Abstract
Background:
The role of maintenance therapy for malignant pleural mesothelioma (MPM) is unknown. We performed a randomized phase 2 trial to determine if continuation of pemetrexed after first-line pemetrexed and platinum would improve progression-free survival (PFS).
Methods:
Eligible patients with unresectable MPM, without disease progression following 4–6 cycles of pemetrexed and platinum were randomized 1:1 to observation or continuation of pemetrexed until progression, stratified by number of cycles (<6 or 6), cisor carboplatin containing regimen, and histology. Study size was calculated based on the assumption that observation would produce a median PFS of 3 months and pemetrexed would yield median PFS of 6 months.
Results:
72 patients were registered from December 2010 to June 2016. The study closed early after 53 patients were randomized; 49 eligible (22 on observation, and 27 on pemetrexed arms) were included in the analysis. Median PFS was 3 months (95% confidence intervals (CI): 2.6–11.9) on observation and 3.4 months (95% CI: 2.8–9.8) on pemetrexed (hazard ratio (HR) 0.99; 95% CI: 0.51–1.90; p=0.9733). Median overall survival (OS) was 11.8 months (95% CI: 9.3–28.7) for observation, and 16.3 months (95% CI: 10.5–26.0) for pemetrexed (HR 0.86; 95% CI: 0.44–1.71; p=0.6737). Grade 3 or 4 toxicities on the pemetrexed arm included anemia (8%), lymphopenia (8%), neutropenia (4%), and fatigue (4%). A higher baseline level of soluble mesothelin-related peptide was associated with worse PFS (HR 1.86, 95% CI: 1.00–3.46, p=0.049).
Conclusion:
Maintenance pemetrexed following initial pemetrexed and platinum chemotherapy does not improve PFS in MPM patients.
Keywords: phase 2 study, pleural mesothelioma, progression-free survival
MicroAbstract
The role of maintenance therapy after first-line platinum and pemetrexed for malignant pleural mesothelioma is unknown. We performed first-to-date randomized trial to determine if continuation of pemetrexed would improve progression-free survival over that of observation and found that primary endpoint was not different between study arms. Therefore, we cannot recommend pemetrexed continuation maintenance for treatment of malignant pleural mesothelioma.
Introduction
Malignant pleural mesothelioma (MPM) is an uncommon tumor afflicting up to 2,800 patients annually in the United States. Most patients present with advanced disease, and treatment is limited to palliative chemotherapy. The only first-line chemotherapy regimen approved by the Food and Drug Administration for mesothelioma is pemetrexed and cisplatin. In a randomized phase III trial, treatment with pemetrexed and cisplatin was better than cisplatin alone with regard to response rates (41% versus 17%), time to progression (6 versus 4 months), and overall survival (OS) (12 versus 9 months).1 The combination of pemetrexed and carboplatin is a reasonable alternative for patients who cannot tolerate cisplatin, based on results from large phase II trials and the expanded access experience showing comparable response rates and survival times.2–4 The optimal duration of first-line chemotherapy has been a long-standing question in the treatment of many solid tumors. Many investigators have argued that if a patient’s cancer is controlled and the toxicities of the treatment are manageable, then discontinuation of the therapy will only result in earlier tumor regrowth. On the other hand, solid tumors ultimately reach a response plateau, at which time additional chemotherapy does not result in further tumor shrinkage. Furthermore, continuation of chemotherapy for prolonged time results in cumulative toxicities.
This study aimed to determine if continuing pemetrexed as “maintenance” therapy after treatment with a pemetrexed and platinum regimen will improve outcomes for patients with MPM. This concept was based on the following reasoning: pemetrexed plus cisplatin is a standard first-line regimen in MPM; a phase III trial in advanced MPM confirmed that treatment with pemetrexed as a second-line regimen resulted in improved progression-free survival (PFS) over best supportive care.5 In a Dutch single-arm observational study of 27 patients with MPM who had at least stable disease after 6 cycles of single agent pemetrexed or carboplatin pemetrexed doublet, 13 received maintenance pemetrexed. Pemetrexed maintenance therapy was well tolerated and resulted in improved time to disease progression and OS in patients who received maintenance therapy versus in patients who did not continue therapy (8.5 and 17.9 months versus 3.4 and 6.0 months, respectively).6
In this study, patients with MPM who had a response or stable disease after four cycles of pemetrexed and cisplatin or carboplatin were randomized to either maintenance pemetrexed or observation.
Patients and Methods
Study Design
Cancer and Leukemia Group B (CALGB; now part of the Alliance for Clinical Trials in Oncology) study 30901, a randomized phase II trial of maintenance pemetrexed versus observation, accrued patients with unresectable MPM without progression after four to six cycles of first-line standard chemotherapy doublet with pemetrexed and cisplatin or carboplatin. After registration to CALGB 30901, patients were randomized to pemetrexed or observation in a 1:1 ratio. The randomization was implemented with a permuted block scheme7 with stratification on first-line regimen cisplatin versus carboplatin, six versus less than six cycles of first-line therapy, and histologic subtype epithelioid versus other. The primary endpoint was PFS, defined as the time from patient randomization to disease progression or death from any cause, whichever comes first. An interim analysis was planned when accrual reached 28 events (or 50% of information) based on the observed hazard ratio in an opposite direction to the expectation.8 Secondary objectives were to determine if maintenance therapy with pemetrexed improves OS, defined as the time from patient randomization to death from any cause; to evaluate frequency of responses to maintenance therapy with pemetrexed; to assess toxicity of maintenance therapy with pemetrexed (using CTCAE version 4); and to assess whether biomarkers correlate with disease status, i.e., PFS and OS. For sample size determination, we assumed that observation arm would produce a median PFS of 3 months during the maintenance phase, and that pemetrexed would have 100% improvement in median PFS to 6 months. Under constant hazards, that corresponded to a 3-month PFS of 50% for observation and 70.7% for the pemetrexed arm. With 57 events to be observed from a total of 63 randomized patients, the study had approximately 91% power to reject the null hypothesis that λA/λB = 1 and accept the alternative hypothesis λA/λB > 1 when the true λA/λB = 2 using a one-sided log-rank test at a significance level of 0.10.
