Abstract
Metabolic labeling, in which substrate analogs containing diminutive tags can infiltrate biosynthetic pathways and generate labeled products in cells, has led to dramatic advancements in the means by which complex biomolecules can be detected and biological processes can be elucidated. Within this realm, metabolic labeling of lipid products, particularly in a manner that is headgroup-specific, brings about a number of technical challenges including the complexity of lipid metabolic pathways as well as the simplicity of biosynthetic precursors to headgroup functionality. As such, only a handful of strategies for metabolic labeling of lipids have thus far been reported. However, these approaches provide enticing examples of how strategic modifications to substrate structures, particularly by introducing clickable moieties, can enable the hijacking of lipid biosynthesis. Furthermore, early work in this field has led to an explosion in diverse applications by which these techniques have been exploited to answer key biological questions or detect and track various lipid-containing biological entities. In this article, we review these efforts and emphasize recent advancements in the development and application of lipid metabolic labeling strategies.
Keywords: metabolic labeling, Phospholipids, lipids, Synthesis, Phosphatidylcholine, Phosphatidic Acid, Phosphatidylinositol
1. Introduction
The ability to selectively label and analyze target biomolecules within their native environments of living cells and organisms has long been a prominent goal of biological research (Prescher and Bertozzi, 2005). This endeavor has faced profound challenges due to the vast complexity of metabolic networks, in which a plethora of variations of each biomolecule are constantly undergoing nuanced transformations that alter structure and thereby dictate function, which are often dysregulated in disease (Cairns et al., 2011; Santos and Schulze, 2012). Therefore, it is exceedingly difficult to focus in on one particular biomolecule or family of compounds for study. Despite these challenges, the potential payoff for this pursuit is very high, as these techniques enable the tracking of key features including biosynthesis, subcellular localization, and trafficking that are at the core of many fundamental biological questions (Best, 2009; Devaraj, 2018; Sletten and Bertozzi, 2009, 2011). Within this realm, lipid metabolic labeling strategies, which employ synthetic substrate analogs bearing diminutive click chemistry tags that are capable of hijacking metabolic pathways and producing tagged products in cells, have emerged as invaluable chemical tools (Scheme 1) (Bumpus and Baskin, 2018). In this arena, strategies for the effective labeling of important lipid targets have begun to be reported, which have paved the way for initial applications of this strategy to address biological questions. These recent advancements provide the subject for this review article.
Scheme 1.
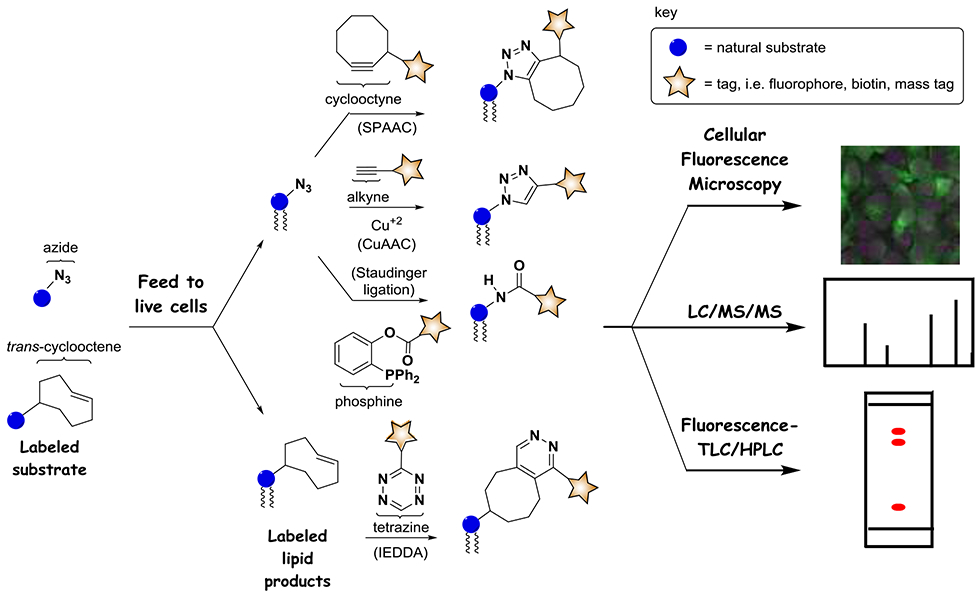
Workflow for lipid metabolic labeling. Derivatized substrate analogs containing clickable groups such as azide, alkyne (not shown) or trans-cyclooctene can be fed to cells to enter biosynthetic pathways and produce tagged products. These products can be derivatized via click reactions including CuAAC, SPAAC, the Staudinger Ligation, and IEDDA to introduce reporter groups such as fluorescent dyes, thereby enabling detection of products by a range of techniques exemplified by fluorescence microscopy, MS and TLC/HPLC.
A key challenge that needs to be overcome to achieve the production of labeled versions of products in cells pertains to issues associated with modifying natural substrates, and particularly that these modifications may suppress entry into metabolic networks. For this reason, many early labeling strategies were fairly conservative, primarily focusing on isotopically labeled versions of native substrates, which have the benefit of most closely resembling native substrates due to the subtle introduction of heavy atoms. As a result, experiments exploiting the production of isotopically labeled lipid products have been invaluable for understanding a variety of aspects of lipid metabolism and activity (Ecker and Liebisch, 2014; Wegener et al., 2016). Nevertheless, particularly in early studies, this approach has been limited in terms of the ability to image the localization of lipid products, although that has now changed due to advancements in mass spectrometry-based imaging methods (Berry et al., 2011; Bowman et al., 2019; Murphy et al., 2009). Cellular fluorescence microscopy is a stalwart technique in interrogating biological systems due to the ability to analyze changes in molecular subcellular localization (Bolte and Cordelieres, 2006). However, this requires the introduction of bulky fluorescent reporters onto labeled products, which for quite some time was not feasible for metabolic labeling strategies due to the dramatic alterations in structure this would necessitate for substrate analogs.
A key advancement in metabolic labeling came with the discovery of biorthogonal strategies for bioconjugation, involving reactions that utilize functional groups that are orthogonal to those that exist in biological systems (Best, 2009; Devaraj, 2018; Sletten and Bertozzi, 2009, 2011). Phrased another way, these reactions exploit probes bearing pairs of reactive tags that do not exist in biological systems (or minimally exist), do not react with other functional groups present in biology (or react as little as possible), but reliably react with one another under ambient conditions to produce stable labeled products. This was a longstanding challenge since, due to the vast complexity of functional groups present in nature, it is quite challenging to discover biorthogonal reactions. A breakthrough came through advancement of the ‘click chemistry’ philosophy, which focused on the development of high-yielding reactions that occur under ambient conditions utilizing ‘spring-loaded’ reactants (Kolb et al., 2001). These attributes translated into strong biorthogonal features for prototypical bioconjugation reactions including the copper catalyzed azide-alkyne cycloaddition (CuAAC) (Hein and Fokin, 2010; Meldal and Tornoe, 2008; Rostovtsev et al., 2002; Tornoe et al., 2002), and the Staudinger ligation between azide and phosphine reagents (Kohn and Breinbauer, 2004; Nilsson et al., 2000; Saxon et al., 2000; van Berkel et al., 2011) (Scheme 1). Please note that while numerous conditions and reagents have been developed for these reactions, we only show generalized reactions in Scheme 1. In addition, while we show specific combinations of probe tags and reagents in these figures, these can often be swapped (i.e. utilizing alkyne-tagged probes and azide-functionalized reagents).
An additional benefit of these biorthogonal conjugation reactions is that many of them employ reactive tags that are very small, such as azide and alkyne, consisting of only three and two atoms, respectively, for the tags themselves. This attribute was key for overcoming the challenge of developing suitable modified substrate analogs. Therefore, minimally modified substrate derivatives strategically altered by the addition of reactive tags such as azide and alkyne could act as effective precursors for biosynthetic machinery when fed to cells so as to produce labeled downstream products via native metabolic pathways. These products bearing clickable tags can then be post-modified with bulky reporter groups such as fluorescent dyes and biotin to enable selective interrogation of the progeny of the target probe. This transformative metabolic labeling procedure was first demonstrated for the labeling of cell surface glycoproteins using azide-modified mannose analogs as precursors for sialic acid-containing glycans (Saxon and Bertozzi, 2000). Since that time, metabolic labeling strategies have been extended to all families of biomolecules, including carbohydrates (Chang et al., 2009; Laughlin and Bertozzi, 2007; Tiwari et al., 2016), nucleotides (El-Sagheer and Brown, 2010; Gierlich et al., 2006; Gramlich et al., 2008; Seo et al., 2003), proteins (Johnson et al., 2010; Kiick et al., 2002; Liu and Schultz, 2010; Ngo and Tirrell, 2011; Wang et al., 2001), and lipids (Bumpus and Baskin, 2018; Jao et al., 2015a; Jao et al., 2009), and have led to dramatic advancements in the ability to scrutinize biological systems.
Concomitantly, numerous improvements have been made to biorthogonal reactions to balance reactant stability with reaction kinetics. A key advancement involved the development of the strain-promoted azide-alkyne cycloaddition (SPAAC) (Agard et al., 2004; Baskin et al., 2007; Jewett and Bertozzi, 2010), which circumvents the need for problematic copper catalyst in click chemistry reactions. In addition, new tags and reactions such as the inverse electron-demand Diels-Alder (IEDDA) cycloaddition reaction between tetrazine and trans-cyclooctene tags (Oliveira et al., 2017; Selvaraj and Fox, 2013; Wu and Devaraj, 2016) have expanded the toolbox for bioconjugation (Scheme 1).
Of the biomolecule families that have been studied, the development of metabolic labeling strategies targeting glycerophospholipids has progressed at a rather sluggish pace. However, these lipids represent a key target group for studies since they act as signaling molecules that regulate critical biological processes, their sub-cellular localization is a key aspect for controlling their function, and due to the vast complexity of lipid metabolic networks consisting of numerous combinations of lipid headgroups and acyl chain compositions that are constantly undergoing enzymatically driven interconversions. These attributes will be briefly introduced herein, and are conveniently covered in great detail in this special issue on lipid signaling.
