Abstract
SARS-CoV-2 emerged in Wuhan in December 2019 and caused the pandemic respiratory disease, COVID-19.1,2 In 2003, the closely related SARS-CoV had been detected in domestic cats and a dog.3 However, little is known about the susceptibility of domestic pet mammals to SARS-CoV-2. Two of 15 dogs from households with confirmed human cases of COVID-19 in Hong Kong SAR were found to be infected using quantitative RT-PCR, serology, sequencing the viral genome, and in one dog, virus isolation. SARS-CoV-2 RNA was detected in a 17 year-old neutered male Pomeranian from five nasal swabs collected over a 13 day period. A 2.5 yo male German Shepherd dog had SARS CoV-2 RNA on two occasions and virus was isolated from nasal and oral swabs. Both dogs had antibody responses detected using plaque reduction neutralisation assays. Viral genetic sequences of viruses from the two dogs were identical to the virus detected in the respective human cases. The animals remained asymptomatic during quarantine. The evidence suggests that these are instances of human-to-animal transmission of SARS-CoV-2. It is unclear whether infected dogs can transmit the virus to other animals or back to humans.
When any human case of COVID-19 is diagnosed in Hong Kong SAR, the person is hospitalised and household contacts, regarded as “close contacts,” are quarantined for COVID-19 in designated centres. Affected pet owners are given the option of having their dogs and cats looked after and isolated by the Hong Kong Agriculture, Fisheries and Conservation Department (AFCD). Specimens are collected from these animals to assess whether they are infected with SARS-CoV-2 and to assist in determining the best methods for managing animals in quarantine, including timing of release back to the owner. Fifteen dogs and seven cats from households with known COVID-19 cases have been quarantined and tested as of 27rd March. During this period, two dogs had virological test results demonstrating they were infected.
Results
Canine case 1 is a 17 year-old neutered male Pomeranian which had a number of pre-existing diseases, including a Grade II heart murmur, systemic and pulmonary hypertension, chronic renal disease, hypothyroidism and a previous history of hyperadrenocorticism (Dr Florence Chan personal communication). The owner, a 60 year-old woman, developed symptoms on the 12th February 2020 and was diagnosed with COVID-19 disease on 24 February 2020. One female domestic helper (secondary case A) in the household developed a fever on 16th February and was subsequently confirmed to be infected. The remaining three members of the household were sent to a quarantine centre on 26th February, and one of them was confirmed to be infected in March 7th (secondary case B). The dog was transferred to a holding facility, managed by AFCD on the 26th February and nasal, oral, and rectal swabs as well as a faecal sample were collected. Additional specimens for virus detection were collected from the dog on six occasions. A blood sample was collected on 3rd March for serological testing (see Fig 1). Throughout the period in quarantine the dog remained bright and alert with no obvious change in clinical condition.