Patients
Eligible patients had a histologically confirmed diagnosis of MPM, epithelial, sarcomatoid or mixed type, not amenable to surgical resection. Patients had to have complete response, partial response, or stable disease following 4, 5, or 6 cycles of first-line chemotherapy with pemetrexed and either cisplatin or carboplatin; they had to be previously registered to CALGB 30901 and then randomized to maintenance therapy with pemetrexed or to observation. Study treatment was to start within 14 days after randomization. Patients were ≥ 18 years of age with an Eastern Clinical Oncology Group (ECOG) performance status of 0 or 1. Other inclusion criteria included adequate bone marrow, hepatic, and renal functions, with calculated creatinine clearance of ≥ 45 mL/min.
Study Treatment and Outcome Measures
This study (ClinicalTrials.gov Identifier: NCT01085630) was approved by local institutional review boards, and all patients provided written informed consent before enrollment. Patients randomized to the treatment arm received 500 mg/m2 of pemetrexed every 3 weeks. Mesothelioma burden was assessed by investigators with computed tomography (CT) and evaluated by modified RECIST criteria9 every 6 weeks for 6 months, then every 9 weeks for 6 months and then every 12 weeks until disease progression for a maximum of 3 years from date of randomization. In addition to a baseline scan, confirmatory scans had to be obtained at least four weeks following initial documentation of objective response. The baseline circulating plasma levels of osteopontin and soluble mesothelin-related peptide (SMRP) in serum were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN; and Elabscience, Houston, UT).
Study Analysis
In March 2015, an interim futility test was conducted after observing 28 progression/death events from 42 randomized patients. The observed hazard ratio of pemetrexed relative to observation was 0.78 (95% CI, 0.32–1.93). The trial was recommended to continue to the full target accrual10, before it was terminated early in July 2016 due to slow accrual.
The primary analysis included all randomized patients but excluded ineligible patients or patients who were canceled from the study before receiving any protocol treatment (Figure 1). The PFS and OS analysis comparing patients treated with pemetrexed relative to the patients on observation was conducted using a stratified log rank test. The product limit estimator developed by Kaplan and Meier was used to graphically describe PFS and OS.11 From these product limit estimates, median PFS, median OS, 3-month PFS, 12-month OS rate and their 95% confidence intervals (CIs) were estimated for patients randomized to each arm. Cox proportional hazards model12 was used to estimate the hazard ratios (HR) and their 95% confidence intervals of the experimental regimen relative to the control group with and without adjusting for baseline prognostic factors. The frequency of best response to each arm was tabulated and its 95% exact binomial confidence intervals was computed. Differences in response rates (including complete and partial response) between treatments was tested using Fisher’s exact test.13
Figure 1.
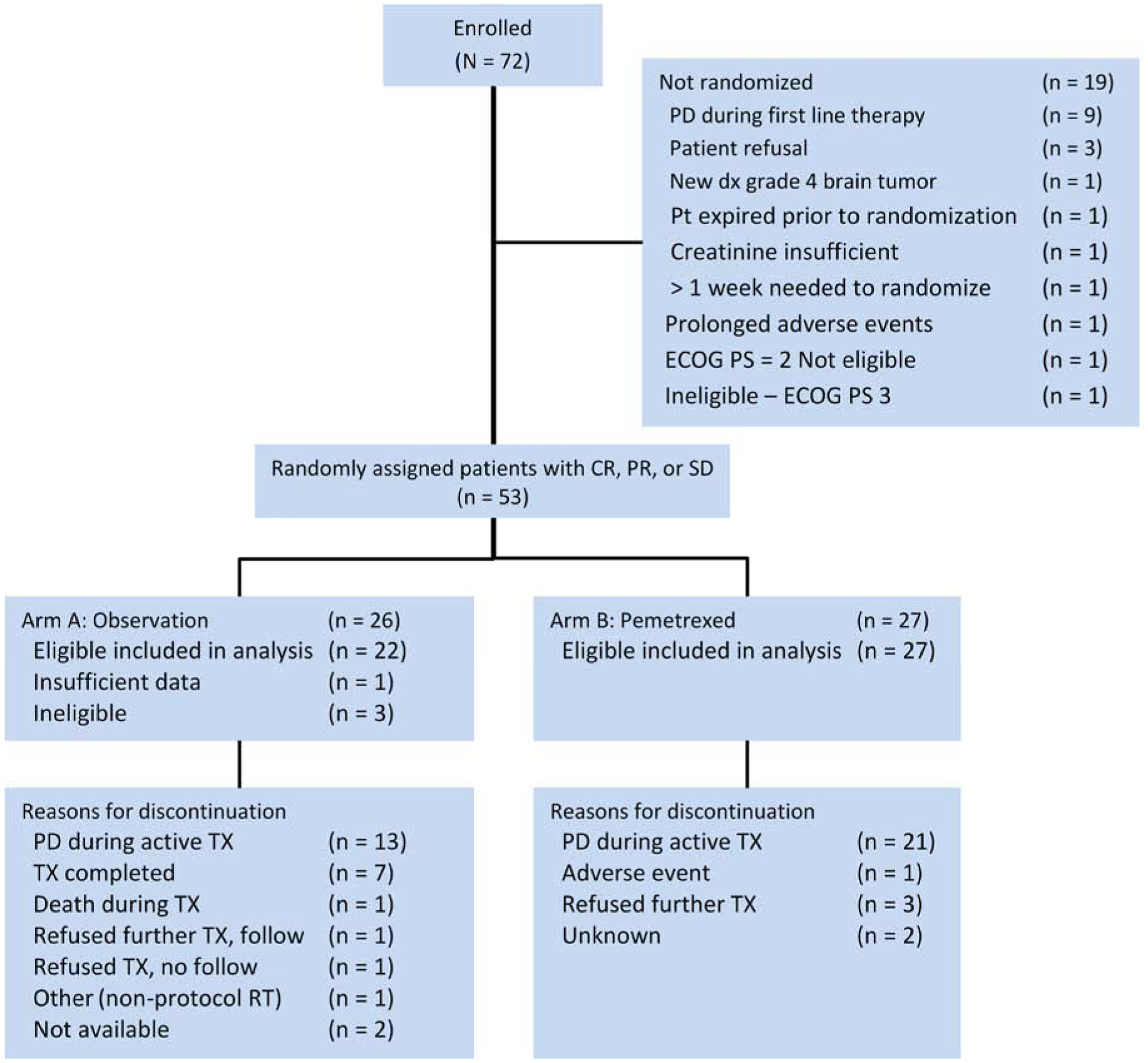
