Abstract
Central nervous system (CNS) injuries are often debilitating, and most currently have no cure. This is due to the formation of a neuroinhibitory microenvironment at injury sites, which includes neuroinflammatory signaling and non-permissive extracellular matrix (ECM) components. To address this challenge, we developed a viscous interfacial self-assembly approach to generate a bio-inspired hybrid three dimensional (3D) porous nanoscaffold platform for delivering anti-inflammatory molecules and establishing a favorable 3D-ECM environment for the effective suppression of the neuroinhibitory microenvironment. By tailoring the structural and biochemical properties of the 3D porous nanoscaffold, we demonstrate enhanced axonal growth from the dual-targeting therapeutic strategy in a human induced pluripotent stem cell (hiPSC)-based in vitro model of neuroinflammation. Moreover, nanoscaffold-based approaches promote significant axonal growth and functional recovery in vivo in a spinal cord injury model through a unique mechanism of anti-inflammation-based fibrotic scar reduction. Given the critical role of neuroinflammation and ECM microenvironments in neuroinhibitory signaling, our developed nanobiomaterial-based therapeutic intervention may pave a new road for treating CNS injuries.
Keywords: biomaterials, inorganic-organic hybrid nanomaterials, nanoscaffolds, neural tissue engineering, spinal cord injury
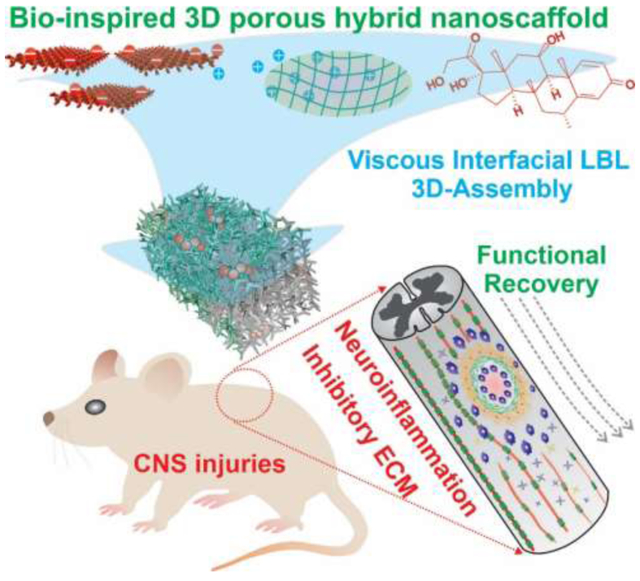
GRAPHICAL ABSTRACT
Current biomaterials-based treatment of CNS injuries has been hampered by the resulting neuroinhibitory microenvironment. By targeting two critical neuroinhibitory factors in a single platform, a biomimetic 3D porous hybrid nanoscaffold is created by developing viscous interfacial self-assembly. The nanoscaffold-based achieved functional recovery through reducing neuroinflammation and fibrotic scarring, thereby paving a new road for the biomaterials-based treatment of CNS injuries.
Neuroinflammation and inhibitory signaling are known to critically affect the progress of many neurological disorders at both acute and chronic phases.[1] For example, the restoration of disrupted neural circuitry after central nervous system (CNS) injuries [e.g., stroke, traumatic brain injury, and spinal cord injury (SCI)] is hampered by inhibitory microenvironments (e.g., chondroitin sulfate proteoglycans deposited in the form of an astroglial and fibrotic scar) that are often initiated by neuroinflammatory processes (e.g., neurotoxic cytokines and metalloproteinase secretion).[2] To this end, considerable effort has been put forth to develop effective biomaterial-based therapeutic approaches for alleviating inflammatory pathways or suppressing scar formation through stimuli-responsive drug delivery, guided immunomodulation, and controlled regulation of neuronal behaviors (e.g., axonal growth and neurite outgrowth).[3] For example, synthetic polymer nanofiber and biomaterial (e.g., fibrin)-based scaffolds have accelerated nerve regeneration in SCI animal models via sustainable delivery of neurotrophic factors and mimicking healthy neural ECM.[4] And, polypeptide-based hydrogels promoted neurogenesis of transplanted stem cells and improved functional outcomes by providing a permissive ECM environment for axonal growth.[5] More recently, nanomaterial (e.g., carbon nanotube and graphene)-based hybrid bioscaffolds have shown multi-functionalities, including high-resolution in vivo imaging/stimulation and drug delivery, which has enabled the enhancement of their therapeutic effects after CNS injuries.[6, 7] Despite their huge potential, limited success in the clinical translation of nano/biomaterials has been achieved.[3, 9] This could be largely attributed to the dynamic and complex nature of the neuroinhibitory microenvironment.[11] For instance, recent evidence strongly suggests that targeting neuroinflammation or inhibitory ECM components alone is insufficient to promote motor function recovery after CNS injuries, but few biomaterials have successfully targeted both inhibitory factors.[12, 13] Also, while a majority of biomaterial-based treatments of SCI have been focused on the reduction of the astroglial scar, researchers have recently drawn contradictory conclusions as to its therapeutic effect while also suggesting the fibrotic scar may play a critical role in the functional recovery of SCI animals.[14] Taken together, it remains an ongoing challenge to design novel multifunctional biomaterials that address SCI therapeutic targets by effectively modulating the dynamic and complex neuroinhibitory microenvironment.