Figure 1:
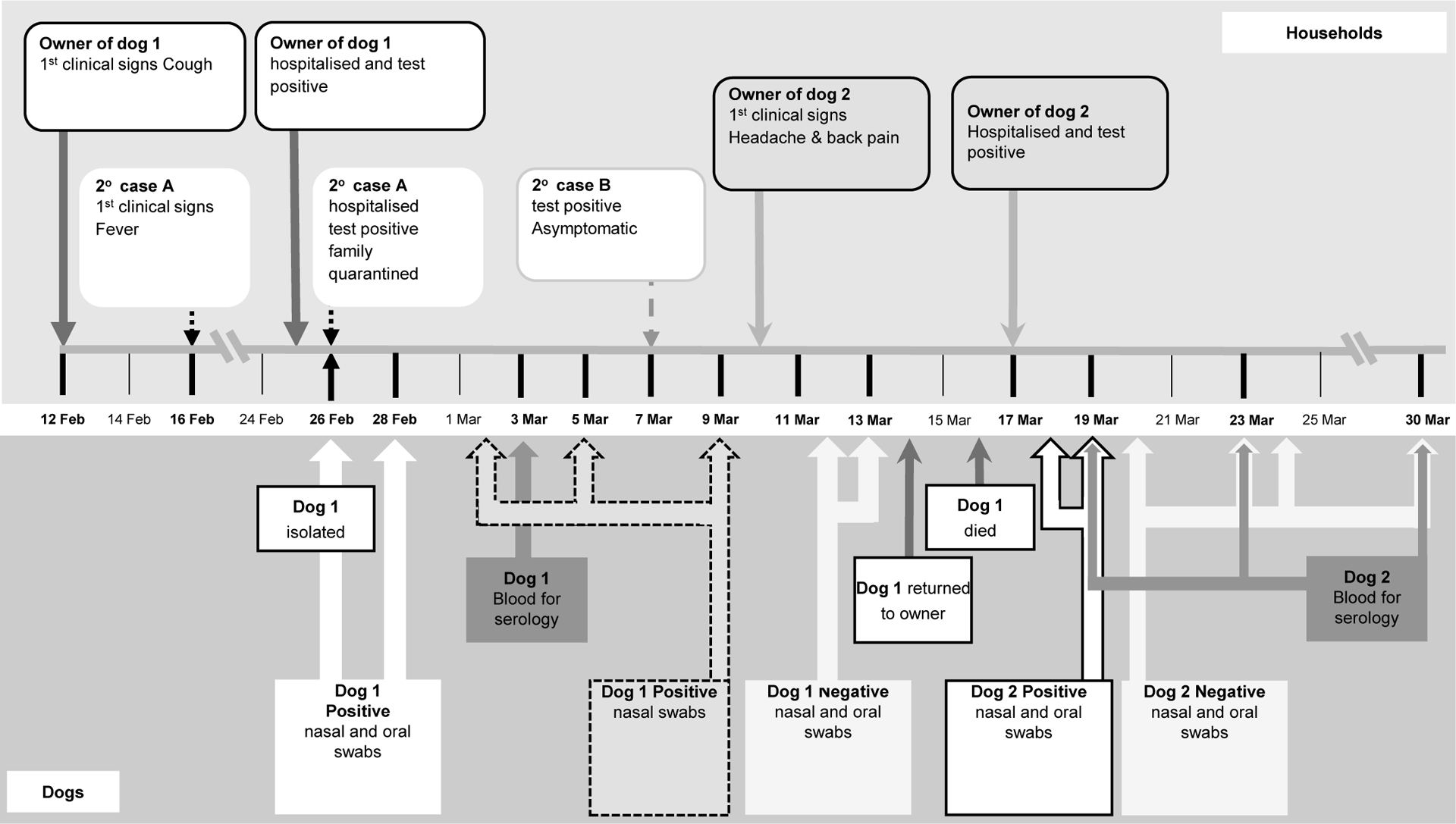
Timeline of events.
SARS-CoV-2 RNA was detected from nasal swabs collected from canine case 1 by quantitative RT-PCR4,5 on 5 consecutive specimens collected on and between 26th February and March 9th (Table 1). Rectal and faecal specimens were negative. Attempt to culture the virus was unsuccessful. Given the low viral load (range 7.5×102 to 2.6 ×104 RNA copies per mL of specimen) it was unlikely that virus culture would be successful as in human patients with COVID-19, virus isolation was less likely to be successful when viral load in the specimen was <106 per mL.6
Table 1:
RT-PCR testing results on nasal and oral swabs of the dogs and serology#
| TLVL laboratory | HKU laboratory | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E gene (Ct) | RdRP gene (Ct) | nsp14 gene (Ct) | N gene (Ct) | nsp16 gene (Ct) | M gene (Ct) | ||||||||||
| Date of collection |
Nasal | Oral | Nasal | Oral | Nasal | Oral | Nasal | N Gene copies/mL nasal swab virus transport medium@ |
Oral | Nasal | Oral | Nasal | Oral | Serum (PRNT90 antibody titre) |
|
| Canin Case 1 (potential exposure 12th-26th Feb) | |||||||||||||||
| 26-Feb-20 | 33.9 | 34.52 | 38.97 | Neg* | 36.76 | 37.96 | 34.71 | 11,741 | 36.48 | 37.94 | 39.25 | 36.91 | 37.95 | ||
| 28-Feb-20 | 31.98 | Neg | 37.44 | Neg | 38.96 | 39.01 | 34.58 | 10,145 | Neg | 38.64 | Neg | 38.97 | Neg | ||
| 2-Mar-20 | 31.69 | Neg | Neg | Neg | 32.49 | Neg | 33.2 | 25,788 | Neg | 32.71 | Neg | 32.41 | Neg | ||
| 3-Mar-20 | 1:80 | ||||||||||||||
| 5-Mar-20 | 33.58 | Neg | 38.53 | Neg | 39.14 | Neg | 38.43 | 751 | Neg | 37.72 | Neg | Neg | Neg | ||
| 9-Mar-20 | 30.07 | Neg | Neg | Neg | 35.86 | Neg | 34.97 | 7,777 | Neg | 36.96 | Neg | 36.24 | Neg | ||
| 12-Mar-20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ||
| 13-Mar-20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ||
| Canin Case 2 (potential exposure 10th-17th Mar | |||||||||||||||
| 18-Mar-20 | 24.85 | 26.60 | 31.19 | 32.63 | 26.74 | 28.72 | 27.31 | 724,500 | 29.33 | 28.26 | 30.29 | 27.73 | 29.49 | ||
| 19-Mar-20 | 28.11 | 31.23 | 36.12 | 38.45 | 32.98 | 36.09 | 32.66 | 62,933 | 36.98 | 33.65 | 36.95 | 32.17 | 35.97 | <1:10 | |
| 20-Mar-20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ||
| 23-Mar-20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 1:40 | |
| 24-Mar-20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ||
