Abstract
Context.—
Sexually transmitted infections (STIs) are among the most common communicable diseases globally and are associated with significant morbidity and mortality worldwide. Point-of-care tests have the potential to revolutionize the prevention and control of STIs by enabling rapid diagnosis and early treatment of infections, thus interrupting transmission and preventing the sequelae of untreated infections. Currently, there are several point-of-care (POC) tests available for the diagnosis of Treponema pallidum, Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis infections, although these tests differ with regard to their performance, turnaround time, and cost.
Objective.—
To provide an updated review of the POC tests available and under development for the diagnosis of T pallidum, C trachomatis, N gonorrhoeae, and T vaginalis infections, to discuss the context for which these tests might be used, and to highlight future directions for test development.
Data Sources.—
We reviewed the literature pertaining to the recent development and performance evaluations of POC tests for the diagnosis of syphilis, chlamydia, gonorrhea, and trichomonas.
Conclusions.—
Recently, there has been rapid development of new POC tests for STIs. Although there are inexpensive, rapid, and accurate POC tests available for syphilis, there are few such tests available for the diagnosis of chlamydia, gonorrhea, or trichomonas, and currently none with the ability to detect antimicrobial resistance in N gonorrhoeae. Research evaluating implementation strategies for the currently available tests and the development of additional POC tests that are rapid, accurate, and affordable are urgently needed to address the rising number of STIs worldwide.
Sexually transmitted infections (STIs) are among the most common communicable diseases worldwide, they are associated with significant morbidity and mortality, and their incidence is increasing globally.1 The World Health Organization (WHO) estimated there were 376 million infections of the 4 curable STIs—chlamydia, gonorrhea, trichomoniasis, and syphilis—in 2016, accounting for more than 1 million infections per day worldwide2 (Figure 1). In the United States, rates of bacterial STIs are increasing,3 with a 63% increase in gonorrhea, 19% for chlamydia, and 71% for syphilis since 2014. Additionally, there were 1306 cases of congenital syphilis in 2018, representing an 185% increase since 2014. Those infections are highest in vulnerable populations, including adolescents, pregnant women, and men who have sex with men.3
Figure 1.
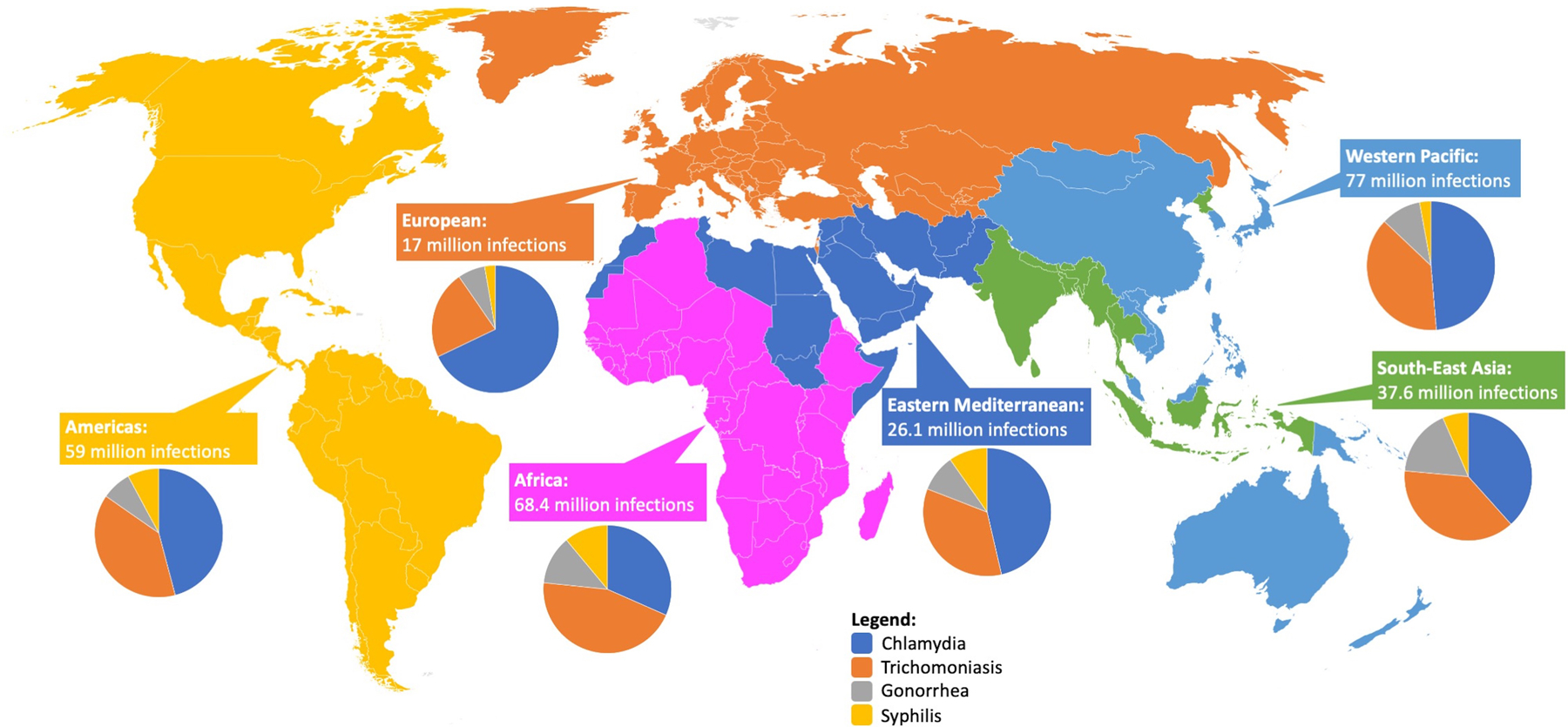
Total number and distribution of new syphilis, gonorrhea, chlamydia, and trichomoniasis infections worldwide in 2016, by World Health Organization Region.62
Sexually transmitted infections are important causes of morbidity and mortality worldwide. Sexually transmitted infections, primarily chlamydia and gonorrhea, are known to cause pelvic inflammatory disease in women, increasing the risk for ectopic pregnancies, tubal factor infertility, miscarriage, and preterm birth.4 Trichomonas vaginalis infections are a major cause of vaginal discharge, and infections during pregnancy have been associated with adverse birth outcomes, including low birth weight and preterm delivery.5 Additionally, infections with Chlamydia trachomatis, Neisseria gonorrhoeae, and T vaginalis can increase the risk for HIV acquisition and, among women living with HIV, can increase the risk for maternal-child transmission of HIV.6–8 Syphilis infections in pregnancy are associated with a number of adverse birth outcomes and are the second leading cause of stillbirth worldwide.9 The highest burden of these infections is typically seen in low- and lower-middle–income countries, which have the highest prevalence of disease and limited access to laboratory diagnostics.1