Many lipids are now known to regulate key biological processes, for which aberrant lipid production correlates with high-profile diseases such as cancer (Bruntz et al., 2014; Griner and Kazanietz, 2007; Katso et al., 2001; Mazhab-Jafari et al., 2015). These signaling roles of lipids are often complex and difficult to study, such as protein-lipid binding interactions that anchor proteins onto the membrane surface and thereby regulate function (Cho and Stahelin, 2005; Lemmon, 2008). Localization is also critical since different lipid species are known to be selectively localized within the membranes of distinct organelles. Furthermore, lipids self-assemble into domains such as lipid rafts, a phenomenon that also elicits key ramifications for function (Lingwood and Simons, 2010; Sezgin et al., 2017). These phenomena result from complex lipid biosynthetic machinery as well as lipid trafficking pathways that remain mysterious. For all of these reasons, lipids are important targets for metabolic labeling since they control key biological processes, they exhibit complex biosynthetic and localization patterns that are linked to their function and the onset of disease, and due to the inherent complexity of biological membranes.
2. Strategies for Metabolic Labeling of Glycerophospholipid Headgroups
Metabolic labeling strategies have built upon prior work in imaging lipids in cells. For example Schultz and co-workers demonstrated the ability to deliver and image clickable PA compounds in cells (Kuerschner and Thiele, 2014; Laguerre and Schultz, 2018). From there, early success was found in lipid metabolic labeling by utilizing fatty acid (FA) analogs bearing clickable tags including azide and alkyne at the terminus of the hydrophobic acyl chain. This approach circumvents the issue of obstructing biosynthetic transformations since these tags can be conveniently hidden at the end of these long hydrocarbon chains. As a result, clickable FAs have provided tremendously important probes for tracking fatty acid metabolism (Robichaud et al., 2016; Thiele et al., 2012) and characterizing proteins that undergo posttranslational lipidation processes by using strategies including post-derivatization via click chemistry, affinity chromatography purification, and mass spectrometry (MS)-based proteomics to identify labeled proteins (Hang and Linder, 2011; Hang et al., 2011). However, many applications of lipid labeling hinge upon the selective labeling of lipid molecules containing a particular headgroup. This is challenging to accomplish using FA probes, although Thiele and co-workers have recently reported a sophisticated multiplexed MS platform for tracking lipid metabolism (Thiele et al., 2019). Alternatively, modified substrate analogs have been developed that alter the lipid head group in order to differentiate between lipid molecules. In this endeavor, it is challenging to develop clickable precursors that can successfully mimic the native substrate for incorporation into specific lipid products. This aspect of lipid metabolic labeling will be the primary focus of this review article.
Before discussing recent labeling advances, we should take a quick peak at lipid metabolic networks, for which we will focus on a simplified picture of transformations in mammalian systems as shown in Scheme 2. As is typical of metabolic networks, here we find a complex series of interconversions highlighted by two major pathways that are linked by feedback loops. The Kennedy Pathway commences with choline and culminates in phosphatidylcholine (PC) (Gibellini and Smith, 2010), while the CDP-DAG pathway converts glycerol 3-phosphate through the intermediate phosphatidic acid (PA) to cytidine diphosphate diacylglycerol (CDP-DAG) (Athenstaedt and Daum, 1999). However, these pathways are linked via the hub of PC, PA, and diacylglycerol (DAG), which are interconverted by phospholipase, phosphatase, and kinase enzymes, which have been key targets of metabolic labeling (Bumpus and Baskin, 2018). These central lipids exhibit significant differences; for example PC is the most abundant phospholipid in mammalian cells while PA and DAG are signaling lipids that are tightly maintained at low concentrations (Merida et al., 2008; Wang et al., 2006). These core pathways feed into the biosynthesis of downstream lipids including phosphatidylinositol (PI), phosphatidylglycerol (PG), and phosphatidylserine (PS). PI acts as the precursor for the phosphatidylinositol polyphosphate (PIPn) family (Best et al., 2010) as well as glycophosphatidylinositol (GPI) anchors (Orlean and Menon, 2007), the latter of which serve to anchor proteins onto the surfaces of cellular membranes. These complex metabolic networks are important to keep in mind when designing substrate analogs to target particular lipid species in order to scrutinize the different pathways by which labeled molecules will be formed and altered. Finally, it should be noted that the pathways shown in Scheme 2 are simplified and only depict transformations in mammalian cells, as lipid metabolic networks are often significantly different in varying organisms.
Scheme 2.
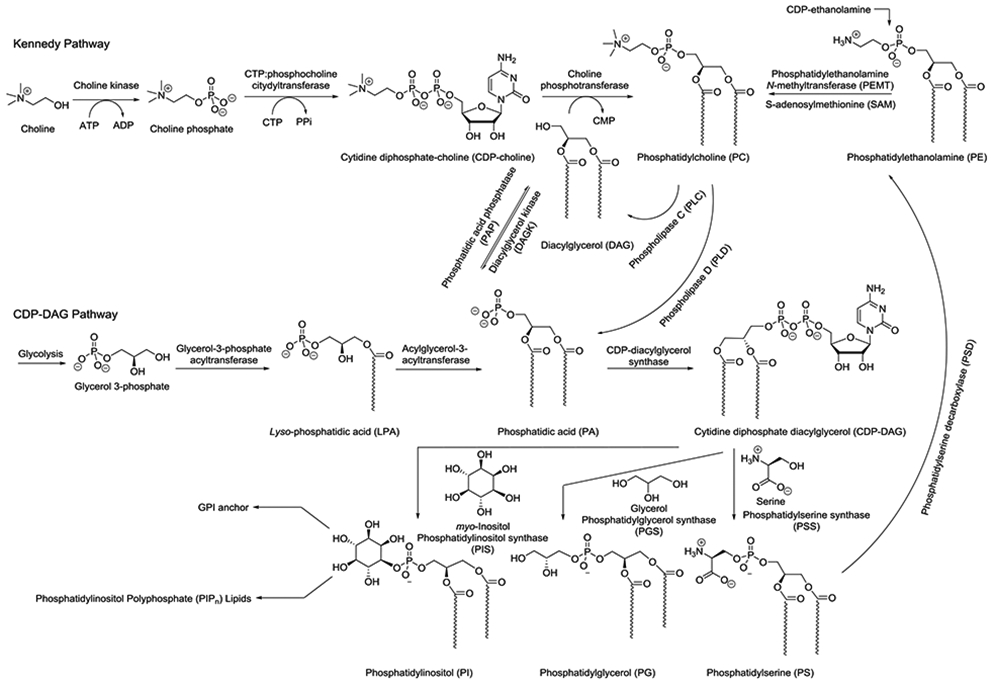
Simplified scheme for metabolic interconversions of glycerophospholipids in mammals in order to understand pathways that have been exploited for lipid metabolic labeling. Lipid tails are generalized to denote mixtures of acyl chain lengths and unsaturation.
2.1. Labeling of PC and PA Products
A pioneering effort in this field was the report of the labeling of PC by Salic and co-workers in 2009 (Jao et al., 2009). To achieve this, the native substrate choline was modified into propargylcholine (PCho) to incorporate a clickable tag into PC products (Figure 1). This modification entails the conversion of one native methyl group into the new propargyl moiety, in essence adding two additional carbons by changing one hydrogen into a CCH unit, culminating in a minor manipulation to minimize potential steric clash in the active sites of biosynthetic enzymes. Previous studies had indicated that a methyl of the choline group could be replaced with an alkyl group of up to 5 carbons to produce metabolically active substrate analogs. Metabolic PC labeling via click chemistry was confirmed to be successful through dose-dependent intensity increases in fluorescence microscopy images of NIH 3T3 cells after treatment with a clickable partner azide-tagged fluorophore. This fluorescence was abrogated by treatment with phospholipase C (PLC) that decomposes PC, but restored upon treatment with PLC and EDTA, the latter of which occupies calcium that is needed for PLC activity. Fluorescence and immuno microscopy imaging experiments also showed that labeled PC molecules matched the subcellular localization of native PC, with products primarily localized at the Golgi and ER (site of biosynthesis) as well as the plasma membrane (PM) and mitochondria (to which PC is trafficked). Additionally, tandem MS experiments were performed to investigate incorporation, and observed mass peaks showed labeled PC products resulting from PCho for which acyl chain compositions closely mirrored those of native PC. Kinetic studies indicated that staining was readily observable within 30 minutes and intensity increased up to 24 hours, with pulse chase studies indicating that the labeled PC population remained stable. Finally, imaging studies were successful in mice. This result provided an exciting initial effort in lipid metabolic labeling. Since this pioneering report, PCho has also been shown to label myelin, as was developed in work aimed at studying remyelination (Aharoni et al., 2016), as well as teichoic acid glycopolymers attached to the peptidoglycan of Streptococcus pneumoniae (Di Guilmi et al., 2017). PCho has also been used to probe phosphorylcholine-derivatized proteins (Snodgrass et al., 2015) and acts as an inhibitor of choline catabolism in bacteria (Fitzsimmons et al., 2011).
Figure 1.
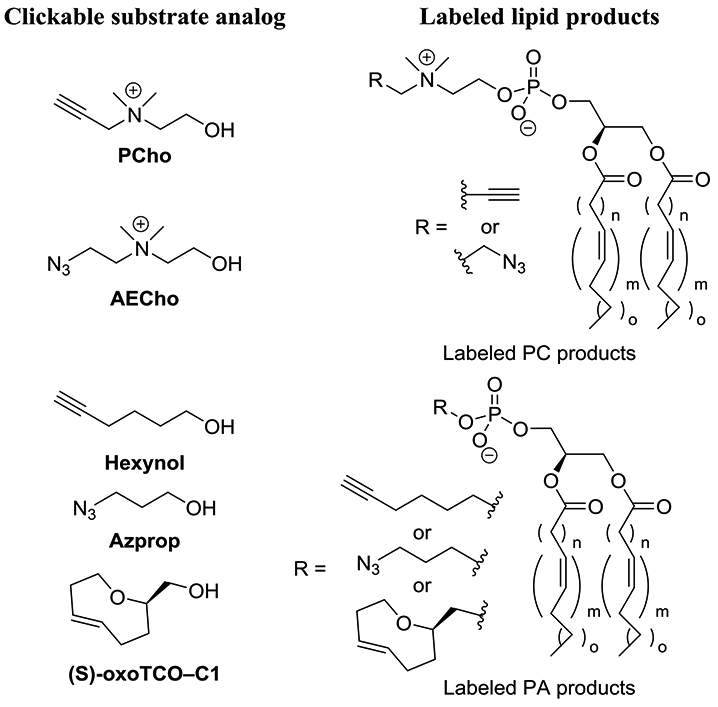
Metabolic labeling strategies for labeling PC and PA using clickable substrate analogs.