CONSORT diagram.
The correlation of PFS and OS with SMRP and osteopontin were examined using Cox proportional hazards model with and without adjustment for treatment and other prognostic factors. The concordances between the observed survival and the predicted survival based on the Cox models were evaluated with C-Stat14,15 and incremental area under the curve (iAUC)16.
All p-values reported are two-sided unless stated otherwise for the test on the primary endpoint PFS, and a p-value less than 0.05 was considered statistically significant. The statistical analyses were performed on SAS 9.4 (SAS Inc., Cary, NC) on data set locked on December 13, 2018. Patient data were reviewed by the study chair (AD) and the data management and statistical analyses were provided by Alliance Statistics and Data Center.
Results
Patients
The study started in December 2010 to enroll patients who had completed first-line pemetrexed and platinum therapy. In March 2015, an interim futility test was conducted after observing 28 progression/death events from 42 randomized patients. The observed hazard of pemetrexed relative to observation was 0.78 (95% CI, 0.32–1.93). The trial was recommended to continue to the full target accrual10. The study closed early in July 2016 due to slow accrual after 72 patients were enrolled from 30 individual sites. Of them, 19 patients were not randomized; 53 patients were randomized (27 to the pemetrexed arm, and 26 to the observation arm) (Figure 1). One randomized patient’s eligibility status was not ascertained due to insufficient data by the time of this analysis, and three randomized patients were deemed ineligible due to progressive disease during the first-line therapy. A total of 49 eligible patients were included in the efficacy and safety analysis (27 on pemetrexed arm, and 22 on observation arm). At the time of analysis, 41 out of 49 patients were deceased. The median follow-up time on the remaining 8 living patients was 34.2 months with a range of 0.9 – 37.7 months since randomization.
Patient demographics and baseline characteristics
Patient demographics and characteristics (Table 1) were evenly distributed. Median age was 70 years old in both arms. Epithelioid histology was seen in 77% of patients on the observation arm, and 70% on the pemetrexed arm. The fraction of patients who received six cycles of front-line therapy was 40.9% in observation and 37% in pemetrexed arm. Most patients received carboplatin and pemetrexed as a first-line treatment; 72.7% on observation arm, and 74.1% on pemetrexed arm. Most patients had performance status of 1; 72.7% and 66.7% on observation and pemetrexed arms, respectively. Median number of cycles of platinum and pemetrexed was 4 on both arms. Median leukocyte count was 6.7 per dL, and 6.0 per dL, and platelets count was 222,000 per dL, and 216,000 per dL, respectively on observation and pemetrexed arms. A median of four cycles of pemetrexed was delivered (range: 1–33). 22.2% of patients required dose modifications. The following reasons for going off treatment were: disease progression during active treatment (77.8%), refusal to further continue treatment on study protocol (11.1%), adverse event (3.7%), unknown reason (7.4%).
Table 1.