| 30-Mar-20 | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 1:160 | |
| Cutoff CT for positive | <36 | <39 | <40 | <40 | <40 | <40 | |||||||||
Abbreviations: Neg - negative
The E gene, nsp14 and N gene RT-PCR assays are reactive with SARS-CoV-2, SARS-CoV and closely related bat-SARS CoV viruses.
The RdRp, nsp16 and M gene RT-PCR assays are specific for SARS-CoV-2
gene copies per mL of original swab specimen with adjustment for virus extraction dilutions.
Assumes no pre-symptomatic shedding of virus from human casesCanine
Canine case 2, was a 2.5 year-old male German Shepherd Dog in good health from a household in which the owner developed symptoms on 10th March and was diagnosed with COVID-19 on 17th of March 2020. Specimens from this dog were collected five times between 18th and 30th March . Both oral and nasal swabs tested positive on the first two occasions (Table 1). Rectal swabs collected on 18th March tested positive in four of the six assays, all with higher Ct values (lower viral load) than those obtained from oral and nasal swabs. A second dog kept in the household was sampled on five occasions from the 18th to 30th March and tested negative for virus RNA.
Serum samples collected from canine case 1 on 3rd March 2020, and from canine case 2 on 19th, 23rd and 30th March 2020 were tested for SARS-CoV-2 antibody by 90% plaque reduction neutralization tests (PRNT90)7. The serum from canine case 1 had a PRNT90 titre of 1:80. The antibody titres from canine case 2 were <1:10 (March 19th), 1:40 (March 23rd) and 1:160 (March 30th). The second dog in the household remained antibody negative on the 30th March. Twenty control canine sera tested negative for PRNT90 neutralizing antibody.
Viral RNA from the nasal swab specimen collected from canine case 1 on 26th and 28th February was genetically sequenced directly from the clinical specimen and compared with the virus found in clinical specimens from the owner and secondary cases A and B. Full virus genome sequence (29,764 nucleotides) was obtained from the index case, secondary cases A and B. Viral sequences of length 27,871 nucleotides (94% of genome) and 26,025 nucleotides (93% of genome) was obtained from the nasal swabs collected on the 26th and 28th February, respectively. The viral sequences from the index case and two secondary cases were identical across the full genome. Viral RNA from the nasal swabs of canine case 2 collected on 18th and 19th March and the human index case from the same household were genetically sequenced and were found to be identical across the full genome (29,764 nucleotides). On the other hand, the viruses from the two households were clearly distinguishable (Fig 2). The genome sequences derived in this study have been deposited in GenBank Accession numbers MT215193, MT215194, MT215195, MT270814, MT270815 and MT276600.