In subsequent work, a two-color labeling protocol was developed for imaging different pools of PC products in cells (Jao et al., 2015b). To do so, azido-choline substrate analogs were analyzed, and while both azidoethyl- and azidopropyl-choline were found to be effective, the former (AECho, Figure 1) was determined to be incorporated more efficiently. Impressively, almost 20% of PC lipids were found to contain an azidoethyl tag at the highest concentration and after 24 hours, even in the presence of 30 μM of natural choline. For 2-color labeling, cells treated with either PCho, AECho or both were first labeled via SPAAC to selectively derivatize and image products labeled by AECho that contain an appended azide moiety. Subsequently, products resulting from PCho were labeled by CuAAC. While these two probes showed similar localization in proof-of-concept studies, this approach opens the door to differentiating pools of PC products generated under different conditions. Duan and co-workers also utilized PCho to develop a ratiometric labeling strategy for MCF-7 cells employing an azido-cresyl violet reagent that is blue-shifted upon CuAAC (Fu et al., 2015).
While the prior effort achieved infiltration of the Kennedy pathway, Baskin and co-workers have explored the labeling of lipid products of PC hydrolysis by phospholipase D (PLD) enzymes, which are important due to their regulation of processes such as cell growth, division, and migration, with aberrant behavior leading to disease. Their initial reports took advantage of the discovery that hydrolysis of PC by PLD, which normally produces PA, can be intercepted using short chain alcohols to produce phosphodiester analogs of PA. Initially, alkynols including 6-hexynol (Hexynol) were identified as substrates for PLD, but this required post-derivatization via CuAAC, which can be problematic due to copper toxicity (Bumpus and Baskin, 2016). Therefore, in follow-up work, 3-azidobutanol (Azprop) was explored as a precursor that is amenable to SPAAC derivatization. This approach was coined IMPACT (Imaging Phospholipase D Activity with Clickable Alcohols via Transphosphatidylation) (Bumpus and Baskin, 2017). Systematic studies including fluorophore labeling and analysis via fluorescence-coupled HPLC, MS analysis of labeled products after clicking on an ammonium group to enhance ionization, and cellular fluorescence microscopy imaging revealed robust labeling of PLD products in HeLa cells. These studies took advantage of the ability to stimulate PLD activity using the small molecule phorbol 12-myristate 13-acetate (PMA) and suppress activity using the PLD inhibitor 5-fluoro-2-indolyl deschlorohalopemide (FIPI) as well as isoform-selective inhibitors. Fluorescence imaging studies in conjunction with colocalization markers also confirmed that labeled products primarily appeared in the ER (attributed to isoform-selective inhibitors to PLD1) and Golgi apparatus (arising from a mixture of PLD1 and 2). The former result was somewhat surprising, as PA synthesis in the ER has primarily been thought to result from acylation of glycerol 3-phosphate (Scheme 2). Fluorescence recovery after photobleaching (FRAP) studies indicated rapid diffusion of labeled lipids in the ER but slower diffusion in the Golgi. Finally, enhanced IMPACT labeling in a subset of puncta led to the discovery that an mCHERRY-tagged PLD1 exhibited similar localization. Overall, these initial reports proved IMPACT to be a powerful tool for characterizing PLD activity.
Baskin and co-workers have recently advanced this work in different directions. First, IMPACT has been modified to develop a real-time imaging protocol for imaging PLD activity (Liang et al., 2019). To do so, it was required to circumvent wash protocols that are typically necessary to remove unreacted fluorophores after click chemistry postderivatization protocols. This was achieved by discovering that probe (S)-oxoTCO–C1 bearing a bulky trans-5-oxocene (oxoTCO) unit was a suitable substrate for PLD enzymes that could be modified by an IEDDA reactions using a fluorogenic tetrazine reagent. Therefore, the fluorescent signal is dramatically enhanced (1,600-fold) following postlabeling, enabling real-time imaging by circumventing the need to remove unreacted dyes. This approach extended imaging of PA production beyond the ER and Golgi apparatus to enable visualization of labeled products at the plasma membrane (PM), which was attributed to the rapid time-scale of labeling. Furthermore, time-lapse movies captured trafficking of products out of the PM and into the ER and then the Golgi apparatus. Additional studies led to the conclusion that activation of PLD through the platelet-derived growth factor receptor (PDGFR) primarily impacted intracellular pools of PLD1, in contrast to activation by PMA or M1 muscarinic receptor (M1R), which acts primarily at the PM.
Additionally, the lab asked the question of whether propargylcholine probe PCho could also serve as a substrate for PLD enzymes (Bumpus et al., 2018). This effort was inspired by the potential of interrogating both PC and PA biosynthesis using a single probe. When HeLa cells were activated with PMA and treated with PCho, labeled PA products were again detected, primarily as a result of PLD1 activity based on isoform-selective inhibitor studies. This was achieved by identifying varying conditions for targeting each pathway; short (20 min) treatment and PMA stimulation to target PA labeling via PLD as opposed to long (24 h) incubation periods in the absence of PMA to produce tagged PC products.
2.1. Labeling of Inositol-containing Lipid Products
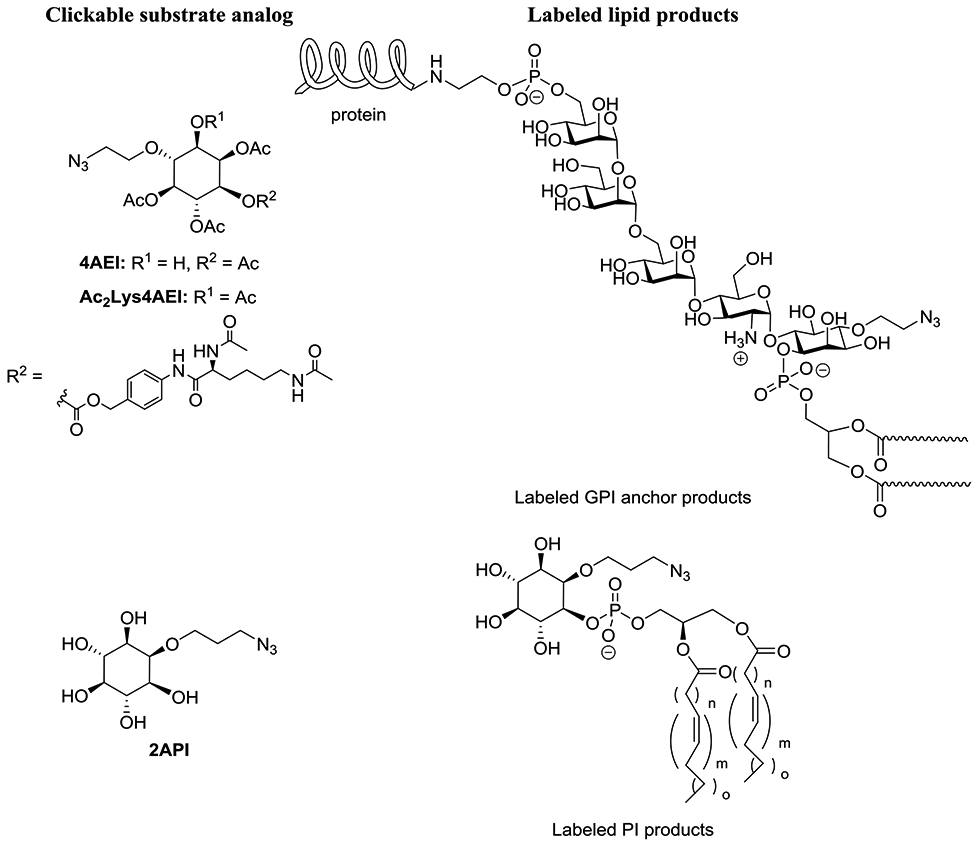
Beyond these core lipids, efforts have also recently emerged to label downstream lipid products. Much of this work has focused on myo-inositol-containing lipids due to the important signaling roles of these molecules. In pioneering work, Guo and co-workers pursued the labeling of glyophosphatidylinositol (GPI) anchors, which cause the membrane-attachment of postranslationally appended proteins (Lu et al., 2015). Doing so required the design, synthesis and study of clickable myo-inositol substrates, during which a variety of probes containing azidoethyl tags attached at the 3-, 4-, and 5-positions were pursued to avoid the 1-, 2-, and 6-positions at which myo-inositol is derivatized in GPI anchors. It should be noted that the synthesis of these particular probes is quite challenging since this requires enantioselective synthesis via the desymmetrization of myo-inositol. While initial studies indicated that these probes were not successful, probes with esterified hydroxyl groups were explored in an effort to increase hydrophobicity in order to cross membranes, after which ester moieties can be removed by intracellular esterase enzymes. Partially acylated probes, exemplified by 4AEI were found to be particularly effective for GPI labeling, as judged by fluorescence imaging and flow cytometry after CuAAC labeling as well as competitive inhibition through treatment with per-acetylated myo-inositol and treatment with phosphatidylinositol-specific phospholipase C (PI-PLC) to detach labeled proteins.
In subsequent work, the Guo group sought a modified strategy by which they could enhance selectivity for labeling of GPI anchored proteins in cancer cells (Jaiswal et al., 2020). To do so, alternate probes were developed, exemplified by Ac2Lys4AIE, in which an Nα,Nε-diacetyl-l-Lysine (Ac2Lys) moiety was linked via a self-immolating p-aminobenzyl alcohol (PABA) tether as a caging group at the 1-position of myo-inositol. This was designed such that the caging group would be removed by two enzymes, histone deacetylase (HDAC) and cathepsin L (CTSL), that are overexpressed in cancer, thereby selectively unmasking the substrate analog in diseased cells. This result was supported by flow cytometry and fluorescence imaging showing that probes of type Ac2Lys4AIE exhibited enhanced labeling of MCF-7 breast cancer cells compared to normal human fibroblast cells (IMR-90), whereas probes of type 4AEI resulted in similar labeling in both cell lines.
Our group has been interested in labeling myo-inositol-containing phospholipids including phosphatidylinositol (PI) and its downstream phosphatidylinositol polyphosphate (PIPn) products, which are critical regulators of several key biological processes (Ricks et al., 2019). In this case, we avoided modification of myo-inositol at the 3-, 4-, and 5-positions where these compounds are phosphorylated in nature to produce the seven core PIPn isomers, along with the 1-position as the site of phospholipid attachment. Of the remaining 2- and 6-positions, the former was selected since inositol-1,4,5-trisphosphate (IP3) probes modified at the 2-position have been found to effectively bind IP3 receptors (Hirata et al., 1989; Hirata et al., 1990; Ozaki et al., 1992; Watanabe et al., 1991). Therefore, probe 2API was synthesized and studied, and found to diminish conversion of radiolabeled myo-inositol to PI in a cell extract PI synthase assay. It also enabled fluorescence imaging of labeled PI products in cells supported by MS studies in which tagged PI products were detected and fluorescence-based thin-layer chromatography (TLC) experiments in which dose-dependent of new spots that correlated with commercial PI and PIPn standards were observed. In our case, probe 2API was found to be superior to peracetylated analogs. We also observed interesting cell growth results in which increasing concentrations of probe 2API enhanced cell growth, in contrast to decreased growth observed when raising myo-inositol concentrations. This could be attributed to the ability of 2API to produce important PI lipids while perhaps not repressing phospholipid biosynthesis, which natural myo-inositol is known to do. In addition to these articles, other clickable inositol probes have been reported as putative substrates for metabolic labeling of inositol-containing lipids (Ausmus et al., 2020; Pasari et al., 2015).