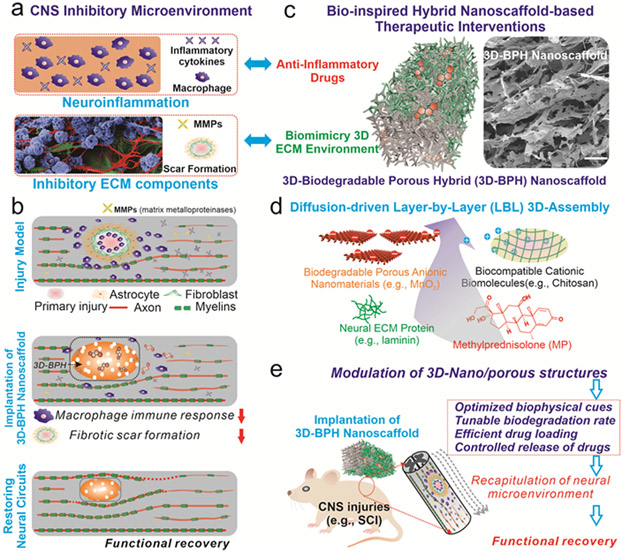
To address the aforementioned issues and facilitate the progress of in vivo drug/cell delivery, herein we developed a 3D-biodegradable porous hybrid (3D-BPH) nanoscaffold to synergistically modulate the inhibitory microenvironment by combining the delivery of anti-inflammatory compounds (methylprednisolone) and reducing fibrotic scarring for the enhanced treatment of SCI [FIGURE 1a-b]. This is achieved through designing a 3D-BPH nanoscaffold that demonstrate several unique structural, biological, and physicochemical properties by: i) creating a 3D-biomimetic matrix permissive to neural growth; ii) releasing therapeutic molecules (e.g., anti-neuroinflammatory drugs) in a spatiotemporally controlled manner; iii) tuning the biodegradation rate of 3D-BPH nanoscaffold precisely; and iv) producing in vivo [magnetic resonance imaging (MRI)] neural imaging modalities in response to the scaffold-degradation process and drug release [FIGURE 1c]. To achieve these ideal properties of nanobioscaffolds, our 3D-BPH nanoscaffold system was carefully designed and synthesized by employing a unique viscous interfacial layer-by-layer (LBL) 3D-electrostatic assembly of biocompatible cationic polymers (e.g., chitosan) and biodegradable nanomaterials [e.g., two-dimensional manganese dioxide (2D-MnO2) nanosheets], which allows the formation of 3D-ordered, porous scaffold structures tailored for the treatment of CNS injuries [FIGURE 1d]. More specifically, we achieved biomimetic Young’s modulus, controllable drug release and tunable biodegradation by modulating the porosity and composition of our 3D-BPH nanoscaffold. Strikingly, this unique 3D-BPH nanoscaffold facilitated the formation of 3D neuronal networks and synergistically promoted axonal growth in a human induced pluripotent stem cell-derived neural stem cell (hiPSC-NSC)-based neuroinflammation model by providing dual-functions of anti-inflammation and 3D-biomimetic neural matrix formation. Moreover, the transplantation of our 3D-BPH nanoscaffold in vivo into a murine SCI model suppressed neuroinflammation and fibrotic scarring, while significantly improving functional recovery and enhancing axonal growth. Taken together, by developing our 3D-BPH nanoscaffold for modulating neuroinhibitory microenvironments, we may provide new insights into the biomaterial-based treatment of CNS injuries.
FIGURE 1. Effective modulation of inhibitory CNS microenvironment for the enhanced treatment of CNS injuries by developing bio-inspired 3D-biodegradable porous hybrid (3D-BPH) nanoscaffold.
(a) A schematic illustration of the inhibitory microenvironment after CNS injury, which includes neuroinflammation and inhibitory ECM. In the “neuroinflammation” panel, blue-colored cells refer to macrophages and blue cross-refer to inhibitory cytokines. In the “inhibitory ECM components” panel, blue, red and green refer to the cell body, neurites and ECM of neurons, respectively. (b) A proposed strategy for the effective modulation of CNS microenvironment after injuries by developing a 3D-BPH nanoscaffold-based therapeutic interventions. (c) A schematic diagram (left) and field-emission scanning electron microscopy (FE-SEM, the image on the right) illustrating the structure and composition of 3D-BPH nanoscaffold designed for overcoming inhibitory microenvironments synergistically. Scale bar in FE-SEM: 100 μm. (d) The 3D-BPH nanoscaffold is assembled from a unique viscous interfacial layer-by-layer (LBL) 3D electrostatic assembly from anionic nanomaterials and cationic polymers, which simultaneously allows loading of an anti-inflammatory drug (methylprednisolone, MP, colored in red) and absorption of favorable neural ECM protein (e.g., laminin, colored in green). (e) A schematic diagram illustrating the implantation of MP-loaded anti-inflammatory 3D-BPH nanoscaffold for the treatment of CNS injury by using a murine hemisection spinal cord injury (SCI) model.
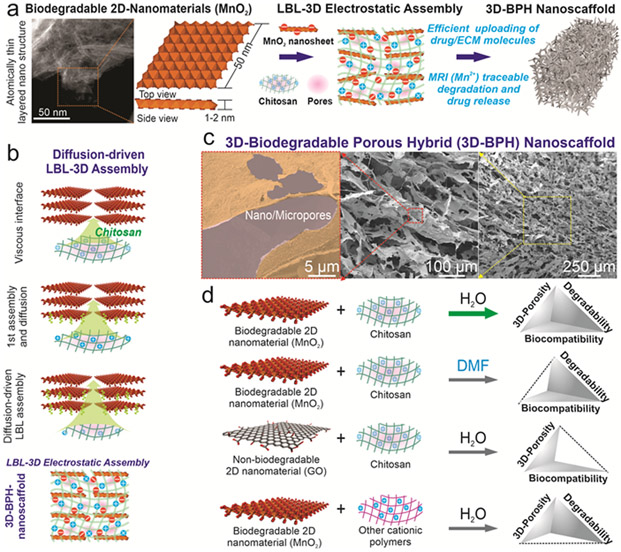
First, we established a viscous interfacial 3D LBL assembly for generating 3D biodegradable porous structures from atomic thin 2D nanomaterials with molding capability, injectability and high biocompatibility. 2D nanomaterial (e.g., graphene)-based hybrid scaffolds developed by our group, as well as others, have shown excellent protein absorption with a high surface-area-to-volume ratio, drug loading, and bioimaging properties that offer clear advantages over conventional bioscaffolds.[8, 15] However, currently, there is a lack of reliable methods to produce the desired biomimetic ordered 3D porous structures from biodegradable 2D MnO2 nanomaterials without compromising their chemical stability, biocompatibility, and bioactivity.[8, 9, 16, 17] Inspired by a conventional electrostatic LBL technique as well as recent advancement of diffusion-driven 3D assembly of graphene nanosheets, we developed a synthetic route to generate our 3D-BPH nanoscaffolds from a biodegradable 2D nanomaterial (i.e., MnO2 nanosheets) and a Food and Drug Administration (FDA)-approved cationic polymer (i.e., chitosan), as a means to provide a clinically-relevant nanomaterial-based bioscaffold [FIGURE 2a].[18] More specifically, 2D-MnO2 nanosheets were first generated through liquid exfoliation [FIGURE S1]. To initiate the 3D electrostatic LBL assembly, a highly viscous droplet of chitosan solution [Molecular weight (Mw): 190,000-310,000 Da, +40 mV zeta potential) was placed and incubated in a nanosheet solution (3 −50 mV) [FIGURE 2a-b, FIGURE S1]. The transparent droplet of chitosan then slowly expanded into a dark-colored gel-like macrostructure via step-by-step complexation between the negatively charged nanosheets and positively charged chitosan macromolecules [FIGURE 2b]. After 2D-MnO2 nanosheets and chitosan underwent 3D-LBL complexation, lyophilization then produced a mechanically robust, flexible, and airy 3D-scaffold, suggesting a highly porous nature of the hybrid scaffold [FIGURE 2b-c, FIGURE S2]. Interestingly, the free-standing structure preserved the 3D tubular shape of the reaction vessel, suggesting a molding ability of our assembly method for potential uses in personalized treatment of patients with SCI [FIGURE S2-S3]. Additionally, we examined the mechanism of LBL 3D electrostatic assembly, by testing a different 2D nanomaterial with a negative surface charge, graphene oxide (GO, −35 mV), and another cationic polymer, polyethyleneimine (PEI, +15 mV). While scaffolds composed of graphene or PEI may also be useful for other applications, we found that structures assembled from GO do not degrade, and PEI induces unhealthy NSC morphology, a sign of poor biocompatibility, both of which are consistent with literature reports [FIGURE 2d, FIGURE S3].[8, 19] Moreover, we found that 3D-BPH nanoscaffolds can be implanted through a clinically relevant syringe needle. Recently developed injectable scaffolds and hydrogels have advantages in terms of minimal invasiveness during surgery.[20] Here, 3D-BPH nanoscaffold can be flowed through the syringe and injected into an artificial soft tissue (calcium-crosslinked alginate hydrogel) [FIGURE S4]. Overall, with these unique properties, our LBL 3D electrostatic assembly strategy could represent an advantageous method to produce 3D-porous nanoscaffolds for general tissue engineering applications.
