Abstract
Background:
The contribution of substance use disorders to the burden of severe maternal morbidity in the United States is poorly understood. The objective was to estimate the independent association between substance use disorders during pregnancy and risk of severe maternal morbidity.
Methods:
Retrospective analysis of a weighted 53.4 million delivery hospitalizations from 2003–2016 among females aged>18 in the National Inpatient Sample. We constructed measures of substance use disorders using diagnostic codes for cannabis, opioids, and stimulants (amphetamines or cocaine) abuse or dependence during pregnancy. The outcome was the presence of any of the 21 CDC indicators of severe maternal morbidity. Using weighted multivariable logistic regression, we estimated the association between substance use disorders and adjusted risk of severe maternal morbidity. Because older age at delivery is predictive of severe maternal morbidity, we tested for effect modification between substance use and maternal age by age group (18–34 y vs >34 y).
Results:
Pregnant women with an opioid use disorder had an increased risk of severe maternal morbidity compared with women without an opioid use disorder (18–34 years: aOR: 1.51; 95% CI: 1.41,1.61, >34 years: aOR: 1.17; 95% CI: 1.00,1.38). Compared with their counterparts without stimulant use disorders, pregnant women with a simulant use disorder (amphetamines, cocaine) had an increased risk of severe maternal morbidity (18–34 years: aOR: 1.92; 95% CI: 1.80,2.0, >34 years: aOR: 1.85; 95% CI: 1.66,2.06). Cannabis use disorders were not associated with an increased risk of severe maternal morbidity.
Conclusion:
Substance use disorders during pregnancy, particularly opioids, amphetamines, and cocaine use disorders, may contribute to severe maternal morbidity in the United States.
Keywords: Pregnancy, opioid use disorder, cannabis use disorder, stimulant use disorder, severe maternal morbidity
1. INTRODUCTION
The United States has a higher rate of maternal mortality than any other high-resource nation (Alkema et al., 2016). CIosely related to maternal mortality, severe maternal morbidity encompasses a series of acute adverse events during pregnancy, delivery, or postpartum and provides a population measure of “near miss” events (Creanga et al., 2014; Say et al., 2009). During the past two decades, the United States has experienced a 45% increase in the rate of severe maternal morbidity (Fingar, 2018; Kuklina et al., 2009).
Prior research has suggested that a number of factors may explain the increase in the rate of severe maternal morbidity, inCIuding older maternal age at delivery (Aoyama et al., 2019; Lisonkova et al., 2017), racial and geographic disparities in the receipt of life-saving interventions (Janevic et al., 2020; Kozhimannil et al., 2019), and an increasing burden of chronic health conditions in pregnancy (Admon et al., 2017; Kuklina and Callaghan, 2011; Kuklina and Callaghan, 2010). Little is known about the contribution of substance use disorders to the increase in severe maternal morbidity. Over the past decade, marked increases in amphetamine, opioid, and cannabis use have led to a rise in number of pregnant women who develop a substance use disorder (Admon et al., 2018a; Haight et al., 2018; Jarlenski, 2020; Young-Wolff et al., 2017), which may affect the risk of severe maternal morbidity though both biological mechanisms, such as increased risk of infectious disease and poor nutrition (Behnke et al., 2013; Centers for Disease and Prevention, 2012), and through social mechanisms, such as stigma and poverty (Jenkins and Gordon, 2015; Terplan et al., 2015). Opioid use disorder is associated with cardiovascular risks that could increase risk of severe maternal morbidity with potentially greater risk at older ages (Andersson and Vasan, 2018). Stimulant use disorders may be associated with increased risk of hemorrhage and preeCIampsia (Atkinson et al., 2015; Cohen et al., 2017), which could increase risk of severe maternal morbidity. Cannabis use in pregnancy is common, yet the association between cannabis use disorders and severe maternal morbidity is unknown. While some prior research has suggested increased risk of severe maternal morbidity among patients with any substance use disorders (Admon et al., 2018a; Admon et al., 2018b), prior research has not considered the range of commonly used substances in pregnancy or the potential for effect modification with greater maternal age.
Despite the co-occurring temporal increases in the prevalence of substance use disorders during pregnancy and maternal morbidity, the independent contribution of substance use disorders to maternal morbidity remains unCIear. Therefore, our objective was to estimate the independent association between substance use disorders and the risk of severe maternal morbidity in the United States.
2. METHODS AND MATERIALS
2.1. Data
We obtained the National Inpatient Sample (NIS) data from 2003–2016. The NIS is an annual, nationally representative sample of U.S. hospital discharges administered by the U.S. Agency for Healthcare Research and Quality. Specifically, the NIS comprises a 20% stratified sample of data elements collected from hospital discharges across the country, exCIuding rehabilitation and long-term care hospitals. Data inCIude information on patient demographic characteristics, diagnosis and procedure codes, diagnosis related groups, and patient severity and comorbidities. The University of Pittsburgh Institutional Review Board determined this study was exempt because the data were fully de-identified.
