Abstract
Objective
We compared the prevalence of frailty by HIV serostatus and related biomarkers to the modified frailty phenotype among older individuals in a rural population in South Africa.
Methods
Questionnaire data were from a cohort of people living with HIV (PWH) on antiretroviral therapy (ART) and HIV-uninfected people aged 50 years and older sampled from the Africa Health Research Institute Demographic Health and Surveillance area in northern KwaZulu-Natal. The prevalence of frailty was compared using five categories: 1) physical activity; 2) mobility; 3) fatigue; 4) gait speed; 5) grip strength and assessed for demographic, clinical, and inflammatory correlates of frailty.
Results
Among 614 individuals in the study, 384(62.5%) were women. The median age at study enrolment was 64 years [Interquartile range (IQR) (58.6–72.0)]. 292 (47.6%) were PWH.499 (81%) were classified as either pre-frail or frail. 43 (7%) were frail and HIV positive,185 (30%) were pre-frail and HIV positive, 57 were frail and HIV negative and 214 (35%) were pre-frail and HIV negative. Frailty was similar for HIV negative and PWH (17.7% vs 14.7%, P-value 0.72). Women were more likely to be frail (18.3% vs. 13.04%, P-value 0.16). The prevalence of frailty increased with age for both HIV groups. In the multivariable analysis, the odds of being frail were higher in those aged 70 years and above than those aged between 50 and 59 years (P<0.001). Females were less likely to be pre-frail than males (P<0.001). There was no association between any of the inflammatory biomarkers and frailty and pre-frailty.
Conclusion
In this population the prevalence of frailty is similar for PWH and people without HIV, but higher for women than men. These data suggest that the odds of developing frailty is similar for PWH over the age of 50 years, who survive into older age, as for people without HIV.
Keywords: frailty measures, aging, ageing
Introduction
Frailty is characterised by a decline in functioning across multiple organ systems and is associated with a greater risk of adverse outcomes (Hoogendijk et al., 2019). Frailty prevalence increases with age, but it occurs independently from chronological age (Dent, Kowal, & Hoogendijk, 2016). With current available therapies, people living with HIV (PWH) are living longer, but are at higher risk of age-related health concerns, including frailty. HIV infection can induce biologically similar mechanisms as aging (Brothers & Rockwood, 2014; Gross et al., 2016), and, historically, the clinical trajectory of HIV disease has been shown to mimic the frailty phenotype in the United States, particularly at advanced stages of HIV infection (Desquilbet et al., 2007). Indeed, data from high-income countries suggests that as PWH age, they experience increased vulnerability to multimorbidity, and appear to be at risk for an earlier onset of the phenotype of frailty (Bloch, 2018; Desquilbet et al., 2007; Fried et al., 2001; Levett, Cresswell, Malik, Fisher, & Wright, 2016; Önen et al., 2009; Thurn & Gustafson, 2017).
Although the exact mechanisms of this increased risk of frailty are not clear, chronic immune activation and cellular senescence related to HIV infection have been indicated (Desai & Landay, 2010; Piggott, Erlandson, & Yarasheski, 2016). Similar causal pathways may be contributing to both frailty and the course of HIV infections - increased levels of circulating cytokines (C-reactive protein [CRP], tumour necrosis factor alpha [TNF-α] and interleukin-6 [IL-6]) are amongst the immunological features related to ageing and frailty previously described (Althoff et al., 2013; Hubbard, O’Mahony, Calver, & Woodhouse, 2008; Li, Manwani, & Leng, 2011). Consequently treated HIV has been classified as a disease associated with both accelerated and accentuated ageing, with effects seen both on cellular ageing and ageing phenotypes (Pathai, Bajillan, Landay, & High, 2014).
Although the majority of work in this area has come from cohort studies in the United States, there has been increasing attention to risks of frailty and disability among the large, and aging population of PWH in sub-Saharan Africa (Mugisha et al., 2016; Nyirenda et al., 2013; Pathai, Gilbert, et al., 2013). Importantly, the clinical spectrum of HIV infection in sub-Saharan Africa has changed greatly in the last decade, with the widespread distribution of antiretroviral therapy (ART). This has resulted in a decrease in AIDS-related morbidity and mortality, and an increase in the number of people living into old age with HIV infection (Negin, Mills, & Bärnighausen, 2012; Vollmer et al., 2017).
Preliminary data from South Africa in the early ART era, showed greater numbers of people with HIV than uninfected comparators, meeting frailty criteria (Pathai, Gilbert, et al., 2013; Pathai, Lawn, et al., 2013). Moreover, chronic immune activation despite treatment-mediated virologic suppression has been demonstrated in the region (Siedner et al., 2018). However, there is a recognized gap in clinical data on frailty among older PWH in the region, particularly among people on long term suppressive ART (Siedner, 2019).
Objectives
In this study we sought to leverage data from a population-based study in rural South Africa to investigate whether HIV infection is associated with the frailty phenotype, particularly among older people on long-term ART. The primary objective was to compare the prevalence of frailty by HIV serostatus among older individuals in a rural population in South Africa with the hypothesis that PWH over the age of 50 years have a higher prevalence of frailty, and that the frailty phenotype manifests at younger ages among those with HIV. The secondary objective was to identify correlates of frailty in this population, including biomarkers of inflammation such as IL-6, D-Dimer and CRP. The hypothesis being that higher levels of these biomarkers would be associated with an increased odds of the presence of frailty.