Early and effective treatment of curable STIs can prevent ongoing transmission and the sequelae of untreated infections. Thus, effective prevention and control strategies for STIs hinge on the availability of diagnostic testing to establish etiologic diagnosis of infections, as well as the provision of effective antimicrobial therapy. A number of molecular tests exist for the diagnosis of STIs, predominantly nucleic acid amplification tests (NAATs), and are primarily used in high-income countries and other well-resourced settings.10 However, establishing an etiologic diagnosis for STIs is difficult in many low-resource settings, where diagnostic tests are typically unavailable because of prohibitive costs and a lack of equipment and trained personnel.11 In response to the limited access to diagnostic testing in low- and middle-income countries, the WHO introduced syndromic case management for STIs, which continues to be used as the standard of care in most low- and middle-income countries.12 Syndromic management for STIs is based on the identification of easily recognized signs and symptoms, organized into syndromes, that guide treatment for the most common organisms causing that syndrome.13 However, many STIs are asymptomatic, and the syndromic approach to treatment does not address these infections.
Although diagnostic tests, like NAATs, have improved the diagnosis of STIs, they suffer from high costs and require laboratory infrastructure, which limits accessibility, particularly in low- and middle-income countries. In addition, many laboratory tests have long turnaround times, which can lead to delays in treatment, resulting in ongoing transmission of STIs. These barriers contribute to the increasing rates of STIs seen worldwide. Point-of-care (POC) tests for STIs can expand access to STI diagnostic testing, improve etiologic diagnosis of STIs, and facilitate a reduction in treatment times, leading to public health benefits by interrupting disease transmission.
POC TESTS
In 2004, the WHO’s Special Program for Research and Training in Tropical Diseases identified an urgent need for new POC diagnostic tests for bacterial STIs and published a set of criteria, known as the ASSURED criteria, to guide the development of tests with utility in low- and high-resource settings.14 The ASSURED criteria establish that the ideal POC tests should be affordable to health systems using the tests; sensitive, to limit false negative test results; specific, to limit false positive test results; user friendly, with simple testing procedures and a limited number of steps that can be performed with minimal training; rapid and robust, to provide results that allow for provision of treatment at the first visit, typically considered to be 60 minutes or less after sample collection, and can withstand diverse conditions of a supply chain without refrigeration; equipment free, not requiring additional laboratory equipment or able to be operated on battery power; and delivered (accessible) to end users (Table). Some have suggested that additional criteria should be added to ASSURED: real-time connectivity to improve mobile health application and ease of specimen collection (using noninvasive specimens), putting forth the REASSURED criteria.15
Table.
The ASSURED criteria, put Forth by the World Health Organization’s Special Program for Research and Training in Tropical Diseases to Guide the Development of Point-of-Care Tests
| Criterion | Description | |
|---|---|---|
| A | Affordable | Tests are affordable to the health system and individuals using the test |
| S | Sensitive | To avoid false negatives |
| S | Specific | To avoid false positives |
| U | User-friendly | Simple to perform, with minimal steps and requiring minimal training |
| R | Rapid and robust | Rapid to enable same-visit treatment |
| Robust to withstand diverse transportation and storage conditions without refrigeration | ||
| E | Equipment-free | Does not require additional equipment for collection or for processing |
| D | Delivered to end users | Accessible to end users |
There are a variety of POC STI diagnostic tests available for syphilis, chlamydia, gonorrhea, and trichomoniasis, with varying assay performance. The WHO has published a set of target product profiles (TPPs) to guide the development of POC tests. The WHO’s TPPs include test sensitivity and specificity for the following infections: gonorrhea, 90%/90%; chlamydia, higher than 90%/98%; syphilis treponemal tests, higher than 80%/higher than 90%; syphilis nontreponemal tests, higher than 95%/higher than 80%; and trichomoniasis, higher than 85%/99%.16 Although those criteria set forth minimal and optimal testing characteristics, modeling studies have shown that integrating existing POC tests, even those with lower sensitivity, can lead to more infections being treated and can be a cost-effective approach.17–19 Most of the treatment benefits are likely to occur in settings with high transmission of infections because of low rates of return for treatment and with high prevalence of asymptomatic infections.
