral prednisone taper and an increase in the standard maintenance immune suppression (CNI and MMF). Her aminotransferase levels slowly trended down to normal range within 6 weeks. The details of the remaining liver biopsies are summarized in Table 5.
Table 5:
Summary of Liver allograft biopsies performed per study group
| Study Group | Time post Liver Transplant that Liver Biopsy performed (post-operative day (POD)) | Key Findings |
|---|---|---|
| Budesonide 3 | POD 4 | Patchy hepatocyte necrosis (25%) with sinusoidal hemorrhage suggestive of ischemic injury. Normal portal tracts |
| Budesonide 4 | POD 5 | Central venule and sinusoidal congestion suggestive of outflow obstruction |
| Budesonide 7* | POD 29 | Acute cellular rejection with plasma rich infiltrates. Rejection activity index score was eight. A C4d stain was negative. |
| Budesonide 9 | POD 74 | Predominant lymphocytic portal inflammation aggregates suggestive of chronic viral hepatitis. Perivenular (zone 3) sinusoidal dilatation, congestion, hepatocellular dropout and fibrosis consistent with vascular outflow obstruction |
| Budesonide 10 | POD 11 | Patchy moderate cholestasis and mild bile ductular reactions (resolving ischemia-reperfusion injury). Mild regenerative hyperplasia-like changes and abnormal portal vein branches suggestive of vascular flow abnormality |
| Budesonide 11 | POD 5 | Mild ischemia reperfusion injury |
| Control 5ǂ | POD 8 | Acute cellular rejection with a rejection activity index score of 5 |
| Control 11 | POD 5 | Perivenular (zone 3) sinusoidal dilatation, congestion, hepatocellular dropout and fibrosis consistent with vascular outflow obstruction |
| Control 16 | POD 4 | Focal mild cholestasis and rare acidophil formation, favor ischemic/reperfusion injury |
The patient in budesonide group that developed acute cellular rejection
The patient in prednisone group that developed acute cellular rejection
NODAT
New Onset Diabetes was not observed in any patient in the budesonide group whereas 3 subjects in prednisone group developed NODAT at week 24 (0 vs 15%, Fischer’s exact, p=0.23). Median HemoglobinA1c levels at weeks 12 and 24 were 5.0 and 5.1 for budesonide group and 5.1 and 5.2 for the prednisone group, respectively.
Adrenal Suppression
Serial cortisol samples were available for 12 subjects in budesonide group. The trend of early morning cortisol level over the 12-week period for these 12 subjects is illustrated in figure 2. Median cortisol level remained steady from 9.3 μg/dl at week 4 to 9.7 μg/dl at week 12. Cosyntropin stimulation test was performed in 15 subjects in budesonide group only. Two subjects (13%) developed adrenal suppression as per the testing criteria without any symptoms or requirement for specific treatment.