Methods
Study population
The WHO multi-country Study on global AGEing and adult health (SAGE) Wellbeing of Older People (WOPS) cohort, at the Africa Health Research Institute (AHRI), gathered data on persons aged 50 years or older living with and/or affected by HIV over three survey waves (2010, 2013 and 2017). The WOPS cohort enrolled individuals selected from the AHRI (previously Africa Centre for Population Health) Demographic Surveillance Area (DSA) (Nyirenda et al., 2012). Data for this current analysis are derived from Wave 3.
In Wave 1 and 2 participants were enrolled to represent 4 groups: 1) HIV positive on ART for ≥1 year; 2) HIV positive not on ART or on ART for ≤3 months; 3) having an adult child aged 18–49 on ART for ≥1 year or for ≤3 months; 4) having an adult child aged 18–49 who died of HIV related causes. However, there has been considerable overlap between these groups as well as changes in availability of ART over time, so in our current analysis we distinguish only by HIV status.
To achieve our target sample size for Wave 3 of WOPS (n=600, equivalent to the total sample size in both Wave 1 and Wave 2), we first recruited all previous WOPS participants, and sampled additional individuals as needed, to achieve full enrolment. All previous WOPS participants were traced and invited to participate in Wave 3. Stratified (by sex and HIV-status) random sampling was used to generate a list of new individuals to be invited. The over-sampling rate was 20%, to allow for refusals and/or individuals who could not be located. Once the target enrolment had been met, recruitment was halted.
A total of 632 people enrolled in prior waves of the study were approached to participate in Wave 3. Of these 104 were deceased, 27 were not contactable, and 34 were no longer residents of the catchment area, leaving a total of 467 individuals for re-engagement in WOPS 3. An additional 366 people (n=833 total), were identified using stratified random sampling to ensure balance by sex and HIV serostatus, from the demographic health surveillance area.
Out of the 833 identified a total of 726 consent visits were conducted yielding a final cohort of 658 participants, which met our sample size target. The remainder were unenrolled (n=68) due to death (n=28), out-migration from the study area (n=11), declined to participate (n=9) or unavailable to participate (n=20).
Study Measures
Frailty classification
The primary outcome of interest was frailty. The Fried Frailty Index defined a clinical syndrome of frailty when three or more of the following were evident: weight loss in the past year, self-reported exhaustion, weak grip strength, slow walking speed and low physical activity (Fried et al., 2001).
In our study a measure of unintentional weight loss was not included in the study design. The authors therefore assessed the degree of correlation between the measure of physical activity and of mobility to ensure that they did not measure the same modality.
The assessment found that the two measures were not highly correlated:
Difficulty in vigorous activity vs. difficulty walking 1 km: correlation = 0.58
Difficulty in vigorous activity vs. time to walk 4 m: correlation = 0.34
We therefore used a modified definition of the Fried Frailty Index consisting of the following measures: 1) physical activity; 2) mobility; 3) fatigue; 4) gait speed; and 5) grip strength.
For the sub-measures of mobility, physical activity, and fatigue, study staff conducted a questionnaire from the World Health Organization’s multi-country study on global AGEing and adult health (SAGE) (Kowal et al., 2012). The questionnaire included questions about:
Mobility: difficulty experienced with mobility, rated from 0 (no difficulty) to 5 (extremely difficult or cannot complete task)
Physical activity: difficulty experienced in walking a long distance rated from 0 (no difficulty) to 5 (extremely difficult or cannot complete task)
Fatigue: reports of long periods of low energy and fatigue (yes/no).
Gait speed
The gait speed in metres per second (m/s) was measured using a stopwatch to time taken for participants to walk a distance of four meters. To note, due to missing data and therefore reduced power calculation, grip strength and gait speed were not adjusted by BMI and height in our analysis.
Grip strength
Grip strength was measured using a dynamometer (Smedley’s Hand Dynamometer Fabrication Enterprises, New York) and results categorized according to the median (25th −75th percentile) by dominant hand, age, sex and region (Leong et al., 2016).
Participants who met at least three of five criteria were classified as frail and participants who met one or two criteria were classified as pre-frail (Fried et al., 2001):
Self-report of severe (or greater) difficulty walking a long distance
Self-report of severe (or greater) difficulty with vigorous activities
Measured grip strength of the dominant hand in the lowest quintile of the cohort (by age, sex, and geographical region)
Measured walking speed in the lowest quintile of the cohort (by sex)
Self-report of an extended time of lack of energy and fatigue
Height and weight (BMI)
Height (centimetres) was measured barefoot with a stadiometer and weight (kilograms) on a flat surface on a calibrated Seca bathroom dial scale (Model SCA726). Body Mass Index was calculated and categorised according to the WHO guidelines of underweight (<18.5 kg/m2), normal weight/healthy (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥30 kg/m2).
Blood pressure
Systolic, diastolic blood pressure and pulse rate were measured on the left upper arm while the participants were seated quietly. Three measurements were taken with short waiting periods between measures, using a digital automatic blood pressure monitor. Participants were categorised as being hypertensive when their systolic BP was ≥140 mmHg and diastolic BP was ≥90mmHg for at least two of the measurements.