SYPHILIS
A number of POC tests are commercially available for the diagnosis of syphilis. The majority of the available syphilis POC tests detect antibodies specific to Treponema pallidum (TP), the causative agent of syphilis, and can be used as screening tests. These tests include the Alere Determine Syphilis TP (Abbott Laboratories, Inc), SD Bioline Syphilis 3.0 (Abbott Laboratories, Inc), Syphicheck (The Tulip Group/Qualpro), and Visitect Syphilis (Omega Diagnostics), which use plasma or whole blood specimens and return results in 5 to 30 minutes. In a recent meta-analysis of those tests, excluding the Syphilis Health Check assay, Jafari et al20 found that most of the tests performed reasonably well in either whole blood or serum specimens, with sensitivities ranging from 74% to 99% and specificities ranging from 94% to 99%. In that analysis, the Alere Determine assay using serum was the best-performing test, with sensitivity of 90% and specificity of 94%.
Another POC test for the diagnosis of syphilis is the Syphilis Health Check (Trinity Biotech USA, Inc), which is the only US Food and Drug Administration (FDA)–cleared, Clinical Laboratory Improvement Amendments–waived POC syphilis test available in the United States. The test is a rapid, qualitative test to determine the presence of TP-specific antibodies. The test is a lateral flow assay that can be done using whole blood or plasma, requires only 2 steps, and returns results in 10 minutes. A recent systematic review and meta-analysis evaluated the performance of the Syphilis Health Check and found that the assay performed very well; the pooled sensitivity was 98.5% and the pooled specificity was 95.9% for laboratory evaluations. In prospective studies, the pooled sensitivity was lower at 87.7%, but this increased to 97.0% with the use of nontreponemal supplemental testing.21
A key challenge for tests using TP-specific antibodies is the ability to differentiate prior treated infections from current infections, as the antibodies remain positive following treatment. A positive TP test can be followed by a rapid plasma reagin (RPR) test, which can be used to detect an active infection. Although this practice is used in most well-resourced settings, uptake of reflex RPR testing is low in resource-limited settings. One assay has been developed for the simultaneous detection of TP-specific and non–TP-specific antibodies at the POC. The DPP Syphilis Screen & Confirm Assay (Chembio Diagnostic Systems) is Conformité Européenne (CE) marked, uses whole blood, can be stored at room temperature, and provides results in 15 to 20 minutes. The assay uses a combination of protein A and anti–human immunoglobulin M antibody, conjugated to colloidal gold particles, with TP and non-TP antigens bound to a solid-phase membrane. A running buffer is used to allow the gold conjugates to migrate and bind to the antibody/antigen complex at the separate testing lines, allowing for a visual readout. The assay performs very well; in a recent meta-analysis, Marks et al22 reported an overall agreement of 85% compared with TP serology reference tests and found a sensitivity 98% or higher for both the TP and non-TP components among patients with a rapid plasma reagin titer 1:16 or higher.
Screening for syphilis is needed among target populations who are also at risk for HIV infection, including pregnant women, men who have sex with men, and sex workers. Therefore, rapid tests that can screen for both HIV and syphilis can be used to increase screening coverage for both these infections. There are a number of dual HIV/syphilis tests available on the market, with syphilis tests detecting TP-specific antibodies and subject to the limitations previously discussed. In a systematic review, Gliddon et al23 found excellent sensitivity for HIV (>94%), but lower sensitivity for syphilis (47%–96%). They also found that dual screening was more cost-effective than single tests, could prevent more adverse birth outcomes, and were acceptable to clients.23
GONORRHEA AND CHLAMYDIA
NAAT-Based Systems
The Xpert CT/NG assay can be performed on the GeneXpert platform (Cepheid) and is a qualitative, in vitro real-time PCR test for automated detection and differentiation of genomic DNA from C trachomatis and/or N gonorrhoeae (Figure 2). The platform is modular and the assay can be performed directly on specimens collected from patients without the need for manual or off-board specimen preparation. The assay also contains a sample adequacy control to confirm adequate patient specimen and appropriate testing. Results are available within 90 minutes of loading the specimen. The assay and platform have been CE marked and FDA cleared for use in urine, endocervical, vaginal, rectal, and pharyngeal specimens.
Figure 2.

The GeneXpert system. Images used with permission from Cepheid.