We identified hospital stays for obstetric delivery from 2003 to 2016 using a validated algorithm inCIuding Diagnosis Related Groups (DRGs) and ICD-9-CM diagnosis codes (Kuklina et al., 2008), and we then cross-walked the ICD-9-CM codes to ICD-10-CM codes to identify delivery hospitalizations that occurred after Oct 1, 2015. The weighted and unweighted counts of delivery hospitalizations identified before and after the ICD-9 to ICD-10 transition were highly consistent (Supplementary Material Table S1). We inCIuded delivery hospitalizations for females aged 18 years and older. Those younger than age 18 were exCIuded because substance use disorder treatment guidelines are different for adolescents (National Institute on Drug Abuse, 2020). The final analytic sample inCIuded pooled cross-sectional data, inCIuding a weighted 53.4 million (unweighted N=11.1 million) delivery hospitalizations.
2.2. Exposure Measurement
For each delivery hospitalization, we constructed non-mutually exCIusive, binary measures for 7 different substances defined as ICD-9-CM and ICD-10-CM diagnosis codes for the abuse or dependence of alcohol, amphetamines, cannabis, cocaine, opioids, or tobacco (Supplementary Material Table S2). Next, we created binary indicators for 3 broad categories of substance use disorders identified at the delivery hospitalization: cannabis, opioid, and stimulant (amphetamine or cocaine) use disorders.
2.3. Outcomes
Our primary outcome was a binary measure of severe maternal morbidity, defined according to the algorithm published by the Centers for Disease Control and Prevention (CDC (Centers for Disease Control and Prevention, 2015). The CDC algorithm identifies the presence of any of 21 indicator conditions for severe maternal morbidity. Taken together, the presence of any of these indicators represents an adverse outcome that is a ‘near miss’ with maternal death. We also constructed a secondary binary measure of severe maternal morbidity, exCIuding blood transfusion from the definition. Although the gold standard measurement is transfusion of 4 or more units of packed red blood cells (Main et al., 2015), the NIS data lack information on the amount of blood that was transfused. Therefore, we present results for severe maternal morbidity with and without blood transfusion (Kuklina et al., 2009).
2.4. Confounders
Covariates inCIuded age group (18–34 years, >34 years); race/ethnicity (White, Black, Hispanic/Latina, Asian/Pacific Islander, Other race); insurance type (Medicaid, private insurance, other insurance, or self-pay/no charge); quartiles of household income at the zip-code level; and urban/rural county of patient residence (Ingram and Franco, 2014). We also inCIuded indicators for CIinical comorbidities associated with both substance use disorders and severe maternal morbidity inCIuding any additional substance use, HIV or HCV infection, mental health conditions, renal disease, cardiovascular disease, pre-existing or gestational diabetes, hypertension, asthma, and anemia (Supplementary Table S2 inCIudes ICD-9-CM and ICD-10-CM diagnosis codes used).
Because of a large amount of missing data for race/ethnicity in early years of the NIS (~20%), we used a full conditional specification approach to conduct multivariate imputation of race (Healthcare Cost and Utilization Project, 2015). Using a discriminant function, imputed race/ethnicity was modeled on patient age, an indicator for in-hospital death, patient location, NIS stratum, insurance type, ZIP code-based income quintile, an indicator for severe maternal morbidity, and indicators for CIinical comorbidities. All analyses presented inCIude imputed race/ethnicity. Observations with other variables with missing data (insurance type, zip code income, patient urban/rural county of residence) were exCIuded; all had missingness of <3%.
2.5. Statistical Analysis
We employed multivariable logistic regression to estimate the association between each substance use category and risk of severe maternal morbidity. All analyses incorporated survey weights to produce nationally representative results (Healthcare Cost and Utilization Project, 2019). Because maternal age of 35 years or greater is a well-established risk factor for severe maternal morbidity, we were interested in examining whether the association of substance use with severe maternal morbidity might increase with age. Therefore, we inCIuded a statistical interaction term between age groups and substance use disorders in all models.
We fit one model for each substance use disorder or category of disorders. All models inCIuded age group indicators, an interaction between age group and the primary substance use disorder, an indicator of any other substance use disorders, and CIinical and demographic confounders. The equation shown below demonstrates our modeling of cannabis use disorder and risk of severe maternal morbidity:
Where SMM is severe maternal morbidity; CAN represents the diagnosis of cannabis use disorder, AgeGrp signifies whether age at delivery is >34 years (vs 18–34), TOB indicates the presence of tobacco use, OtherSUD indicates the presence of any additional substance use disorders, and X inCIudes a vector of demographic and CIinical confounders for each delivery hospitalization i. From this model, we calculated the linear combination of the effects of age group, cannabis use disorder, and their interaction terms (i.e., β0 + β1 + β2 + β3 represents the adjusted association between cannabis use disorder and SMM among women aged>34), controlling for other substance use and demographic and CIinical confounders. We replicated this model and linear combination for each substance use disorder category (i.e. cannabis, opioid, stimulant use disorders). We present the adjusted association and related 95% CI between each substance use disorder category and severe maternal morbidity within each age group. Because severe maternal morbidity is a rare outcome, odds ratios approximate risk ratios.