HIV serostatus
Participants in Wave 3 were offered a rapid HIV test (using Abon HIV 1/2/O Triline HIV rapid Test (Alere Healthcare) and ONE STEP Anti HIV (1&2) test for repeat test (InTec Xiamen, China)), along with counselling and referral for those who tested positive. In addition, following consent, a Dried Blood Spot (DBS) specimen was collected on a filter card (Whatman 903™, GE Healthcare, Cardiff, UK) for research purposes. HIV serostatus was determined by an enzyme-linked immunosorbent assay (ELISA) test of the DBS using the GenScreen UItra HIV Ag-Ab assay (Biorad, Marnes-la-Coquette, France) which has previously been adapted and validated in-house for use on DBS.
Inflammatory markers
Interleukin 6 (IL-6), D-Dimer and C-reactive protein (CRP) biomarkers were tested using ELISAs designed to measure the biomarkers in serum, plasma and cell culture supernatant for research purposes only D-Dimer Human SimpleStep ELISA Kit; (Abcam, Cambridge, UK), Quantikine Human IL-6 ELISA (R&D Systems, Minneapolis, Minnesota, USA), and Quantikine human CRP ELISA (R&D Systems) were used to measure antibody responses to D-Dimer, IL-6 and CRP respectively from the DBS specimens collected from each of the participants.
Each ELISA assay was adapted to use a 6mm punched DBS spot eluted overnight at 4°C in the assay diluent provided in the respective kits and each protocol from this point onwards was followed as per the package insert. The optical densities (OD) were read and calculations of analyte concentrations were performed using the Gen5 V3.03 Software package on an ELx800 Universal microplate reader (BioTek, Vermont, USA). All settings and calculations were programmed as per the instructions in the package inserts of each kit and the results were reported in concentrations (ng/ml). The OD values in these cases exceed the limit of the instrument for determining a specific OD i.e. any sample with a concentration > 31.500 (for D-Dimer) or >52.500 for CRP is reported as these values. Participants were classified as having a low, medium or high concentration of each biomarker based on tertiles of each biomarker within the cohort. The thresholds used for the classification of D-Dimer were 7.6325ng/ml and 16.21ng/ml; for IL6 the values were 0.1ng/ml and 1.48ng/ml; and for CRP the thresholds were 13.1ng/ml and 34.8ng/ml.
Statistical methods
Participants with fewer than three frailty criterion measurements available in the dataset were excluded from the analysis. Values for frailty and all frailty components were tabulated and compared between PWH and those who were HIV negative. Multivariable logistic regression was performed on the primary outcome of frailty and on the secondary outcome of pre-frailty. As a sensitivity analysis, we performed an ordered logistic regression (proportional odds model) on the three level outcomes of non-frail (no frailty measures), pre-frail and frail. All models included HIV status, sex, and age group (categorized as 50–59, 60–69, 70+ years). ART status was not included as the vast majority (95%) of PWH were receiving treatment. To assess for associations between biomarkers and pre-frailty and frailty, a minimally adjusted logistic regression model (including age category and HIV serostatus) was conducted allowing a linear trend across the three tertiles of each biomarker. All point estimates are presented with 95% confidence intervals (CI). Analysis was performed in Stata version 15 (College Station, Texas, USA).
Ethical review and Informed Consent
Approval for the study was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (BF36/09). All participants provided written informed consent to participate in the study, as well as providing biological samples for storage and testing.
Results
A total of 614 out of 658 participants were included in this analysis (44 participants [6.7%] were excluded because of insufficient frailty measurements). Compared to those who were included in the analysis, those excluded were more likely to be male (51.4% vs 37.5%). The age of excluded participants was similar to those included (median 67.0 vs 66.3 years). Only 10, 5, and 10 of those excluded had data on D-Dimer, IL6 and CRP levels, respectively.
Of those in the analytic cohort, the majority (n=384, 62.5%) were female with an overall median age of 64.3 years (IQR 58.6–72.0) and approximately half were HIV positive (n=292, 47.6%). HIV negative respondents were slightly older (median age 68.7 years vs 61.0, Table 1).
Table 1.