While beyond the scope of glycerophospholipid labeling, we will note that success has also been achieved with sphingolipid probe development. For example, Cairo and co-workers developed clickable sphingomyelin probes and showed that sphingomyelinase enzymes still accept these compounds and their products of click chemistry derivatization as substrates for hydrolysis (Sandbhor et al., 2009). Penno, Thiele and co-workers developed alkynyl-1-deoxysphingolipid analogs to detect restrictions in metabolism and links to mitochondrial dysfunction (Alecu et al., 2017). Wittman and co-workers have reported clickable analogs of glycosphingolipids containing mono- and disaccharides at the headgroup (Dauner et al., 2016). Finally, incorporation of azido-sugars into liposomes, either through lipidation or encapsulation, has also been shown to enhance metabolic labeling of carbohydrates (Shen et al., 2019).
These initial reports of lipid metabolic labeling showcase wide-ranging strategies that can be invoked to successfully label important and widely variable lipid products in order to detect and study metabolism in cells. The common theme is that careful manipulation of substrate architecture, benefitting from diminutive click tags, can enable infiltration of biosynthetic machinery to produce labeled products in cells. In certain cases enzymes can be surprisingly tolerant to substrate modifications. While these initial reports of labeling strategies have provided insights into lipid biosynthesis and trafficking, as was discussed in the prior summaries, they have also inspired numerous subsequent studies in which these techniques have been applied for a wide range of studies aimed at understanding lipid production and activity. The next section will focus on these recent applications of the lipid metabolic labeling technique.
3. Applications Involving Detection and Imaging of Labeled Lipids
The utility of lipid metabolic labeling techniques for tracking metabolism and answering biological questions was already demonstrated in the first reports in this field. Since those initial studies, demonstrations of the applicability of this approach have rapidly progressed, and the examples that will be subsequently discussed showcase the power of metabolic labeling for studying biological processes at the molecular, cellular, and organismal levels. A primary benefit of the lipid metabolic movement is its versatility, as this technique enables a plethora of studies in which wide-ranging tags can be introduced onto labeled lipid products for detection and manipulation by a variety of techniques. Labeling of PC lipids using choline probes Pcho and AECho, the first of this type to be reported, has been particularly mined for applications.
3.1. Investigations of Lipid Metabolism, Trafficking, Localization, and Protein Binding
An intrinsic benefit of lipid metabolic labeling is that it enables direct imaging of the synthesis and localization of lipid products, which is invaluable for probing the complex processes of lipid metabolism and trafficking. Multiple reports have showcased the efficacy of labeling strategies for elucidating these complex processes. Cairo and co-workers followed up on lipid metabolic labeling using PCho, AECho and a ketone- containing choline (Li et al., 2014). In doing so, they explored various live cell imaging protocols and showed that lipid dynamics could be studied via fluorescence recovery by photobleaching, and that cells could be attached onto functionalized surfaces by click chemistry. Lang, Thiele, and co-workers used PC imaging by PCho to rule out exchange of lipid content at contact sites between the ER and PM (Merklinger et al., 2016). In work that showed that lipid droplets (LDs) in nuclei of hepatocytes are derived from apiloprotein B-free luminal LDs, Fujimoto and co-workers used fluorescence imaging of PCho labeling to demonstrate that perilipin-3-V5 decreased PC synthesis, which was reversed by a modified protein (perilipin-3-NES-V5) containing an appended nuclear exclusion signal driving localization to the cytoplasm (Soltysik et al., 2019). PCho labeling has also been used to image the localization of PC biosynthesis during mitosis (Sawicki et al., 2019). This helped show that PC and FA synthesized at the mitotic exit end up in the nuclear envelope, and that FA and PC biosynthesis, but not decreases in LPC, are necessary for the cell cycle to proceed past the G2 and M phases. These results pointed to FA biosynthesis as a means for anti-cancer intervention.
Schrick and co-workers investigated fluorescence imaging of PC labeling in plants by subjecting Arabidopsis thaliana seedlings to propargylcholine probe PCho followed by CuAAC (Paper et al., 2018). They initially observed no difference between plants grown in the presence or absence of PCho. Seedlings and mature plants subjected to labeling and confocal microscopy exhibited fluorescence in all tissues examined, with especially strong signal observed in the roots and guard cells of the epidermis of leaves, respectively. This signal was abrogated by treatment with PLC, as expected due to hydrolysis and removal of the click-tagged choline headgroup. In fluorescence colocalization studies, fluorescence-tagged PC was found to be primarily excluded from the cell wall and nuclei, but present in regions including the ER, PM, and tonoplast, along with variable inclusion in mitochondria. MS studies comparing labeled to unlabeled PC also supported greater mole percent incorporation in the roots (~50%) compared to seedlings, leaves, stems, cotyledons, and siliques (~18-28%)
Recently, Griese and co-workers employed PC labeling using PCho to study the activity of ATP-binding cassette sub-family A member 3 (ABCA3) in the formation and trafficking of lipids into the lamellar body (LB), processes that are dysregulated in chronic interstitial lung diseases (Li et al., 2019b). This labeling protocol was first validated for the fluorescence imaging of PC lipid products in ABCA3+ vesicles, with all chosen vesicles labeled after 24 hours. Next, transport of tagged PC products was found to be ATP-dependent based on the decrease in fluorescence signal upon treatment with the inhibitor orthovanadate. Experiments performed in the presence of choline kinase inhibitors MN58B and miltefosine (MLF) indicated that PC synthesis took place in the first 12 hours of experiments in which ABCA3+ vesicle labeling was reduced, but this was restored when inhibitors were introduced after that time. Additionally, two mutants of ABCA3 known to correlate with neonatal surfactant deficiency were found to result in smaller ABCA3+ vesicles than wild type, although the time curves for lipid synthesis and transportation were found to be similar. This report highlights the utility of lipid metabolic labeling for elucidating the details of disease-related defects in protein machinery that controls lipid synthesis and transport.
In an intriguing example, Yao, Wenk and co-workers applied lipid metabolic labeling to aid in the global identification of lipid-binding proteins (Wang et al., 2017). A recent strategy to this end has entailed the development of bifunctional lipid probes containing both a cross-linking group by which the probe becomes attached to bound proteins as well as a secondary tag that enables their enrichment and identification (Best, 2014; Best et al., 2011; Laguerre and Schultz, 2018). A common concern with this approach is that the probe itself could enter metabolism to generate modified probes that correspond to different lipids. These modified probes could then label proteins that are not bound by the original probe target, leading to false positives. This article reported an ingenious solution to this issue by exploiting metabolic labeling using PCho to introduce the clickable alkyne tag for post-derivatization (Scheme 3). Therefore, while downstream probe analogs could still be produced and label proteins, the clickable tag used for postderivatization would only be present for PC probe products resulting from metabolic labeling. Using this approach, FAs containing diazirine crosslinking groups were treated alongside PCho to label and identify a number of PC-binding proteins.
Scheme 3.
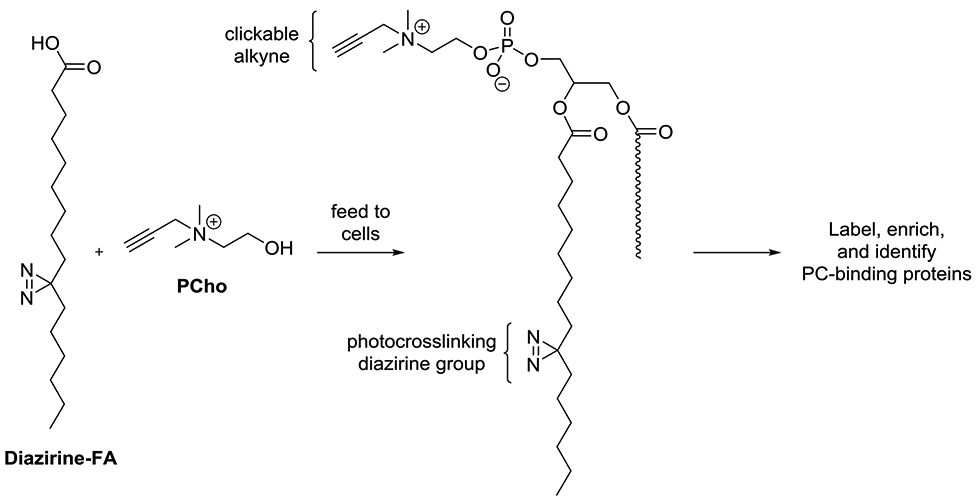
Approach using metabolic labeling to produce specific bifunctional PC probes for the global identification of PC-binding proteins. Note: the diazirine-containing acyl chain could be incorporated at different positions in labeled products.
Liu and co-workers exploited PC lipid metabolic labeling as a tool for imaging the production and cell entry of exosomes, which are important mediators of communication and transport between cells. This was performed through both PCho derivatization of PC (Zhang et al., 2018b) as well as PLD-mediated Hexynol (Jiang et al., 2020) labeling of PA. To do so, exosomes of larger sizes derived from breast cancer cell lines were enriched by centrifugation followed by fluorescence labeling. In the former study, exosomes produced by different cell lines were found to exhibit distinct variations in uptake in different organs. In the latter study, comparison of PA metabolic labeling with Dil staining showed that fluorophores that were not covalently appended to lipids were successfully removed by washes, prior to which the two fluorescence signals were colocalized in exosomes. Overall, transmission electron microscopy (TEM), dynamic light scattering (DLS), Western blot, and cell migration studies showed that labeling did not affect exosome morphology, size, zeta potential, protein inclusion in exosomes, cell scratch healing efficacy, and promotion of cell proliferation. Studies also showed this approach to be superior to Dil dye incorporation in terms of the stability of the fluorescence signal over time. Finally, systematic fluorescence microscopy studies enabled a series of insights regarding EV entry into RAW264.7 cells, including that this occurs in a time-dependent manner (beginning at ~1 hour and ending after ~ 8 hours) and is driven by interactions with filopodia and subsequently the PM. Temperature-dependence was also a sign of active endocytosis events, and a panel of selective inhibitors indicated that EV cell entry was primarily driven by micropinocytosis and phagocytosis.