Figure 2:
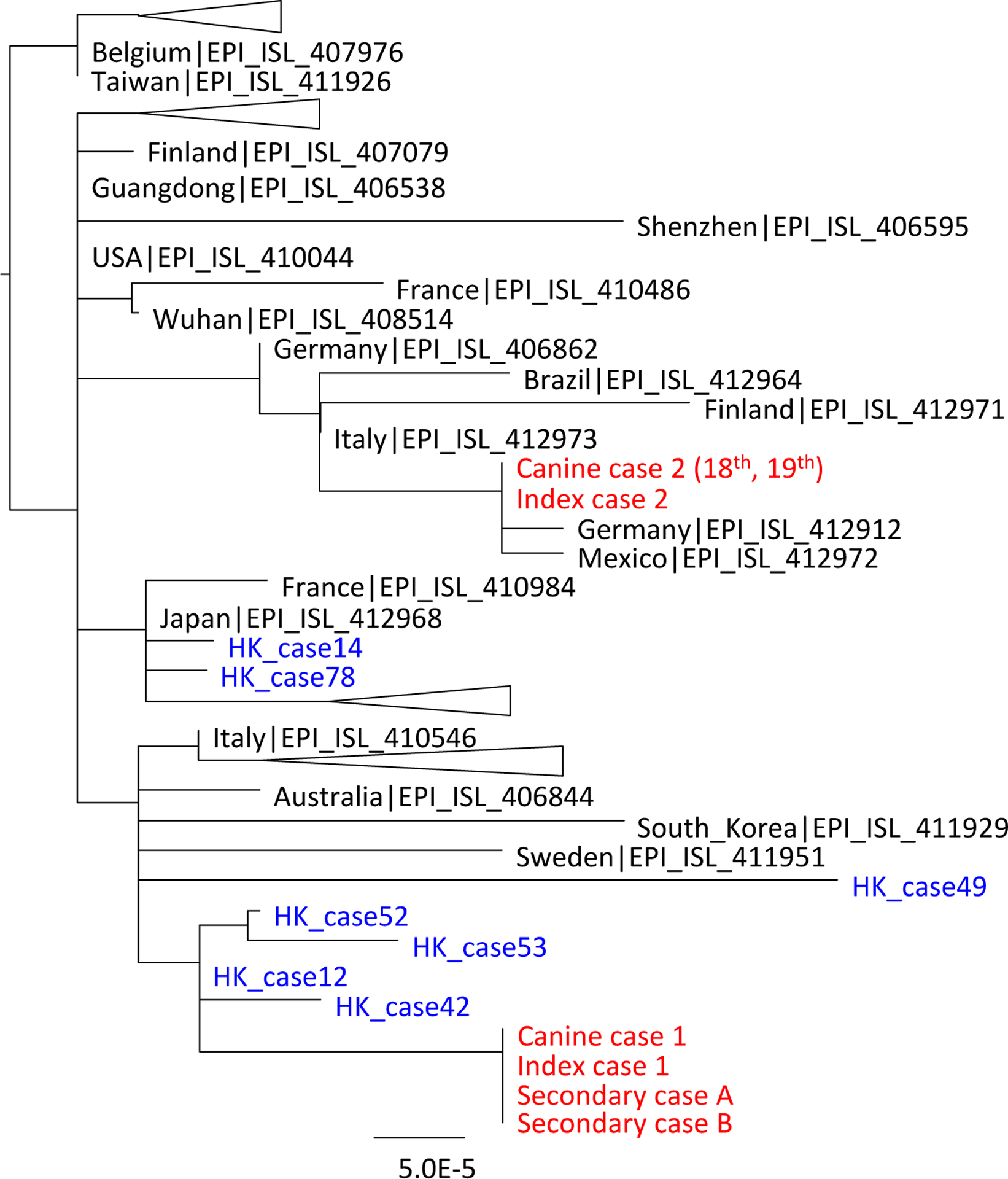
A phylogenetic tree of SARS-CoV-2 showing viruses from infected dogs and humans in Hong Kong. Virus sequences from humans and animals from the two affected households are indicated in red font. Other human virus sequences in Hong Kong were indicated in blue. Other selected full and partial (length longer that 23k nucleotides) virus genomes from the GISAID data base were included in this analysis. The tree is unrooted and was constructed by maximum likelihood method using PhyML.
Discussion and Conclusions
Our results demonstrate infection of two dog by SARS-CoV-2. ACE-2 is known to be the receptor for this virus and canine ACE-2 is similar to that of humans (Extended data Figure). Of 18 amino acids that are known to be involved in interaction between ACE-2 and the spike receptor binding domain (RBD) of SARS-CoV-2, there are five that differ between humans and dogs, but none of these are in regions known to disrupt the interaction between the RBD of SARS-CoV and ACE-2.8
The evidence suggests that human-to-animal transmission of SARS-CoV-2 can occur. We do not have information on whether this virus can cause illness in dogs but no specific signs were seen in either of the infected dogs during the time they were shedding virus. The Pomeranian died two days after release from isolation, very likely due to the previous underlying diseases, but there was no permission from the owner for post mortem examination. Whether infected dogs could transmit the virus to other animals or back to humans remains unknown. In addition to the German Shepherd dog, index case 2 kept a second cross breed dog in which neither viral RNA nor antibody responses were detected, suggesting that transmission had not occurred between the two dogs within the household.