Cohort Profile by serostatus
| Characteristic | HIV negative | HIV positive | Total |
|---|---|---|---|
| Number of participants (row percentage) | 322 (52.4%) | 292 (47.6%) | 614 |
| Age; Median (IQR) | 68.7 (61.2 – 77.5) | 61.0 (57.8 – 65.7) | 64.3 (58.6 – 72.0) |
| Sex=Female | 182 (56.5%) | 202 (69.2%) | 384 (62.5%) |
| Weight in kg; Median (IQR) (17 missing) | 71.0 (60.0 – 85.0) | 67.0 (57.0 – 80.0) | 70.0 (58.0 – 82.0) |
| Systolic blood pressure; Median (IQR) | 134.5 (120.5 – 151.0) | 121.8 (112.0 – 138.0) | 128.5 (115.5 – 145.5) |
| Diastolic blood pressure; Median (IQR) | 82.0 (74.5 – 90.5) | 78.0 (70.8 – 86.0) | 80.5 (73.0 – 89.0) |
| Hypertensive | 144 (55.3%) | 78 (26.7%) | 222 (36.2%) |
| D-Dimer (19 missing) | |||
| Low | 103 (32.8) | 97 (34.5) | 200 (33.6) |
| Medium | 97 (30.9%) | 99 (35.2%) | 196 (32.9%) |
| High | 114 (36.3%) | 85 (30.2%) | 199 (33.4%) |
| IL1 (78 missing) | |||
| High | 273 (100.0%) | 263 (100.0%) | 536 (100.0%) |
| IL6 (82 missing) | |||
| Low | 95 (34.9%) | 82 (31.5%) | 177 (33.3%) |
| Medium | 99 (36.4%) | 81 (31.2%) | 180 (33.8%) |
| High | 78 (28.7%) | 97 (37.3%) | 175 (32.9%) |
| CRP (16 missing) | |||
| Low | 125 (39.4%) | 74 (26.3%) | 199 (33.3%) |
| Medium | 105 (33.1%) | 97 (34.5%) | 202 (33.8%) |
| High | 87 (27.4%) | 110 (39.1%) | 197 (32.9%) |
Prevalence of Frailty
The prevalence of pre-frailty and frailty by serostatus is given in Table 2. In total, 100 participants (16.3%) were classified as frail having met at least three of the five criteria, with a similar proportion among HIV negative (17.7%) and PWH (14.7%, P=0.72) respondents.
Table 2.
Outcomes profile by serostatus
| Characteristic | HIV negative | HIV positive | Total |
|---|---|---|---|
| Time to walk 4 metres; Median (IQR) (57 missing) | 8.9 (7.7 – 9.9) | 8.6 (7.5 – 9.7) | 8.7 (7.6 – 9.8) |
| Difficulty walking 1 km (16 missing) | |||
| None | 43 (13.9%) | 38 (13.1%) | 81 (13.5%) |
| Mild | 104 (33.7%) | 115 (39.8%) | 219 (36.6%) |
| Moderate | 73 (23.6%) | 69 (23.9%) | 142 (23.7%) |
| Severe | 42 (13.6%) | 38 (13.1%) | 80 (13.4%) |
| Extreme | 47 (15.2%) | 29 (10.0%) | 76 (12.7%) |
| Difficulty in vigorous activity | |||
| None | 136 (42.2%) | 121 (41.4%) | 257 (41.9%) |
| Mild | 51 (15.8%) | 53 (18.2%) | 104 (16.9%) |
| Moderate | 60 (18.6%) | 53 (18.2%) | 113 (18.4%) |
| Severe | 56 (17.4%) | 59 (20.2%) | 115 (18.7%) |
| Extreme/cannot do | 19 (5.9%) | 6 (2.1%) | 25 (4.1%) |
| Grip strength (dominant hand); Median (IQR) | 11.5 (8.5 – 15.0) | 11.5 (8.5 – 14.8) | 11.5 (8.5 – 15.0) |
| Decreased energy in last 12 months | 32 (9.9%) | 25 (8.6%) | 57 (9.3%) |
| Frailty | |||
| Frailty (overall) | 57 (17.7%) | 43 (14.7%) | 100(16.3%) |
| Pre-frail | 214 (66.5%) | 185 (63.4%) | 399 (65.0%) |
| Frailty indicators | |||
| Frail: grip strength | 228 (70.8%) | 183 (62.7%) | 411 (66.9%) |
| Frail: walking speed (4m) (57 missing) | 61 (21.6%) | 47 (17.1%) | 108 (19.4%) |
| Frail: walking long distance (16 missing) | 89 (28.8%) | 67 (23.2%) | 156 (26.1%) |
| Frail: vigorous activity | 75 (23.3%) | 65 (22.3%) | 140 (22.8%) |
| Frail: energy in last 12 months | 32 (9.9%) | 25 (8.6%) | 57 (9.3%) |
| Frailty by age | |||
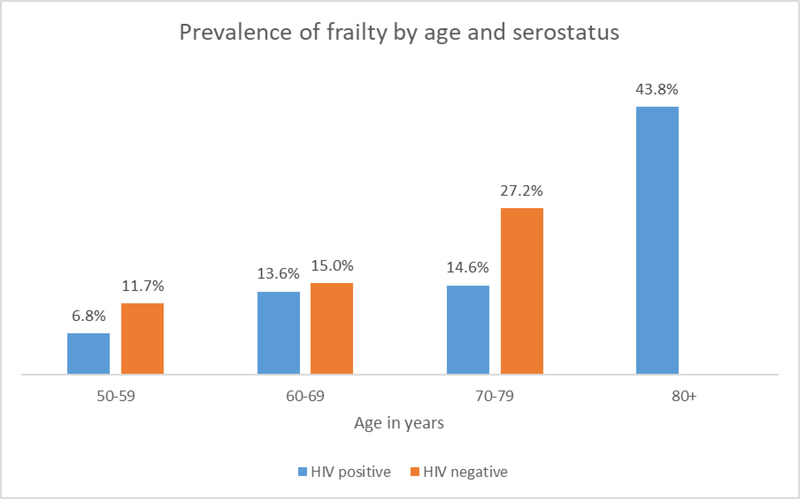
| 50–59 | 5 (6.8%) | 15 (11.7%) | 20 (10.0%) |
| 60–69 | 14 (13.6%) | 19 (15.0%) | 33 (14.3%) |
| 70–79 | 13 (14.6%) | 9 (27.2%) | 22 (18.3%) |
| 80+ | 25 (43.8%) | 0 / 4 | 25 (41.0%) |
| Frailty by BMI* | |||
| Underweight | 0 / 11 | 6 (26.1%) | 6 (17.6%) |
| Healthy | 18 (17.3%) | 12 (10.6%) | 30 (13.8%) |
| Overweight | 5 (6.2%) | 8 (11.4%) | 13 (8.7%) |
| Obese | 24 (21.0%) | 15 (18.3%) | 39 (19.9%) |
17 respondents missing data on BMI N=614
The frailty component with the highest level of observed deficit was grip strength in 66.9% of respondents.