FIGURE 2. Multifunctional 3D-BPH nanoscaffold synthesized by viscous interfacial 3D electrostatic layer-by-layer (LBL) self-assembly of biodegradable 2D nanomaterials and cationic polymers.
(a) A Schematic diagram showing the rationale for the synthesis of 3D-BPH nanoscaffold by assembling a biocompatible cationic polymer (chitosan) with biodegradable 2D nanosheets. (b) A schematic diagram showing the detailed mechanism of viscous interfacial LBL 3D electrostatic assembly between negatively charged MnO2 nanosheets and cationic polymer to form a 3D-hybrid hydrogel-like microstructure. By incubating a viscous droplet of chitosan solution in a nanosheet solution, the cationic polymer diffuses across the boundary between two solutions and binds to anionic nanosheets layer by layer until reach a balance. The scheme on the bottom shows the structure of the 3D-BPH nanoscaffold after lyophilization. Pink color represents micropores. (c) FE-SEM images characterizing the generation of an ordered hierarchical porous network of 3D-BPH nanoscaffolds. (d) Generalized synthesis of 3D-porous nanoscaffolds using differently charged macromolecules based on the LBL 3D electrostatic assembly as well as the solvent effects on the porosity formation. Among all the building blocks, the 3D-BPH nanoscaffold assembled from MnO2 nanosheets, and chitosan with aqueous solvent has all desired properties for modulating neuroinhibitory microenvironments.
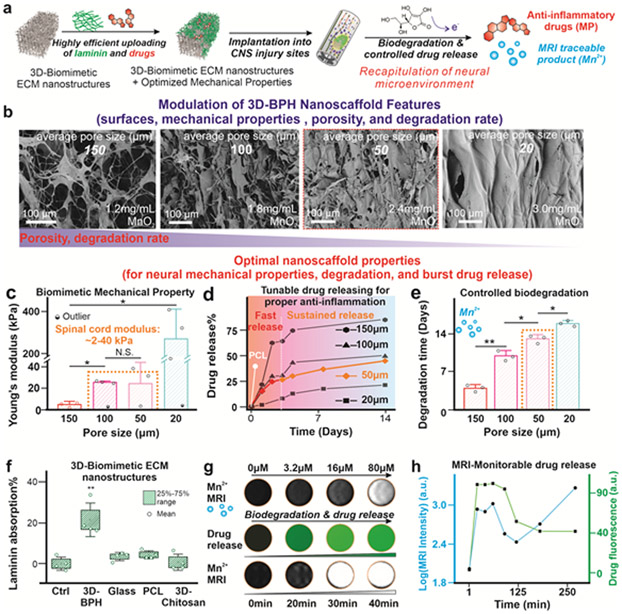
Next, we sought to manipulate the porosity of 3D-BPH nanoscaffolds to achieve biomimetic mechanical properties, and to control their drug release profiles and biodegradation speeds, all of which are critical for modulating the dynamic and complex neuroinhibitory microenvironments [FIGURE 3a].[3, 21] Although the control over these critical biomaterial properties has been demonstrated in several polymer-based systems, it is still an ongoing challenge for inorganic-organic hybrid 3D nanoscaffolds.[22] Based on the mechanism of viscous interfacial LBL assembly, the pore size in 3D-BPH nanoscaffolds can be reduced by gradually decreasing the hydration layer trapped between two layers of electrostatic assembly, which is realized by merely increasing concentrations of 2D-MnO2 nanosheets stepwise. Field emission scanning electron microscope (FE-SEM), mercury intrusion porosimetry, fluorescence microscopy as well as liquid cell AFM techniques all confirmed the expected trend of pore size distributions [FIGURE 3b, S2, S5]. Afterward, we further showcase the control over the physiochemical properties of 3D-BPH nanoscaffold. First, a proper porosity range (between 100 μm and 50 μm conditions) was identified to correlate with the stiffness of a spinal cord tissue (around 20kPa within a 2-40 kPa range), which is desired to avoid a mechanical mismatch and potential exacerbated immune response [FIGURE 3c].[23] In parallel, we found that a higher porosity leads to accelerated biodegradation, and results in a faster drug release from the nanoscaffold. More specifically, to match the time-frame of the inflammatory response post- SCI, we demonstrated the feasibility of 3D-BPH nanoscaffold to deliver anti-inflammatory drugs in the scenario of SCI within an acute therapeutic window (24-72 hours) at pore sizes of 50 μm which rapidly release drugs in the first 3 days, followed by a more sustainable release, that matches the typical inflammatory timescale in SCI for in vivo applications [FIGURE 3d].[24] Also, we adjusted biodegradation rates (1-2 weeks decomposition) of 3D-BPH nanoscaffolds for neural regeneration, since fast erosion of the scaffold may not allow sufficient time for axonal growth, while slow biodegradation can result in chronic immune reactions from the host spinal cord tissue [FIGURE 3e, FIGURE S4].[3] Our simulation indicated that fluid exchange within the microporous structures of 3D-BPH nanoscaffolds might contribute to the scaffold’s overall exposure to extracellular reductants, thereby inducing biodegradation as well [FIGURE S4]. Moreover, 3D-BPH nanoscaffold showed several distinctive properties compared to conventional polymeric scaffolds. First, the 3D-BPH nanoscaffold (average pore size of 50 μm) showed a high loading efficiency toward small molecule drugs with long-term maintenance of drug stability, likely mediated through metal-π and hydrophobic interactions [FIGURE S4, S6].[15] Second, we demonstrated higher ECM protein (i.e., laminin) and growth factor absorption onto 3D-BPH nanoscaffolds, which could be also facilitated by their high specific surface areas and suggests the potential to improve biomimicry of favorable neural microenvironments [FIGURE 3f, S7].[25] Most importantly, we were able to check drug release from 3D-BPH nanoscaffolds through the use of MRI [FIGURE 3g-h, S4]. This process can be an attractive approach to non-invasively monitor both drug release and scaffold degradation in vivo for potential applications in personalized medicine. Taken together, we established a reliable approach for uniquely modulating biochemical properties of inorganic-organic 3D hybrid scaffolds with a variety of advantages over conventional scaffold biomaterials.