These two cases in Hong Kong demonstrate that dogs can acquire infection in households with SARS-CoV-2 infected humans. A survey of 4000 specimens from dogs, cats and horses from places where community transmission of SARS-CoV-2 was occurring in humans, did not detect any positive results suggesting the virus is not widely circulating in pet animals.9 This study did not specifically investigate dogs from households of COVID-19 patients as was done in our study. A challenge study in five six-week-old beagles conducted elsewhere demonstrated seroconversion in two dogs and detection of viral RNA (up to log106.5 copies) in rectal swabs two days after challenge and one dog had viral RNA in a rectal swab on day 6. No detectable virus was found in oropharyngeal swabs, but nasal swabs were not collected.10 Our results suggest higher viral load and longer viral shedding in nasal swabs compared to oral swabs. The experimental challenge study reported that cats had large quantities of virus in nasal mucosa and other tissues and shed sufficient virus to allow cat-to-cat transmission.10 It has recently been reported that a cat in Belgium in contact with a confirmed case tested positive for the virus.11 . SARS-CoV-2 virus RNA was detected in one cat in Hong Kong SAR, after the cut-off date for the present study. This cat was from a household with a confirmed case of COVID-19.
These findings and the results from animal testing during the SARS outbreak in 20033 have potential implications for the management of mammalian pets owned by persons who develop SARS-CoV-2 infection. There was no evidence that domestic animals played any role in onward transmission of the SARS outbreak.3 However, from a precautionary point of view, pets belonging to COVID-19 cases could be isolated and tested virologically, as is being done in Hong Kong SAR.
The findings also have implications for future zoonotic transmission events by the precursor virus of SARS-CoV-2. Rhinolophid bats are considered a likely reservoir of the precursor of SARS-CoV-2.12 However, based on experiences with SARS virus, it is likely that intermediate hosts serve to bridge transmission from bats to humans. Dogs, other canids, and felids can be sold in or found in the vicinity of wild-game animal markets, the presumed source for the initial zoonotic spill-over of SARS-CoV-2. They should be tested during investigations into the origin of this virus to determine if they play any role in spillover events.
Methods
Specimen collection
Specimens from dogs and cats were collected by veterinarians from animals sent to the AFCD isolation centre and included deep oropharyngeal and nasal swabs and a sample of fresh faeces and/or a rectal swab, placed in virus transport medium and kept on cool-packs until arrival in the laboratory. Virus Transport Medium comprised of Medium 199 (sigma M0393) as basal medium, 0.5% Bovine Serum Albumin, Antibiotics (Penicillin G, Streptomycin sulfate, Polymyxin B sulfate, Sulfamethoxazole, Nystatin, Gentamicin sulfate, Ofloxacin). Specimens were collected on at least 3 occasions (on arrival in the isolation centre and in the two days prior to release). Any animal that had a positive test was retested until no positive results were obtained. Owners provided written consent at the time their pets were moved to isolation to allow specimens to be collected and tested.
Control specimens including nasal, oral, rectal swabs and faeces were collected from 21 stray dogs soon after euthanasia. Stored residual sera from 20 dogs collected for diagnostic purposes from veterinary clinics during 2017–18 were used as controls for serology.
Specimens from humans were collected and tested by RT-PCR as part of routine clinical care and the viruses genetically sequenced as part of the routine public health response (Institutional Review Board approval UW20–168).
Quantitative RT-PCR
At the AFCD laboratory, RNA from 200uL specimen in virus transport medium was extracted using NucliSENS* easyMag* extraction kit (BioMerieux, Marcy-l’Étoile, France) following instructions provided by the manufacturer and eluted into 60uL. The RNA was tested for SARS-CoV-2 RNA in a commercial assay RT-PCR assay for the E and RdRp gene sequences (TIB Molbiol Lightmix® Modular Assays, Berlin, Germany) based on published RT-PCR assay for SARS-CoV-2.5 Positive, negative and inhibitor controls were included in each RT-PCR run and work-flow precautions were in place to minimise PCR contamination. Positive samples were sent to the HKU as an independent reference laboratory for confirmation.
