Abstract
Purpose
Patients with localized breast cancer have a 5-year survival rate >99% compared to patients with metastatic breast cancer (MBC) that have a 5-year survival rate of ~27%. Unregulated PI3K/AKT signaling is a common characteristic of MBC, making it a desirable therapeutic target for tumors with activating mutations in this pathway. Interestingly, inhibition of the PI3K/AKT pathway can affect signaling in immune cells, which could potentially alter the immune phenotype of patients undergoing therapy with these drugs. The purpose of this study is to evaluate how PI3K inhibition affects the immune cells of MBC patients during treatment.
Methods
We investigated the effects of PI3K inhibition on the immune cell populations in peripheral blood of MBC patients enrolled in 4 different clinical trials utilizing PI3K inhibitors. Peripheral blood was drawn at different points in patient treatment cycles to record immune cell fluctuations in response to therapy.
Results
MBC patients who responded to treatment with a positive fold-change in cytotoxic T-cell population, had an average duration of treatment response of 31.4 months. In contrast, MBC patients who responded to treatment with a negative fold-change in cytotoxic T-cell population, had an average duration of therapeutic response of 5 months. These data suggest that patients with a more robust, initial anti-tumor T-cell response may have a longer therapeutic response compared to patients who do not have a robust, initial anti-tumor T-cell response
Conclusions
These results highlight the potential for PI3K inhibition to sensitize tumors to immune checkpoint inhibitors, thus providing additional therapeutic options for patients with MBC.
Keywords: breast, metastatic, PI3K, leukocyte, immunotherapy
Introduction
Breast cancer is a major contributor to cancer-related deaths in women worldwide 1. In particular, metastatic spread of the primary breast tumor is the major driver of cancer-related mortality 40. Metastatic breast cancer (MBC) can be difficult to treat due to the location and/or the characteristics of the metastatic disease. A common characteristic of MBC is the over-activation of the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling pathway, which can result from PIK3CA activating mutation, AKT amplification or activating mutation, and/or functional loss of the tumor suppressor, PTEN 6, 9, 18, 26. PI3Ks form an evolutionarily conserved family of lipid kinases whose members include Class IA catalytic subunits (p110α, β, δ), Class 1B catalytic subunit (p110γ), Class I regulatory domains (p85α and β, p55α and γ, p50α, p101, p84, p87), Class II subunits (C2α, C2β, C2γ), and Class III catalytic protein (Vps34) and regulatory protein (Vps15) 15. Interestingly, the Class IA PI3K known as PIK3CA (p110α) plays a critical role in a wide range of carcinomas, and over 33% of somatic gene mutations in breast cancer patient tumors occur in this kinase family 28. The PI3K/AKT signaling pathway is responsible for numerous pro-tumorigenic characteristics including, but not limited to, cell proliferation, survival, and angiogenesis 12, 13, 25. Unfortunately, breast cancers harboring PIK3CA and/or AKT mutations or amplifications have shown unfavorable prognosis and increased drug resistance 27. Moreover, breast tumors with high PIK3CA mRNA exhibit significantly lower relapse free survival (RFS; p<0.001) (Supplemental Figure 1a), and high PIK3CA protein expression significantly decreases overall survival (OS; p<0.05) (Supplemental Figure 1b).
Due the oncogenic nature of this signaling pathway, many studies are investigating the effects of pharmacological inhibition of targets in this pathway 2–5, 11, 14, 16, 21–23, 33, 34. Recently, two PI3K inhibitors, alpelisib and taselisib (Supplemental Figures 2a–b), were utilized in clinical trials (Supplemental Table 1) for the treatment of MBC. Alpelisib (BY719) inhibits the PI3Kα isoform, and taselisib (GDC-0032, RG7604) is a pan-PI3K (β-sparing) inhibitor 24. PIK3CA-mutant human breast cancer cell lines show increased sensitivity to both alpelisib (p<0.001) (Supplemental Figure 2a) and taselisib (p<0.001) (Supplemental Figure 2b), compared to human breast cancer cells without mutation (cancerrxgene.org) 22. Accordingly, the phase III randomized clinical trial SOLAR-1 data revealed that patients with metastatic hormone receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) breast cancer harboring PIK3CA mutations significantly benefited from the addition of alpelisib to endocrine therapy (fulvestrant) when compared to endocrine therapy only 2, which led to FDA approval of alpelisib. This trial, which evaluated 300 mg/day alpelisib combined with fulvestrant, demonstrated a progression-free survival of 11 months for as compared to 5.7 months for fulvestrant plus placebo. The overall response for patients with PIK3CA mutation was 26.6% versus 12.8% in alpelisib versus placebo treated patients, respectively. The Sandpiper Phase III trial showed that PIK3CA mutant postmenopausal women with HR+/HER2- locally advanced, or MBC patients receiving both taselisib plus fulvestrant demonstrated a PFS of 7.4 months in contrast to 5.4 months for patients receiving fulvestrant with placebo 5, 30. This minimal response combined with severe adverse events led to withdrawal of taselisib from the market.