Figure 2:
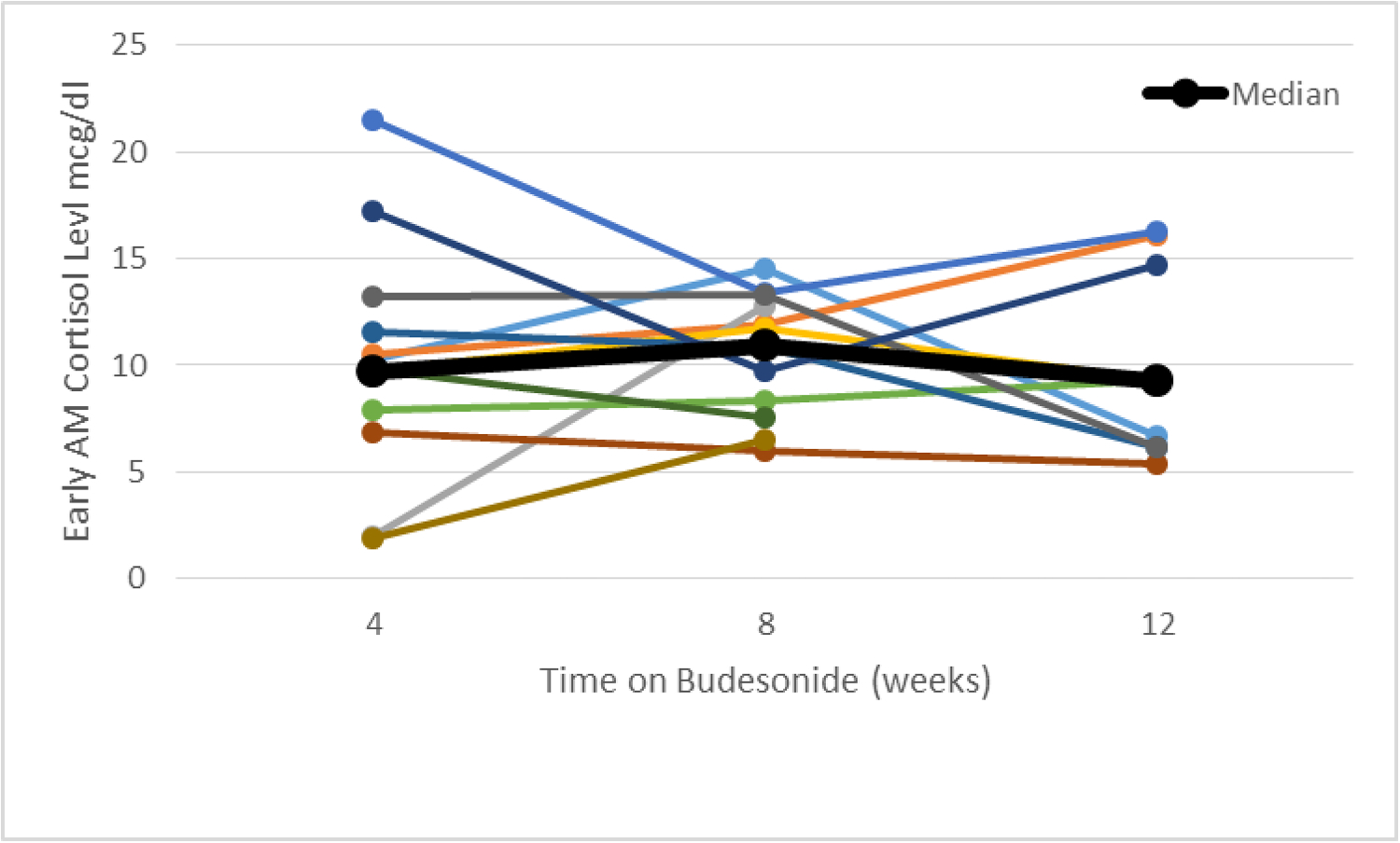
Trend of early morning serum cortisol level in individual subjects along with the median value.
Infections
The overall rate of infections was zero in budesonide group and 30% in prednisone group (p = 0.02). Three subjects in prednisone group developed bacteremia, two subjects developed CMV infection and one subject developed bacteremia as well as fungal blood stream infection. The CMV risk status for two subjects who developed CMV infection were high (donor positive/recipient negative), and intermediate (donor positive/recipient positive). Both subjects had CMV viremia which was successfully treated with antiviral medications.
Adverse Events and Severe Adverse Events:
There were no deaths or graft failures in either group. In budesonide group grade 3 or 4 adverse events included acute kidney injury (20%), ascites and/or pleural effusion (15%), sinus tachycardia (5%), biliary leak (10%) and biliary stricture (5%). None of these adverse events were attributed to the study drug.
Discussion
In a pilot, proof of concept, non-randomized prospective trial, we have shown that Budesonide has the potential to be used in a safe and effective way for LT immune suppression. The rates of biopsy proven ACR in the budesonide group were comparable to that of prednisone group. Both subjects that developed ACR in our study would be considered high risk for rejection based on the baseline characteristics (i.e. age and autoimmune liver disease) and subtherapeutic CNI trough levels at time of ACR. Both groups were matched based on risk of ACR. However, future studies need to further categorize the subjects based on the risk of rejection to further identify the specific patient population that will benefit from the use of budesonide based immune suppression. Overall rate of ACR was lower in our study as compared to historical data. 46. This could be by chance due to small sample size. Two subjects in our study (one in each group) received anti-thymocyte globulin induction therapy as per our renal sparing protocol. In addition, budesonide group had more patients (20% vs 5%) with hepatocellular carcinoma (HCC). This was most likely due to chance. We do not believe that this affected the outcomes in our study since there is no literature to support that having HCC at the time of LT has any impact on the risk of rejection or diabetes. If immune suppression is changed or lowered to reduce the risk of HCC recurrence, this could affect rejection risk. However, we used standard immune suppression with similar CNI levels in both groups (Table 3).