FIGURE 3. Achieving biomimetic mechanical property, and tailoring the degradation and drug release of 3D-BPH nanoscaffolds by tuning porous structures.
(a) A schematic diagram illustrating the development steps of the 3D-BPH nanoscaffold-based therapeutic platform and the critical scaffold considerations for our in vivo applications. (b) FE-SEM images confirming the decrease of microporosities of 3D-BPH nanoscaffolds by manipulating the 3D-LBL self-assembly via increasing the concentrations of MnO2 nanosheets. (c) Modulation of Young’s modulus of 3D-BPH nanoscaffolds with increasing MnO2 nanosheet concentration characterized by AFM. Error bars are standard deviation around the mean. n=3-4 experimental replicates. Outliers are tested by Grubb’s method. *P<0.05, N.S. means no significance, by one-way ANOVA with Tukey post-hoc analysis. (d) Tunable and sustainable drug delivery release profiles to match the resolution of inflammation post-SCI. The tunable release profiles were controlled by modulating the porosity of 3D-BPH nanoscaffolds. A control PCL nanoscaffold with burst release of drugs is shown in a white-colored line. (e) The robust control of biodegradation speeds via tuning the porosity of 3D-BPH nanoscaffolds in the presence of an endogenous reductant at a concentration similar to the blood [ascorbic acid (10 μg/ml)]. A higher porosity was found to lead to a faster degradation speed. Error bars are standard deviation around the mean. n=3 experimental replicates. *P<0.05, **P<0.01 by one-way ANOVA with Tukey post-hoc analysis. (f) BCA assay-based characterization of enhanced absorption towards laminin from 3D-BPH nanoscaffold compared to control scaffolds [glass and polymer (PCL and 3D-chitosan scaffolds)], which helps the creation of biomimicry ECM microenvironment. Error bars are standard deviation. Box represent 25%-75% data range around the mean. n=4 experimental replicates. **P<0.01 by one-way ANOVA with Tukey post-hoc analysis. (g) MRI monitoring of drug release from 3D-BPH nanoscaffold demonstrated by first establishing a standard Mn2+ concentration (top row), drug release (show in green fluorescence in the middle row) and MRI signal of released Mn2+ (bottom row). (h) Quantifications of the MRI-monitorable drug release in g.
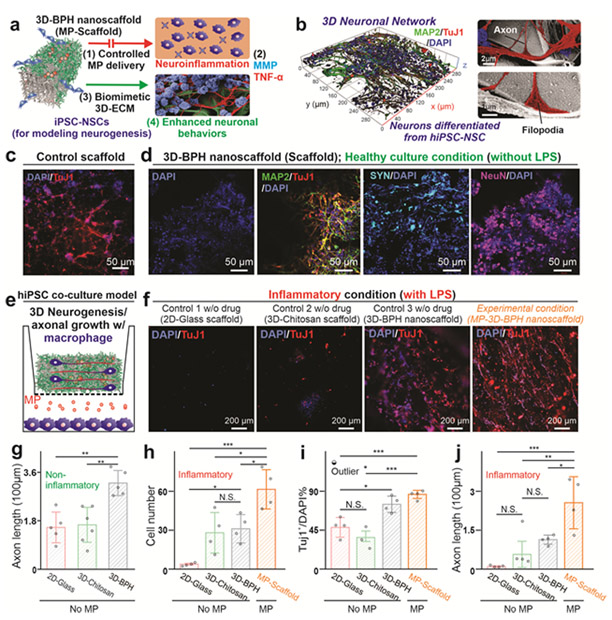
The robust therapeutic effects of the developed 3D-BPH nanoscaffold for synergistically modulating the neuroinhibitory microenvironment were then demonstrated through a hiPSC-NSC-based neuroinflammation model [FIGURE 4a-b]. The neuroinhibitory microenvironment is typically composed of both neuroinflammation and inhibitory ECM components, which have not been well addressed by previous biomaterial-based approaches.[1] [12] As such, we hypothesized that the 3D-BPH nanoscaffold could more effectively enhance neural regeneration, with its capabilities of tailoring drug-releasing profiles for anti-inflammation, and strongly adsorbing neural favorable ECM proteins. To demonstrate this, we first showcased the robust anti-inflammatory effects from the drug-loaded 3D-BPH nanoscaffold in a Transwell®-based model by co-culture with human monocyte-derived and lipopolysaccharide (LPS)-activated macrophages. For the selection of anti-inflammatory drugs, we loaded methylprednisolone (MP) onto 3D-BPH nanoscaffold as a proof-of-concept, as it is currently the only clinically prescribed treatment for acute SCI [FIGURE S8].[26] We proved the robust anti-inflammatory effect from the MP-loaded 3D-BPH nanoscaffold, based on the substantially reduced expression of pro-inflammatory markers tumor necrosis factor (TNF), interleukin (IL), chemokine ligands (CCL) genes IL-4, and IL-13 [FIGURE S8]. In addition, as degradation of laminin by metalloproteinase (MMPs) have been associated with inhibition of axonal growth after SCI, we also confirmed the reduced expression of MMP-1, MMP-2 and MMP-9 upon activation by LPS through the treatment of MP-loaded 3D-BPH nanoscaffold (MP-scaffold).[26] This result could imply a unique synergy between anti-inflammation and the creation of a favorable ECM environment for promoting axonal growth by effectively downregulating the expression of pro-inflammatory cytokines and upregulating anti-inflammatory cytokines in activated macrophages. While direct treatment of macrophages by MP also showed anti-inflammatory effects, our nanoscaffold can offer spatially controlled release [FIGURE S6] that can potentially reduce side effects from MP, such as global immune suppression, and can prolong therapeutic effects at SCI sites in vivo.[28] Next, to demonstrate the synergistic effects between the creation of a permissive 3D ECM environment and reduction of neuroinflammation, we further performed a hiPSC-NSC-based in vitro assay on drug-loaded and laminin-functionalized 3D-BPH nanoscaffold. In this in vitro model, hiPSC-NSCs can provide an excellent platform that closely mimics the human CNS for disease modeling and immune modulation.[29] We hypothesized that 3D-BPH nanoscaffold alone could enhance positive neuronal behaviors (e.g., axonal growth) by providing a biomimicry 3D ECM microenvironment, due to its significantly higher absorption towards laminin and the 3D biomimicry porous structures tailored for neural applications [FIGURE S9]. We confirmed our hypothesis by observing a nearly two-fold increase of axonal lengths as well as the formation of 3D neuronal networks of hiPSC-NSC-differentiated neurons on the 3D-BPH nanoscaffold under normal culture conditions (without inflammation) [FIGURE S10, FIGURE 4c-d, g]. Then, we verified that 3D-BPH nanoscaffold could be further combined with anti-inflammatory approaches for synergistically enhancing cell survival, neuronal differentiation, and axonal growth in our hiPSC-NSC-based neuroinflammation co-culture model [FIGURE 4e-j]. As control groups, 3D-BPH nanoscaffold without the loaded drug (scaffold), laminin-coated chitosan, and laminin-coated glass substrates were seeded with hiPSC-NSCs cultured in the upper layer of the Transwell® system. In this system, secreted pro-inflammatory cytokines and chemokines interacted with hiPSC-NSCs in the top chamber, mimicking a post-injury inflammatory microenvironment. After seven days in neuronal differentiation media, both laminin-coated chitosan and 3D-BPH nanoscaffolds showed similar enhancement of cell survival compared to the glass substrates (significant with P<0.05) [FIGURE 4f, h], which is consistent with the laminin absorption assay [FIGURE 3f], as laminin is known to play a critical role in supporting the survival of neural stem cells.[4] However, neuronal differentiation shows a similar trend in the healthy culture (non-inflammatory) condition, with enhancement only existent in the 3D-BPH nanoscaffolds but not in the laminin-coated chitosan scaffolds. Most importantly, the enhancement of axonal growth is only significant in MP-3D-BPH nanoscaffold condition, thereby proving the additional therapeutic effects from the sustainable delivery of the anti-inflammatory drug MP (P<0.001, 0.01, and 0.05 compared to the laminin-coated glass, chitosan and 3D-BPH nanoscaffolds, respectively) [FIGURE 4g, i-j]. Therefore, in the neuroinflammation model used by our nanoscaffold platform, a synergistic effect from anti-inflammation and creation of biomimetic 3D-ECM environment on promoting neuronal behaviors (e.g., neurogenesis and axonal growth) were strongly supported, suggesting a potential for in vivo applications.