Human breast cancer cell lines harboring PTEN mutations are more sensitive to either paclitaxel (p=0.12) (Supplemental Figure 2c) or docetaxel (p<0.05) (Supplemental Figure 2d) (cancerrxgene.org), suggesting that combining either alpelisib or taselisib with paclitaxel or docetaxel could exert synergistic anti-tumor effects for patients with PIK3CA mutant MBC. While initial trials with alpelisib revealed considerable toxicity at doses of 300 or 250 mg alpelisib once daily with paclitaxel (80mg/m2), and the maximum tolerated dose (MTD) was established at 150mg daily when combined with paclitaxel. 31 However, another study found that compared to MBC patients without PIK3CA mutation, those with PIK3CA mutation had significantly better progression free survival (PFS) (7m versus 13m; p<0.05) when treated with alpelisib plus paclitaxel compared to PI3K inhibitor plus placebo, and the treatment was tolerable 36. Alpelisib is currently being examined for treatment of HER2+ and TNBC by Novartis in Phase III clinical trials. (Novartis. Novartis global pipeline. 2019. https://www.novartis.com/our-science/novartis-global-pipeline).
Orditura et al. generated ex-vivo three-dimensional cultures from 25 human breast cancer patients for selection of patients that were sensitive to PI3K/AKT inhibitors 27. This study found that PIK3CA mutant human breast cancer cells isolated from patient tumors were more sensitive to anti-PI3K agents than the non-PIK3CA mutant counterparts 27. Their findings also showed an additive synergy of anti-PI3K agent in combination with a microtubule stabilizer (e.g. paclitaxel), resulting in augmented killing of breast cancer patient isolated tumor cells paclitaxel on human breast cancer cells 23. The combination of taselisib and paclitaxel completely suppressed PI3K/AKT signaling pathway activation 23.
Altogether, these findings formed the basis for moving agents targeting PI3K/AKT signaling pathway into clinical trials testing their efficacy in MBC. The landscape of current clinical trials investigating the effects of PI3K/AKT signaling inhibition in MBC is quite large. Currently, reports show 15 clinical trials [(1) available, (2) recruiting, (6) active, not recruiting, (5) completed, (1) unknown] investigating the effects of alpelisib on MBC and 6 clinical trials [(1) recruiting and (3) active, not recruiting, (2) completed] investigating taselisib’s effects on MBC (clinicaltrials.gov) (Supplemental Table 1).
In pre-clinical studies, we and others have shown that inhibition of PI3K enhances the response to immune checkpoint inhibitors (ICIs) in murine cancer models 10, 17, 32. These reports show that either inhibition of PI3K with a pan inhibitor or by inhibition of only PI3Kγ can enhance response to ICIs. Taselisib will inhibit PI3Kα,γ,δ while alpelisib is PI3Kα specific. PI3Kα and β are broadly expressed in multiple cell types, while immune cell functions rely more heavily on PI3Kγ and PI3Kδ-mediated signaling 13, 39. Thus, general inhibition of PI3K could potentially inhibit tumor growth, but also lead to the impairment of anti-tumor immune cell recruitment into the tumor.
MBC patients whose tumors are infiltrated with a high content of CD8+ T effector cells have an improved PFS and/or improved overall survival 7. Studies have found that enhanced T-cell activation in the peripheral blood reflects T-cell activation in the spleen 38, and this finding could be translated to measuring intratumoral T-cell activation by analyzing T-cell activation in the peripheral blood. Moreover, there are now multiple reports showing that PI3K inhibitors enhance the activation state of CD8+ T effector cells, and this can be detected in both peripheral blood and in the tumor microenvironment 10, 32. Analysis of leukocytes in peripheral blood is one way to access the effects of therapy on immune cells when biopsy tissue is not available during treatment 38. To determine how inhibition of PI3K α,γ,δ with taselisib compares to PI3Kα inhibition with alpelisib affects the peripheral blood leukocytes in breast cancer patients treated with these inhibitors plus fulvestrant, we analyzed the peripheral blood of MBC patients enrolled in clinical trials with alpelisib or taselisib. Here we report data obtained from multiple time points on trial from 11 patients enrolled in (4) clinical trials investigating alpelisib and taselisib treatments for metastatic breast cancer (Table 1).