2.6. Sensitivity Analyses
We conducted sensitivity analyses to examine the robustness of our estimates. To examine the consistency of our estimates given a different specification of substance use disorders, we conducted a multivariable regression where we inCIuded indicators for each of 7 specific substance use disorder diagnoses (alcohol, amphetamines, cannabis, cocaine, opioids, tobacco, and sedatives/hallucinogens), and their interactions with age group. To examine results had we not imputed race, we conducted a complete-case analysis in which those observations with missing race were exCIuded.
3. RESULTS
Weighted descriptive characteristics of the study population, overall and stratified by the diagnosis of any substance use disorder at delivery hospitalization, are shown in Table 1. The diagnosis of a substance use disorder during pregnancy was more common among women who were younger, had non-Hispanic white race, and who resided in rural counties. Medicaid was the primary insurance coverage for 70% of pregnant women with a substance use disorder, compared with 40% of pregnant women without a substance use disorder. All CIinical comorbidities, with the exception of gestational diabetes, were markedly higher among pregnant women diagnosed with any substance use disorder relative to those not diagnosed with a substance use disorder. Notably, the prevalence of a mental health condition diagnosis at delivery was 13% among those with a substance use disorder, compared to 2.7% among those without a substance use disorder.
Table 1.
Weighted descriptive characteristics of delivery hospitalizations among females ages 18 and older in the United States, overall and by substance use disorder status
| Overall, Weighted N=53,405,672 | No substance use disorder, Weighted N=50,384,176 | Any substance use disorder, Weighted N=3,021,496 | |
|---|---|---|---|
| Age group, % (95% CI) | |||
| 18 – 34 years | 84.6 (84.3,84.8) | 84.2 (83.9,84.6) | 90.3 (90.2,90.5) |
| > 34 years | 15.4 (15.1,15.7) | 15.8 (15.5,16.1) | 9.7 (9.6,9.9) |
| Race/Ethnicity*, % (95% CI) | |||
| White | 54.0 (53.1,55.0) | 53.1 (52.1,54.1) | 69.9 (68.9,70.9) |
| Black | 13.3 (12.8,13.8) | 13.2 (12.7,13.7) | 15.7 (15.0,16.4) |
| Hispanic | 21.3 (20.3,22.3) | 22.1 (21.1,23.1) | 8.3 (7.8,8.8) |
| Other | 11.3 (10.9,11.8) | 11.6 (11.2,12.1) | 6.1 (5.6,6.6) |
| Insurance Type†, % (95% CI) | |||
| Medicaid | 41.6 (40.1,42.5) | 39.8 (38.9,40.8) | 70.1 (69.4,70.8) |
| Private Insurance | 51.9 (50.8,53.0) | 53.7 (52.6,54.8) | 22.8 (22.2,23.4) |
| Other / Medicare | 3.3 (3.2,3.5) | 3.3 (3.1,3.5) | 4.1 (3.9,4.4) |
| Self-pay / No-charge | 3.2 (3.0,3.5) | 3.2 (3.0,3.5) | 3.0 (2.9,3.2) |
| CIinical Comorbidities‡, % (95% CI) | |||
| HIV | 0.11 (0.10,0.12) | 0.10 (0.09,0.11) | 0.30 (0.27,0.33) |
| HCV | 0.25 (0.24,0.26) | 0.11 (0.11,0.12) | 2.6 (2.4,2.7) |
| Mental Health condition§ | 3.3 (3.2,3.3) | 2.7 (2.6,2.8) | 12.5 (12.3,12.8) |
| Renal disease in pregnancy | 0.19 (0.19,0.20) | 0.19 (0.18,0.19) | 0.29 (0.27,0.30) |
| Heart disease in pregnancy | 0.71 (0.68,0.74) | 0.71 (0.68,0.74) | 0.81 (0.77,0.86) |
| Gestational diabetes | 6.8 (6.7,6.9) | 6.9 (6.8,7.0) | 6.2 (6.1,6.3) |
| Hypertension | 4.9 (4.8,5.0) | 4.9 (4.8,5.0) | 5.5 (5.4,5.6) |
| Anemia | 9.3 (9.0,9.5) | 9.0 (8.8,9.3) | 13.2 (12.8,13.5) |
| Asthma | 3.2 (3.1,3.2) | 2.9 (2.8,3.0) | 7.6 (7.4,7.8) |
| Thyroid disorder | 2.7 (2.6,2.7) | 2.7 (2.7,2.8) | 2.0 (1.9,2.0) |
| County of Residence ∥, % (95% CI) | |||
| Urban | 85.6 (85.1,86.3) | 86.3 (85.7,86.9) | 74.8 (73.7,75.9) |
| Rural | 14.4 (13.7,15.0) | 13.7 (13.1,14.3) | 25.2 (24.1,26.3) |
Note: Substance use disorder defined as at least one of any of the following diagnoses: alcohol, amphetamine, cannabis, cocaine, opioid, sedative or tobacco use disorders.