3.2. Lipid Metabolic Labeling for Viral Studies
A prominent family of targets has involved viruses, either by labeling of viral particles themselves or by elucidating changes in lipid metabolism in host cells caused by viral infection. Xie and co-workers have reported several strategies showcasing the ability to label lipids present within materials ranging from viruses to nanoparticles for drug delivery, efforts that have been recently reviewed (Huang et al., 2020). For the former, attention was turned to metabolic production of azide-tagged PC in host cells using AECho for labeling by SPAAC. PC was selected since it is the most abundant lipid in mammalian membranes and is enriched in the outer PM, providing suitable presentation of the tag. This approach was used to label vaccinia virus (VACV) membranes by exploiting the phenomenon of host-derived viral membranes (Huang et al., 2013). The resulting viral particles showed similar titers to unlabeled control virus and induced the cytopathic effect. This approach was combined with viral nucleic acid imaging to achieve two-color labeling of the virus, which enabled single particle tracking. Systematic studies showed that VACV Tiantan strain entered vero cells through pinocytosis rather than membrane fusion, which was dependent on ATP and actin but not on caveolin. Wang and co-workers showed that the measles virus could be decorated with quantum dots by SPAAC following PCho treatment (Zhao et al., 2016). Finally, Cai and co-workers showed this approach to be effective for the labeling and imaging of H5N1 pseudotype virus in a manner that preserves virus infectivity and immunogenicity (Pan et al., 2017).
Mota and co-workers studied PCho labeling in malaria and found that Plasmodia hijacked host enzymes to enhance the biosynthesis of PC as well as neutral lipids. The upregulated PC products were found to be taken up by the parasites and localized to parasite PMs, parasitophorous vacuole membrane, and exo-erythrocytic forms, which were determined to be vital for parasite survival (Itoe et al., 2014). Wang and co-workers used PCho labeling to show that all positive-strand RNA viruses, including poliovirus, hepatitis C virus, and brome mosaic virus, exploit host enzymes that synthesize PC to support viral replication (Zhang et al., 2016). In addition, it has been demonstrated that the enzyme CTP:phosphocholine cytidylyltransferaseα (CCTα) translocates from the nucleus to the cytoplasm upon infection of poliovirus, which results in conversion of neutral lipids from lipid droplets (LDs) into phospholipids (Viktorova et al., 2018). In doing so, Belov and co-workers used PCho to demonstrate that inhibition of LD-associated lipases by diethylumbelliferyl phosphate (DEUP) severely inhibited phospholipid synthesis infected cells, while inhibitors of other phospholipid biosynthesis pathways did not.
3.3. Applications of Lipid Metabolic Labeling to Cancer Imaging and Study
Due to the important roles that lipids play in cell growth and proliferation, applications of lipid metabolic labeling to image and study cancer onset have emerged, as have potential avenues for therapeutic intervention. Nitin and co-workers have applied PCho labeling for a series of studies aimed at cancer imaging and treatment. For example, this technique has been used to develop an optical molecular imaging approach to visualize changes associated with oral neoplasia. Here it was found that cell mean fluorescence intensities resulting from PCho fluorescence labeling of neoplastic tissues were 4-5-fold greater than normal samples (Luo et al., 2013). This labeling protocol was also used to determine the effects of different anti-cancer drugs on choline metabolism, and indicated that the inhibitors cisplatin (Luo et al., 2012), gefitinib, and carboplatin (Luo et al., 2016) were detectable based on decreased PCho uptake. The latter cases showcased decreased labeling in drug-sensitive cell lines while not in drug-resistant cell lines. The probe AECho has also been exploited to radiolabel PC lipids through SPAAC using 99mTc chelates (Chen and Chu, 2016). As will be discussed in the next section, the Xie group has been interested in developing clickable nanoparticles for drug delivery using lipid metabolic labeling strategies. Recently, they exploited the enhanced presentation of labeled PC products resulting from AECho treatment on cancer cell surfaces for the targeting of DBCO-containing nanoparticles (Lu et al., 2020). This report showcases exciting prospects for the use of metabolic labeling techniques for targeted drug delivery.
3.4. Studies involving Nanoparticle Drug Delivery Platforms
The Xie group has also been interested in using this technique to engineer nanoparticle surfaces using natural or metabolically labeled lipids for cell targeting in biomedical delivery applications. This approach was initially used to modify magnetic nanoclusters (MNCs) that were camouflaged with cancer cell membranes to develop immunomagnetosomes (IMSs) (Xiong et al., 2016). This strategy was applied to detect circulating tumor cells (CTC) by decorating IMSs with membranes containing metabolically labeled azido-PC followed by SPAAC to introduce the antibody of epithelial cell adhesion molecule (EpCAM) as a CTC biomarker. This enabled capture of ~90% of CTCs from blood in 15 minutes. This was later enhanced by switching to platelet-leukocyte membranes (Rao et al., 2018) and through detection using a microfluidic system (Zhang et al., 2019b).
Similar nanoparticles camouflaged with clickable PC have been applied for different goals, highlighting the versatility of this strategy. Adsorption of siRNA onto MNCs followed by decoration of azide-tagged PC with RGD peptides enabled a highly effective gene delivery platform that delivers siRNA directly into the cytoplasm (Zhang et al., 2018a). Decoration of MNCs with T-cell stimulating peptides produced artificial antigen-presenting cells (aAPCs) that were highly effective for stimulating the production of CD8+ T cells (Nie et al., 2019; Zhang et al., 2017). MNCs were also developed to trigger ferroptosis in cancer cells by decorating the membrane with PD-1 antibody while incorporating the hydrophobic TGF-b inhibitor Pa, generating a macrophage-mediated immune response (Zhang et al., 2019a). Finally, an approach to a cancer vaccine was developed by appending anti-CD205 antibodies to MNPs onto which the Toll-like receptor CpG-ODN was adsorbed. The former enhanced recognition of CD8+ cells, while the latter was used to activate an immune response (Zhu et al., 2017).
3.5. Lipid Metabolic Labeling in Conjunction with Raman Spectroscopy Techniques
Another tool for lipid detection that has been enhanced through lipid metabolic labeling is the technique of stimulated Raman scattering (SRS), which enables high-sensitivity detection of biomolecules such as lipids without requiring bulky dyes to be appended (Hu et al., 2019; Liu et al., 2020; Wei et al., 2016). Expanding beyond the use of traditional isotope labels (Matthaus et al., 2012; Shi et al., 2018), the alkyne moiety in particular is beneficial since it produces Raman peaks that correlate with silent regions of signals produced by cells, further enhancing resolution. Min and co-workers pursued lipid imaging in reports that also included analysis of labeled nucleic acids and proteins (Hu et al., 2016; Wei et al., 2014). Here, propargylcholine probe PCho enabled the labeling of PC lipids in cellular membranes of hippocampal neurons while treatment of C. elegans with tagged fatty acid 17-octadecynoic acid was found to primarily result in incorporation into LDs in the form of triglycerides. Additionally, Raman studies of PCho (Zhang and Min, 2017) and alkyne-tagged fatty acid probes (Hong et al., 2014; Li et al., 2019a) have been used to investigate lipid metabolism and cholesterol probes have been used to assess cholesterol storage (Lee et al., 2015). Lipid metabolic labeling has also been detected using the technique of surface-enhanced Raman scattering (SERS) (Xiao et al., 2014).
3.6. Electron Microscopy Efforts Exploiting Labeled Lipids
Lipid metabolic labeling in conjunction with click chemistry derivatization has also recently emerged as a powerful tool for conducting electron microscopy imaging experiments. Fujimoto, Salic, and co-workers developed a technique of this type to analyze the distribution of PC lipids in the inner and outer leaflets of different membranes using a freeze-fracture protocol employing PCho (Iyoshi et al., 2014). Following metabolic labeling of PC, CuAAC was used to introduce biotin, which was then detected using antibiotin primary antibodies followed by colloidal gold-tagged secondary antibodies. This approach facilitated imaging of membranes at a resolution in which membrane leaflets could be differentiated. This enabled the authors to observe surprising results; budding yeast Golgi and PM showed asymmetry with PC exhibiting cytoplasmic leaflet dominance, while other organelles were found to be evenly distributed. In mammalian cells, the PM instead showed symmetric distributions. Lipid subcellular localization is critical for controlling lipid activity and membrane properties, and these results provide important insights into these phenomena at the molecular level as well as insights into variations between organisms.
EM has also been enhanced for lipids and other molecules by using click chemistrybased post-derivatization to introduce tags that catalyze the photooxidation-based polymerization of diaminobenzene (DAB) for detection purposes (Ngo et al., 2016). This has been performed through PC labeling using AECho followed by SPAAC to introduce IRDye700X that acts as an antenna to catalyze this process. This enabled imaging of intracellular membranes, and particularly the ER membrane due to its high PC content. This technique also enabled visualization of detailed features such as ER-mitochondrial contacts and mitochondrial folds.
4.0. Conclusions
This review article showcases the vast possibilities for biological studies that are enabled by lipid metabolic labeling. Despite this, it is clear that lipid studies of this type have lagged behind the metabolic labeling of other molecules. Of the pathways shown in Scheme 1, successful labeling strategies have only been reported for a handful of these molecules. One might surmise that this coincides with the woeful history of the lipid molecule as being the biomolecule that is most commonly overlooked. However, upon closer inspection, intrinsic challenges of lipid metabolic labeling strategies emerge. First off, the fact that lipid molecules are interconverted among one another through a web of transformations leads to problems when one attempts to label one particular lipid. For example, in our investigation of PI labeling using 2API, we obtained evidence suggesting the additional labeling of downstream PIPn molecules. This makes sense since the myo-inositol headgroup is retained among these molecules, but as a result it is not clear how one would selectively target one particular inositol lipid family member and not the others. Another concern is the simplicity of the substrates that are used to introduce the headgroups, such as glycerol, ethanolamine, and serine, which leads to concern about how these diminutive molecules can be modified to produce viable analogs for metabolic labeling. Nevertheless, the recent successes in labeling multiple lipids promotes enthusiasm that that these strategies can work in the context of lipids. In particular, the successful metabolic labeling of PA, which is formed as a result of the nucleophilic attack of water, provides confidence that lipid labeling can be tackled with some ingenuity.