Viral RNA from the original swabs referred by the AFCD laboratory were independently extracted at the HKU using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. 160uL of swab supernatant was used for RNA extraction with the final elution volume being 60uL. One-step quantitative RT-PCR assays were run for previously published nsp14 and N gene that detect SARS-CoV, SARS-CoV-2 and bat SARS-CoV.4 In addition, RT-PCR assays for nsp16 and M gene that are specific for SARS-CoV-2 with no cross-reaction with SARS-CoV were also used. The forward primer (5’-GGWCAAATCAATGATATGATTTT), reverse prime (5’-GTTGTTAACAAGAACATCACTAGA) and probe (5’-FAM-AAGTCTRCCTTTACTAAGAAGAGA-TAMRA-3’) were used for the ORF1b-nsp16 assay and forward primer (5’-GGYTCTAARTCACCCATTCA-3’), reverse prime (5’-TGATACTCTARAAAGTCTTCATA-3’) and probe (5’-FAM-AATTTAGGTTCCTGGCAATTAATT-TAMRA-3’) were used for the M gene assay. The thermal cycling conditions were identical to those published for the nsp14 and N gene assays.4 Positive, negative and inhibitor controls were included in each RT-PCR run and work-flow precautions were in place to minimise PCR contamination.13
Nasal, oral, rectal swabs and faecal samples from 21 control dogs were run by all six RT-PCR assays with negative results. No evidence of PCR inhibition was seen in any of these RNA extract.
Sequencing the viral genomes
To amplify the virus genome, reverse transcription reactions were set up using superscript IV reverse transcriptase (Thermo Fisher Scientific, Massachusetts, USA) with multiple gene specific primers targeting different regions of the viral genome (Supplementary data table). The synthesised cDNA was then subjected to multiple overlapping PCRs using Platimum Taq DNA polymerase (Thermo Fisher Scientific, Massachusetts, USA) using the protocol provided by the manufacturer. The PCRs performed were in sizes of around 2000bp designed to cover the whole virus genome. PCR amplicons were visualised by agarose gel electrophoresis. Nested PCRs were performed when necessary for genome amplification. Aliquots of 5ul PCR products and DNA ladder were loaded into wells in 2% agarose gel. Electrophoresis was run at 120V for 20 minutes in TAE buffer and the DNA band was visualised with SYBR safe DNA gel stain.
PCR amplicons obtained from the same specimens were pooled and sequenced using MiSeq sequencing platform (Illumina, California, USA). Sequencing library was prepared by Nextera XT DNA library prep Kit (Illumina) following standard protocols. Generated sequencing reads were mapped to a reference virus genome by BWA14 and genome consensus was generated by Geneious version 11.1.4 (https://www.geneious.com) with a minimal coverage depth of 20. Percentage of nucleotides at each position of the genome was calculated by bam-readcount (https://github.com/genome/bam-readcount) with minimal base quality score of 20 and minimum mapping quality score of 20.
Plaque reduction neutralization tests
BetaCoV/Hong Kong/VM20001061/2020 isolated from the nasopharynx aspirate and throat swab of a COVID-19 patient in Hong Kong was grown in Vero E6 cells (ATCC CRL-1586). Stock virus was prepared and aliquoted and stored at -80 °C until use. The virus stock was titrated in quadruplicate in Vero-E6 cells in 24-well tissue culture plates (TPP Techno Plastic Products AG, Trasadingen, Switzerland) in a biosafety level 3 facility. After one hour incubation in 5% CO2 incubator, the plates were overlaid with 1% agarose in cell culture medium and incubated for 3 days when the plates were fixed and stained and plaque forming units per mL of the virus stock was determined. Serial dilutions of serum samples were then incubated with 30–40 plaque-forming units of virus for 1 h at 37°C. The virus-serum mixtures were added on to Vero cell monolayers, incubated, overlayed and stained as above. Antibody titers were defined as the highest serum dilution that resulted in >90% (PRNT90) reduction in the number of plaques.7
Virus isolation
Fresh nasal and oral swab fluid collected from SARS-CoV-2 PCR confirmed dogs in viral transport media were used as the inoculum for virus isolation. Briefly, Vero E6 (ATCC CRL-1586) cells were cultured for 24 hours in a 24 well plate format (TPP Techno Plastic Products AG, Trasadingen, Switzerland) before inoculation. Culture medium was minimal essential medium containing 2% fetal bovine serum, 100 units/mL penicillin and 100 μg/mL streptomycin. The swab fluids were centrifuged at 5000 rpm for 10 mins at 4°C in a benchtop centrifuge and the supernatant was separated and inoculated on to Vero E6 cells in alternative wells of the 24 well plate. After two hours incubation for adsorption in a 37°C incubator containing 5% CO2, fresh virus growth medium was added to a final volume of 1ml and then incubated in a 37°C incubator containing 5% CO2 for six days. The presence of cytopathic effect (CPE) was looked for daily. Additionally, the aliquots of culture supernatant samples was collected into AVL buffer at 0hr, 24hr, 48hr and 72hrs post inoculation for PCR. The culture medium was replaced as required with fresh culture medium. Cell cultures that were negative for virus growth was blind-passaged again after six days. The culture that were positive for virus growth as judged by cytopathic effect and increasing viral load by RT-PCR were harvested and passed on to new cull culture wells in 24 well plates and then progressively onto cells in T25 culture flasks (Greiner Bio-one, Kremsmunster, Austria). Mock inoculated Vero E6 cells were used as negative control for each isolation experiment.