The prevalence of frailty increased with age for both HIV negative and PWH (Fig. 1). there was clear evidence that women were less likely to be pre-fail or frail than men in this population (OR=0.43, 95% CI 0.26–0.70 Tables 3 and 4). In secondary analyses, there was no evidence that the risk of being either pre-frail or frail differed according to HIV status (OR=0.85, 95% CI 0.541.33 and being hypertensive (OR=0.94,95% CI 0.61–1.47).
Figure 1.
Prevalence of frailty by age category and HIV serostatus
Table 3.
Prevalence of frailty by sex
| Frailty category | Male | Female | Total |
|---|---|---|---|
| Healthy | 26 (11.30%) | 89 (23.18%) | 115 (18.73%) |
| Pre-frail | 174 (75.65%) | 225 (58.59%) | 399 (64.98%) |
| Frail | 30 (13.04%) | 70 (18.23%) | 100 (16.29%) |
| Total | 230 (100%) | 384 (100%) | 614 (100%) |
Table 4.
Univariate and Multivariable logistic regression models for frailty and pre-frailty
| Univariable Models | Multivariable models | |||
| Frail | ||||
| OR* (95% CI) | P-value | AOR** | P-value | |
| HIV | ||||
| Negative | Reference | Reference | ||
| Positive | 0.80 (0.52 – 1.24) | 0.319 | 1.06 (0.62 – 1.82) | 0.828 |
| Age 50–59 years | Reference | Reference | ||
| 60 – 69 years | 1.52 (0.84 – 2.74) | 0.168 | 1.59(0.83 – 3.05) | 0.16 |
| 70+ years | 3.13 (1.77 -, 5.52) | 0 | 3.49(1.75 – 6.95) | <0.001 |
| Sex | ||||
| Female Sex | 1.49 (0.94 – 2.36) | 0.093 | 1.67 (0.98 – 2.86) | 0.059 |
| IL-6 | ||||
| Lowest Tertile | 1.45 (0.84 – 2.51) | 0.187 | 1.61 (0.91 – 2.86) | 0.103 |
| Middle Tertile | Reference | Reference | ||
| Highest Tertile | 0.90 (0.50 – 1.63) | 0.73 | 0.90 (0.49 – 1.68) | 0.749 |
| D-dimer | ||||
| Lowest Tertile | 0.94 (0.53 – 1.64) | 0.822 | 1.09 (0.59 – 2.01) | 0.793 |
| Middle Tertile | Reference | Reference | ||
| Highest Tertile | 1.27 (0.75 – 2.17) | 0.378 | 1.09 (0.61 – 1.95) | 0.78 |
| CRP | ||||
| Lowest Tertile | 0.63 (0.36 – 1.11) | 0.108 | 0.84 (0.46 – 1.53) | 0.563 |
| Middle Tertile | Reference | Reference | ||
| Highest Tertile | 1.00 (0.60 – 1.66) | 0.988 | 1.08 (0.61– 1.91) | 0.79 |
| Pre-Frail | ||||
| Univariable Models | Multivariable models | |||
| HIV | OR (95% CI) | P-value | AOR | P-value |
| Negative | Reference | Reference | ||
| Positive | 0.67 (0.45 – 1.01) | 0.055 | 0.72 (0.43 – 1.19) | 0.196 |
| Age | ||||
| Age 50–59 years | Reference | Reference | ||
| 60– 69 years | 0.69 (0.43 – 1.11) | 0.129 | 0.73 (0.43 – 1.23) | 0.233 |
| 70+ years | 1.45 (0.83 – 2.53) | 0.198 | 1.29 (0.66 – 2.52) | 0.46 |
| Sex | ||||
| Female sex | 0.42 (0.26 – 0.68) | <0.001 | 0.35 (0.20 – 0.60) | <0.001 |
| IL-6 | ||||
| Lowest Tertile | 0.71 (0.41 – 1.24) | 0.23 | 0.70 (0.39 – 1.25) | 0.231 |
| Middle Tertile | Reference | Reference | ||
| Highest Tertile | 0.57 (0.33 – 0.98) | 0.043 | 0.58 (0.33 – 1.03) | 0.063 |
| D-dimer | ||||
| Lowest Tertile | 0.64 (0.39 – 1.03) | 0.066 | 0.61 (0.36 – 1.05) | 0.077 |
| Middle Tertile | Reference | Reference | ||
| Highest Tertile | 1.38 (0.80 – 2.39) | 0.243 | 1.34 (0.74 – 2.44) | 0.337 |
| CRP | ||||
| Lowest Tertile | 0.95 (0.58 – 1.56) | 0.842 | 0.91 (0.52 – 1.59) | 0.74 |
| Middle Tertile | Reference | Reference | ||
| Highest Tertile | 1. 04 (0.63 – 1.72) | 0.89 | 1.25 (0.71 – 2.19) | 0.439 |
OR – odds ratio
AOR adjusted odds ratio.