FIGURE 4. 3D-BPH nanoscaffolds promote 3D-neurogenesis and axonal growth under inflammatory microenvironments by providing a favorable 3D ECM environment and controlled anti-inflammatory drug delivery.
(a) A schematic diagram illustrating that macrophage-mediated inflammation produces neurotoxic cytokines (e.g., TNF) and ECM-degrading enzyme (e.g., MMP). The MP-loaded 3D-BPH nanoscaffold reduces this inhibitory signaling by reducing inflammation and providing a favorable 3D-ECM environment. (b-c) A 3D-confocal image (b, on the left) and cell FE-SEM characterizations (b, on the right) showing the successful formation of the 3D neuronal network in the 3D-BPH nanoscaffold seeded with hiPSC-NSCs. (c) A representative immunostaining image from hiPSC-NSCs differentiated on a control group (laminin-coated chitosan, 7 days after differentiation). (d) Representative immunostaining images showing the expression of early (TuJ1, Day7) and mature (MAP2, SYN, NeuN, Day14) neuronal markers on the 3D neuronal network formed on 3D-BPH nanoscaffold under healthy culture (non-inflammatory) condition. (e) A schematic diagram of neuroinflammation co-culture model. (f) Representative immunostaining images confirming the improved neurogenesis and 3D-like neuronal network formation 7 days after differentiation from the anti-inflammatory MP-loaded 3D-BPH nanoscaffold (MP-3D-BPH) compared to the controls. (g-j) Quantifications of the immunostaining results from control, 3D-BPH, and MP-3D-BPH nanoscaffolds under normal (g) or inflammatory (h-j) conditions in terms of survival (h), neurogenesis (i) and axon growth (g, j) Error bars are standard deviation around the mean, n=3-5 experimental replicates. *p<0.05, **p<0.01 and ***p<0.001 by one-way ANOVA with Tukey post-hoc analysis. N.S. means no significance.
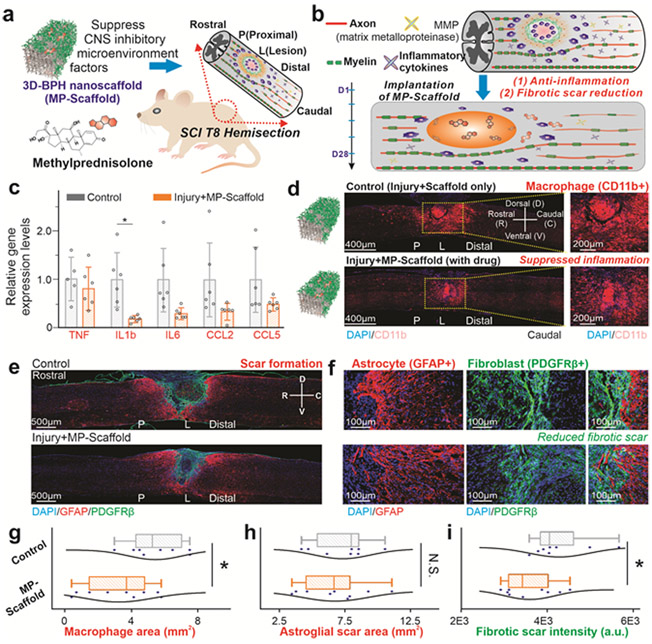
With the encouraging results from the in vitro neuroinflammation assay, we went on to study the 3D-BPH nanoscaffold-facilitated enhanced axonal growth and functional recovery in a spinal cord injury model in vivo. Systemic delivery of MP is currently the only clinical standard for treating acute SCI, but it is no longer widely used due to complications resulting from global immunosuppression.[26] Although several nanobiomaterial-based drug delivery approaches have been developed for reducing astroglial scarring and improving the treatment of CNS injuries, recent evidence suggests it is essential to overcome multiple therapeutic targets within the neuroinhibitory microenvironment, which includes the fibrotic scarring that is often overlooked in terms of their critical role in the functional recovery of SCI. [12-14] To this end, we performed a Thoracic 8 (T8) spinal cord dorsal hemisection injury in mice and immediately implanted the laminin-coated MP-loaded 3D-BPH nanoscaffold (MP-scaffold) or the laminin-coated scaffold-only control (scaffold) in the lesion site to investigate the therapeutic effects of 3D-BPH nanoscaffold. We hypothesized that the MP-scaffold treatment would lead to improved pathology and functional recovery of SCI. First, we used a quantitative real time-polymerase chain reaction (qRT-PCR) to investigate the effects of MP-scaffold on neuroinflammation in vivo and demonstrated the reduced expression of a subset of pro-inflammatory cytokine genes (TNF, IL1b, IL6, CCL2, and CCL5) [FIGURE 5c, TABLE S1-S2]. The anti-inflammatory effects in the MP-scaffold group were also indicated by a significant reduction in the density of infiltrating macrophages, as measured by the area of the cluster of differentiation molecule 11b (CD11b+) immunoreactivity [FIGURE 5d, g]. Our findings are well supported by literature and demonstrate the therapeutic effect of local scaffold-mediated MP release without global immune suppression.[30] Next, we investigated the effects of MP-scaffold on fibrotic scarring post-SCI. Non-resolved SCI pathology is characterized by the formation and persistence of an astrocyte-rich border (astrocytic scar) surrounding the fibroblast dense lesion core (fibrotic scar).[30, 31] Recent reports have suggested the reduction of the astroglial scar does not aid the functional recovery of SCI animals.[14][32] As such, we could effectively reduce the fibrotic scar and study its role in promoting functional recovery.[31] We verified this based on a significant attenuation of the fibrotic scar, as measured by the intensity of platelet-derived growth factor subunit β (PDGFRβ+), in the MP-scaffold group [FIGURE 5e-i, FIGURE S11]. Notably, MP-scaffold visibly reduced the dense fibrotic border adjacent to the astroglial scar. Meanwhile, evaluation of the glial scar formation revealed that the MP-scaffold implantation did not affect the reactivity of astrocytes and lesion size as measured by the glial fibrillary acidic protein (GFAP)-negative area compared to the scaffold-only group. Taken together, our results show that the local delivery of MP through the 3D-BPH nanoscaffold reduces the inflammatory response, as well as the fibrotic scarring after SCI.