Patient Demographics and Baseline Characteristics
| Observation (N=22) |
Pemetrexed (N=27) |
Total (N=49) |
p value | |
|---|---|---|---|---|
| Age at Randomization (years) | 0.30711 | |||
| Median (range) | 70 (39–85) | 70 (52–87) | 70 (39–87) | |
| Gender | 0.44922 | |||
| Male | 15 (68.2%) | 21 (77.8%) | 36 (73.5%) | |
| Female | 7 (31.8%) | 6 (22.2%) | 13 (26.5%) | |
| Race | 0.44992 | |||
| Unknown | 1 (4.5%) | 3 (11.1%) | 4 (8.2%) | |
| White | 21 (95.5%) | 23 (85.2%) | 44 (89.8%) | |
| Black/African American | 0 (0.0%) | 1 (3.7%) | 1 (2.0%) | |
| Performance status | 0.64712 | |||
| 0 | 6 (27.3%) | 9 (33.3%) | 15 (30.6%) | |
| 1 | 16 (72.7%) | 18 (66.7%) | 34 (69.4%) | |
| Histology | 0.40262 | |||
| Mixed Type | 2 (9.1%) | 6 (22.2%) | 8 (16.3%) | |
| Sarcomatoid | 3 (13.6%) | 2 (7.4%) | 5 (10.2%) | |
| Epithelial | 17 (77.3%) | 19 (70.4%) | 36 (73.5%) | |
| White blood cell count | 0.59571 | |||
| Median (range) | 6.7 (4.1–12.5) | 6.0 (2.9–15.9) | 6.1 (2.9–15.9) | |
| Platelets | 0.29331 | |||
| Median (range) | 222 (48–759) | 216 (101–599) | 216 (48–759) | |
| First-line chemotherapy | 0.91542 | |||
| Pemetrexed + Cisplatin | 6 (27.3%) | 7 (25.9%) | 13 (26.5%) | |
| Pemetrexed + Carboplatin | 16 (72.7%) | 20 (74.1%) | 36 (73.5%) | |
| Number of Cycles Administered | 0.94412 | |||
| 4 | 12 (54.5%) | 16 (59.3%) | 28 (57.1%) | |
| 5 | 1 (4.5%) | 1 (3.7%) | 2 (4.1%) | |
| 6 | 9 (40.9%) | 10 (37.0%) | 19 (38.8%) | |
| Prior surgery? | 0.64712 | |||
| No | 16 (72.7%) | 18 (66.7%) | 34 (69.4%) | |
| Yes | 6 (27.3%) | 9 (33.3%) | 15 (30.6%) | |
| Prior radiotherapy? | 0.43402 | |||
| No | 20 (90.9%) | 26 (96.3%) | 46 (93.9%) | |
| Yes | 2 (9.1%) | 1 (3.7%) | 3 (6.1%) | |
| Prior intracavitary cytotoxic or sclerosing therapy | 0.90672 | |||
| No | 19 (86.4%) | 23 (85.2%) | 42 (85.7%) | |
| Yes | 3 (13.6%) | 4 (14.8%) | 7 (14.3%) | |
| Has the patient had exposure to asbestos? | 0.61712 | |||
| No | 8 (36.4%) | 8 (29.6%) | 16 (32.7%) | |
| Yes | 14 (63.6%) | 19 (70.4%) | 33 (67.3%) | |
| Has the patient previously been a smoker? | 0.94362 | |||
| No | 10 (45.5%) | 12 (44.4%) | 22 (44.9%) | |
| Yes | 12 (54.5%) | 15 (55.6%) | 27 (55.1%) | |
| Chest pain | 0.44012 | |||
| No | 19 (86.4%) | 21 (77.8%) | 40 (81.6%) | |
| Yes | 3 (13.6%) | 6 (22.2%) | 9 (18.4%) | |
| Dyspnea | 0.80362 | |||
| No | 13 (59.1%) | 15 (55.6%) | 28 (57.1%) | |
| Yes | 9 (40.9%) | 12 (44.4%) | 21 (42.9%) | |
| Duration of initial symptoms | 0.94362 | |||
| None - <3 months | 12 (54.5%) | 15 (55.6%) | 27 (55.1%) | |
| ≥ 3 months | 10 (45.5%) | 12 (44.4%) | 22 (44.9%) | |
| Weight loss in previous 6 months | 0.44702 | |||
| 0 – < 5% | 14 (63.6%) | 18 (66.7%) | 32 (65.3%) | |
| 5 – < 10% | 3 (13.6%) | 5 (18.5%) | 8 (16.3%) | |
| 10 – < 20% | 3 (13.6%) | 4 (14.8%) | 7 (14.3%) | |
| ≥ 20% | 2 (9.1%) | 0 (0.0%) | 2 (4.1%) |
Unequal Variance T-Test
Chi-Square
Efficacy
The primary endpoint of the study was PFS. The median PFS was similar on both arms; 3 months on the observation arm (95% CI: 2.6–11.9), and 3.4 months on the pemetrexed arm (95% CI:2.8–9.8), with HR of 0.99 (95% CI: 0.51–1.90, p-value: 0.9733), with a corresponding one-sided p-value 0.4867 (Figure 2A).
Figure 2.