Concerns about the challenges associated with developing successful lipid labeling strategies are mitigated by the vast array of applications that we now see are enabled by this technology. Since the labeling of PC lipid products using clickable tags was the first approach to be reported by Salic and co-workers, this technique has inspired the greatest amount of subsequent work thus far. In the slightly more than 10 years since that protocol was initially reported, it has been applied by a number of labs for myriad studies including: elucidation of lipid metabolism, trafficking, subcellular localization, and identification of protein-lipid binding interactions, exosome tracking, detection and investigation of the mechanism of viral particles, cancer imaging, study of disease onset, characterization of inhibitor activity, and tracking and targeting of nanoparticle-based drug delivery platforms. These varied studies showcase the immense versatility of this approach. In addition, lipids labeled by these strategies have been detected and investigated using a range of techniques including fluorescence spectroscopy and microscopy, mass spectrometry, chromatography, different Raman spectroscopy techniques, and electron microscopy. All of this work points to a bright future for lipid metabolic labeling that will be further enhanced by the development of strategies for labeling other glycerophospholipid molecules based on their head groups.
Figure 2.
Strategies for metabolic labeling of myo-inositol-containing phospholipids.
Acknowledgments
This material is based upon work supported by the National Institutes of Health under grant number 1R15GM120705-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Agard NJ, Prescher JA, and Bertozzi CR 2004. A strain-promoted 3+2 azide-alkyne cycloaddition for covalent modification of blomolecules in living systems. J. Am. Chem. Soc 126, 15046–15047. [DOI] [PubMed] [Google Scholar]
- Aharoni R, Rosen C, Shezen E, Bar-Lev DD, Golani O, Reisner Y, Sela M, and Arnon R 2016. Assessing remyelination - metabolic labeling of myelin in an animal model of multiple sclerosis. J. Neuroimmunol 301, 7–11. [DOI] [PubMed] [Google Scholar]
- Alecu I, Tedeschi A, Behler N, Wunderling K, Lamberz C, Lauterbach MAR, Gaebler A, Ernst D, Van Veldhoven PP, Al-Amoudi A, et al. 2017. Localization of 1-deoxysphingolipids to mitochondria induces mitochondrial dysfunction. J. Lipid Res 58, 42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athenstaedt K, and Daum G 1999. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem 266, 1–16. [DOI] [PubMed] [Google Scholar]
- Ausmus AP, Hogue M, Snyder JL, Rundell SR, Bednarz KM, Banahene N, and Swarts BM 2020. Ferrier Carbocyclization-Mediated Synthesis of Enantiopure Azido Inositol Analogues. J. Org. Chem 85, 3182–3191. [DOI] [PubMed] [Google Scholar]
- Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, and Bertozzi CR 2007. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U.S.A 104, 16793–16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry KAZ, Hankin JA, Barkley RM, Spraggins JM, Caprioli RM, and Murphy RC 2011. MALDI Imaging of Lipid Biochemistry in Tissues by Mass Spectrometry. Chem. Rev 111, 6491–6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MD 2009. Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Biochemistry 48, 6571–6584. [DOI] [PubMed] [Google Scholar]
- Best MD 2014. Global approaches for the elucidation of phosphoinositide-binding proteins. Chem. Phys. Lipids 182, 19–28. [DOI] [PubMed] [Google Scholar]
- Best MD, Rowland MM, and Bostic HE 2011. Exploiting Bioorthogonal Chemistry to Elucidate Protein-Lipid Binding Interactions and Other Biological Roles of Phospholipids. Acc. Chem. Res 44, 686–698. [DOI] [PubMed] [Google Scholar]
- Best MD, Zhang HL, and Prestwich GD 2010. Inositol polyphosphates, diphosphoinositol polyphosphates and phosphatidylinositol polyphosphate lipids: Structure, synthesis, and development of probes for studying biological activity. Nat. Prod. Reports 27, 1403–1430. [DOI] [PubMed] [Google Scholar]
- Bolte S, and Cordelieres FP 2006. A guided tour into subcellular colocalization analysis in light microscopy. J. Microscopy 224, 213–232. [DOI] [PubMed] [Google Scholar]
- Bowman AP, Heeren RMA, and Ellis SR 2019. Advances in mass spectrometry imaging enabling observation of localised lipid biochemistry within tissues. Trends Anal. Chem 120. [Google Scholar]
- Bruntz RC, Lindsley CW, and Brown HA 2014. Phospholipase D signaling pathways and phosphatidic acid as therapeutic targets in cancer. Pharmacol. Rev 66, 1033–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus TW, and Baskin JM 2016. A Chemoenzymatic Strategy for Imaging Cellular Phosphatidic Acid Synthesis. Angew. Chem., Int. Edit 55, 13155–13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus TW, and Baskin JM 2017. Clickable Substrate Mimics Enable Imaging of Phospholipase D Activity. ACS Cent. Sci 3, 1070–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus TW, and Baskin JM 2018. Greasing the Wheels of Lipid Biology with Chemical Tools. Trends Biochem. Sci 43, 970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus TW, Liang FJ, and Baskin JM 2018. Ex Uno Plura: Differential Labeling of Phospholipid Biosynthetic Pathways with a Single Bioorthogonal Alcohol. Biochemistry 57, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, and Mak TW 2011. Regulation of cancer cell metabolism. Nat. Rev. Cancer 11, 85–95. [DOI] [PubMed] [Google Scholar]
- Chang PV, Chen X, Smyrniotis C, Xenakis A, Hu TS, Bertozzi CR, and Wu P 2009. Metabolic Labeling of Sialic Acids in Living Animals with Alkynyl Sugars. Angew. Chem. Int. Edit 48, 4030–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QW, and Chu TW 2016. A two-step strategy to radiolabel choline phospholipids with Tc-99m in S180 cell membranes via strain-promoted cyclooctyne-azide cycloaddition reaction. Bioorg. Med. Chem. Lett 26, 5472–5475. [DOI] [PubMed] [Google Scholar]
- Cho W, and Stahelin RV 2005. Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct 34, 119–151. [DOI] [PubMed] [Google Scholar]
- Dauner M, Batroff E, Bachmann V, Hauck CR, and Wittmann V 2016. Synthetic Glycosphingolipids for Live-Cell Labeling. Bioconjugate Chem. 27, 1624–1637. [DOI] [PubMed] [Google Scholar]
- Devaraj NK 2018. The Future of Bioorthogonal Chemistry. ACS Cent. Sci 4, 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guilmi AM, Bonnet J, Peissert S, Durmort C, Gallet B, Vernet T, Gisch N, and Wong YS 2017. Specific and spatial labeling of choline-containing teichoic acids in Streptococcus pneumoniae by click chemistry. Chem. Commun 53, 10572–10575. [DOI] [PubMed] [Google Scholar]
- Ecker J, and Liebisch G 2014. Application of stable isotopes to investigate the metabolism of fatty acids, glycerophospholipid and sphingolipid species. Prog. Lipid Res 54, 14–31. [DOI] [PubMed] [Google Scholar]
- El-Sagheer AH, and Brown T 2010. Click chemistry with DNA. Chem. Soc. Rev 39, 1388–1405. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons LF, Flemer S, Wurthmann AS, Deker PB, Sarkar IN, and Wargo MJ 2011. Small-Molecule Inhibition of Choline Catabolism in Pseudomonas aeruginosa and Other Aerobic Choline-Catabolizing Bacteria. Appl. Environ. Microbiol 77, 4383–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HX, Li YR, Sun LB, He P, and Duan XR 2015. Ratiometric Fluorescence Azide-Alkyne Cycloaddition for Live Mammalian Cell Imaging. Anal. Chem 87, 11332–11336. [DOI] [PubMed] [Google Scholar]
- Gibellini F, and Smith TK 2010. The Kennedy Pathway-De Novo Synthesis of Phosphatidylethanolamine and Phosphatidylcholine. IUBMB Life 62, 414–428. [DOI] [PubMed] [Google Scholar]
- Gierlich J, Burley GA, Gramlich PME, Hammond DM, and Carell T 2006. Click chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA. Org. Lett 8, 3639–3642. [DOI] [PubMed] [Google Scholar]
- Gramlich PME, Wirges CT, Manetto A, and Carell T 2008. Postsynthetic DNA Modification through the Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction. Angew. Chem., Int. Edit 47, 8350–8358. [DOI] [PubMed] [Google Scholar]
- Griner EM, and Kazanietz MG 2007. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 7, 281–294. [DOI] [PubMed] [Google Scholar]
- Hang HC, and Linder ME 2011. Exploring Protein Lipidation with Chemical Biology. Chem. Rev 111, 6341–6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang HC, Wilson JP, and Charron G 2011. Bioorthogonal Chemical Reporters for Analyzing Protein Lipidation and Lipid Trafficking. Acc. Chem. Res 44, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein JE, and Fokin VV 2010. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev 39, 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M, Watanabe Y, Ishimatsu T, Ikebe T, Kimura Y, Yamaguchi K, Ozaki S, and Koga T 1989. Synthetic inositol trisphosphate analogs and their effects on phosphatase, kinase, and the release of Ca-2+. J. Biol. Chem 264, 20303–20308. [PubMed] [Google Scholar]
- Hirata M, Yanaga F, Koga T, Ogasawara T, Watanabe Y, and Ozaki S 1990. Stereospecific recognition of inositol 1,4,5-trisphosphate analogs by the phosphatase, kinase, and binding-proteins. J Biol. Chem 265, 8404–8407. [PubMed] [Google Scholar]
- Hong SL, Chen T, Zhu YT, Li A, Huang YY, and Chen X 2014. Live-Cell Stimulated Raman Scattering Imaging of Alkyne-Tagged Biomolecules. Angew. Chem., Int. Edit 53, 5827–5831. [DOI] [PubMed] [Google Scholar]
- Hu FH, Lamprecht MR, Wei L, Morrison B, and Min W 2016. Bioorthogonal chemical imaging of metabolic activities in live mammalian hippocampal tissues with stimulated Raman scattering. Sci. Rep 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FH, Shi LX, and Min W 2019. Biological imaging of chemical bonds by stimulated Raman scattering microscopy. Nat. Meth 16, 830–842. [DOI] [PubMed] [Google Scholar]