FIGURE 5. 3D-BPH nanoscaffolds-mediated in vivo modulation of CNS inhibitory microenvironments post-SCI.
(a-b) Schematic diagrams illustrating the design of in vivo scaffold transplantation assay in a T8 dorsal hemisection mouse model (a) to modulate the inhibitory microenvironment, which includes neuroinflammation and scar formation (b). (c) Short-term (24 hours) suppression of inflammatory genes in vivo by MP-loaded 3D-BPH nanoscaffold (MP-scaffold) compared to the control (injury followed by scaffold implantation only). Error bars are standard deviation around the mean. n=5 and 6 biological replicates for the control and experimental group, respectively. *p<0.05 by Student’s t-test. (d) Long-term (28 days) suppression of inflammation in vivo by the MP-scaffold based on a macrophage marker, CD11b. (e-f) Immunostaining images on tissue sections at the SCI sites characterizing the glial scar (GFAP, in red) and fibrotic (PDGFRβ, in green) scar suppressed by the implantation of MP-scaffold. (g) Quantification of immune cell infiltration into the lesion based on the macrophage marker CD11b. n=8 and 9 biological replicates for the control and experimental group, respectively. 4-5 sections of each animal were analyzed and averaged to obtain the individual data points. (h-i) Quantification of the astroglial (h) and fibrotic scar (i) showing a decreased fibrotic scar formation in the MP-scaffold-treated conditions. Error bars are standard deviation around the mean. *p<0.05 by Student’s t-test. N.S. means no significance.
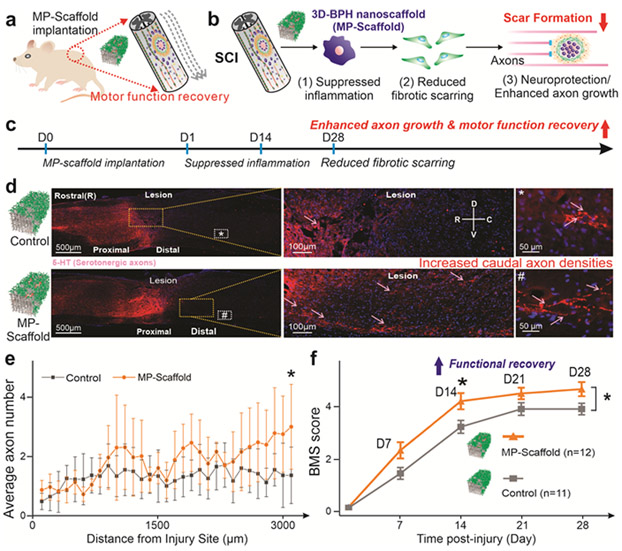
Moreover, we investigated the axonal growth and functional recovery from the suppressed neuroinflammation and selective reduction of fibrotic scarring [FIGURE 6a-b]. Fibroblasts that accumulate in the lesion core after SCI are non-permissive for axonal growth, and reducing fibrotic scarring leads to increased axonal growth.[32] We hypothesized that by reducing the fibrotic scarring, axonal growth could be promoted effectively. This was confirmed by quantifying the number of serotonergic axons in the caudal region based on 5-hydroxytryptamine (5-HT+) immunostaining [FIGURE 6c-d, FIGURE S12-13]. Among various sub-types of neurons in the spinal cord, serotonergic (5-HT) axons are descending fibers that have been reported to play essential roles in locomotor recovery after SCI.[33] More specifically, while in both groups, we observed 5-HT+ axons caudal to the lesion site, a significantly higher number of 5-HT+ axons were found in the MP-scaffold group, suggesting a combined neuroprotective and regenerative effect from the MP-scaffold [FIGURE 6e]. To study whether the enhanced axonal growth further improved locomotor behavioral recovery, Basso Mouse Scale (BMS) scoring was performed blindly on animals from the control and MP-scaffold group.[34] Strikingly, we found that the MP-scaffold experimental group showed significantly better hindlimb locomotion than the scaffold-only group throughout the 1-month duration of this study. More specifically, MP-scaffold implantation produced significantly improved functional outcomes from as early as 14 days after SCI [FIGURE 6f]. Taken together, our data demonstrates that local MP delivery to the injured spinal cord using 3D-BPH nanoscaffolds significantly decreased inflammatory cytokine expression, macrophage infiltration, and fibrotic scarring; thereby leading to improved functional outcomes after SCI. Both neuroinflammation and scar formation have been considered as critical factors for impeding functional recovery after CNS injuries.[1] As such, our 3D-BPH nanoscaffold has excellent potential to treat CNS injuries by effectively tuning drug delivery rates and enhancing drug stability, while minimizing the side effects from systemic administration of drugs. Additionally, the enhanced functional recovery from the selective reduction of fibrotic scarring also indirectly supports recent findings undergoing the debate that reduced astroglial scarring does not aid the functional recovery of SCI.[14] Taken together, the current work highlights the robust therapeutic potential of 3D-BPH nanoscaffolds to modulate the inhibitory SCI microenvironments and improve functional recovery, which is clinically relevant for developing novel treatments of SCI.
FIGURE 6. Reduced inhibitory microenvironment leads to enhanced caudal axonal densities and promotes functional recovery after SCI.