The Xpert CT/NG assay is one of the best-performing POC assays, with sensitivities higher than 97% and specificities higher than 95% in genitourinary specimens.24 The assay has also received clearance from the FDA for testing in pharyngeal specimens, with sensitivity and specificity of higher than 94% and higher than 98%, and rectal specimens, with sensitivity and specificity higher than 86% and higher than 99%.25 There have been several studies showing how the GeneXpert system can be used in a variety of health care settings, in both high- and low-resource areas. A study at the Dean Street Express clinic in London, United Kingdom, demonstrated how the platform could be used to screen asymptomatic patients using self-collection to provide same-day results and reduce time to treatment.26 A study among gay, bisexual, transgender, and homeless adolescents in 2 US cities showed that the platform could be used to provide same-day treatment in these populations, which are typically difficult to treat.27 A study evaluating the Xpert CT/NG assay in antenatal clinics in rural Papua New Guinea reported high proportions of C trachomatis, N gonorrhoeae, and T vaginalis and found the assay was feasible for use in this setting.28 The assay was used among pregnant women living with HIV in South Africa, where STI prevalence was also reported to be high.29 Another study in South Africa demonstrated how the use of the assay could improve same-day treatment, facilitate expedited partner therapy, and reduce reinfection at 6 months.30 A recent study in Rwanda established that the assay could be used to provide targeting screening to improve the WHO’s syndromic treatment approach and resulted in a reduction in overtreatment of STIs.31
The binx health io CT/NG Assay (binx health, Inc) is a rapid qualitative PCR-based diagnostic system that consists of a small, benchtop, fully integrated instrument that uses single-use, assay-specific cartridges. Patient samples are loaded directly into the cartridge and no sample preparation is needed. Amplified target DNA is detected by hybridization of electrochemically labeled probes and cleavage of the label. The assay incorporates an internal process control. The turnaround time from inserting a patient sample to a result is about 30 minutes. The test is CE marked and received FDA clearance in August 2019 for use in vaginal swab specimens.
A prospective study of diagnostic accuracy of the C trachomatis assay was performed on self-collected vaginal specimens in 4 STI clinics in the United Kingdom showed promising results, with a sensitivity and specificity of 96.1% and 97.7%, respectively.32 A previous evaluation done in 2 settings in the United States reported a test sensitivity of 92.9% and specificity of 98.8%, while finding that most women found self-collection acceptable and easy, and that they were willing to wait for test results if they could receive same-day treatment.33
The Truelab Real Time micro PCR system (Molbio Diagnostics Pvt Ltd) is a rapid, portable, semiquantitative PCR assay. The testing process requires sample collection and an automated extraction process, followed by transfer of extracted nucleic acid onto the analyzer chip. Amplification of target nucleic acid releases fluorophores, which are captured by the optic sensor. Results are available in less than 1 hour, inclusive of the 20-minute extraction step. Positive or negative results, as well as semiquantitative values, are reported and an internal process control is used for validity. The platform has not been CE marked or FDA cleared.
The STI Array (Randox Biosciences) has been developed to be performed on a Vivalytic Analyzer (Bosch Healthcare Solutions), which is a small, fully automated device platform that is capable of quantitative PCR and uses microfluidics to achieve rapid results. The STI array cartridge contains all reagents needed for testing and does not require sample preparation. The cartridge contains internal controls that also integrate with the Vivalytic Analyzer. The STI array is CE marked.
There are a number of promising POC diagnostic tests that are currently under development. On such test, the mobiNAAT, is a mobile phone–based NAAT that uses a magnetofluidic cartridge, the size of a USB device, to perform loop-mediated isothermal amplification that is powered and operated through the mobile phone. The platform delivers results in approximately 1 hour. A prototype of the device testing for C trachomatis was evaluated in an emergency room setting and the results showed 100% concordance with the standard-of-care NAAT (2 of 30 patients positive for C trachomatis).34 Another promising test is the TwistDx assay, which uses an isothermal recombinase polymerase amplification to detect C trachomatis and N gonorrhoeae in approximately 15 minutes and can be run on battery power. Sample preparation includes a brief immersion in a lysis buffer for 90 seconds and mixing of a neutralization buffer. A prototype of the TwistDx assay recently underwent a diagnostic evaluation on genital specimens and was found to have a sensitivity ranging from 94.3% to 100% and a specificity from 99.7% to 100%, with results being available in 20 minutes.35 Related to the GeneXpert platform, the GeneXpert Omni is a single-cartridge, battery-powered platform that uses low power and offers connectivity for secure data transfer and storage. Mobile devices can control the testing module, allowing for improved scalability and flexibility. The platform is currently under development, and is being evaluated for tuberculosis testing. Future application for STIs is in development.