NODAT adversely affects patient and graft survival and its management presents additional challenges. 2 Results of NODAT incidence from our study although did not reach statistical significance, most likely due to the small sample size, but the trends are encouraging. In addition to lower rates of NODAT, insulin requirement and overall glycemic control also seemed to be improved within in budesonide group. All subjects in the prednisone group required outpatient management of hyperglycemia after being discharged from index hospitalization for LT. In comparison, only four subjects in budesonide group required very short-term use of insulin after being discharged from index hospitalization. Post-transplant hyperglycemia has been associated with increased risk of ACR as well as other morbidities.47 In addition, hyperglycemia management after LT utilizes significant resources48 and can result in additional laboratory testing, office and emergency room visits as well as hospital admissions. Improvement in glycemic control post LT can result in better clinical outcomes. The scope of our study did not allow us to collect detailed data regarding use of insulin, hypoglycemic agents and more sophisticated measures of glycemia control. Future studies looking at Budesonide need to incorporate more sensitive measures of glycemic control as well as longer follow up LT outcomes.
Infectious complications were lower in budesonide group as compared to prednisone group in our study. These include bacterial, fungal as well as CMV infections. The results are very encouraging since infections in the immediate post-LT period can complicate clinical course, can cause renal dysfunction and can affect patient and graft survival. Results from our cohort are mimicking the infection rates seen in steroid free arms of steroid free trials.13 However our data needs validation since the control group (prednisone) in our study was not matched based on the basis of infection risk. In addition, the mechanisms leading to lower infection rates need exploration. Systemic steroids are just one component of LT immune suppression. Is minimizing this component enough to reduce the rates of infections or is this a combined effect of less systemic exposure and improved glycemic control. Budesonide appears to provide both these benefits. Two patients in prednisone group developed CMV viremia, one was high risk for CMV infection based on serologies (donor positive/recipient negative) and the other patient was intermediate risk (donor positive/recipient positive). Our institutional protocol recommends a lower dose of Valganciclovir (450 mg orally daily) for LT recipients with intermediate risk of CMV infection. Lower dose of Valganciclovir is shown to be as effective in preventing CMV infection in solid organ transplant recipients as the standard dose (900 mg daily) but with less side effects.49
Lastly, our data shows low rates of adrenal suppression after 12 weeks use of Budesonide. Other studies have shown that Budesonide has minimal effect on hypothalamus-pituitary-adrenal axis when used in patients with chronic liver disease for up to 2 years.34 In patients with advance liver disease and portal hypertension, portsosystemic shunts are common and can result in bypassing the first-pass hepatic metabolism of budesonide; thus increasing systemic bioavailability and affecting the hypothalamus-pituitary and adrenal axis. Although these shunts improve/resolve after a successful LT and resolution of portal hypertension, the timing and extent of improvement is unclear. Our institutional practice is that we ligate large portosystemic shunts at the time of LT if portal venous flow after graft reperfusion is low (less than 500 cc/minute). None of the patients in our study met these criteria. Twenty percent of patients in each group had prior history of portal venous thrombosis while only 15% of patients in budesonide group and 10% in the control group required thrombectomy at the time of LT. Our study is limited in terms of any additional measurements of portosystemic shunting that might have raised the systemic bioavailability of budesonide. More sophisticated measurements and analyses in future studies can help answer this question. On a similar account, incidence of hepatic artery thrombosis or primary graft non-function can alter graft’s ability to metabolize budesonide and potentially increase systemic bioavailability. These conditions are uncommon and our study was not powered to control for these parameters. However, if similar findings of lower rates of adrenal suppression can be reproduced in LT recipients, Budesonide can be used for long term immune suppression as compared to prednisone; the use of which is limited to few months after LT, mainly due to systemic toxicity. In addition, long term use of budesonide may allow us to lower our dependence on CNIs and might prove to be another option for an effective renal sparing strategy. In addition, incidence of metabolic bone disease rises significantly in first few months after LT and immune suppressive agents, mainly corticosteroids, are the major contributing factor to bone loss.50 Based on data from Inflammatory Bowel Disease studies, rate of bone loss with budesonide is significantly lower when compared to prednisone.37 Similar data in LT population can further establish superior safety profile of budesonide over prednisone.