(a) A schematic diagram illustrating the transplantation of MP-scaffold in a mouse T8 hemisection model. (b) Proposed mechanism for MP-scaffold-enhanced functional recovery by suppressed inflammation, reduced fibrotic scarring and enhanced axonal growth. (c) Timeline of the long-term in vivo experiments. (d) Representative immunostaining images of the spinal cord from the control (SCI with scaffold implantation) and experimental (SCI with MP-scaffold implantation) conditions illustrating the overall serotonergic (5-HT) axonal density 28 days after the injury (left panel). Magnified views of the immunohistochemical staining images are shown in the middle and right panels, demonstrating the presence of axonal growth caudal to the lesion site (arrows indicate 5-HT labeled axons). (e) Quantification of the distance-dependent distribution of axonal numbers (per section) 1-month post-injury show higher axonal density in the MP-scaffold-treated animal groups. Error bar represents standard deviation around the mean. *p<0.05 by Student’s t-test, n=7 animals for both groups. 4-5 tissue sections were analyzed and then averaged for each animal. (f) BMS scores of the control and experimental conditions indicating an accelerated functional recovery from the experimental condition compared to the control. Whereas most animals in the control group only achieved scores at dorsal stepping, the experimental group present significantly higher functional scores. n=12 and 11 animals for the experimental group and control group, respectively. Error bar represents standard error around the mean. *p<0.05 by student’s t-test.
In summary, treating CNS injuries has been a significant challenge for decades due to the limited regenerative capacity of neurons and the neuroinhibitory microenvironment. To achieve an effective modulation of the multi-faceted neuroinhibitory microenvironment, we developed a 3D-BPH nanoscaffold through establishing a viscous interfacial 3D LBL self-assembly method. The 3D-BPH nanoscaffold achieved a biomimetic Young’s modulus and showed tunable biochemical properties for anti-inflammatory applications as well as promoted neural cell behaviors through the formation of favorable neural ECM. We explored the synergistic effects of targeting both neuroinhibitory factors (neuroinflammation and inhibitory ECM) on the promotion of neural regeneration in vitro and demonstrated the enhanced treatment of SCI in vivo. It was found that a robust suppression of inflammation and a selective reduction of fibrotic scarring, promoted by a drug-loaded 3D-BPH nanoscaffold, directly improved axonal growth and functional recovery in vivo, implying an active role of scaffold-mediated suppression of the inhibitory microenvironment and functional recovery after CNS injuries. Moving forward, a comprehensive study on the mechanistic pathways in vivo would be essential to confirm and understand the synergistic effect to improve the design of biomaterials in the treatment of SCI. The correlation of scaffold degradation to the release of MRI signals as well as the regeneration of the spinal cord in vivo could also encourage us to establish our 3D-BPH nanoscaffold as a precise therapeutic platform for personalized treatment of SCI. Furthermore, a comprehensive study on the systemic cytotoxic effects of the 3D-BPH nanoscaffold in a more relevant large animal model would be essential for the clinical translation of our therapeutic platform. Broadly, considering that neuroinflammation and deposition of inhibitory ECM components commonly exist in many different CNS injuries, a thorough investigation on the therapeutic effects of multifunctional biomaterials in different CNS injury models could provide novel perspectives on understanding the crosstalk among inflammation, ECM, and biomaterials that are crucial to treating CNS injuries.
Supplementary Material
ACKNOWLEDGMENT
Prof. Ki-Bum Lee acknowledges the partial financial support from the NSF (CHE-1429062), the New Jersey Commission on Spinal Cord (CSCR17IRG010; CSCR16ERG019), NIH R21 (R21AR071101), and NIH R01 (1R01DC016612, 3R01DC016612-01S1, and 5R01DC016612-02S1). We acknowledge Shavin Patel, Yunlong Zhang, and Ha-Yeong Jeon, for assistance in the synthesis of nanoscaffolds. We are also grateful for Jeffery Luo, Dr. Hyeon-Yeol Cho, Dr. Jinho Yoon, and Christopher Rathnam for their help in the characterization of the scaffold.
Footnotes
SUPPORTING INFORMATION
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Letao Yang, Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, 123 Bevier Road, Piscataway, NJ 08854, USA.
Brian M. Conley, Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, 123 Bevier Road, Piscataway, NJ 08854, USA
Susana R. Cerqueira, Miami Project to Cure Paralysis, Department of Neurological Surgery, University of Miami School of Medicine, 1095 NW 14th Terrace, LPLC 4-19, Miami, FL 33136, United States of America.
Thanapat Pongkulapa, Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, 123 Bevier Road, Piscataway, NJ 08854, USA.
Shenqiang Wang, Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, 123 Bevier Road, Piscataway, NJ 08854, USA.
Jae K. Lee, Miami Project to Cure Paralysis, Department of Neurological Surgery, University of Miami School of Medicine, 1095 NW 14th Terrace, LPLC 4-19, Miami, FL 33136, United States of America.
Ki-Bum Lee, Department of Chemistry and Chemical Biology, Rutgers, The State University of New Jersey, 123 Bevier Road, Piscataway, NJ 08854, USA.
REFERENCES:
- [1].a) Fawcett JW, Neurochem. Res 2020, 45, 144; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, Zachariou V, Guo L, Zhang B, He XJ, Friedel RH, Zou HY, Nat. Neurosci 2020, 23, 337; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Walsh JG, Muruve DA, Power C, Nat. Rev. Neurosci 2014, 15, 84. [DOI] [PubMed] [Google Scholar]
- [2].a) Simon DW, McGeachy MJ, Bayir H, Clark RSB, Loane DJ, Kochanek PM, Nat. Rev. Neurol 2017, 13, 171; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yiu G, He ZG, Nat. Rev. Neurosci 2006, 7, 617; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Milich LM, Ryan CB, Lee JK, Acta Neuropathol. 2019, 137, 785; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Ong W, Pinese C, Chew SY, Adv. Drug Deliver. Rev 2019, 149, 19; [DOI] [PubMed] [Google Scholar]; b) Rocha LA, Silva D, Barata-Antunes S, Cavaleiro H, Gomes ED, Silva NA, Salgado AJ, Adv. Funct. Mat, 2020, 26. [Google Scholar]
- [4].Donaghue IE, Tam R, Sefton MV, Shoichet MS, J. Control. Release, 2014, 190, 219. [DOI] [PubMed] [Google Scholar]