Non–NAAT-Based Systems
In addition to the NAAT-based POC tests for C trachomatis mentioned above, there are also a number of POC tests that can detect C trachomatis antigen. The antigen detection tests are rapid, inexpensive, simple to use, lateral flow assays. A recent systematic review of the ACON Chlamydia (ACON Laboratories), aQcare Chlamydia TRF kit (Medisensor Inc), BioRapid Chlamydia Ag Test (Biokit SA), Chlamydia Rapid Test SAS (Diagnostics for the Real World), Clearview Chlamydia (Abbott Laboratories, Inc), Chlamydia test card (Ultimed Products GmbH), HandiLab-C (HandiLab), and QuickVue (Quidel Corporation) found low pooled sensitivities of the tests (37%–63%), despite excellent specificities (≥97%).36 However, the aQcare Chlamydia TRF kit performed on par with NAATs, with a sensitivity ranging from 88.2% in cervical specimens to 93.8% in urine specimens and specificities ranging from 94.7% to 96.8%.36
There are fewer antigen-detection POC tests available for N gonorrhoeae. There are 4 immunoassay tests available for N gonorrhoeae: ACON Duo and NG ACON Plate (ACON Laboratories), BioStar Optical ImmunoAssay-Gonorrhea (BioStar, Inc), and the One Step Gonorrhea RapiCard InstaTest (Cortez Diagnostics). Most of these assays have only undergone preliminary testing. Among those, the BioStar Optical ImmunoAssay shows the most promise, with sensitivity of 100% and specificity of 98% on urine specimens in symptomatic men, although it has been evaluated only in a pilot study, with a very small sample size.37
Although many of the available lateral flow assay tests for C trachomatis and N gonorrhoeae show promise, they generally underperform compared with NAAT-based POC tests and are not suitable for use in screening populations. There is an ongoing need for better-performing assays and more rigorous evaluations to inform their potential use.
TRICHOMONIASIS
Traditionally, T vaginalis infections were diagnosed at the POC through wet-mount microscopy, which, although inexpensive and rapid, suffers from poor sensitivity, ranging from 36% to 75%.38 However, several molecular tests are now available for the diagnosis of T vaginalis infections at the POC, as identified by Gaydos et al39 in a recent systematic review.
The Xpert TV assay (Cepheid) is FDA cleared to detect T vaginalis infections. The sensitivity ranges from 95% in patient-collected vaginal swabs to 100% in symptomatic endocervical swabs, with specificities of 99% or higher.40 A benefit of this assay is that it can be used in settings already using the GeneXpert platform. Indeed, the assay was found to be feasible in antenatal care clinics in Papua New Guinea and among pregnant women living with HIV in South Africa.28,29
Another available assay is the Solana Trichomonas Assay (Quidel), which is a qualitative NAAT that can be run on Solana’s platform and is FDA cleared for clinician-collected vaginal swabs and urine specimens. The assay is designated as a moderately complex POC assay by the FDA, requires a short specimen preparation step, and can produce results in 35 minutes. The sensitivity of the assay ranges from 92.9% in urine specimens of asymptomatic women to 100% in vaginal swabs in asymptomatic women, with specificities 98% or higher.41