- Huang LL, Lu GH, Hao J, Wang HZ, Yin DL, and Xie HY 2013. Enveloped Virus Labeling via Both Intrinsic Biosynthesis and Metabolic Incorporation of Phospholipids in Host Cells. Anal. Chem 85, 5263–5270. [DOI] [PubMed] [Google Scholar]
- Huang LL, Nie WD, Zhang JF, and Xie HY 2020. Cell-Membrane-Based Biomimetic Systems with Bioorthogonal Functionalities. Acc. Chem. Res 53, 276–287. [DOI] [PubMed] [Google Scholar]
- Itoe MA, Sampaio JL, Cabal GG, Real E, Zuzarte-Luis V, March S, Bhatia SN, Frischknecht F, Thiele C, Shevchenko A, et al. 2014. Host Cell Phosphatidylcholine Is a Key Mediator of Malaria Parasite Survival during Liver Stage Infection. Cell Host Microbe 16, 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoshi S, Cheng JL, Tatematsu T, Takatori S, Taki M, Yamamoto Y, Salic A, and Fujimoto T 2014. Asymmetrical Distribution of Choline Phospholipids Revealed by Click Chemistry and Freeze-Fracture Electron Microscopy. ACS Chem. Biol 9, 2217–2222. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Zhu SY, Jiang WJ, and Guo ZW 2020. Synthesis and evaluation of N-alpha,N-epsilondiacetyl-l-lysine-inositol conjugates as cancer-selective probes for metabolic engineering of GPIs and GPI-anchored proteins. Org. Biomol. Chem 18, 2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao CY, Nedelcu D, Lopez LV, Samarakoon TN, Welti R, and Salic A 2015a. Bioorthogonal Probes for Imaging Sterols in Cells. ChemBioChem 16, 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao CY, Roth M, Welti R, and Salic A 2009. Metabolic labeling and direct imaging of choline phospholipids in vivo. Proc. Natl. Acad. Sci. U.S.A 106, 15332–15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao CY, Roth M, Welti R, and Salic A 2015b. Biosynthetic Labeling and Two-Color Imaging of Phospholipids in Cells. ChemBioChem 16, 472–476. [DOI] [PubMed] [Google Scholar]
- Jewett JC, and Bertozzi CR 2010. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev 39, 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang L, Zhang PJ, Liu XH, Di HX, Yang J, Liu SL, Pang DW, and Liu DB 2020. Chemoenzymatic labeling of extracellular vesicles for visualizing their cellular internalization in real time. Anal. Chem 92, 2103–2111. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Lu YY, Van Deventer JA, and Tirrell DA 2010. Residue-specific incorporation of noncanonical amino acids into proteins: recent developments and applications. Curr. Opin. Chem. Biol 14, 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, and Waterfield MD 2001. Cellular function of phosphoinositide 3-kinases: Implications for development, immunity, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol 17, 615–675. [DOI] [PubMed] [Google Scholar]
- Kiick KL, Saxon E, Tirrell DA, and Bertozzi CR 2002. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl. Acad. Sci. U.S.A 99, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn M, and Breinbauer R 2004. The Staudinger ligation - A gift to chemical biology'. Angew. Chem., Int. Edit 43, 3106–3116. [DOI] [PubMed] [Google Scholar]
- Kolb HC, Finn MG, and Sharpless KB 2001. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem., Int. Edit 40, 2004–2005. [DOI] [PubMed] [Google Scholar]
- Kuerschner L, and Thiele C 2014. Multiple bonds for the lipid interest. Biochim. Biophys. Acta 1841, 1031–1037. [DOI] [PubMed] [Google Scholar]
- Laguerre A, and Schultz C 2018. Novel lipid tools and probes for biological investigations. Curr. Opin. Cell Biol 53, 97–104. [DOI] [PubMed] [Google Scholar]
- Laughlin ST, and Bertozzi CR 2007. Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via Staudinger ligation. Nat. Protocols 2, 2930–2944. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Zhang WD, Zhang DL, Yang Y, Liu B, Barker EL, Buhman KK, Slipchenko LV, Dai MJ, and Cheng JX 2015. Assessing Cholesterol Storage in Live Cells and C-elegans by Stimulated Raman Scattering Imaging of Phenyl-Diyne Cholesterol. Sci. Rep 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA 2008. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol 9, 99–111. [DOI] [PubMed] [Google Scholar]
- Li CS, Key JA, Jia F, Dandapat A, Hur S, and Cairo CW 2014. Practical Labeling Methodology for Choline-Derived Lipids and Applications in Live Cell Fluorescence Imaging. Photochem. Photobiol 90, 686–695. [DOI] [PubMed] [Google Scholar]
- Li XS, Li Y, Jiang MJ, Wu WJ, He SC, Chen CP, Qin ZY, Tang BZ, Mak HY, and Qu JAY 2019a. Quantitative Imaging of Lipid Synthesis and Lipolysis Dynamics in Caenorhabditis elegans by Stimulated Raman Scattering Microscopy. Anal. Chem 91, 2279–2287. [DOI] [PubMed] [Google Scholar]
- Li Y, Kinting S, Hoppner S, Forstner ME, Uhl O, Koletzko B, and Griese M 2019b. Metabolic labelling of choline phospholipids probes ABCA3 transport in lamellar bodies. Biochim. Biophys. Acta 1864, 10. [DOI] [PubMed] [Google Scholar]
- Liang DJ, Wu KN, Tei R, Bumpus TW, Ye J, and Baskin JM 2019. A real-time, click chemistry imaging approach reveals stimulus-specific subcellular locations of phospholipase D activity. Proc. Natl. Acad. Sci. U.S.A 116, 15453–15462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, and Simons K 2010. Lipid Rafts As a Membrane-Organizing Principle. Science 327, 46–50. [DOI] [PubMed] [Google Scholar]
- Liu CC, and Schultz PG (2010). Adding New Chemistries to the Genetic Code. In Annu. Rev. Biochem, Kornberg RD, Raetz CRH, Rothman JE, and Thorner JW, eds., pp. 413–444. [DOI] [PubMed] [Google Scholar]
- Liu XH, Liu XM, Rong PF, and Liu DB 2020. Recent advances in background-free Raman scattering for bioanalysis. Trends Anal. Chem 123, 14. [Google Scholar]
- Lu GH, Zuo LP, Zhang JF, Zhu HS, Zhuang WR, Wei W, and Xie HY 2020. Two-step tumor-targeting therapy via integrating metabolic lipid-engineering with in situ click chemistry. Biomater. Sci 8, 2283–2288. [DOI] [PubMed] [Google Scholar]
- Lu LL, Gao J, and Guo ZW 2015. Labeling Cell Surface GPIs and GPI-Anchored Proteins through Metabolic Engineering with Artificial Inositol Derivatives. Angew. Chem., Int. Edit 54, 9679–9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Loja M, Farwell DG, Luu QC, Donald PJ, Amott D, Gandour-Edwards R, and Nitin N 2013. High-resolution optical molecular imaging of changes in choline metabolism in oral neoplasia. Transl. Oncol 6, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Samadzadeh KM, and Nitin N 2016. Rapid assessment of drug resistance of cancer cells to gefitinib and carboplatin using optical imaging. Anal. Biochem 504, 50–58. [DOI] [PubMed] [Google Scholar]
- Luo Z, Tikekar RV, Samadzadeh KM, and Nitin N 2012. Optical molecular imaging approach for rapid assessment of response of individual cancer cells to chemotherapy. J. Biomed. Optics 17, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaus C, Krafft C, Dietzek B, Brehm BR, Lorkowski S, and Popp J 2012. Noninvasive Imaging of Intracellular Lipid Metabolism in Macrophages by Raman Microscopy in Combination with Stable Isotopic Labeling. Anal. Chem 84, 8549–8556. [DOI] [PubMed] [Google Scholar]
- Mazhab-Jafari JT, C.B. M, Smith MJ, Gasmi-Seabrook GM, Stathopoulos PB, Inagaki F, Kay LE, Neel BG, and Ikura M 2015. Oncogenic and RASopathy-associated K-RAS mutations relieve membranedependent occlusion of the effector-binding site. Proc. Natl. Acad. Sci. U. S. A 112, 6625–6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldal M, and Tornoe CW 2008. Cu-catalyzed azide-alkyne cycloaddition. Chem. Rev 108, 2952–3015. [DOI] [PubMed] [Google Scholar]
- Merida I, Avila-Flores A, and Merino E 2008. Diacylglycerol kinases: at the hub of cell signalling. Biochem. J 409, 1–18. [DOI] [PubMed] [Google Scholar]
- Merklinger E, Schloetel JG, Spitta L, Thiele C, and Lang T 2016. No Evidence for Spontaneous Lipid Transfer at ER-PM Membrane Contact Sites. J. Membrane Biol 249, 41–56. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Hankin JA, and Barkley RM 2009. Imaging of lipid species by MALDI mass spectrometry. J. Lipid Res 50, S317–S322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo JT, Adams SR, Deerinck TJ, Boassa D, Rodriguez-Rivera F, Palida SF, Bertozzi CR, Ellisman MH, and Tsien RY 2016. Click-EM for imaging metabolically tagged nonprotein biomolecules. Nat. Chem. Biol 12, 459–U128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo JT, and Tirrell DA 2011. Noncanonical Amino Acids in the Interrogation of Cellular Protein Synthesis. Acc. Chem. Res 44, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie WD, Wei W, Zuo LP, Lv CL, Zhang F, Lu GH, Li F, Wu GH, Huang LL, Xi XB, et al. 2019. Magnetic nanoclusters armed with responsive PD-1 antibody synergistically improved adoptive T-cell therapy for solid tumors. ACS Nano 13, 1469–1478. [DOI] [PubMed] [Google Scholar]
- Nilsson BL, Kiessling LL, and Raines RT 2000. Staudinger ligation: A peptide from a thioester and azide. Org. Lett 2, 1939–1941. [DOI] [PubMed] [Google Scholar]
- Oliveira BL, Guo Z, and Bernardes GJL 2017. Inverse electron demand Diels-Alder reactions in chemical biology. Chem. Soc. Rev 46, 4895–4950. [DOI] [PubMed] [Google Scholar]
- Orlean P, and Menon AK 2007. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J. Lipid Res 48, 993–1011. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Watanabe Y, Ogasawara T, Hirata M, and Kanematsu T 1992. Synthesis and biological properties of 2-substituted myo-inositol 1,4,5-trisphosphate analogs directed toward affinitychromatography and photoaffinity-labeling. Carbohydr. Res 234, 189–206. [DOI] [PubMed] [Google Scholar]
- Pan H, Li WJ, Yao XJ, Wu YY, Liu LL, He HM, Zhang RL, Ma YF, and Cai LT 2017. In Situ Bioorthogonal Metabolic Labeling for Fluorescence Imaging of Virus Infection In Vivo. Small 13, 8. [DOI] [PubMed] [Google Scholar]