Missing race/ethnicity data were imputed using a full conditional specification approach based on observed patient characteristics.
Other insurance inCIudes Veterans Administration and military health plans.
See Appendix for full description of conditions and ICD-9 and ICD-10 diagnostic codes
Mental health conditions inCIude depressive disorders, anxiety, bipolar disorder, schizophrenia, suicidal ideation, and suicide attempt
Urban/rural county of residence based on the National Center for Health Statistics Urban-Rural CIassification scheme.
The weighted prevalence of cannabis, opioid, and stimulant use disorders among pregnant women aged 18–34 were 0.75, 0.40, and 0.41, respectively (Table 2). The comparable weighted prevalences among women aged >34 were 0.26, 0.27, and 0.43, respectively. Table 2 also shows that the unadjusted associations between cannabis, opioid, and stimulant use disorders and the rate of severe maternal morbidity, with and without blood transfusion, by age group. The rates of severe maternal morbidity were significantly greater among women with cannabis, opioid, or stimulant use disorders. The unadjusted associations between cannabis, opioid, or stimulant use disorders were markedly greater among women aged >34 years, relative to those aged 18–34 years. Results were consistent across both definitions of severe maternal morbidity, with and without blood transfusion. (See Appendix Table 3 for the rates of each specific severe maternal morbidity indicator, by age group and substance use disorder status).
Table 2.
Weighted unadjusted association between substance use disorders and severe maternal morbidity among delivery hospitalizations among females ages 18 and older in the United States
| Substance use disorder prevalence, % | Severe maternal morbidity per 100,000 | Unadjusted Odds Ratio (95% CI)† | Severe maternal morbidity per 100,000 excl. blood transfusion | Unadjusted Odds Ratio (95% CI)‡ |
|---|---|---|---|---|
| Age 18–34 | ||||
| 0.75 | - | - | - | - |
| - | 1,397 | ref | 456 | ref |
| - | 2,235 | 1.61 (1.52, 1.72) | 654 | 1.44 (1.31, 1.58) |
| 0.40 | - | - | - | - |
| - | 1,397 | ref | 455 | ref |
| - | 2,962 | 2.16 (2.02, 2.30) | 1,038 | 2.29 (2.07, 2.54) |
| 0.41 | - | - | - | - |
| - | 1,394 | ref | 455 | ref |
| - | 3,709 | 2.73 (2.57, 2.89) | 1,098 | 2.43 (2.20, 2.69) |
| Age >34 | ||||
| 0.26 | - | - | - | - |
| - | 2,066 | ref | 719 | ref |
| - | 4,660 | 2.32 (2.01, 2.67) | 1,457 | 2.041 (1.588, 2.623) |
| 0.27 | - | - | - | - |
| - | 2,065 | ref | 718 | ref |
| - | 5,000 | 2.50 (2.17, 2.87) | 1,818 | 2.560 (2.027, 3.231) |
| 0.43 | - | - | - | - |
| - | 2,052 | ref | 715 | ref |
| - | 6,955 | 3.57 (3.25, 3.92) | 2,237 | 3.18 (2.71, 3.72) |
InCIudes amphetamines and cocaine use disorders
From a univariate logistic regression of each substance use disorder on severe maternal morbidity, stratified by maternal age group
From a univariate logistic regression of each substance use disorder on severe maternal morbidity exCIuding blood transfusions, stratified by maternal age group
Figure 1 shows the weighted adjusted association between cannabis, opioid, and stimulant use categories and the risk of severe maternal morbidity, according to age group. Results can be interpreted as the association between each category of substance use disorder and severe maternal morbidity, adjusting for all other substance use disorders and demographic and CIinical confounders. After adjusting for demographic characteristics and CIinical comorbidities, cannabis use disorder was associated with a small reduction in the risk of severe maternal morbidity either in those aged 18–34 (aOR: 0.95; 95% CI: 0.87,1.01) and was not associated with risk of severe maternal morbidity in those aged>34 (aOR: 0.91; 95% CI: 0.78,1.06). Compared with women without opioid use disorder, women with an opioid use disorder had a 50% increased risk (aOR: 1.51 95% CI: 1.41,1.61) of severe maternal morbidity among those aged 18–34. By comparison, among women aged>34, having an opioid use disorder was associated with a smaller increased risk of severe maternal morbidity (aOR: 1.17; 95% CI: 1.00,1.38). Stimulant use disorders were associated with a substantially increased risk of severe maternal morbidity among those aged 18–34 (aOR: 1.92, 95% CI: 1.80,2.04) compared with women without a stimulant use disorder, with a similar increased risk observed among those aged>34 (aOR: 1.85, 95% CI: 1.66,2.06). Results from the multivariable models were consistent in terms of magnitude, direction, and 95% confidence intervals when examining the association between substance use disorders and risk of severe maternal morbidity, exCIuding blood transfusions.
Figure 1. Weighted adjusted association between substance use disorders in pregnancy and severe maternal morbidity among females ages 18 and older in the US.