The OSOM Trichomonas Rapid Test (Sekisui Diagnostics) is a rapid antigen test that uses an immunochromatographic capillary-flow immunoassay that can provide results in 10 minutes. The assay is FDA cleared, is Clinical Laboratory Improvement Amendments waived, and compares favorably with NAAT-based assays, with sensitivities ranging from 83% to 90%.39 This assay is relatively inexpensive and does not require additional equipment, differentiating itself from the other platforms. These aspects also make the assay appealing for use in low-resource settings, and studies have demonstrated its potential to improve diagnosis of T vaginalis infections in Rwanda and South Africa.30,31
The timelines for the available POC tests for STIs and those under development are displayed in Figure 3.42
Figure 3.
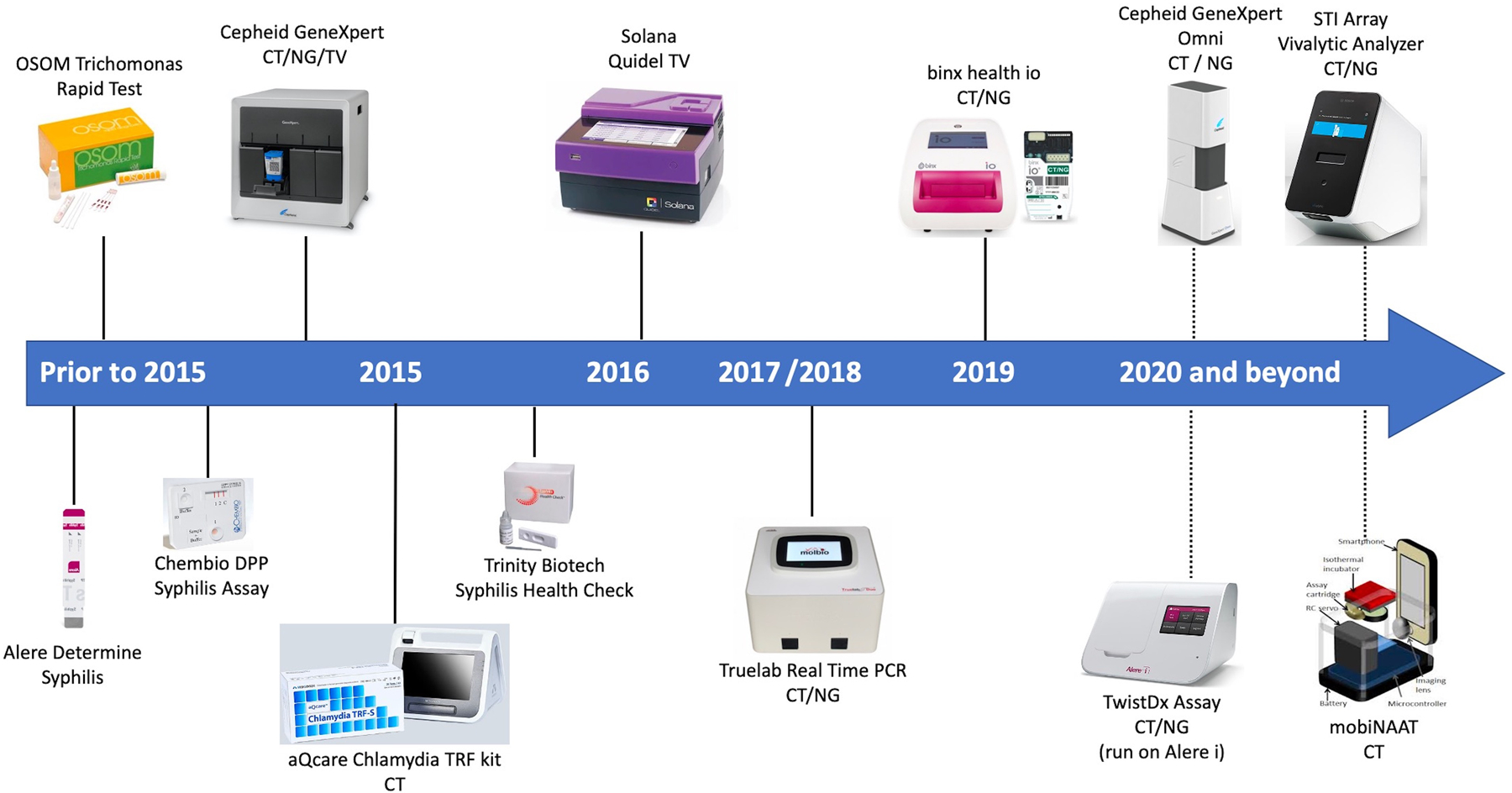
Point-of-care tests, available and under development, for the diagnosis of syphilis, chlamydia, gonorrhea, and trichomoniasis. Dotted lines indicate tests that are not yet commercially available. Figure adapted from prior report and used with author’s permission.42 Abbreviations: CT, Chlamydia trachomatis; NG, Neisseria gonorrhoeae; PCR, polymerase chain reaction; TV, Trichomonas vaginalis.
ABILITY TO DETECT DRUG RESISTANCE
The emergence and spread of antimicrobial resistance (AMR) in N gonorrhoeae is an urgent global public health concern, and N gonorrhoeae has been listed as a priority pathogen by the WHO.43 The US Centers for Disease Control and Prevention estimates that approximately half of all gonorrhea infections in the United States are resistant to at least 1 antibiotic.3 Recently, several extensively resistant strains of gonorrhea, so-called superbugs, that are highly resistant to azithromycin and resistant to ceftriaxone—the last line of empiric therapy—have emerged.44–46 Additionally, the spread of ceftriaxone-resistant strains has been found in Canada and Denmark.45 The increasing identification of highly resistant strains of N gonorrhoeae highlights the impending threat of untreatable gonorrhea.
Given the remarkable ability for N gonorrhoeae to develop resistance to every class of antibiotics used for treatment, diagnostic tests that can detect AMR in N gonorrhoeae are needed.47 Point-of-care tests allow for rapid ascertainment of etiologic diagnosis and can help guide clinical decisions, reduce treatment time, and allow for more targeted antimicrobial therapy, thereby slowing the spread of antibiotic resistance.48 In addition, POC tests that can rapidly detect determinants of AMR promise to provide personalized, targeted therapy, thereby allowing for repurposing of older antimicrobial treatment and reducing the use of last-line antibiotics. However, there are currently no commercially available POC tests that can predict AMR in N gonorrhoeae.
Modeling studies have shown that the introduction of POC tests for N gonorrhoeae can reduce presumptive treatment and lead to more targeted therapy.49,50 However, there are also concerns that higher treatment rates can increase the spread of resistance.51,52 Several modeling studies have investigated the potential impact of introducing a POC test with the ability to detect AMR in N gonorrhoeae. The models generally found that the introduction of a POC test with resistance testing could result in delayed emergence of resistance and extend the use of existing antimicrobial therapies.53–55 Nevertheless, the models also contained cautionary findings. The Fingerhuth et al54 model showed that the introduction of a POC without resistance detection could accelerate the spread of resistance, whereas the Tuite et al55 model found that a POC test with resistance testing limited to a single antibiotic accelerated the emergence of isolates with resistance to all 3 antibiotics tested. These models can provide a framework for the development and eventual implementation of new POC tests.