- [5].a) Rochford AE, Carnicer-Lombarte A, Curio VF, Williams GG, Barone DG, Adv. Mat 2020, 17; [DOI] [PubMed] [Google Scholar]; b) Li SR, Nih LR, Bachman H, Fei P, Li YL, Nam E, Dimatteo R, Carmichael ST, Barker TH, Segura T, Nat. Mat 2017, 16, 953; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Cell 2012, 150, 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI, Science 2004, 303, 1352. [DOI] [PubMed] [Google Scholar]
- [7].a) Srikanth M, Kessler JA, Nat. Rev. Neurol 2012, 8, 307; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ledesma HA, Li XJ, Carvalho-de-Souza JL, Wei W, Bezanilla F, Tian BZ, Nat. Nanotechnology 2019, 14, 645; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tian BZ, Liu J, Dvir T, Jin LH, Tsui JH, Qing Q, Suo ZG, Langer R, Kohane DS, Lieber CM, Nat. Mat 2012, 11, 986. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Zhang BB, Yan W, Zhu YJ, Yang WT, Le WJ, Chen BD, Zhu RR, Cheng LM, Adv. Mat 2018, 30, 23. [Google Scholar]
- [8].Yang LT, Chueng STD, Li Y, Patel M, Rathnam C, Dey G, Wang L, Cai L, Lee KB, Nat. Comm 2018, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gong LL, Cao LN, Shen ZM, Shao L, Gao SR, Zhang C, Lu JF, Li WD, Adv. Mat 2018, 30. [DOI] [PubMed] [Google Scholar]
- [11].Tran AP, Silver J, Science 2015, 348, 285; [DOI] [PubMed] [Google Scholar]; Kipnis J, Science 2016, 353, 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He ZG, Courtine G, Sofroniew MV, Nature 2018, 561, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ji B, Li M, Budel S, Pepinsky RB, Walus L, Engber TM, Strittmatter SM, Relton JK, European Journal of Neurosci. 2005, 22, 587; [DOI] [PMC free article] [PubMed] [Google Scholar]; Olson L, Exp. Neurol 2013, 248, 309. [DOI] [PubMed] [Google Scholar]
- [14].Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV, Nature 2016, 532, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].a) Solanki A, Chueng STD, Yin PT, Kappera R, Chhowalla M, Lee KB, Adv. Mat 2013, 25, 5477; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shah S, Yin PT, Uehara TM, Chueng STD, Yang L, Lee KB, Adv. Mat 2014, 26, 3673; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tang W, Fan WP, Zhang WZ, Yang Z, Li L, Wang ZT, Chiang YL, Liu YJ, Deng LM, He LC, Shen ZY, Jacobson O, Aronova MA, Jin A, Xie J, Chen XY, Adv. Mat 2019, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dey G, Yang L, Lee K-B, Wang L, J. Phys. Chem. C 2018, 122, 29017. [Google Scholar]
- [16].Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen SC, Tuszynski MH, Nat. Med 2019, 25, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Liu ZM, Tang ML, Zhao JP, Chai RJ, Kang JH, Adv. Mat 2018, 30, 20. [Google Scholar]
- [18].a) Ichinose I, Mizuki S, Ohno S, Shiraishi H, Kunitake T, Polym. J 1999, 31, 1065; [Google Scholar]; b) Zou J, Kim F, Nat. Comm 2014, 5, 5254. [DOI] [PubMed] [Google Scholar]
- [19].Molla MR, Levkin PA, Adv. Mat 2016, 28, 1159. [DOI] [PubMed] [Google Scholar]
- [20].a) Bencherif SA, Sands RW, Ali OA, Li WA, Lewin SA, Braschler TM, Shih T-Y, Verbeke CS, Bhatta D, Dranoff G, Nat. Comm 2015, 6, 7556; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu J,Fu TM, Cheng ZG, Hong GS, Zhou T, Jin LH, Duvvuri M, Jiang Z, Kruskal P, Xie C, Suo ZG, Fang Y, Lieber CM, Nat. Nanotech 2015, 10, 629. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bencherif SA, Warren Sands R, Ali OA, Li WA, Lewin SA, Braschler TM, Shih T-Y, Verbeke CS, Bhatta D, Dranoff G, Mooney DJ, Nat. Comm 2015, 6, 7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].a) Thuret S, Moon LDF, Gage FH, Nat. Rev. Neurosci 2006, 7, 628; [DOI] [PubMed] [Google Scholar]; b) Popovich PG, Longbrake EE, Nat. Rev. Neurosci 2008, 9, 481; [DOI] [PubMed] [Google Scholar]; c) David S, Kroner A, Nat. Rev. Neurosci 2011, 12, 388. [DOI] [PubMed] [Google Scholar]
- [22].Hollister SJ, Nat. Mat 2005, 4, 518. [DOI] [PubMed] [Google Scholar]
- [23].a) Place ES, Evans ND, Stevens MM, Nat. Mat 2009, 8, 457; [DOI] [PubMed] [Google Scholar]; b) Karimi A, Shojaei A, Tehrani P, J. Chem. Neuroanat 2017, 86, 15. [DOI] [PubMed] [Google Scholar]
- [24].Pineau I, Lacroix S, J. Comp. Neurol 2007, 500, 267. [DOI] [PubMed] [Google Scholar]
- [25].Tang M, Song Q, Li N, Jiang Z, Huang R, Cheng G, Biomaterials 2013, 34, 6402. [DOI] [PubMed] [Google Scholar]; Lee WC, Loh KP, Lim CT, Biomaterials 2018, 155, 236. [DOI] [PubMed] [Google Scholar]; Chen M, Patra PK, Lovett ML, Kaplan DL, Bhowmick S, J Tissue Eng Regen M, 2009, 3, 269. [DOI] [PubMed] [Google Scholar]
- [26].a) Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J, N. Eng. J. Med 1990, 322, 1405; [DOI] [PubMed] [Google Scholar]; b) Kiraz M, Demir E, World Neurosurg. 2020, 136, E504. [DOI] [PubMed] [Google Scholar]
- [27].a) Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY, Lipton SA, J. Neurosci 2005, 25, 6401; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Busch SA, Horn KP, Silver DJ, Silver J, J. Neurosci 2009, 29, 9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].a) Orive G, Anitua E, Pedraz JL, Emerich DF, Nat. Rev. Neurosci 2009, 10, 682; [DOI] [PubMed] [Google Scholar]; b) Cerqueira SR, Oliveira JM, Silva NA, Leite‐Almeida H, Ribeiro‐Samy S, Almeida A, Mano JF, Sousa N, Salgado AJ, Reis RL, Small 2013, 9, 738; [DOI] [PubMed] [Google Scholar]; c) Kim Y.-t., Caldwell J-M, Bellamkonda RV, Biomaterials 2009, 30, 2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rowe RG, Daley GQ, Nat. Rev. Genet 2019, 20, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].a) Bartholdi D, Schwab ME, Brain Res. 1995, 672, 177; [DOI] [PubMed] [Google Scholar]; b) Fu ES, Saporta S, J. Neurosurg. Anesthes 2005, 17, 82; [DOI] [PubMed] [Google Scholar]; c) Park J, Zhang Y, Saito E, Gurczynski SJ, Moore BB, Cummings BJ, Anderson AJ, Shea LD, PNAS. 2019, 116, 14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].a) Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Science 2011, 331, 928; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Vogelaar CF, König B, Krafft S, Estrada V, Brazda N, Ziegler B, Faissner A, Müller HW, PloS one 2015, 10, e0134371; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bradbury EJ, Burnside ER, Nat. Comm. 2019, 10, 3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].a)Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK, Neurobio. Disease 2015, 74, 114; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shearer MC, Fawcett JW, Cell Tissue Res. 2001, 305, 267. [DOI] [PubMed] [Google Scholar]
- [33].Soderblom C, Lee DH, Dawood A, Carballosa M, Santamaria AJ, Benavides FD, Jergova S, Grumbles RM, Thomas CK, Park KK, Guest JD, Lemmon VP, Lee JK, Tsoulfas P, Eneuro 2015, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG, J. Neurotrauma 2006, 23, 635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.