Our study had certain limitations. First, our sample size was small; however, the aim of this pilot study was to assess the safety and efficacy of budesonide in LT recipients since data in this patient population is scarce; and to generate data for sample size calculation for larger trial. Secondly, due to limited availability of resources and funding, we could not randomize the control group. The control group was matched only based on the risk of ACR; however, to minimize the selection bias, we enrolled the control group in prospective fashion. In addition to the methods detailed previously, we tried to remain blinded to the outcomes in the control group by identifying and enrolling them within 8 weeks of their LT. However, lack of randomization could have caused an unintentional measurement bias that resulted in more liver biopsies performed in the budesonide group. Lastly, some of the outcomes, such as, effect of prednisone on hypothalamic-pituitary- adrenal axis and adverse events could not be studied in prednisone group; however, the role of prednisone in causing adrenal suppression, even after a 4-week course, is well documented in literature. 51
In conclusion, use of Budesonide, in place of prednisone for LT immune suppression appears to be safe and effective in our pilot study. It is associated with similar rates of ACR but lower rates NODAT and infectious complications. Larger, randomized, controlled trial is needed to validate these findings before wide spread clinical use of Budesonide in combination with CNI and MMF post LT can be recommended.
Financial Support:
2016 ACG Clinical Research Award, American College of Gastroenterology.
National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations:
- CNI
Calcineurin inhibitors
- MMF
mycophenolate mofetil
- LT
Liver Transplantation
- ACR
Acute cellular rejection
- NODAT
New Onset Diabetes After Transplant
- CMV
Cytomegalovirus
- POD
post-transplant day
- HbA1c
glycated hemoglobin
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- HCC
hepatocellular carcinoma
REFERENCES
- 1.Mukherjee S, Mukherjee U. A comprehensive review of immunosuppression used for liver transplantation. J Transplant. 2009;2009:701464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon JI, Barbeito R, Faradji RN, Gaynor JJ, Tzakis AG. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: Long-term follow up. Transplantation. 2006;82(12):1625–1628. [DOI] [PubMed] [Google Scholar]
- 3.Castedal M, Skoglund C, Axelson C, Bennet W. Steroid-free immunosuppression with low-dose tacrolimus is safe and significantly reduces the incidence of new-onset diabetes mellitus following liver transplantation. Scand J Gastroenterol. 2018;53(6):741–747. [DOI] [PubMed] [Google Scholar]
- 4.De Carlis L, Belli LS, Rondinara GF, et al. Early steroid withdrawal in liver transplant patients: final report of a prospective randomized trial. Transplant Proc. 1997;29(1–2):539–542. [DOI] [PubMed] [Google Scholar]
- 5.Lerut JP, Pinheiro RS, Lai Q, et al. Is minimal, [almost] steroid-free immunosuppression a safe approach in adult liver transplantation? Long-term outcome of a prospective, double blind, placebo-controlled, randomized, investigator-driven study. Ann Surg. 2014;260(5):886–891; discussion 891–882. [DOI] [PubMed] [Google Scholar]
- 6.Padbury RT, Gunson BK, Dousset B, et al. Steroid withdrawal from long-term immunosuppression in liver allograft recipients. Transplantation. 1993;55(4):789–794. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier SJ, Nadig SN, Lee DD, et al. A prospective, randomized trial of complete avoidance of steroids in liver transplantation with follow-up of over 7 years. HPB (Oxford). 2013;15(4):286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Punch JD, Shieck VL, Campbell DA, Bromberg JS, Turcotte JG, Merion RM. Corticosteroid withdrawal after liver transplantation. Surgery. 1995;118(4):783–786; discussion 786–788. [DOI] [PubMed] [Google Scholar]