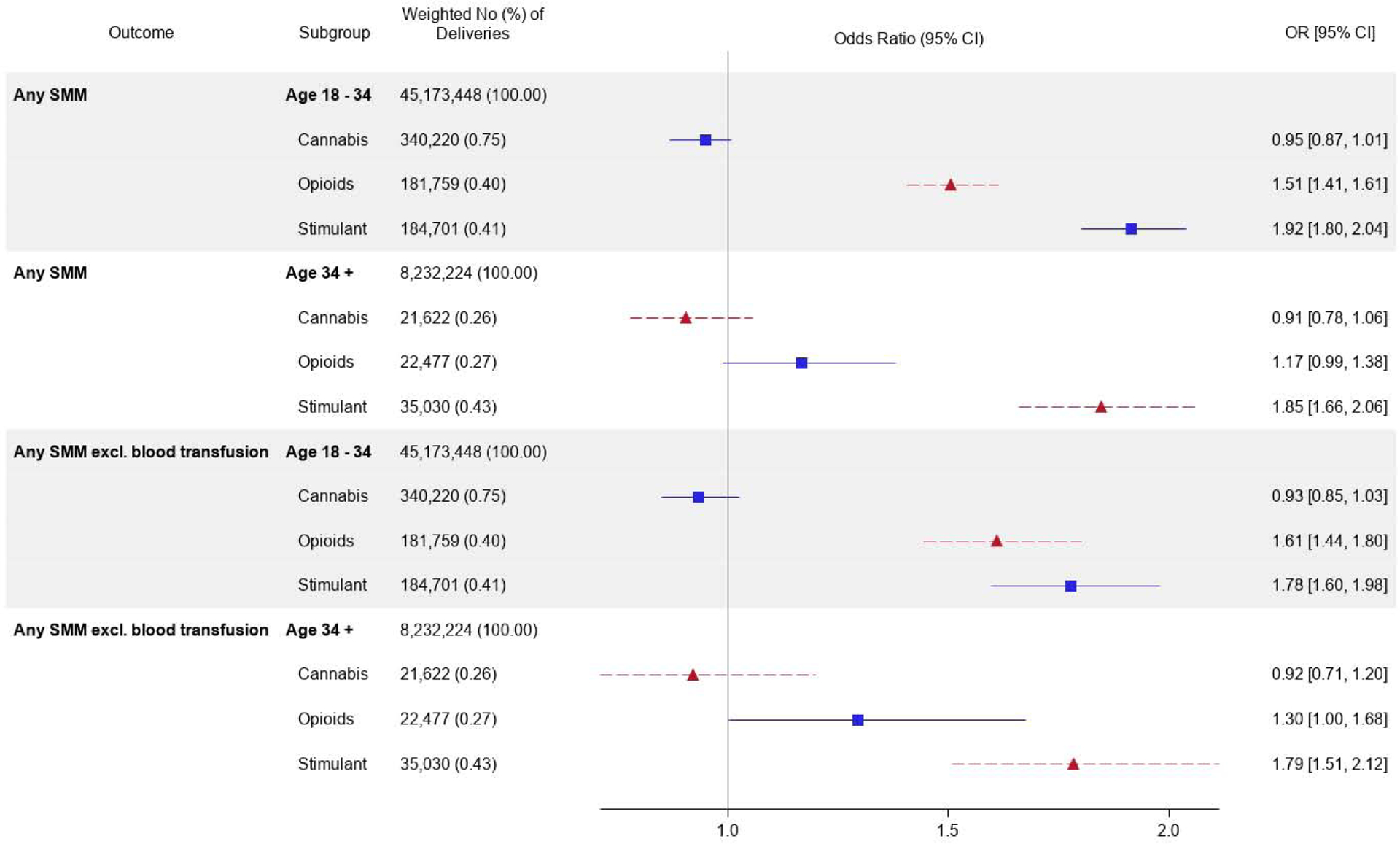
Adjusted odds ratios represent the linear combination of age group, substance use disorders, and their interaction terms. Models control for age, race/ethnicity, patient insurance, rural/urban residence, and all CIinical comorbidities (HIV, HCV, mental health conditions, renal or cardiovascular disease in pregnancy, diabetes or gestational diabetes, hypertension, anemia, asthma, and thyroid disease). Models also control for use of tobacco, alcohol, or any substance use other than the primary substance.
3.1. Sensitivity Analyses
Results from a model inCIuding indicators for specific substances were highly consistent with the primary results (Supplementary Material Table S4). There were no meaningful differences in our results when we inCIuded only women with complete data for race and ethnicity (Supplementary Material Table S5).
4. DISCUSSION
In this nationally representative study, we found that pregnant women with either an opioid or stimulant use disorder have a significantly increased risk of severe maternal morbidity compared with women who did not have these disorders, and that cannabis use was not associated with an increased risk of severe maternal morbidity. The magnitude and direction of associations we observed were consistent across maternal age groups and were consistent when considering severe maternal morbidity with and without inCIuding blood transfusions. These findings indicate that the rise in the prevalence of substance use disorders among pregnant women may accompany other chronic conditions such as hypertension and diabetes as a contributing factor to the rise in severe maternal morbidity in the United States.