- Paper JM, Mukherjee T, and Schrick K 2018. Bioorthogonal click chemistry for fluorescence imaging of choline phospholipids in plants. Plant Meth. 14, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasari S, Ismail SM, Wenk MR, and Lear MJ 2015. Preparation of azide biosynthetic surrogates of myo-inositol. Tetrahedron Letters 56, 2597–2601. [Google Scholar]
- Prescher JA, and Bertozzi CR 2005. Chemistry in living systems. Nat. Chem. Biol 1, 13–21. [DOI] [PubMed] [Google Scholar]
- Rao L, Meng QF, Huang QQ, Wang ZX, Yu GT, Li A, Ma WJ, Zhang NG, Guo SS, Zhao XZ, et al. 2018. Platelet-Leukocyte Hybrid Membrane-Coated Immunomagnetic Beads for Highly Efficient and Highly Specific Isolation of Circulating Tumor Cells. Adv. Funct. Mater 28, 9. [Google Scholar]
- Ricks TJ, Cassilly CD, Carr AJ, Alves DS, Alam S, Tscherch K, Yokley TW, Workman CE, Morrell-Falvey JL, Barrera FN, et al. 2019. Labeling of Phosphatidylinositol Lipid Products in Cells through Metabolic Engineering by Using a Clickable myo-Inositol Probe. ChemBioChem 20, 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud PP, Poirier SJ, Boudreau LH, Doiron JA, Barnett DA, Boilard E, and Surette ME 2016. On the cellular metabolism of the click chemistry probe 19-alkyne arachidonic acid. J. Lipid Res 57, 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtsev VV, Green LG, Fokin VV, and Sharpless KB 2002. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew. Chem., Int. Edit 41, 2596–2597. [DOI] [PubMed] [Google Scholar]
- Sandbhor MS, Key JA, Strelkov IS, and Cairo CW 2009. A Modular Synthesis of Alkynyl-Phosphocholine Headgroups for Labeling Sphingomyelin and Phosphatidylcholine. J. Org. Chem 74, 8669–8674. [DOI] [PubMed] [Google Scholar]
- Santos CR, and Schulze A 2012. Lipid metabolism in cancer. FEBS J. 279, 2610–2623. [DOI] [PubMed] [Google Scholar]
- Sawicki LR, Garcia KA, Corsico B, and Scaglia N 2019. De novo lipogenesis at the mitotic exit is used for nuclear envelope reassembly/expansion. Implications for combined chemotherapy. Cell Cycle 18, 1646–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon E, Armstrong JI, and Bertozzi CR 2000. A "traceless" Staudinger ligation for the chemoselective synthesis of amide bonds. Org. Lett 2, 2141–2143. [DOI] [PubMed] [Google Scholar]
- Saxon E, and Bertozzi CR 2000. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010. [DOI] [PubMed] [Google Scholar]
- Selvaraj R, and Fox JM 2013. trans-Cyclooctene - a stable, voracious dienophile for bioorthogonal labeling. Curr. Opin. Chem. Biol 17, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo TS, Li ZM, Ruparel H, and Ju JY 2003. Click chemistry to construct fluorescent oligonucleotides for DNA sequencing. J. Org. Chem 68, 609–612. [DOI] [PubMed] [Google Scholar]
- Sezgin E, Levental I, Mayor S, and Eggeling C 2017. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Molec. Cell Biol 18, 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Cai KM, Yu J, and Cheng JJ 2019. Novel Liposomal Azido Mannosamine Lipids on Metabolic Cell Labeling and Imaging via Cu-Free Click Chemistry. Bioconjugate Chem. 30, 2317–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi LY, Zheng CG, Shen YH, Chen ZX, Silveira ES, Zhang LY, Wei M, Liu C, de Sena-Tomas C, Targoff K, et al. 2018. Optical imaging of metabolic dynamics in animals. Nat. Commun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten EM, and Bertozzi CR 2009. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem., Int. Edit 48, 6974–6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten EM, and Bertozzi CR 2011. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc. Chem. Res 44, 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass CJ, Burnham-Marusich AR, Meteer JC, and Berninsone PM 2015. Conserved ion and amino acid transporters identified as phosphorylcholine-modified N-glycoproteins by metabolic labeling with propargylcholine in Caenorhabditis elegans cells. Glycobiology 25, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltysik K, Ohsaki Y, Tatematsu T, Cheng JL, and Fujimoto T 2019. Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nat. Commun 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C, Papan C, Hoelper D, Kusserow K, Gaebler A, Schoene M, Piotrowitz K, Lohmann D, Spandl J, Stevanovic A, et al. 2012. Tracing Fatty Acid Metabolism by Click Chemistry. ACS Chem. Biol 7, 2004–2011. [DOI] [PubMed] [Google Scholar]
- Thiele C, Wunderling K, and Leyendecker P 2019. Multiplexed and single cell tracing of lipid metabolism. Nat. Methods 16, 1123-+. [DOI] [PubMed] [Google Scholar]
- Tiwari VK, Mishra BB, Mishra KB, Mishra N, Singh AS, and Chen X 2016. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem. Rev 116, 3086–3240. [DOI] [PubMed] [Google Scholar]
- Tornoe CW, Christensen C, and Meldal M 2002. Peptidotriazoles on solid phase: 1,2,3 -triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem 67, 3057–3064. [DOI] [PubMed] [Google Scholar]
- van Berkel SS, van Eldijk MB, and van Hest JCM 2011. Staudinger Ligation as a Method for Bioconjugation. Angew. Chem., Int. Edit 50, 8806–8827. [DOI] [PubMed] [Google Scholar]
- Viktorova EG, Nchoutmboube JA, Ford-Siltz LA, Iverson E, and Belov GA 2018. Phospholipid synthesis fueled by lipid droplets drives the structural development of poliovirus replication organelles. PLoS Pathog. 14, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DY, Du SB, Cazenave-Gassiot A, Ge JY, Lee JS, Wenk MR, and Yao SQ 2017. Global mapping of protein-lipid interactions by using modified choline-containing phospholipids metabolically synthesized in live cells. Angew. Chem., Int. Edit 56, 5829–5833. [DOI] [PubMed] [Google Scholar]
- Wang L, Brock A, Herberich B, and Schultz PG 2001. Expanding the genetic code of Escherichia coli. Science 292, 498–500. [DOI] [PubMed] [Google Scholar]
- Wang XM, Devalah SP, Zhang WH, and Welti R 2006. Signaling functions of phosphatidic acid. Progr. Lipid Res. 45, 250–278. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Hirata M, Ogasawara T, Koga T, and Ozaki S 1991. Synthesis and characterization of a photoaffinity probe possessing biotinyl and azidobenzoyl moieties for IP3-affiniated protein. Bioorg. Med. Chem. Lett 1, 399–402. [Google Scholar]
- Wegener G, Kellermann MY, and Elvert M 2016. Tracking activity and function of microorganisms by stable isotope probing of membrane lipids. Curr. Opin. Biotechnol 41, 43–52. [DOI] [PubMed] [Google Scholar]
- Wei L, Hu FH, Chen ZX, Shen YH, Zhang LY, and Min W 2016. Live-Cell Bioorthogonal Chemical Imaging: Stimulated Raman Scattering Microscopy of Vibrational Probes. Acc. Chem. Res 49, 1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Hu FH, Shen YH, Chen ZX, Yu Y, Lin CC, Wang MC, and Min W 2014. Live-cell imaging of alkyne-tagged small biomolecules by stimulated Raman scattering. Nat. Meth 11, 410–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HX, and Devaraj NK 2016. Inverse Electron-Demand Diels-Alder Bioorthogonal Reactions. Topics Curr. Chem 374. [DOI] [PubMed] [Google Scholar]
- Xiao M, Lin L, Li ZF, Liu J, Hong SL, Li YY, Zheng ML, Duan XM, and Chen X 2014. SERS Imaging of Cell-Surface Biomolecules Metabolically Labeled with Bioorthogonal Raman Reporters. Chem. Asian J 9, 2040–2044. [DOI] [PubMed] [Google Scholar]
- Xiong K, Wei W, Jin YJ, Wang SM, Zhao DX, Wang S, Gao XY, Qiao CM, Yue H, Ma GH, et al. 2016. Biomimetic Immuno-Magnetosomes for High-Performance Enrichment of Circulating Tumor Cells. Adv. Mater 28, 7929–7935. [DOI] [PubMed] [Google Scholar]
- Zhang F, Li F, Lu GH, Nie WD, Zhang LJ, Lv YL, Bao WE, Gao XY, Wei W, Pu KY, et al. 2019a. Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS Nano 13, 5662–5673. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wu LL, Nie WD, Huang LL, Zhang JF, Li F, and Xie HY 2019b. Biomimetic microfluidic system for fast and specific detection of circulating tumor cells. Anal. Chem 91, 15726–15731. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhao LJ, Wang SM, Yang J, Lu GH, Luo NN, Gao XY, Ma GH, Xie HY, and Wei W 2018a. Construction of a biomimetic magnetosome and its application as a siRNA carrier for high-performance anticancer therapy. Adv. Funct. Mater 28, 9. [Google Scholar]
- Zhang JT, Zhang ZL, Chukkapalli V, Nchoutmboube JA, Li JH, Randall G, Belov GA, and Wang XF 2016. Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites. Proc. Natl. Acad. Sci. U.S.A 113, E1064–E1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LY, and Min W 2017. Bioorthogonal chemical imaging of metabolic changes during epithelialmesenchymal transition of cancer cells by stimulated Raman scattering microscopy. J. Biomed. Optics 22, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PJ, Dong B, Zeng EZ, Wang FC, Jiang Y, Li DQ, and Liu DB 2018b. In Vivo Tracking of Multiple Tumor Exosomes Labeled by Phospholipid-Based Bioorthogonal Conjugation. Anal. Chem 90, 11273–11279. [DOI] [PubMed] [Google Scholar]
- Zhang QB, Wei W, Wang PL, Zuo LP, Li F, Xu J, Xi XB, Gao XY, Ma GH, and Xie HY 2017. Biomimetic magnetosomes as versatile artificial antigen-presenting cells to potentiate T-cell-based anticancer therapy. ACS Nano 11, 10724–10732. [DOI] [PubMed] [Google Scholar]
- Zhao X, Shen Y, Adogla EA, Viswanath A, Tan R, Benicewicz BC, Greytak AB, Lin Y, and Wang Q 2016. Surface labeling of enveloped virus with polymeric imidazole ligand-capped quantum dots via the metabolic incorporation of phospholipids into host cells. J. Mater. Chem. B 4, 2421–2427. [DOI] [PubMed] [Google Scholar]
- Zhu GZ, Zhang FW, Ni QQ, Niu G, and Chen XY 2017. Efficient nanovaccine delivery in cancer immunotherapy. ACS Nano 11, 2387–2392. [DOI] [PubMed] [Google Scholar]