Extended Data
Extended data figure:
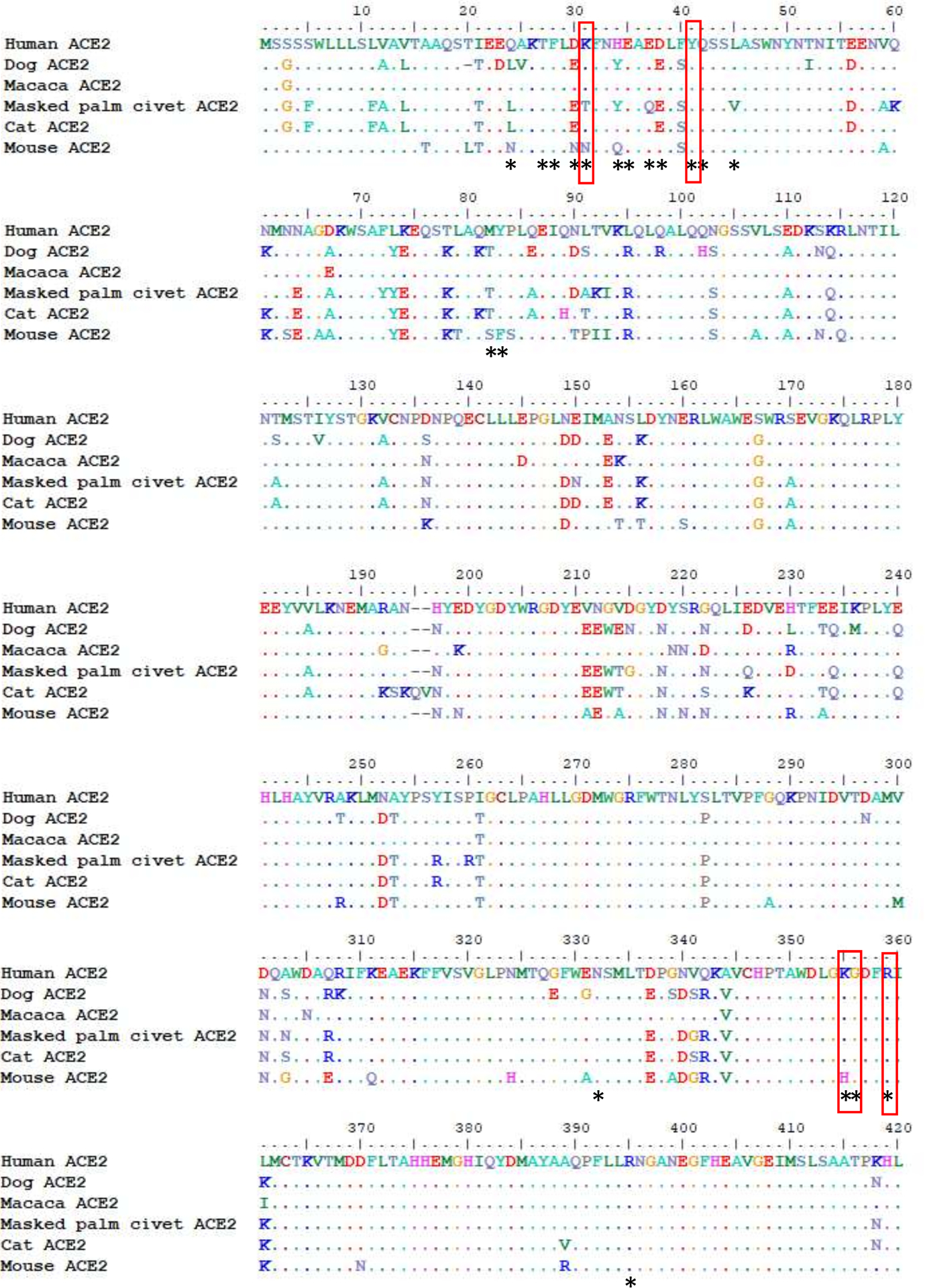
Sequence alignment of ACE2 proteins from human, dog, macaca, masked palm civet, cat and mouse. Amino acid residues of human ACE2 that are experimentally shown to interact with the receptor binding domain (RBD) of SARS-CoV-28 are denoted by *. Mutations known to disrupt the interaction between human ACE2 and RBD of SARS-CoV are highlighted in red boxes and these amino acid residues are all conserved between human and dog ACE2 proteins.
Supplementary Material
Acknowledgement
The authors acknowledge the work done by staff of the Hong Kong SAR Centre for Health Protection who undertook investigations of the human cases in the affected household, Dr Eric Tai for information on infection in pet animals during the SARS outbreak and Mr Elvis Yiu for design and preparation of the time line. We greatly appreciate the support given by the dog owners to allow the material on these cases to be published. We acknowledge research funding from the US National Institute of Allergy and Infectious Diseases (NIAID) under Centers of Excellence for Influenza Research and Surveillance (CEIRS) contract no. HHSN272201400006C. We thank Dr Hui-ling Yen for providing control canine sera and acknowledge expert technical assistance from Pavithra Krishnan and Daisy Yuet Mei Ng. We thank the core facility at the Centre for PanorOmic Sciences at the University of Hong Kong for the Illumina MiSeq sequencing of viral genomes. We gratefully acknowledge the Originating and Submitting Laboratories for sharing genetic sequences and other associated data through the GISAID Initiative for SARS-CoV-2 genome sequences.
Footnotes
Competing financial interests:
Authors declare no competing financial interests.
Reference:
- 1.Zhu N, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 382: 727–733 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 50. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200310-sitrep-50-covid-19.pdf?sfvrsn=55e904fb_2.