Table 4 shows associations between a range of inflammatory biomarkers and pre-frailty and frailty as outcomes. In the multivariable analysis, there was no evidence of association between any of the inflammatory biomarkers and frailty and pre-frailty (Table 4).
The odds of being frail were higher in those aged 70 years and above than those aged between 50 and 59 years (OR=3.49, 95% CI 1.75–6.95). Females were less likely to be pre-frail than males (OR=0.35, 95% CI 0.20–0.60). However, there was no association between any of the inflammatory biomarkers and frailty and pre-frailty..
Discussion
In this analysis comparing the prevalence of frailty by HIV serostatus among community-dwelling older individuals in a rural population in South Africa, we found a similar prevalence of frailty between older-aged PLWH and HIV- uninfected comparators. Moreover, we found no differences by HIV serostatus for specific sub-components of the frailty index measures. Although these results are contrary to our primary hypothesis, the observation that PWH on ART have a similar prevalence of frailty as those without HIV corroborates data from elsewhere in the region. An analysis of data from rural South Africans over the age of 40 years found a range of prevalence of frailty between 5.4% and13.2%, with no difference noted by HIV serostatus (Payne et al., 2017).
Several studies from the United States and Europe have demonstrated both higher rates of frailty in those with HIV and the development of the frailty phenotype at earlier ages than for HIV uninfected individuals (Brothers et al., 2014; Desai & Landay, 2010; Desquilbet et al., 2007; Levett et al., 2016). In contrast, data from sub-Saharan Africa suggest that older people taking ART have similar or better health profiles than their counterparts living without HIV, including lower prevalence of hypertension (Kwarisiima et al., 2016; Manne-Goehler et al., 2019), obesity (Malaza, Mossong, Bärnighausen, & Newell, 2012; Okello et al., 2017), and carotid atherosclerosis (Feinstein et al., 2017; Muiru et al., 2018; Nonterah et al., 2019). The reasons for the similar prevalence of frailty in those with HIV on ART and those without HIV infection in sub-Saharan Africa remain unclear. It is possible that access to the HIV care system provides primary care in a timely fashion (because of regular clinic appointments), which is not always accessed by older people without HIV-infection (Feinstein et al., 2017; Negin, Nyirenda, Seeley, & Mutevedzi, 2013). Alternatively, older people with HIV might represent a unique group of resilient survivors of the epidemic, from within the larger cohort of PWH, and such data might reflect a survivor effect.
The overall prevalence of frailty (16.3% ) noted in this study was higher than that in the Agincourt study (frailty prevalence of 5.4% to 13.2% across nine frailty measure scores) and reported by Payne et al. (2017). However, the prevalence was lower than that found in 2012 in a Cape Town population (where people with HIV were found to be more likely to be frail than those without (18%)) (Pathai, Lawn, et al., 2013). There are several possible explanations for these differences. The Agincourt study (Payne et al., 2017) included 5,059 people of 40 years and older (both living with and without HIV), 72% of whom were aged less than 70 years. The sample in our study included individuals aged 50 years and older and the highest rates of frailty were noted among those individuals over 70 years of age. In addition, the Agincourt study team measured frailty using a different set of criteria and a larger number of measures (nine) than in our study (five) which could account for differences in the results. The age range was also different for the Cape Town study: 504 individuals were recruited aged 30 years and older, half of the participants were attending an HIV treatment centre. Matched (by gender and age) controls were recruited from an HIV-prevention trial site in the same area. Of note, the data were collected in 2011, before national guidelines changed to recommend provision of ART first to individuals with CD4 counts of ≤350 cells/μl, then ≤500 cells/μl and finally, from 2016, universal access to ART for all individuals living with HIV regardless of CD4 cell count (or WHO stage). While older age, socio-economic status and alcohol consumption were associated by Pathai, Gilbert, et al. (2013, p. 47) with higher odds of frailty for those living with HIV; a CD4 cell count ≥500 cells /μl was also associated with increased rates of frailty. Our data were collected in 2017.
Finally, we found no relationship between biomarkers of inflammation or coagulopathy and the frailty phenotype. Future work should more exhaustively explore these relationships, and particularly whether there are diagnostic biomarkers which could help detect early stage frailty as a warning indicator.
This study had a number of strengths, including a relatively large sample size of people 50 years and older, community-based enrolment, matching by HIV serostatus, and the use of biomarkers of inflammation to assess mechanisms of frailty. The primary limitation, however, was the use of cross-sectional sampling for frailty measurement, which did not support an analysis of directionality or causality of effects. Another limitation to be emphasized is that the Fried Frailty Index that we implemented was an modified version where we excluded the measure of unintentional weight loss because we did not have previous weight measures. The adapted version therefore only used 4 of the 5 measures to calculate frailty. This limits direct comparisons between our dataset and the data sets of similar studies in South Africa (Pathai, Gilbert, et al., 2013; Payne et al., 2017). The adaptation further decreases the strength of predictions of the presence of frailty.