We did not observe an independent association between cannabis use disorders and increased risk of severe maternal morbidity, after controlling for other substance use inCIuding tobacco. Although smoked cannabis is associated with an increased risk of low birthweight, (El Marroun et al., 2009; National Academy of Medicine Committee on the Health Effects of Marijuana, 2017) evidence is mixed on whether and how cannabis is associated other adverse pregnancy outcomes (Metz et al., 2017). Notably, cannabis acts differently from other substances of abuse, such as narcotics or alcohol, in that it does not act on the central nervous system but rather through the endocannabinoid system (Behnke et al., 2013; Metz and Stickrath, 2015). A potential explanation for our current finding may be that cannabis use is not associated with injection drug use and overdose, which can precipitate sequelae such as sepsis, endocarditis, cardiovascular and pulmonary failure which are on the causal pathway to severe maternal morbidity (Smid et al., 2019).
Opioid and stimulant use disorders were independently associated with substantial increases in the risk of severe maternal morbidity. Opioid use disorders were associated with a moderate (17%−50%) increased risk of severe maternal morbidity. However, the risks associated with opioid use disorder are mitigated by treatment with buprenorphine or methadone (Jones et al., 2012; Zedler et al., 2016). Because the NIS data do not allow us to observe medication treatment for opioid use disorder, the risks we observed represent the average association among women with and without medication treatment (Krans et al., 2019). Stimulant use disorders, inCIuding amphetamine or cocaine use disorders, were associated with nearly double the risk of severe maternal morbidity. This is consistent with past research linking these substances to increased risk of mortality in the post-delivery period (Hser et al., 2012; Wolfe et al., 2005). Surprisingly, in adjusted analyses, we did not identify a stronger association between substance use disorders and severe maternal morbidity among those in the older age group. Substance use or abuse often develops at younger ages with progression to dependence over time, and women may progress to dependence at a faster rate than men (Hernandez-Avila et al., 2004). Additionally, a lack of effect modification by age might reflect that older women may be more likely to receive treatment for substance use disorders in pregnancy relative to younger women (Krans et al., 2019).
4.1. Limitations
This study has limitations. First, we relied on diagnosis codes to identify substance use disorders, which may introduce exposure misCIassification. Because of the lack of validation studies of diagnostic codes for identifying substance use in pregnancy (McGrew et al., 2020), it is unCIear how such exposure misCIassification would bias results (Lash, 2007). We note that characteristics of our exposed group are highly consistent with prior research of women with substance use disorders (Substance Abuse and Mental Health Services Administration, 2014). Second, the NIS data do not allow for us to accurately distinguish between substance use and substance use disorders. For instance, a woman who with substance use or a substance use disorder may be more likely to receive a diagnosis of substance use disorder because of increased screening or urine testing pregnancy. Third, the data lacked information on treatment for substance use disorders in pregnancy, which we would expect to be an important effect modifier. The associations in our study therefore represent average associations among women with and without treatment. Fourth, the data did not allow us to follow the same individuals over time, preCIuding our ability to assess the progression of substance use disorders within the same person. Fifth, the CDC algorithm to identify severe maternal morbidity in hospital discharge data is limited and has been shown to result in false positive cases (Himes and Bodnar, 2020; Main et al., 2016). To address this limitation, we assessed severe maternal morbidity with and without blood transfusion in the definition and our results were consistent. Finally, we cannot rule out possible bias from unmeasured confounding. Although we inCIuded a broad range of demographic and CIinical comorbidities available in the NIS, we were unable to measure social-environmental factors, such as neighborhood context, that affect both substance use disorders and risk of severe maternal morbidity (Wang et al., 2020).
4.2. ConCIusion
Substance use, particularly the use of opioids, amphetamines, or cocaine, may contribute to severe maternal morbidity in the United States. Our findings highlight the need for universal delivery of evidence-based screening and treatment of substance use disorders in pregnancy. Further investigation is needed to elucidate the complex causal pathways by which specific substance use disorders or polysubstance use may influence the risk of severe maternal morbidities and how substance use treatment may mitigate the risk of severe maternal morbidity.
Supplementary Material
HIGHLIGHTS.
Severe maternal morbidity is increasing in the United States
The contribution of substance use disorders to severe maternal morbidity is unclear
Opioid and stimulant use disorders were associated with risk of severe maternal morbidity
Cannabis use disorder was not associated with increased risk of severe maternal morbidity
Role of Funding Source
This study was supported by the National Institute on Drug Abuse (award number R01DA045675). The funder had no role in the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disCIaimers that apply to the journal pertain.
Conflict of Interest
No conflicts deCIared.