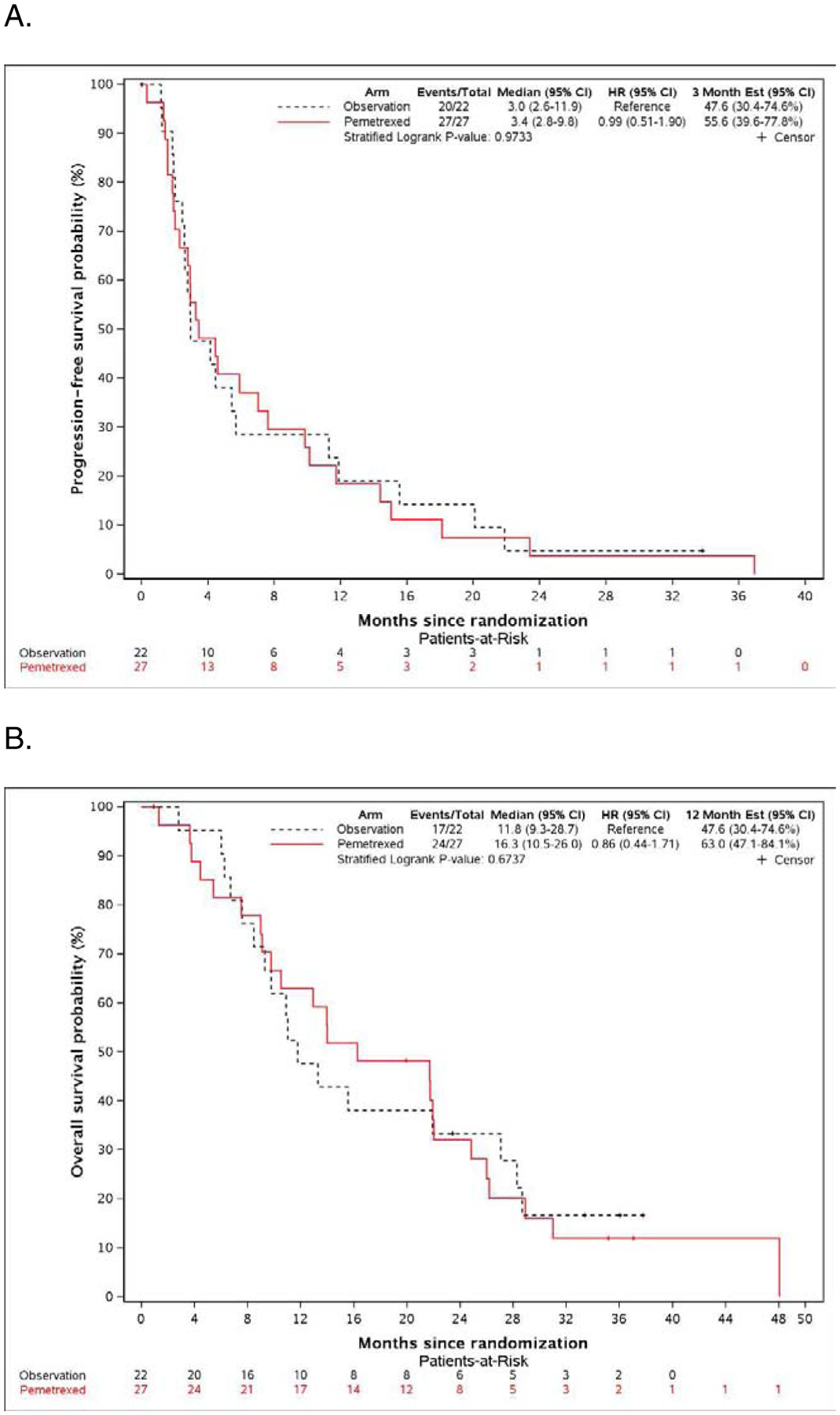
A. Kaplan-Meier plot of progression-free survival in the pemetrexed and observation arms. B. Kaplan-Meier plot of overall survival in the pemetrexed and observation arms.
The median OS was similar on both arms: 11.8 months on the observation arm (95% CI: 9.3–28.7), and 16.3 months on the pemetrexed arm (95% CI: 10.5–26.0), with HR of 0.86 (95% CI: 0.44–1.71, p-value: 0.6737) (Figure 2B).
The following patients’ demographic and baseline clinical characteristics were included in the Cox proportional hazard models: treatment arm (pemetrexed versus observation), histological type (epithelial versus others), first-line chemotherapy agents (pemetrexed and cisplatin versus pemetrexed and carboplatin), number of cycles of first-line chemotherapy received (less than 6 cycles versus 6 cycles), age at randomization, race (White versus. non-White), gender (female versus male), ECOG performance status (PS=1 versus PS=0), presence of prior surgery, prior radiotherapy, prior chemotherapy, prior intracavitary cytotoxic or sclerosing therapy, exposure to asbestos, history of smoking, chest pain, dyspnea, duration of initial symptoms prior to diagnosis (≥ 3 months versus < 3 months including no symptoms), weight loss in previous 6 months (< 5% versus ≥ 5%), baseline leukocyte count, and platelet count. Backward selection method was used to conduct Cox proportional hazard analysis with four covariates (treatment arm, histological type, chemotherapy agents and number of cycles of first-line therapy) forced into the models, the entry level set to 0.10, and the stay level set to 0.05.
Cox proportional hazard modelling on PFS showed performance status of 1 as compared to 0, male gender, first-line cisplatin, rather than carboplatin, prior radiotherapy, and higher platelet count, were associated with shorter PFS (Table 2A). Cox proportional hazard modelling on OS showed performance status of 1 as compared to 0, younger age at randomization, histology, absence of chest pain, shorter duration of initial symptoms, and exposure to asbestos were associated with higher risk of shorter OS (Table 2B).
Table 2 A.