Conclusion
Treated HIV infection does not appear to be associated with the frailty phenotype in older age, community-dwelling individuals in rural KwaZulu-Natal, South Africa. Future work should explore whether improved accessibility to primary care through HIV services might offer an avenue towards improved well-being in functioning older age people in the region.
Acknowledgements
We are grateful to all the participants for giving us their time and sharing their information for this study. We thank the wider community in uMkhanyakude for their support of our research. We are grateful to Sithembile Msane and Ntombi Mkwanazi for data collection and for their enthusiasm for the study and the support of research with older people. We thank Dr Somnath Chatterji and Nirmala Naidoo who lead the study at WHO and Dr Ties Boerma former lead for the study, for their help and support. We also thank Kathy Baisley, LSHTM for help with the sampling. Lastly, we are grateful to the AHRI Diagnostic Laboratory staff for assistance with kit preparation; transporting, storage and testing of samples; as well as sample data analysis.
Funding
This study was funded by the US National Institutes of Health AG (WHO 2016/583903-0) through WHO.
Stephen Nash and Melissa Neuman are supported by an award jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement, which is also part of the EDCTP2 programme supported by the European Union. Grant reference: MR/R010161/1
References
- Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, … Multicenter AIDS Cohort Study. (2013). Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 69(2), 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M (2018). Frailty in people living with HIV. AIDS research and therapy, 15(1), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, & Rockwood K (2014). Frailty in people aging with human immunodeficiency virus (HIV) infection. The Journal of infectious diseases, 210(8), 1170–1179. [DOI] [PubMed] [Google Scholar]
- Brothers TD, & Rockwood K (2014). Biologic aging, frailty, and age-related disease in chronic HIV infection. Current Opinion in HIV and AIDS, 9(4), 412–418. [DOI] [PubMed] [Google Scholar]
- Dent E, Kowal P, & Hoogendijk EO (2016). Frailty measurement in research and clinical practice: a review. European journal of internal medicine, 31, 3–10. [DOI] [PubMed] [Google Scholar]
- Desai S, & Landay A (2010). Early Immune Senescence in HIV Disease. Current HIV/AIDS Reports, 7(1), 4–10. doi: 10.1007/s11904-009-0038-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, & Margolick JB (2007). HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 62(11), 1279–1286. [DOI] [PubMed] [Google Scholar]
- Feinstein MJ, Kim J-H, Bibangambah P, Sentongo R, Martin JN, Tsai AC, … Siedner MJ (2017). Ideal Cardiovascular Health and Carotid Atherosclerosis in a Mixed Cohort of HIV-Infected and Uninfected Ugandans. AIDS research and human retroviruses, 33(1), 49–56. doi: 10.1089/AID.2016.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, … Cardiovascular Health Study COllaborative Research Group. (2001). Frailty in older adults: evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56(3), M146–M157. [DOI] [PubMed] [Google Scholar]
- Gross Andrew M., Jaeger Philipp A., Kreisberg Jason F., Licon K, Jepsen Kristen L., Khosroheidari M, … Ideker T (2016). Methylome-wide Analysis of Chronic HIV Infection Reveals Five-Year Increase in Biological Age and Epigenetic Targeting of HLA. Molecular Cell, 62(2), 157–168. doi: 10.1016/j.molcel.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, & Fried LP (2019). Frailty: implications for clinical practice and public health. The Lancet, 394(10206), 1365–1375. [DOI] [PubMed] [Google Scholar]
- Hubbard RE, O’Mahony MS, Calver BL, & Woodhouse KW (2008). Plasma esterases and inflammation in ageing and frailty. European journal of clinical pharmacology, 64(9), 895. [DOI] [PubMed] [Google Scholar]
- Kowal P, Chatterji S, Naidoo N, Biritwum R, Fan W, Lopez Ridaura R, … SAGE Collaborators. (2012). Data resource profile: the World Health Organization Study on global AGEing and adult health (SAGE). International journal of epidemiology, 41(6), 1639–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwarisiima D, Balzer L, Heller D, Kotwani P, Chamie G, Clark T, … Kamya MR (2016). Population-Based Assessment of Hypertension Epidemiology and Risk Factors among HIV-Positive and General Populations in Rural Uganda. PloS one, 11(5), e0156309–e0156309. doi: 10.1371/journal.pone.0156309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong DP, Teo KK, Rangarajan S, Kutty VR, Lanas F, Hui C, … Noorhassim I (2016). Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: a prospective urban rural epidemiologic (PURE) study. Journal of cachexia, sarcopenia and muscle, 7(5), 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett TJ, Cresswell FV, Malik MA, Fisher M, & Wright J (2016). Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. Journal of the American Geriatrics Society, 64(5), 1006–1014. [DOI] [PubMed] [Google Scholar]
- Li H, Manwani B, & Leng SX (2011). Frailty, inflammation, and immunity. Aging and disease, 2(6), 466–473. [PMC free article] [PubMed] [Google Scholar]