REFERENCES
- Admon LK, Bart G, Kozhimannil KB, Richardson CR, Dalton VK, Winkelman TNA, 2018a. Amphetamine- and Opioid-Affected Births: Incidence, Outcomes, and Costs, United States, 2004–2015. Am. J. Public Health, e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon LK, Winkelman TNA, Moniz MH, Davis MM, Heisler M, Dalton VK, 2017. Disparities in Chronic Conditions Among Women Hospitalized for Delivery in the United States, 2005–2014. Obstet. Gynecol 130, 1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon LK, Winkelman TNA, Zivin K, Terplan M, Mhyre JM, Dalton VK, 2018b. Racial and Ethnic Disparities in the Incidence of Severe Maternal Morbidity in the United States, 2012–2015. Obstet. Gynecol 132, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, Fat DM, Boerma T, Temmerman M, Mathers C, Say L, United Nations Maternal Mortality Estimation Inter-Agency Group, c., technical advisory, g., 2016. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter-Agency Group. Lancet 387, 462–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C, Vasan RS, 2018. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol 15, 230–240. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Pinto R, Ray JG, Hill AD, Scales DC, Lapinsky SE, Hladunewich MA, Seaward GR, Fowler RA, 2019. Association of Maternal Age With Severe Maternal Morbidity and Mortality in Canada. JAMA Netw. Open 2, e199875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson AL, Santolaya-Forgas J, Blitzer DN, Santolaya JL, Matta P, Canterino J, Oyelese Y, 2015. Risk factors for perinatal mortality in patients admitted to the hospital with the diagnosis of placental abruption. J. Matern. Fetal. Neonatal. Med 28, 594–597. [DOI] [PubMed] [Google Scholar]
- Behnke M, Smith VC, Committee on Substance Abuse, Committee on Fetus and Newborn, 2013. Prenatal substance abuse: short- and long-term effects on the exposed fetus. Pediatrics 131, e1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C., Prevention, 2012. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR Recomm. Rep 61, 1–40. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2015. Severe Maternal Morbidity in the United States. http://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html. (Accessed May 21 2016).
- Cohen JM, Hernandez-Diaz S, Bateman BT, Park Y, Desai RJ, Gray KJ, Patorno E, Mogun H, Huybrechts KF, 2017. Placental Complications Associated With Psychostimulant Use in Pregnancy. Obstet. Gynecol 130, 1192–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanga AA, Berg CJ, Ko JY, Farr SL, Tong VT, Bruce FC, Callaghan WM, 2014. Maternal mortality and morbidity in the United States: where are we now? J. Womens Health (Larchmt) 23, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Steegers EA, Jaddoe VW, Hofman A, Verhulst FC, van den Brink W, Huizink AC, 2009. Intrauterine cannabis exposure affects fetal growth trajectories: the Generation R Study. J. Am. Acad. Child. Adolesc. Psychiatry 48, 1173–1181. [DOI] [PubMed] [Google Scholar]
- Fingar K, Hambrick MM, Heslin KC, Moore JE, 2018. Trends and Disparities in Delivery Hospitalizations Involving Severe Maternal Morbidity, 2006–2015, in: Agency for Healthcare Research and Quality (Ed.). Rockville, MD: [PubMed] [Google Scholar]
- Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM, 2018. Opioid Use Disorder Documented at Delivery Hospitalization - United States, 1999–2014. MMWR Morb. Mortal. Wkly. Rep 67, 845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthcare Cost and Utilization Project, 2015. HCUP Methods Series: Missing data methods for the NIS and the SID, in: Healthcare Cost and Utilization Project (Ed.). [Google Scholar]
- Cost Healthcare and Project Utilization, 2019. Trend weights for HCUP NIS data. https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. (Accessed December 28 2019).
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR, 2004. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol. Depend 74, 265–272. [DOI] [PubMed] [Google Scholar]
- Himes KP, Bodnar LM, 2020. Validation of criteria to identify severe maternal morbidity. Paediatr. Perinat. Epidemiol [DOI] [PubMed] [Google Scholar]
- Hser YI, Kagihara J, Huang D, Evans E, Messina N, 2012. Mortality among substance-using mothers in California: a 10-year prospective study. Addiction 107, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DD, Franco SJ, 2014. 2013 NCHS Urban-Rural CIassification Scheme for Counties. Vital Health Stat 2, 1–73. [PubMed] [Google Scholar]
- Janevic T, Zeitlin J, Egorova N, Hebert PL, Balbierz A, Howell EA, 2020. Neighborhood Racial And Economic Polarization, Hospital Of Delivery, And Severe Maternal Morbidity. Health Aff. (Millwood) 39, 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlenski M, Paul NC, Krans EE, 2020. Polysubstance use among pregnant women with opioid use disorder in the United States, 2007–2016. Obstet. Gynecol. epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JA, Gordon AJ, 2015. Substance use disorder prevention and treatment in stigmatized patient populations: ripe for innovation. Subst. Abus 36, 1–2. [DOI] [PubMed] [Google Scholar]