Cox Proportional Hazards Modeling on Progression-free Survival. Table 2 B. Cox Proportional Hazards Modeling on Overall Survival
| Table 2 A | HR | 95% CI | P value | |
|---|---|---|---|---|
| Observation (ref = pemetrexed) |
1.055 | 0.564 | 1.973 | 0.8658 |
| Epithelial (ref = other) |
0.780 | 0.380 | 1.599 | 0.4974 |
| First-line Cisplatin (ref = carboplatin) |
2.294 | 1.080 | 4.873 | 0.0307 |
| First-line <6 cycles (ref = 6 cycles) |
1.206 | 0.609 | 2.388 | 0.5914 |
| Gender (ref=Male) |
0.416 | 0.197 | 0.879 | 0.0217 |
| Performance status (ref = 0) |
2.441 | 1.161 | 5.132 | 0.0186 |
| Prior radiotherapy (ref=none) |
3.917 | 1.012 | 15.162 | 0.0480 |
| Platelets (unit=103/ul) | 1.002 | 1.000 | 1.005 | 0.0396 |
| Table 2 B | HR | 95% CI | P value | |
| Observation (ref = pemetrexed) |
1.619 | 0.787 | 3.332 | 0.1904 |
| Epithelial (ref = other) |
0.269 | 0.112 | 0.648 | 0.0034 |
| First-line Cisplatin (ref = carboplatin) |
0.847 | 0.356 | 2.016 | 0.7070 |
| First-line <6 cycles (ref = 6 cycles) |
1.142 | 0.546 | 2.385 | 0.7245 |
| Age at Randomization | 0.947 | 0.909 | 0.987 | 0.0090 |
| Performance status (ref = 0) |
9.411 | 3.178 | 27.868 | <.0001 |
| Chest pain (ref= no chest pain) |
0.196 | 0.062 | 0.618 | 0.0054 |
| Exposure to asbestos (ref= no exposure) |
4.689 | 1.873 | 11.742 | 0.001 |
| Duration of initial symptoms (ref= < 3 months) |
0.410 | 0.179 | 0.941 | 0.0354 |
Table 3 shows best responses during maintenance on pemetrexed and observation; there were 3.7% complete responses and 7.4% partial responses on pemetrexed arm and no responses on observation arm. On the pemetrexed arm 44.4% progressed as best response versus 28.6% on the observation arm.
Table 3.
Best Response on Pemetrexed and Observation Arm
| Best Response | Pemetrexed (N=27) |
Observa6on† (N=21) |
Total (N=48) |
|---|---|---|---|
| Complete | 1 (3.7%) | 0 (0%) | 1 (2.1%) |
| Partial | 2 (7.4%) | 0 (0%) | 2 (4.2%) |
| Stable | 12 (44.4%) | 14 (66.6%) | 26 (54.2%) |
| Progression | 12 (44.4%) | 6 (28.6%) | 18 (37.5%) |
| Non CR/ Non PD | 0 (0.0%) | 1 (4.8%) | 1 (2.1%) |
| Response Rate (95% CI) * | 11.1 (2.4, 29.2) | 0 (0, 0) | 6.3 (1.3, 17.2) |
One pa6ent’s tumor response is unavailable due to withdrawal of consent for follow-up
Fisher’s exact test on response rate between two arms, two-sided: p =0.2460
Abbreviations: Non CR = non complete response; non PD = non progressive disease
Safety
Twenty-seven patients randomized to pemetrexed were evaluable for adverse events (AEs) analyses. AEs on this study were reported using CTCAE version 4. Pemetrexed was well tolerated with no grade 5 events. The only grade 4 toxicity was a decreased neutrophil count in one patient (4%). Table 4 shows maximum grade per patient and per event, grade 3 or higher AEs possibly, probably, or definitely attributed to treatment. AEs on the observation arm were not required to be collected.
Table 4.
Listing of at Least Possibly Related to Pemetrexed Grade 3 and 4 Adverse Events.
| Adverse Events | Grade 3 | Grade 4 | ||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Hematologic Adverse Events | ||||
| Anemia | 2 | (7%) | 0 | (0%) |
| Lymphocyte count decreased | 2 | (7%) | 0 | (0%) |
| Neutrophil count decreased | 0 | (0%) | 1 | (4%) |
| Platelet count decreased | 1 | (4%) | 0 | (0%) |
| Non-Hematologic Adverse Events | ||||
| Fatigue | 1 | (4%) | 0 | (0%) |
| Anorectal infection | 1 | (4%) | 0 | (0%) |
| Skin infection | 1 | (4%) | 0 | (0%) |
| Hyperglycemia | 2 | (7%) | 0 | (0%) |
| Hypernatremia | 1 | (4%) | 0 | (0%) |
| Peripheral sensory neuropathy | 1 | (4%) | 0 | (0%) |
Exploratory analysis for osteopontin and SMRP
Specimen were collected from randomized patients who consented to the voluntary correlative study. Valid pre-therapy osteopontin and SMRP values were harvested from sixteen patients’ samples in observation arm and seventeen patients’ samples in pemetrexed arm. Analysis were done by Dr. Kaoru Terai at HealthPartners Institute in St. Paul, MN.
Supplemental Table 1 shows the association of serum biomarkers at baseline for PFS evaluated in hazard ratios from different survival models. Larger SMRP value was associated with worse PFS (hazard ratio=1.86, 95%CI: 1.00–3.46, p-value: 0.049) in the model with SMRP and treatment arm as predictors), and osteopontin did not have a significant effect on PFS in a similar model (p-value: 0.3630). The concordance statistics estimated from the same model with treatment arm and SMRP as predictors with Harrell’s C-Stat=0.6012, Uno’s C-Stat=0.6012, iAUC=0.6226, indicating a moderate prediction accuracy of SMRP with PFS. The last model with osteopontin, SMRP and treatment arm as predictors had slightly higher prediction accuracy for PFS.