- Malaza A, Mossong J, Bärnighausen T, & Newell M-L (2012). Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PloS one, 7(10), e47761–e47761. doi: 10.1371/journal.pone.0047761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne-Goehler J, Siedner MJ, Montana L, Harling G, Geldsetzer P, Rohr J, … Bärnighausen TW (2019). Hypertension and diabetes control along the HIV care cascade in rural South Africa. Journal of the International AIDS Society, 22(3), e25213–e25213. doi: 10.1002/jia2.25213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugisha JO, Schatz EJ, Randell M, Kuteesa M, Kowal P, Negin J, & Seeley J (2016). Chronic disease, risk factors and disability in adults aged 50 and above living with and without HIV: findings from the Wellbeing of Older People Study in Uganda. Global health action, 9(1), 31098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muiru AN, Bibangambah P, Hemphill L, Sentongo R, Kim J-H, Triant VA, … Siedner MJ (2018). Distribution and Performance of Cardiovascular Risk Scores in a Mixed Population of HIV-Infected and Community-Based HIV-Uninfected Individuals in Uganda. Journal of Acquired Immune Deficiency Syndromes, 78(4), 458–464. doi: 10.1097/QAI.0000000000001696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin J, Mills EJ, & Bärnighausen T (2012). Aging with HIV in Africa: the challenges of living longer. AIDS (London, England), 26(0 1), S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin J, Nyirenda M, Seeley J, & Mutevedzi P (2013). Inequality in health status among older adults in Africa: the surprising impact of anti-retroviral treatment. Journal of cross-cultural gerontology, 28(4), 491–493. [DOI] [PubMed] [Google Scholar]
- Nonterah EA, Boua PR, Klipstein-Grobusch K, Asiki G, Micklesfield LK, Agongo G, … as members/collaborators of AWI-Gen of the H3A Consortium. (2019). Classical Cardiovascular Risk Factors and HIV are Associated With Carotid Intima-Media Thickness in Adults From Sub-Saharan Africa: Findings From H3Africa AWI-Gen Study. Journal of the American Heart Association, 8(14), e011506–e011506. doi: 10.1161/JAHA.118.011506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirenda M, Chatterji S, Falkingham J, Mutevedzi P, Hosegood V, Evandrou M, … Newell M-L (2012). An investigation of factors associated with the health and well-being of HIV-infected or HIV-affected older people in rural South Africa. BMC Public Health, 12(1), 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirenda M, Newell M-L, Mugisha J, Mutevedzi PC, Seeley J, Scholten F, & Kowal P (2013). Health, wellbeing, and disability among older people infected or affected by HIV in Uganda and South Africa. Global health action, 6(1), 19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello S, Ueda P, Kanyesigye M, Byaruhanga E, Kiyimba A, Amanyire G, … Danaei G (2017). Association between HIV and blood pressure in adults and role of body weight as a mediator: Cross-sectional study in Uganda. Journal of Clinical Hypertension, 19(11), 1181–1191. doi: 10.1111/jch.13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Önen N, Agbebi A, Shacham E, Stamm KE, Önen AR, & Overton ET (2009). Frailty among HIV-infected persons in an urban outpatient care setting. Journal of Infection, 59(5), 346–352. [DOI] [PubMed] [Google Scholar]
- Önen N, Patel P, Baker J, Conley L, Brooks J, Bush T, … Overton, E. (2014). Frailty and pre-frailty in a contemporary cohort of HIV-infected adults. The Journal of Frailty and Aging, 3(3), 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Bajillan H, Landay AL, & High KP (2014). Is HIV a model of accelerated or accentuated aging? The journals of gerontology. Series A, Biological sciences and medical sciences, 69(7), 833–842. doi: 10.1093/gerona/glt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker L-G, & Lawn SD (2013). Frailty in HIV-infected adults in South Africa. Journal of Acquired Immune Deficiency Syndromes, 62(1), 43–51. doi: 10.1097/QAI.0b013e318273b631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathai S, Lawn SD, Gilbert CE, McGuinness D, McGlynn L, Weiss HA, … Shiels PG (2013). Accelerated biological ageing in HIV-infected individuals in South Africa: a case-control study. AIDS (London, England), 27(15), 2375–2384. doi: 10.1097/QAD.0b013e328363bf7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CF, Wade A, Kabudula CW, Davies JI, Chang AY, Gomez-Olive FX, … Witham MD (2017). Prevalence and correlates of frailty in an older rural African population: findings from the HAALSI cohort study. BMC geriatrics, 17(1), 293–293. doi: 10.1186/s12877-017-0694-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott DA, Erlandson KM, & Yarasheski KE (2016). Frailty in HIV: Epidemiology, Biology, Measurement, Interventions, and Research Needs. Current HIV/AIDS Reports, 13(6), 340–348. doi: 10.1007/s11904-016-0334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedner MJ (2019). Aging, health, and quality of life for older people living with HIV in sub-Saharan Africa: a review and proposed conceptual framework. Journal of aging and health, 31(1), 109–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedner MJ, Zanni M, Tracy RP, Kwon DS, Tsai AC, Kakuhire B, … Okello S (2018). Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. The Journal of infectious diseases, 218(6), 922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurn M, & Gustafson DR (2017). Faces of Frailty in Aging with HIV Infection. Current HIV/AIDS Reports, 14(1), 31–37. doi: 10.1007/s11904-017-0348-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer S, Harttgen K, Alfven T, Padayachy J, Ghys P, & Bärnighausen T (2017). The HIV epidemic in sub-Saharan Africa is aging: evidence from the demographic and health surveys in sub-Saharan Africa. AIDS and Behavior, 21(1), 101–113. [DOI] [PubMed] [Google Scholar]