Table 1.
Clinical characteristics of patients enrolled in clinical trials at VUMC.
| Patient ID | Gender | Age | Initial Diagnosis | Stage | Grade | Mutation status | Hormone recepor status | Time on study (months) | Disease progression on study | Reason for withdrawal | Deceased | PFS (months) | OS (months) | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RS001 | Female | 68 | 2012 | III | High | PIK3CA, CCND1, TP53, CHD2, CYLD, FGF19, FGF3, FGF4 | AR+ | 11 | Yes (lungs) | Drug-related adverse event | Yes | 11 | 11.1 | G2 endocrine |
| RS002 | Female | 35 | 2011 | High | PIK3CA, CDK, HER2 | ER+/ PR+/HER2- | 2 | No | Quality of life | res | 4 | 7.8 | G3 vascular | |
| RS004 | Female | 64 | 2004 | II/III | High | Unknown | HER2+/ER+ | 6.5 | No | Drug-related adverse event | No | 55+ | NR | G3 respiratory, G1 neurologic, G2 dermatologic, G2 fatigue |
| RS006 | Female | 58 | 2009 | I | Intermediate | None | HER2+/ER+ (hi)/PR- | 8.5 | Yes (adrenal,bone) | Disease progression | Yes | 8.5 | 38.5 | G1 hepatic, G2 mucositis |
| RS007 | Female | 53 | 2014 | IV | Intermediate | No info | TNBC | 2.5 | Yes (bone, brain) | Disease progression | Yes | 2.5 | 3.3 | none |
| RS009 | Female | 57 | 2001 | Low | PIK3CA H1047R, APC exon 16/I10307K | HER2-/ER+/PR+/AR+ | 22 | Yes (bone,liver) | Disease progression | Yes | 22 | 24.3 | G1 hepatic, G1 gastrointestinal, G1 mucositis, G1 endocrine | |
| RS010 | Female | 59 | 1999 | IV | Low | PIK3CA | HER2-/ER+/PR+ | 6.5 | Yes (liver) | Disease progression | Yes | 6.5 | NR | G1 gastrointestinal, G2 fatigue, G2 mucositis, G1 dermatologic |
| RS011 | Female | 69 | 1989 | III | CHEK2 | HER2-/ER+/PR+ | 32.5 | Yes (bone) | Disease progression | No | 32.5 | NR | G1 gastrointestinal, G1 vascular, G2 endocrine, G1 hepatic | |
| RS012 | Female | 59 | 2009 | Intermediate | PIK3CA | HER2-/ER+/PR+ | 60+ | No | *Still on trial | No | 60+ | NR | G1 gastrointestinal, G1 fatigue, G1 endocrine, G1 dermatologic, G1 cardiac | |
| RS013 | Female | 56 | 2013 | III | High | AKT, MYC, MCCL1, NRAS amplification | HER2-/ER+ (lo)/PR- | 1.5 | No | Deceased | Yes | Unknown | 1.9 | G3 hepatic, G1 endocrine, G1 gastrointestinal, G2 fatigue, G2 hematologic |
| RS014 | Female | 70 | 2016 | II | Intermediate | PIK3CA | HER2-/ER+/PR- | 5.5 | No | Completed adjuvant therapy | No | 44+ | NR | G1 endocrine, G1 fatigue, G1 mucositis |
Patients excluded from correlation analysis (RS006 and RS007 - cycle 1 data incomplete; RS014 - non-metastatic BC).
Methods
Patients
This study was conducted according to IRB Approval number 130489. Data was collected from (11) breast cancer patients who were HR+/HER2+, HR+/HER2-, or HR-/HER2- enrolled in clinical trials NCT01862081, NCT01791478, NCT01872260, or NCT02379247 at Vanderbilt University Medical Center. Efforts were made to collect blood at each cycle of therapy, though in some cases it was not possible to obtain blood at every cycle.