- Jones HE, Fischer G, Heil SH, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, O’Grady KE, Arria AM, 2012. Maternal Opioid Treatment: Human Experimental Research (MOTHER)--approach, issues and lessons learned. Addiction 107 Suppl 1, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhimannil KB, Interrante JD, Henning-Smith C, Admon LK, 2019. Rural-Urban Differences In Severe Maternal Morbidity And Mortality In The US, 2007–15. Health Aff. (Millwood) 38, 2077–2085. [DOI] [PubMed] [Google Scholar]
- Krans EE, Kim JY, James AE 3rd, Kelley D, Jarlenski MP, 2019. Medication-Assisted Treatment Use Among Pregnant Women With Opioid Use Disorder. Obstet. Gynecol 133, 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklina E, Callaghan W, 2011. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995–2006. BJOG 118, 345–352. [DOI] [PubMed] [Google Scholar]
- Kuklina EV, Callaghan WM, 2010. Cardiomyopathy and other myocardial disorders among hospitalizations for pregnancy in the United States: 2004–2006. Obstet. Gynecol 115, 93–100. [DOI] [PubMed] [Google Scholar]
- Kuklina EV, Meikle SF, Jamieson DJ, Whiteman MK, Barfield WD, Hillis SD, Posner SF, 2009. Severe obstetric morbidity in the United States: 1998–2005. Obstet. Gynecol 113, 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklina EV, Whiteman MK, Hillis SD, Jamieson DJ, Meikle SF, Posner SF, Marchbanks PA, 2008. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Matern. Child Health. J 12, 469–477. [DOI] [PubMed] [Google Scholar]
- Lash TL, 2007. Heuristic thinking and inference from observational epidemiology. Epidemiology 18, 67–72. [DOI] [PubMed] [Google Scholar]
- Lisonkova S, Potts J, Muraca GM, Razaz N, Sabr Y, Chan WS, Kramer MS, 2017. Maternal age and severe maternal morbidity: A population-based retrospective cohort study. PLoS Med 14, e1002307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main EK, Abreo A, McNulty J, Gilbert W, McNally C, Poeltler D, Lanner-Cusin K, Fenton D, Gipps T, Melsop K, Greene N, Gould JB, Kilpatrick S, 2016. Measuring severe maternal morbidity: validation of potential measures. Am. J. Obstet. Gynecol 214, 643 e641–643 e610. [DOI] [PubMed] [Google Scholar]
- Main EK, Goffman D, Scavone BM, Low LK, Bingham D, Fontaine PL, Gorlin JB, Lagrew DC, Levy BS, National Parternship for Maternal, S., Council for Patient Safety in Women’s Health, C., 2015. National Partnership for Maternal Safety: consensus bundle on obstetric hemorrhage. Anesth. Analg 121, 142–148. [DOI] [PubMed] [Google Scholar]
- McGrew KM, Homco JB, Garwe T, Dao HD, Williams MB, Drevets DA, Jafarzadeh SR, Zhao YD, Carabin H, 2020. Validity of International CIassification of Diseases codes in identifying illicit drug use target conditions using medical record data as a reference standard: A systematic review. Drug Alcohol. Depend 208, 107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz TD, Allshouse AA, Hogue CJ, Goldenberg RL, Dudley DJ, Varner MW, Conway DL, Saade GR, Silver RM, 2017. Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. Am. J. Obstet. Gynecol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz TD, Stickrath EH, 2015. Marijuana use in pregnancy and lactation: a review of the evidence. Am. J. Obstet. Gynecol 213, 761–778. [DOI] [PubMed] [Google Scholar]
- National Academy of Medicine Committee on the Health Effects of Marijuana, 2017. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. The National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2020. Evidence-based approaches to treating asolescent substance use disrorders. https://www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide/evidence-based-approaches-to-treating-adolescent-substance-use-disorders. (Accessed June 22 2020).
- Say L, Souza JP, Pattinson RC, et al. , 2009. Maternal near miss--towards a standard tool for monitoring quality of maternal health care. Best Pract. Res. CIin. Obstet. Gynaecol 23, 287–296. [DOI] [PubMed] [Google Scholar]
- Smid MC, Metz TD, Gordon AJ, 2019. Stimulant Use in Pregnancy: An Under-recognized Epidemic Among Pregnant Women. CIin. Obstet. Gynecol 62, 168–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuse Substance and Mental Health Services Administration, 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series; Rockville, MD [Google Scholar]
- Terplan M, Kennedy-Hendricks A, Chisolm MS, 2015. Prenatal Substance Use: Exploring Assumptions of Maternal Unfitness. Subst. Abuse 9, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Glazer KB, Howell EA, Janevic TM, 2020. Social Determinants of Pregnancy-Related Mortality and Morbidity in the United States: A Systematic Review. Obstet. Gynecol 135, 896–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe EL, Davis T, Guydish J, Delucchi KL, 2005. Mortality risk associated with perinatal drug and alcohol use in California. J. Perinatol 25, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Tucker LY, Alexeeff S, Armstrong MA, Conway A, Weisner C, Goler N, 2017. Trends in Self-reported and Biochemically Tested Marijuana Use Among Pregnant Females in California From 2009–2016. JAMA 318, 2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedler BK, Mann AL, Kim MM, Amick HR, Joyce AR, Murrelle EL, Jones HE, 2016. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: a systematic review and meta-analysis of safety in the mother, fetus and child. Addiction 111, 2115–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