Supplemental Table 2 shows the association of serum biomarkers at baseline for OS evaluated in hazard ratios from different survival models. Larger osteopontin value was associated with worse OS (hazard ratio=1.01 per one unit of osteopontin increase) based on the estimates with osteopontin and treatment arm as predictors, even though the relationship was only suggestively significant (p-value: 0.0659). Evidenced from another model with SMRP and treatment arm as predictors, SMRP did not have a significant effect on OS (p-value: 0.5497). The concordance statistics estimated from the same model with treatment arm and osteopontin as predictors, with Harrell’s C-Stat=0.6354, Uno’s C-Stat=0.6293, iAUC=0.5615, indicated a moderate prediction accuracy of SMRP with OS. The last model with osteopontin, SMRP and treatment arm as predictors had slightly higher prediction accuracy for OS.
In all models evaluating the prediction accuracy of SMRP and osteopontin, the interactions between treatment and biomarker were tested and none of them were significant at the level of 0.05, indicating that higher baseline SMRP and osteopontin had mostly prognostic value for survival but they had no predictive value for greater benefit of pemetrexed.
Discussion
This randomized phase 2 trial was the first to investigate a continuation maintenance treatment strategy for MPM. Its main limitations were slow accrual leading to premature closure and small sample size. Difficulties in accrual were seen starting from very beginning of study and continued even when study was open at additional study sites. Initial statistical design was looking for 67% improvement of median PFS of 3 months to 5 months and had to be amended on January 15, 2015 to seek 100% improvement in order to make accrual of smaller number of patients more feasible.
During first years of study difficulties in patient participation was thought to be due to perception of benefit of maintenance pemetrexed by treating oncologists, later due to accrual to seemingly more attractive competing studies at leading institutions, changes in front-line therapy with increasing use of bevacizumab, and finally difficulties in registering patients after the fourth cycle of first-line chemotherapy. Pemetrexed did not improve PFS compared to observation. Low, only 11.1% response rate generated by this chemotherapy, after initial platinum-based pemetrexed combination treatment, could possibly explain lack of later impact of this therapy on disease control. Certainly, low toxicity of pemetrexed did not lead to early discontinuation of treatment on experimental arm. Median OS on the observation arm of 11.8 months is similar to that originally reported in the phase 3 trial with cisplatin and pemetrexed.1 Although median OS on the pemetrexed arm of 16.3 months was higher than on observation, survival curves on both arms were overlapping (Figure 2B).
Interestingly, second line therapy with pemetrexed and best supportive care after failure of first-line non-pemetrexed containing regimen showed superior PFS of 3.6 months versus 1.5 months on best supportive care but resulted in no difference in OS.5
Addition of inhibitor of vascular endothelial growth factor (VEGF) receptors 1, 2, and 3, nintedanib, to six cycles of induction with pemetrexed and cisplatin, and then maintenance with nintedanib, resulted in improved PFS, and trend toward better OS than without nintedanib in induction and maintenance. Contribution of continuous maintenance of nintedanib versus placebo was not evaluated by authors.17 Recently switch maintenance strategy was tested with defactinib versus placebo after first-line therapy; where defactinib did not show benefit compared to placebo regardless of merlin expression.18
Addition of bevacizumab to front-line therapy with cisplatin and pemetrexed is now being adopted as standard of care based on results of randomized phase 3, Mesothelioma Avastin Cisplatin Pemetrexed study, showing that triplet therapy generated significantly longer OS than the control doublet.19 In this study, patients who were treated with bevacizumab containing combination could continue maintenance bevacizumab. Study was not designed to assess whether continuation of VEGF inhibition has contributed to OS benefit of triplet therapy. In this context, as of today, role of maintenance therapy of mesothelioma, remains undefined.
Re-challenge with pemetrexed after progression of disease following disease control has resulted in 66% disease control, and could be an alternative to maintenance, strategy in controlling mesothelioma after break following induction, first-line treatment.20–22
Osteopontin and SMRP were studied as exploratory biomarkers in the present study based on two previous studies in which serum osteopontin levels could suggest presence of mesothelioma in individuals who had exposure to asbestos23 and could be used together with SMRP to monitor responses to treatment in patients with epithelioid mesothelioma24. In our study we used plasma level of osteopontin rather than serum based on previous reports of better accuracy of plasma levels.25,26 In our study higher baseline level of SMRP in patients with epithelioid mesothelioma, but not osteopontin, was associated with worse PFS (HR 2.007, 95% CI 1.068–3.771, p=0.05) (Supplemental Table 1). Uno’s C-Stat estimator of 0.6012, and iAUC of 0.6226 indicate a moderate prediction accuracy of SMRP with PFS. Higher levels of osteopontin, but not SMRP suggested worse OS (HR 1.005, 95% CI 1.000–1.011, p=0.0676) (Supplemental Table 2). Harrell’s C-Stat value of 0.6354, Uno’s C-Stat estimator of 0.6293, and iAUC of 0.5615 indicated a moderate prediction accuracy of osteopontin with OS. These findings were not dependent on the treatment arm, indicating they have no predictive marker for greater benefit of pemetrexed. This was an exploratory analysis and these results need to be prospectively validated in a larger cohort.
Conclusions
In summary, continuation of maintenance pemetrexed after initial platinum and pemetrexed therapy in unselected patients does not provide additional benefit over observation in patients with mesothelioma.
Supplementary Material
Clinical Practice Points.
It is unknown whether continuation of pemetrexed after completion of four to six cycles of therapy with pemetrexed and platinum for unresectable malignant pleural mesothelioma provides clinical benefit.