Patients enrolled in Phase I/II (NCT01791478) were given, 2.5mg letrozole daily for 28 days, and alpelisib 250–600mg (escalating dose) daily for 28 days. Secondary outcome measurements included clinical benefit rate, overall response (OR), progression free survival (PFS), and toxicities. Patients enrolled in NCT01862081 were given taselisib 3–10mg (escalating dose) daily and 75mg/mΔ2 docetaxel on day 1of each 218-day cycle. Primary outcome measurements included incidence of adverse advents and dose limiting toxicities. Secondary outcome measurements included area under the curve from time 0 to the last measurable concentration, time to maximum observed plasma concentration, maximum observed plasma concentration, minimum observed plasma concentration, objective response, duration of response, and PFS. Patients enrolled in NCT01872260 Arm 2 received alpelisib 250–600mg (escalating dose) daily for 28 days and 2.5mg letrozole daily. Primary outcome measurements included incidence of dose limiting toxicities, safety and tolerability. Secondary outcome measurements included plasma concentration-time profiles, overall response rate (ORR), duration of response (DOR), and PFS. Patients enrolled in NCT02379247 were given alpelisib 250–600mg (escalating dose) daily and 100mg/kg nab-paclitaxel on days 1, 8, 15 of each 28-day cycle. Primary outcome measurements included incidence of dose limiting toxicities, safety and tolerability. Secondary outcome measurements included plasma concentration-time profiles, ORR, DOR, and PFS.
Patient Peripheral Blood Leukocyte (PBL) Isolation
Peripheral blood was collected from patients at several points in each cycle of patient treatment. Leukocytes were isolated from peripheral blood samples. Because the number of cycles on therapy varied considerably among patients, we present here the data from cycle 1 where 8/11 patients had peripheral blood collected at the beginning and end of cycle (21–28 day cycles). Patients RS006 and RS007 did not have complete cycle 1 data and patient RS014 did not have metastatic disease.
Multicolor FACS Analysis
Isolated leukocytes from 8/11 MBC patients, with complete cycle 1 data, were stained with Biolegend antibodies including (A Group) CD66b (Pacific Blue), CD163 (FITC), CD336 (PE), CD123 (Alexa Fluor 647), CD20 (PE/Cy7), and CD45 (Alexa Fluor 700), (B Group) CD25 (Alexa Fluor 647), CD69 (APC/Cy7), CD154 (Alexa Fluor 488), CD4 (PE within CD45 Alexa Fluor 700 population), and (C Group) CD107a (Alexa Fluor 488), PD-1 (Alexa Fluor 700), CD137 (PE/Cy7), and CD45RO (Pacific Blue within CD8 APC population [CD8 APC within CD45 PE population]). Cell populations were identified as CD45+CD66b+ (Neutrophil), CD45+CD163+ (Macrophage), CD45+CD336+ (Natural Killer cell), CD45+CD123+ (Dendritic cell), CD45+CD20+ (B-cell), CD45+CD25+ (Treg), CD45+CD69+ (T-cell), CD45+CD8+ (T-cell), and CD45+CD107a (T-cell).
Killing Assay
Supplemental peripheral blood was collected from patients RS013 and RS010, and CD8+ T-cells were isolated from each sample. The Dynabeads CD8+ Isolation Kit (Invitrogen) was used to capture CD8+ T-cells (cultured in HBSS with Ca2+/Mg2+ with 2% HAS media), and they were then co-cultured at a 30:1 ratio with MDA-MB-231-Fluc-GFP2 (cultured in DMEM with 10% FBS and 1% pen/strep media), human triple-negative breast cancer cell line, for 18 hours followed by measurement of MDA-MB-231-Fluc-GFP2 cell death.
Statistics
Fold change was calculated for the change in CD45+/CD8+/CD107a+ cell population in the peripheral blood from the 8 MBC patients with complete cycle 1 data. In addition, duration of response was calculated for each of the 8 patients, and a correlation analysis was performed on the fold change of CD45+/CD8+/CD107a+ cell population in relation to the duration of patient response.
Results
MBC patient population characteristics
All patients enrolled in the four clinical trials harbored metastatic disease at the time of trial enrollment except patient RS014, who did not have metastatic disease at the start of the trial. Somatic mutations of the tumors were obtained for the 8 MBC patient tumors and 6/8 harbored mutations in PI3K/AKT signaling pathway, 1/8 harbored CHEK2 mutation, and for 1/8 the PI3K mutational status was unknown (Table 1). Interestingly, we observed that patients whose tumors exhibited increased somatic mutations exhibited longer therapeutic responses (Table 1). However, we did not observe a significant correlation between the number of somatic mutations and duration of therapeutic response across all clinical trials.