- 9.Wei Q, Xu X, Wang C, et al. Efficacy and Safety of a Steroid-Free Immunosuppressive Regimen after Liver Transplantation for Hepatocellular Carcinoma. Gut Liver. 2016;10(4):604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing T, Huang L, Yu Z, Zhong L, Wang S, Peng Z. Comparison of steroid-free immunosuppression and standard immunosuppression for liver transplant patients with hepatocellular carcinoma. PLoS One. 2013;8(8):e71251. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Segev DL, Sozio SM, Shin EJ, et al. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14(4):512–525. [DOI] [PubMed] [Google Scholar]
- 12.Sgourakis G, Dedemadi G. Corticosteroid-free immunosuppression in liver transplantation: an evidence-based review. World journal of gastroenterology. 2014;20(31):10703–10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sgourakis G, Radtke A, Fouzas I, et al. Corticosteroid-free immunosuppression in liver transplantation: a meta-analysis and meta-regression of outcomes. Transplant international : official journal of the European Society for Organ Transplantation. 2009;22(9):892–905. [DOI] [PubMed] [Google Scholar]
- 14.Langrehr JM, Neumann UP, Lang M, et al. First results from a prospective randomized trial comparing steroid-free induction therapy with tacrolimus and MMF versus tacrolimus and steroids in patients after liver transplantation for HCV. Transplant Proc. 2002;34(5):1565–1566. [DOI] [PubMed] [Google Scholar]
- 15.Margarit C, Bilbao I, Castells L, et al. A prospective randomized trial comparing tacrolimus and steroids with tacrolimus monotherapy in liver transplantation: the impact on recurrence of hepatitis C. Transplant international : official journal of the European Society for Organ Transplantation. 2005;18(12):1336–1345. [DOI] [PubMed] [Google Scholar]
- 16.Moench C, Barreiros AP, Schuchmann M, et al. Tacrolimus monotherapy without steroids after liver transplantation--a prospective randomized double-blinded placebo-controlled trial. Am J Transplant. 2007;7(6):1616–1623. [DOI] [PubMed] [Google Scholar]
- 17.Samonakis DN, Mela M, Quaglia A, et al. Rejection rates in a randomised trial of tacrolimus monotherapy versus triple therapy in liver transplant recipients with hepatitis C virus cirrhosis. Transpl Infect Dis. 2006;8(1):3–12. [DOI] [PubMed] [Google Scholar]
- 18.Tisone G, Angelico M, Orlando G, et al. Retrospective analysis of 30 patients who underwent liver transplantation without use of steroids. Transplant Proc. 1999;31(7):2908–2909. [DOI] [PubMed] [Google Scholar]
- 19.Tisone G, Angelico M, Palmieri G, et al. A pilot study on the safety and effectiveness of immunosuppression without prednisone after liver transplantation. Transplantation. 1999;67(10):1308–1313. [DOI] [PubMed] [Google Scholar]
- 20.Washburn K, Speeg KV, Esterl R, et al. Steroid elimination 24 hours after liver transplantation using daclizumab, tacrolimus, and mycophenolate mofetil. Transplantation. 2001;72(10):1675–1679. [DOI] [PubMed] [Google Scholar]
- 21.Boillot O, Mayer DA, Boudjema K, et al. Corticosteroid-free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11(1):61–67. [DOI] [PubMed] [Google Scholar]
- 22.Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE, Jr. Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75(8):1396–1399. [DOI] [PubMed] [Google Scholar]
- 23.Klintmalm GB, Washburn WK, Rudich SM, et al. Corticosteroid-free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1-year interim results of the HCV-3 study. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;13(11):1521–1531. [DOI] [PubMed] [Google Scholar]
- 24.Llado L, Xiol X, Figueras J, et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: results from a prospective multicenter randomized study. Journal of hepatology. 2006;44(4):710–716. [DOI] [PubMed] [Google Scholar]
- 25.Pageaux GP, Calmus Y, Boillot O, et al. Steroid withdrawal at day 14 after liver transplantation: a double-blind, placebo-controlled study. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2004;10(12):1454–1460. [DOI] [PubMed] [Google Scholar]
- 26.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 Annual Data Report: Liver. Am J Transplant. 2019;19 Suppl 2:184–283. [DOI] [PubMed] [Google Scholar]
- 27.Ryrfeldt A, Andersson P, Edsbacker S, Tonnesson M, Davies D, Pauwels R. Pharmacokinetics and metabolism of budesonide, a selective glucocorticoid. Eur J Respir Dis Suppl. 1982;122:86–95. [PubMed] [Google Scholar]
- 28.2001; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-324_Entocort.cfm.