Despite the urgent need for POC tests with the ability to detect AMR is N gonorrhoeae, there are no commercially available tests. Neisseria gonorrhoeae has developed numerous molecular mechanisms to evade antimicrobials, and this creates a challenge for the development of POC tests to predict AMR.48,56 Some mutations that confer AMR are limited to specific single-nucleotide polymorphisms, as is the case with resistance to fluoroquinolones, whereas other genetic determinants of resistance are more complex, existing as highly variable regions of DNA, termed mosaics, and undergo constant evolution, which create further challenges to the development of POC tests, particularly for the extended-spectrum cephalosporins.48 One proposed strategy to overcome that challenge includes a test that would determine the absence of any resistance determinants, effectively identifying a wild-type infection, although this approach would be limited in settings with high fluoroquinolone resistance.56
Despite those challenges, the availability of such tests could revolutionize treatment of N gonorrhoeae infection by enabling resistance-guided therapy, and development of these tests is underway.56,57 Recently, the WHO, the Foundation for Innovative New Diagnostics, and the Global Antibiotic Research and Development Partnership convened an expert panel to put forth 2 TPPs for the detection of Neisseria gonorrhoeae at the POC.58 One TPP is for a test for etiologic diagnosis of N gonorrhoeae and C trachomatis, and the other is for a test that predicts AMR in N gonorrhoeae. The proposed TPP for the POC test to detect N gonorrhoeae and C trachomatis infection would have a clinical sensitivity of higher than 80% for a nonmolecular test (eg, a lateral flow assay that would operate like a “pregnancy test”) or higher than 95% for a molecular test, a clinical specificity of higher than 95%, a result time of 30 minutes or less, and a price lower than US $3. The proposed TPP for the POC test to identify genetic markers of antibiotic resistance and susceptibility in N gonorrhoeae would have a clinical sensitivity of higher than 98% and clinical specificity of higher than 95% to predict resistance, with a result time of 60 minutes or less and a cost lower than US $25. Assays that meet these criteria are being supported and are in the early stages of research and development.59
CONCLUSIONS
During the past decade, there has been rapid development of new POC tests for STIs, with more tests in the development pipeline. The availability of those tests has the potential to improve the treatment and prevention of STIs globally.10,60 However, although the available POC tests for syphilis have performed well, there is need for additional POC tests for C trachomatis and N gonorrhoeae, the 2 most common bacterial STIs worldwide. The number of those tests has increased, but there is considerable variability in their performance. Additionally, POC tests with the ability to simultaneously detect AMR in N gonorrhoeae will be a critical area of future development, as AMR in N gonorrhoeae has become an increasing global health threat and there are no such tests currently available.
Research and development of new, improved POC tests for STIs requires considerable investment, which can be a primary barrier to bringing these tests to market. The research and development of new and improved POC tests can be burdensome to companies, as many tests fail to make it through the early stages of development, which highlights an opportunity for funding organizations to provide additional funding toward the early-stage development of novel assays. That is an approach being taken by the Foundation for Innovative New Diagnostics and Global Antibiotic Research and Development Partnership partnerships and will hopefully catalyze the development of improved POC tests.61 Additional public-private partnerships, for example between industry and public health programs, might also play an important role in the development and testing of new assays, particularly in low-resource settings where there is an urgent need for diagnostic tests and where the development and introduction of POC tests can be key to addressing health equity in settings that might lack extensive laboratory infrastructure.60 In addition, the burden of undiagnosed STIs is greatest in low-resource settings, where the unmet need in POC tests for STIs is driving the development of new assays.10
The regulatory process can be another barrier to the development and introduction of new POC tests for STIs. Currently, regulatory approval processes for the introduction of diagnostic tests are varied, with processes that vary by country and region. Those processes can present a barrier on both ends of the spectrum: a regulatory system that is too strict can impede development and introduction of products, whereas one that is too weak allows for introduction of inaccurate and ineffective tests into the marketplace.61 Standardizing the scattered regulatory approval frameworks has been suggested as one way to accelerate the development and evaluation of new diagnostic POC tests for STIs.57
Even with the tests that are currently available, there exist many barriers to the implementation of STI testing in key target populations. Costs are one key implementation barrier, as the costs of many of the available NAAT-based POC tests are prohibitive in many resource-limited settings where the burden of STIs is greatest.10 Still, studies done in these settings have shown that the use of NAAT-based POC tests is acceptable and feasible, but additional cost-effectiveness studies will be essential to justify the investment by health systems.28–31 Cost-effectiveness studies in high-income settings have demonstrated that the tests can be cost-effective in different testing scenarios.18,51 Ensuring adherence to the ASSURED criteria is one way to maximize the impact of new POC tests.
Additionally, it is important to determine the local context for which POC tests and testing strategies can be used in different health care settings (eg, primary care clinics, antenatal clinics, STI clinics) and within different health care systems (eg, resource-rich or resource-limited settings) in order to achieve different health outcomes (eg, prevention of congenital syphilis, decreasing pelvic inflammatory disease, addressing the threat of AMR). The fields of implementation and programmatic sciences can play important roles in researching ways to address those issues, by adapting the POC tests to the local context and by bridging the divide between research and practice. Additional funding and research into these disciplines will be critical as the availability of new assays increases.
In summary, there is an urgent need for POC testing to combat the rise in STIs globally. Recent years have seen remarkable advances in new diagnostic POC tests and the future development pipeline is promising, but still more is needed. Collaborations among industry, funding bodies, and public health systems can catalyze the development of new tests and ensure that these POC tests reach their target populations around the world where testing is most needed.