The duration of therapeutic response (months) for patients varied. The average duration of therapeutic response for each respective clinical trial was 7.5m for letrozole and alpelisib, 22m for nab-paclitaxel and alpelisib, 30.7m for ribociclib, letrozole, and alpelisib, and 5.2m for docetaxel and taselisib (Figure 1). The two clinical trials with the longest therapeutic responses were (1) ribociclib, letrozole, and alpelisib and (2) nab-paclitaxel and alpelisib. The clinical trial with the shortest therapeutic response administered taselisib with docetaxel. Two patients exhibited duration of response >30 months, including one patient with PIK3CA mutation who continues to respond (60 months at time of publication).
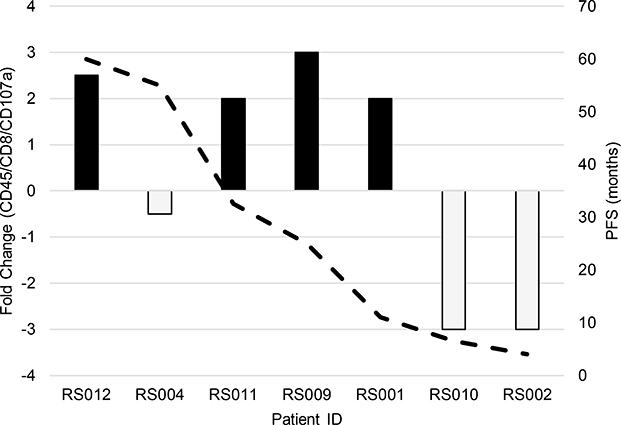
Fig. 1.
Correlation between CD45/CD8/CD107a T-cells fold-change during cycle 1 treatment, and duration of therapeutic response in MBC patients enrolled in various clinical trials
Immune response to alpelisib and taselisib therapy in MBC patients
In order to examine the effects of PI3K inhibition on leukocyte composition in the 8 MBC patients with complete cycle 1 data, peripheral blood of patients was collected at the beginning and end of cycle 1 of treatment, and multicolor-fluorescence-activated cell sorting (FACS) was used to identify leukocyte subpopulations. We did not find any significant trends in early neutrophil, macrophage, dendritic, natural killer, B-, or T-regulatory cells over cycle 1 of treatment. However, we found a strong correlation (correlation coefficient = 0.84; p<0.05) between the cytotoxic T-cell (CD45/CD8/CD107a) population relative to duration of therapeutic response (months) (Figure 1). Of the MBC patients who responded to treatment with a positive fold-change in cytotoxic T-cell population (5/8 – black bars: RS012, RS013, RS011, RS009, and RS001), there was an average duration of treatment response of 31.4 months (Figure 1). In contrast, the patients who responded to treatment with a negative fold-change in cytotoxic T-cell population (3/8 – grey bars: RS010, RS004, and RS002) had an average duration of therapeutic response of 5 months (Figure 1). The data suggest that increased cytotoxic T-cell response increases patient duration of therapeutic response.
In addition, we found a positive correlation (correlation coefficient = 0.48; p=0.10) between the cytotoxic T-cell (CD45/CD8/CD107a) population relative to progression-free survival (PFS) (months) (Figure 2). Of the MBC patients who responded to treatment with a positive fold-change in cytotoxic T-cell population (4/8 – black bars: RS012, RS011, RS009, and RS001), there was an average PFS of 32.1 months (Figure 2). In contrast, the patients who responded to treatment with a negative fold-change in cytotoxic T-cell population (3/8 – grey bars: RS010, RS004, and RS002) had an average PFS of 21.8 months (Figure 2). The data suggest that increased cytotoxic T-cell response increases patient PFS.
Fig. 2.
Fold change in the killing ability of CD45/CD8/CD107a T-cells on metastatic breast cancer (MBC) cells in relation to duration of therapeutic response
While all the patients except patient 7 had some evidence of toxicity, it is clear that the group of patients who responded to treatment with a positive fold-change in cytotoxic T cells did not exhibit more severity in adverse events than those patients who responded to treatment with a negative fold-change in cytotoxic T cells (Table 1). For example, patient 12 (stronger T cell response) did have G1 diarrhea, G1 fatigue, G1 hyperglycemia, G1 brittle nails (but no rash), G2 cramping of hands/feet, G1 alopecia, and G1 QTc prolongation. In comparison, patient 4 and patient 10, who both had a negative fold- change in cytotoxic T cell population, experienced toxicity as follows: Patient 4: G1 peripheral neuropathy, G2 paronychia, G2 pnuemonitis, G2 fatigue, G1 onycholysis; Patient 10: G1 nausea, G2 fatigue, G1 diarrhea, G1 rash, G2 mucositis.