This is first randomized study designed to detect difference in progression-free survival between maintenance pemetrexed and observation. This study failed to demonstrate clinical benefit of additional pemetrexed beyond initial platinum and pemetrexed treatment.
Results of this study does not support current practice of continuation maintenance of pemetrexed for pleural mesothelioma that was based on data derived from treatment of non-small cell lung cancer. Participation in clinical trial or observation remains the best treatment strategy for these patients.
Acknowledgments
We would like to thank James P. Zacny, PhD for assistance in manuscript preparation.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA233253, and UG1CA233327 (https://acknowledgments.alliancefound.org), and the Engdahl Family Foundation . The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
List of supplementary material
Supplemental Tables 1 and 2.doc
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21:2636–44. [DOI] [PubMed] [Google Scholar]
- 2.Ceresoli GL, Castagneto B, Zucali PA, et al. Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer 2008; 99:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008; 19:370–3. [DOI] [PubMed] [Google Scholar]
- 4.Santoro A, O’Brien ME, Stahel RA, et al. Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: results of the International Expanded Access Program. J Thorac Oncol 2008; 3:756–63. [DOI] [PubMed] [Google Scholar]
- 5.Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 2008; 26:1698–704. [DOI] [PubMed] [Google Scholar]
- 6.van den Bogaert DP, Pouw EM, van Wijhe G, et al. Pemetrexed maintenance therapy in patients with malignant pleural mesothelioma. J Thorac Oncol 2006; 1:25–30. [PubMed] [Google Scholar]
- 7.Zelen M The randomization and stratification of patients to clinical trials. J Chronic Dis 1974; 27:365–75. [DOI] [PubMed] [Google Scholar]
- 8.Wieand S, Schroeder G, O’Fallon JR. Stopping when the experimental regimen does not appear to help. Stat Med 1994; 13:1453–18. [DOI] [PubMed] [Google Scholar]
- 9.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004; 15:257–60. [DOI] [PubMed] [Google Scholar]
- 10.Wieand S, Schroeder G, O’Fallon JR. Stopping when the experimental regimen does not appear to help. Stat Med 1994; 13:1453–8. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 12.Cox DR. Regression models and life-tables. J R Stat Soc 1972; 34:187–220. [Google Scholar]
- 13.Agresti A Categorical data analysis. 2nd ed New York, NY: Wiley; 2002. [Google Scholar]
- 14.Harrell FE. The PHGLM Procedure In SUGI Supplemental Library Guide, Version 5 Edition Cary, NC; 1986. [Google Scholar]
- 15.Uno H, Cai T, Pencina MJ, et al. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med 2011; 30:1105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uno H, Cai T, Tian L, et al. Evaluating Prediction Rules for t-Year Survivors with Censored Regression Models. J Am Stat Assoc 2007; 102:527–37. [Google Scholar]
- 17.Grosso F, Steele N, Novello S, et al. Nintedanib Plus Pemetrexed/Cisplatin in Patients With Malignant Pleural Mesothelioma: Phase II Results From the Randomized, Placebo-Controlled LUME-Meso Trial. J Clin Oncol 2017; 35:3591–600. [DOI] [PubMed] [Google Scholar]
- 18.Fennell DA, Baas P, Taylor P, et al. Maintenance Defactinib Versus Placebo After First-Line Chemotherapy in Patients With Merlin-Stratified Pleural Mesothelioma: COMMAND-A Double-Blind, Randomized, Phase II Study. J Clin Oncol 2019; 37:790–8. [DOI] [PubMed] [Google Scholar]
- 19.Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016; 387:1405–14. [DOI] [PubMed] [Google Scholar]
- 20.Bearz A, Talamini R, Rossoni G, Santo A, de Pangher V, Fasola G, Rosetti F, Favaretto A, Gregorc V, Berretta M, Santarossa S, Berto E, Tirelli U. Re-challenge with pemetrexed in advanced mesothelioma: a multi-institutional experience. BMC Res Notes. 2012. September 3;5:482. doi: 10.1186/1756-0500-5-482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrini I, Lucchesi M, Puppo G, Chella A Medical treatment of malignant pleural mesothelioma relapses. J Thorac Dis. 2018. January;10(Suppl 2):S333–S341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrelli F, Ardito R, Conti B, Coinu A, Cabiddu M, Ghilardi M, Borgonovo K, Barni S, Ghidini A. A systematic review and meta-analysis of second-line therapies for treatment of mesothelioma. Respir Med. 2018. August;141:72–80. [DOI] [PubMed] [Google Scholar]
- 23.Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med 2005; 353:1564–73. [DOI] [PubMed] [Google Scholar]
- 24.Bonotti A, Simonini S, Pantani E, et al. Serum mesothelin, osteopontin and vimentin: useful markers for clinical monitoring of malignant pleural mesothelioma. Int J Biol Markers 2017; 32:e126–e31. [DOI] [PubMed] [Google Scholar]
- 25.Creaney J, Yeoman D, Musk AW, et al. Plasma versus serum levels of osteopontin and mesothelin in patients with malignant mesothelioma--which is best? Lung Cancer 2011; 74:55–60. [DOI] [PubMed] [Google Scholar]
- 26.Cristaudo A, Foddis R, Bonotti A, et al. Comparison between plasma and serum osteopontin levels: usefulness in diagnosis of epithelial malignant pleural mesothelioma. Int J Biol Markers 2010; 25:164–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