- 29.Csepregi A, Rocken C, Treiber G, Malfertheiner P. Budesonide induces complete remission in autoimmune hepatitis. World journal of gastroenterology. 2006;12(9):1362–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leuschner M, Maier KP, Schlichting J, et al. Oral budesonide and ursodeoxycholic acid for treatment of primary biliary cirrhosis: results of a prospective double-blind trial. Gastroenterology. 1999;117(4):918–925. [DOI] [PubMed] [Google Scholar]
- 31.Pagan B, Erdozain JC, Comas C, Martin-Chavarri S, Lopez M, Gomez-Cerezo JF. Budesonide combined with ursodeoxycholic acid in primary biliary cirrhosis with advanced liver damage. European journal of internal medicine. 2006;17(7):508–510. [DOI] [PubMed] [Google Scholar]
- 32.Peiseler M, Liebscher T, Sebode M, et al. Efficacy and Limitations of Budesonide as a Second-Line Treatment for Patients With Autoimmune Hepatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(2):260–267.e261. [DOI] [PubMed] [Google Scholar]
- 33.Rabahi N, Chretien Y, Gaouar F, et al. Triple therapy with ursodeoxycholic acid, budesonide and mycophenolate mofetil in patients with features of severe primary biliary cirrhosis not responding to ursodeoxycholic acid alone. Gastroenterologie clinique et biologique. 2010;34(4–5):283–287. [DOI] [PubMed] [Google Scholar]
- 34.Rautiainen H, Karkkainen P, Karvonen AL, et al. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology (Baltimore, Md). 2005;41(4):747–752. [DOI] [PubMed] [Google Scholar]
- 35.Wiegand J, Schuler A, Kanzler S, et al. Budesonide in previously untreated autoimmune hepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2005;25(5):927–934. [DOI] [PubMed] [Google Scholar]
- 36.Manns MP, Woynarowski M, Kreisel W, et al. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139(4):1198–1206. [DOI] [PubMed] [Google Scholar]
- 37.Schoon EJ, Bollani S, Mills PR, et al. Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn’s disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2005;3(2):113–121. [DOI] [PubMed] [Google Scholar]
- 38.Rautiainen H, Farkkila M, Neuvonen M, et al. Pharmacokinetics and bone effects of budesonide in primary biliary cirrhosis. Alimentary pharmacology & therapeutics. 2006;24(11–12):1545–1552. [DOI] [PubMed] [Google Scholar]
- 39.Ozcay N, Fryer J, Grant D, Freeman D, Garcia B, Zhong R. Budesonide, a locally acting steroid, prevents graft rejection in a rat model of intestinal transplantation. Transplantation. 1997;63(9):1220–1225. [DOI] [PubMed] [Google Scholar]
- 40.Ruers TJ, Daemen MJ, Thijssen HH, van der Linden CJ, Buurman WA. Sensitivity of graft rejection in rats to local immunosuppressive therapy. Transplantation. 1988;46(6):820–825. [DOI] [PubMed] [Google Scholar]
- 41.Weber T, Kalbhenn T, Herrmann G, Hanisch E. Local immunosuppression with budesonide after liver transplantation in the rat: a preliminary histomorphological analysis. Transplantation. 1997;64(5):705–708. [DOI] [PubMed] [Google Scholar]
- 42.Bhat M, Ghali P, Wong P, et al. Immunosuppression with budesonide for liver transplant recipients with severe infections. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18(2):262–263. [DOI] [PubMed] [Google Scholar]
- 43.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology (Baltimore, Md). 1997;25(3):658–663. [DOI] [PubMed] [Google Scholar]
- 44.Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14(9):1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kline GA, Buse J, Krause RD. Clinical implications for biochemical diagnostic thresholds of adrenal sufficiency using a highly specific cortisol immunoassay. Clin Biochem. 2017;50(9):475–480. [DOI] [PubMed] [Google Scholar]
- 46.Levitsky J, Goldberg D, Smith AR, et al. Acute Rejection Increases Risk of Graft Failure and Death in Recent Liver Transplant Recipients. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2017;15(4):584–593 e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paka P, Lieber SR, Lee RA, Desai CS, Dupuis RE, Barritt AS. Perioperative glucose management and outcomes in liver transplant recipients: A qualitative systematic review. World J Transplant. 2018;8(3):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallia A, Parikh ND, Molitch ME, et al. Posttransplant hyperglycemia is associated with increased risk of liver allograft rejection. Transplantation. 2010;89(2):222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalil AC, Mindru C, Florescu DF. Effectiveness of valganciclovir 900 mg versus 450 mg for cytomegalovirus prophylaxis in transplantation: direct and indirect treatment comparison meta-analysis. Clin Infect Dis. 2011;52(3):313–321. [DOI] [PubMed] [Google Scholar]
- 50.Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2006;12(9):1390–1402. [DOI] [PubMed] [Google Scholar]
- 51.Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: A systematic review. Semin Arthritis Rheum. 2016;46(1):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]