Furthermore, CD8+ T-cells isolated from patient RS013 exhibited higher killing capacity of human triple-negative breast cancer cells compared to CD8+ T-cells isolated from patient RS010 (Figure 3). These data suggest that patients with a more robust, initial anti-tumor T-cell response may have a longer therapeutic response compared to patients who do not have a robust, initial anti-tumor T-cell response. However, this will need to be confirmed in a larger data set. Overall, alpelisib-treated MBC patients had a more robust initial anti-tumor T-cell response, which was associated with a longer therapeutic response.
Fig. 3.
Fold change in the killing ability of CD45/CD8/CD107a T-cells on metastatic breast cancer (MBC) cells in relation to duration of therapeutic response
Discussion
Elucidating tumor and microenvironment characteristics that are associated with therapeutic response and/or resistance is a major goal in the current era of personalized cancer medicine. Conventional cancer treatments such as cytotoxic and targeted chemotherapy can have immunomodulatory effects, and many studies have investigated their clinical utility with ICIs 41. Common ICIs include anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death protein 1 (anti-PD-1), and anti-programmed death-ligand 1 (anti-PD-L1) 10. Typically, breast cancers are considered immunologically cold, and recent studies show that this phenotype can be switched through the use of certain targeted therapies 32. In late 2018, PD-L1 inhibitor atezolizumab became the first ICI FDA-approved for use in combination with nab-paclitaxel in un-resectable or metastatic triple-negative breast cancer patients35.
De Henau et al. reported that ICI resistance was directly related to PI3K-associated immune suppressive function of infiltrating cells, and that targeting PI3K increased ICI sensitivity in murine melanoma, breast, and colon cancer models 10. Additionally, Kaneda et al. found that PI3K inhibition synergizes with ICI therapy to promote tumor regression and increase survival in various murine cancer models 17. This synergy is made possible via the reprogramming of pro-tumor infiltrating cells via PI3K inhibition that ablates their immune suppressive function, thus increasing the ability of ICI therapy to work efficiently 17.
Importantly, Sai et al. showed that PI3K inhibition reduces/abrogates breast cancer tumor growth and increases activated T-cells in the tumor microenvironment (TME) compared to vehicle treatment 32. Altogether, this study found that ICI therapy in combination with PI3K inhibition, synergistically inhibited breast cancer tumor growth and enhanced anti-tumor immunity 32. In addition, Schmid et al. found that paclitaxel plus ICI therapy significantly increased PFS compared to paclitaxel plus placebo (7.2m versus 5.5m; p<0.01) 35. In addition, this study found that of the patients who were PD-L1 positive, PFS was 7.5m (paclitaxel plus ICI therapy) compared to 5m (paclitaxel plus placebo; p<0.01) 35.
Evaluation of the patients enrolled in the four clinical trials in our above study suggests that real-time assaying of a patient’s cytotoxic T cells from peripheral blood could serve as a predictive biomarker of response to therapeutic regimens that treat with PI3K pathway antagonists. The results obtained here suggest that an increase in patient’s initial anti-tumor T-cell population in peripheral blood in response to alpelisib treatment may correlate with increased progression free survival or duration of therapeutic response. However, since we were able to study only a limited number of patients, our results will need to be validated in a larger study examining the therapeutic response to alpelisib in combination with is other indicated therapeutics for treatment of MBC patients.
Data from our analysis of peripheral immune cells in breast cancer patients undergoing treatment with PI3K inhibitors combined with other therapies suggest that if PI3K inhibition promotes an early anti-tumor T-cell response there is longer duration of therapeutic response and PFS. Previously, studies have shown significant increases in PFS of MBC patients when treated with either (1) paclitaxel plus ICI, (2) PI3K inhibitor plus ICI, or (3) alpelisib plus paclitaxel 32, 35, 36. Moreover, it has been previously demonstrated that therapy induced changes in T cell populations in peripheral blood of patients has a predictive value for prognosis. Increased levels of CD8+CD28+ T cells in peripheral blood was an independent predictor for increased overall survival (OS) of non-small cell lung cancer patients who were treated with chemo-radio)therapy, while elevated CD8+CD28- T cells was associated with poorer OS20. Likewise, in metastatic breast cancer in the course of chemotherapy elevated CD8+CD28- T cells predict shorter PFS 37. In addition, increased peripheral regulatory T cells in NSCL cancer patients correlated with poor PFS in response to radiotherapy 19. In contrast in a melanoma study, Treg frequency in PBMCs did not correlate with prognosis, but increased suppressive activity of Tregs after ipilimumab therapy did correlate with poorer patient outcomes 29. Patients with increased CD4+ and CD8+ T cell ratios had better PFS and OS after chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma 8. Altogether these data indicate that when response to therapy is associated with an increase in circulating T effector cells, there is an indication for heightened immune surveillance of tumor cells, which may contribute to the enhanced progression free survival in patients. In contrast, reduction in CD8+T cells and increased populations of suppressor cells in response to therapy is associated with poorer prognosis, as we observed in this study of breast cancer patients treated with therapies that target the PI3K pathway combined with chemotherapy. Moving forward, a larger cohort of patients need to undergo similar studies to prove concept is valid.