- 3.WHO (2003) Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) available at https://apps.who.int/iris/bitstream/handle/10665/70863/WHO_CDS_CSR_GAR_2003.11_eng.pdf85
- 4.Chu DKW, et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin Chem. pii: hvaa029. doi: 10.1093/clinchem/hvaa029. [Epub ahead of print] (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corman VM, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020. Jan;25(3). doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel R, et al. Virological assessment of hospitalized patients COVID-19. Nature. E pub ahead of print. doi: 10.1038/s41586-020-2196-x (2020). [DOI] [PubMed] [Google Scholar]
- 7.Choe PG, et al. MERS-CoV Antibody Responses 1 Year after Symptom Onset, South Korea, 2015. Emerg Infect Dis. 23:1079–1084 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan J et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. E pub ahead of print doi: 10.1038/s41586-020-2180-5 (2020) [DOI] [PubMed] [Google Scholar]
- 9.IDEXX SARS-CoV-2 (COVID-19) RealPCR Test. https://www.idexx.com/en/veterinary/reference-laboratories/idexx-sars-cov-2-covid-19-realpcr-test/
- 10.Shi J et al. Susceptibility of ferrets, cats, dogs, and different domestic animals to SARS-coroanvirus-2. Science 2020; Published online 10th April 2020 doi: 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SciCom Risque zoonotique du SARS-CoV2 (Covid-19) associé aux animaux de compagnie : infection de l’animal vers l’homme et de l’homme vers l’animal. http://www.afsca.be/comitescientifique/avis/2020/_documents/Conseilurgentprovisoire04-2020_SciCom2020-07_Covid-19petitsanimauxdomestiques_27-03-20_001.pdf.
- 12.Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579:270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustin SA, et al. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol 21;11:74 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 25:1754–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