Supplementary Material
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Breast Cancer Statistics. In: Control DoCPa (ed), vol. 2019: CDC Website, 2019. [Google Scholar]
- 2.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med 2019; 380: 1929–1940. [DOI] [PubMed] [Google Scholar]
- 3.Baird RD, van Rossum AGJ, Oliveira M, Beelen K, Gao M, Schrier M et al. POSEIDON Trial Phase 1b Results: Safety, Efficacy and Circulating Tumor DNA Response of the Beta Isoform-Sparing PI3K Inhibitor Taselisib (GDC-0032) Combined with Tamoxifen in Hormone Receptor Positive Metastatic Breast Cancer Patients. Clin Cancer Res 2019; 25: 6598–6605. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017; 18: 904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baselga J, Dent SF, Cortés J, Im Y-H, Diéras V, Harbeck N et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. Journal of Clinical Oncology 2018; 36: LBA1006–LBA1006. [Google Scholar]
- 6.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res 2004; 64: 7678–7681. [DOI] [PubMed] [Google Scholar]
- 7.Chan A TILs in metastatic breast cancer-no surprises, but more questions. Lancet Oncol 2017; 18: 5–6. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Zhang W, Qian D, Guan Y, Wang Y, Zhang H et al. Chemoradiotherapy-Induced CD4(+) and CD8(+) T-Cell Alterations to Predict Patient Outcomes in Esophageal Squamous Cell Carcinoma. Front Oncol 2019; 9: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat 2018; 169: 397–406. [DOI] [PubMed] [Google Scholar]
- 10.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature 2016; 539: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickler MN, Saura C, Richards DA, Krop IE, Cervantes A, Bedard PL et al. Phase II Study of Taselisib (GDC-0032) in Combination with Fulvestrant in Patients with HER2-Negative, Hormone Receptor-Positive Advanced Breast Cancer. Clin Cancer Res 2018; 24: 4380–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 1998; 67: 481–507. [DOI] [PubMed] [Google Scholar]
- 13.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K Pathway in Human Disease. Cell 2017; 170: 605–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoeflich KP, Guan J, Edgar KA, O’Brien C, Savage H, Wilson TR et al. The PI3K inhibitor taselisib overcomes letrozole resistance in a breast cancer model expressing aromatase. Genes Cancer 2016; 7: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jean S, Kiger AA. Classes of phosphoinositide 3-kinases at a glance. J Cell Sci 2014; 127: 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juric D, Krop I, Ramanathan RK, Wilson TR, Ware JA, Sanabria Bohorquez SM et al. Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors. Cancer Discov 2017; 7: 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S et al. Corrigendum: PI3Kgamma is a molecular switch that controls immune suppression. Nature 2017; 542: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997; 275: 1943–1947. [DOI] [PubMed] [Google Scholar]
- 19.Liu C, Wu S, Meng X, Liu G, Chen D, Cong Y et al. Predictive value of peripheral regulatory T cells in non-small cell lung cancer patients undergoing radiotherapy. Oncotarget 2017; 8: 43427–43438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Hu Q, Hu K, Su H, Shi F, Kong L et al. Increased CD8+CD28+ T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer. J Transl Med 2019; 17: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kalpha-Specific Inhibitor, with Letrozole in ER+/HER2- Metastatic Breast Cancer. Clin Cancer Res 2017; 23: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore HM, Savage HM, O’Brien C, Zhou W, Sokol ES, Goldberg ME et al. Predictive and Pharmacodynamic Biomarkers of Response to the Phosphatidylinositol 3-Kinase Inhibitor Taselisib in Breast Cancer Preclinical Models. Mol Cancer Ther 2019. [DOI] [PubMed] [Google Scholar]
- 23.Morgillo F, Della Corte CM, Diana A, Mauro CD, Ciaramella V, Barra G et al. Phosphatidylinositol 3-kinase (PI3Kalpha)/AKT axis blockade with taselisib or ipatasertib enhances the efficacy of anti-microtubule drugs in human breast cancer cells. Oncotarget 2017; 8: 76479–76491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2–4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1, 2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}−2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem 2013; 56: 4597–4610. [DOI] [PubMed] [Google Scholar]
- 25.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in Cancer: Impact on Tumor Cells, Their Protective Stroma, Angiogenesis, and Immunotherapy. Cancer Discov 2016; 6: 1090–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ooms LM, Binge LC, Davies EM, Rahman P, Conway JR, Gurung R et al. The Inositol Polyphosphate 5-Phosphatase PIPP Regulates AKT1-Dependent Breast Cancer Growth and Metastasis. Cancer Cell 2015; 28: 155–169. [DOI] [PubMed] [Google Scholar]
- 27.Orditura M, Della Corte CM, Diana A, Ciaramella V, Franzese E, Famiglietti V et al. Three dimensional primary cultures for selecting human breast cancers that are sensitive to the anti-tumor activity of ipatasertib or taselisib in combination with anti-microtubule cytotoxic drugs. Breast 2018; 41: 165–171. [DOI] [PubMed] [Google Scholar]
- 28.Polyak K, Metzger Filho O. SnapShot: breast cancer. Cancer Cell 2012; 22: 562–562 e561. [DOI] [PubMed] [Google Scholar]
- 29.Retseck J, VanderWeele R, Lin HM, Lin Y, Butterfield LH, Tarhini AA. Phenotypic and functional testing of circulating regulatory T cells in advanced melanoma patients treated with neoadjuvant ipilimumab. J Immunother Cancer 2016; 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinnerthaler G, Gampenrieder SP, Greil R. ASCO 2018 highlights: metastatic breast cancer. Memo 2018; 11: 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodon J, Curigliano G, Delord JP, Harb W, Azaro A, Han Y et al. A Phase Ib, open-label, dose-finding study of alpelisib in combination with paclitaxel in patients with advanced solid tumors. Oncotarget 2018; 9: 31709–31718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sai J, Owens P, Novitskiy SV, Hawkins OE, Vilgelm AE, Yang J et al. PI3K Inhibition Reduces Mammary Tumor Growth and Facilitates Antitumor Immunity and Anti-PD1 Responses. Clin Cancer Res 2017; 23: 3371–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saura C, Roda D, Rosello S, Oliveira M, Macarulla T, Perez-Fidalgo JA et al. A First-in-Human Phase I Study of the ATP-Competitive AKT Inhibitor Ipatasertib Demonstrates Robust and Safe Targeting of AKT in Patients with Solid Tumors. Cancer Discov 2017; 7: 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saura C, Hlauschek D, Oliveira M, Zardavas D, Jallitsch-Halper A, de la Pena L et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2019; 20: 1226–1238. [DOI] [PubMed] [Google Scholar]
- 35.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med 2018; 379: 2108–2121. [DOI] [PubMed] [Google Scholar]
- 36.Sharma P, Abramson VG, O’Dea A, Pathak HB, Pessetto ZY, Wang YY et al. Clinical and biomarker results from phase I/II study of PI3K inhibitor BYL 719 (alpelisib) plus nab-paclitaxel in HER2-negative metastatic breast cancer. Journal of Clinical Oncology 2018; 36: 1018–1018. [Google Scholar]
- 37.Song G, Wang X, Jia J, Yuan Y, Wan F, Zhou X et al. Elevated level of peripheral CD8(+)CD28(−) T lymphocytes are an independent predictor of progression-free survival in patients with metastatic breast cancer during the course of chemotherapy. Cancer Immunol Immunother 2013; 62: 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teixeira-Carvalho A, Martins-Filho OA, Andrade ZA, Cunha-Mello JR, Wilson RA, Correa-Oliveira R. The study of T-cell activation in peripheral blood and spleen of hepatosplenic patients suggests an exchange of cells between these two compartments in advanced human Schistosomiasis mansoni infection. Scand J Immunol 2002; 56: 315–322. [DOI] [PubMed] [Google Scholar]
- 39.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 2010; 11: 329–341. [DOI] [PubMed] [Google Scholar]
- 40.Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005; 5: 591–602. [DOI] [PubMed] [Google Scholar]
- 41.Yan Y, Kumar AB, Finnes H, Markovic SN, Park S, Dronca RS et al. Combining Immune Checkpoint Inhibitors With Conventional Cancer Therapy. Front Immunol 2018; 9: 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.