Footnotes
Dr Loeffelholz is an employee and receives salary from Cepheid. Dr Klausner is a member of the Cepheid advisory board. Dr Adamson has no relevant financial interest in the products or companies described in this article.
Contributor Information
Paul C. Adamson, Division of Infectious Diseases, David Geffen School of Medicine at UCLA, Los Angeles, California.
Michael J. Loeffelholz, Cepheid, Sunnyvale, California.
Jeffrey D. Klausner, Division of Infectious Diseases, David Geffen School of Medicine at UCLA, Los Angeles, California; Fielding School of Public Health, University of California, Los Angeles.
References
- 1.World Health Organization. Report on global sexually transmitted infection surveillance. Geneva, Switzerland: World Health Organization; 2018. https://www.who.int/reproductivehealth/publications/stis-surveillance-2018/en/. Accessed Feb 23, 2020. [Google Scholar]
- 2.Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548–562P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2018. Atlanta, GA: US Dept of Health and Human Services; 2019. [Google Scholar]
- 4.Wynn A, Bristow CC, Cristillo AD, et al. Sexually transmitted infections in pregnancy and reproductive health. Sex Transm Dis. 2020;47(1):5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meites E, Gaydos CA, Hobbs MM, et al. A review of evidence-based care of symptomatic trichomoniasis and asymptomatic Trichomonas vaginalis infections. Clin Infect Dis 2015;61(suppl 8):S837–S848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet 1998;351:S5–S7. [DOI] [PubMed] [Google Scholar]
- 7.King CC, Ellington SR, Kourtis AP. The role of co-infections in mother-to-child transmission of HIV. Curr HIV Res 2013;11(1):10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adachi K, Xu J, Yeganeh N, et al. Combined evaluation of sexually transmitted infections in HIV-infected pregnant women and infant HIV transmission. PLoS One. 2018;13(1):e0189851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korenromp EL, Rowley J, Alonso M, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes—estimates for 2016 and progress since 2012. PLoS One. 2019;14(2):e0211720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wi TE, Ndowa FJ, Ferreyra C, et al. Diagnosing sexually transmitted infections in resource-constrained settings: challenges and ways forward. J Int AIDS Soc 2019;22(suppl 6):e25343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristillo AD, Bristow CC, Peeling R, et al. Point-of-care sexually transmitted infection diagnostics: proceedings of the STAR Sexually Transmitted Infection–Clinical Trial group programmatic meeting. Sex Transm Dis 2017;44(4):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Progress report of the implementation of the global strategy for prevention and control of sexually transmitted infections: 2006–2015: document for the World Health Assembly. Geneva, Switzerland: World Health Organization; 2015. https://apps.who.int/iris/handle/10665/183117. Accessed April 27, 2020. [Google Scholar]
- 13.World Health Organization. Sexually transmitted and other reproductive tract infections: a guide to essential practice. Geneva, Switzerland: World Health Organization; 2005. https://apps.who.int/iris/handle/10665/43116. Accessed April 27, 2020. [Google Scholar]
- 14.UNICEF/UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases. Mapping the landscape of diagnostics for sexually transmitted infections. https://www.who.int/tdr/publications/documents/mapping-landscape-sti.pdf. Published 2004. Accessed Feb 23, 2020.
- 15.Land KJ, Boeras DI, Chen X-S, Ramsay AR, Peeling RW. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 2019;4(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Technical consultation on point-of-care tests for sexually transmitted infections. Geneva, Switzerland: World Health Organization; 2014. https://www.who.int/reproductivehealth/POTC-TPPs-2016.pdf. Accessed Feb 23, 2020. [Google Scholar]
- 17.Gift TL, Pate MS, Hook EW 3rd, Kassler WJ. The rapid test paradox: when fewer cases detected lead to more cases treated: a decision analysis of tests for Chlamydia trachomatis. Sex Transm Dis 1999;26(4):232–240. [DOI] [PubMed] [Google Scholar]
- 18.Huntington SE, Burns RM, Harding-Esch E, et al. Modelling-based evaluation of the costs, benefits and cost-effectiveness of multipathogen point-of-care tests for sexually transmitted infections in symptomatic genitourinary medicine clinic attendees. BMJ Open. 2018;8(9):e020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vickerman P Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex Transm Infect 2003;79(5):363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafari Y, Peeling RW, Shivkumar S, Claessens C, Joseph L, Pai NP. Are Treponema pallidum specific rapid and point-of-care tests for syphilis accurate enough for screening in resource limited settings?: evidence from a meta-analysis. PLoS One. 2013;8(2):e54695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristow CC, Klausner JD, Tran A. Clinical test performance of a rapid point-of-care syphilis treponemal antibody test: a systematic review and meta-analysis. Clin Infect Dis In press. [DOI] [PMC free article] [PubMed]
- 22.Marks M, Yin Y-P, Chen X-S, et al. Metaanalysis of the performance of a combined treponemal and nontreponemal rapid diagnostic test for syphilis and yaws. Clin Infect Dis 2016;63(5):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gliddon HD, Peeling RW, Kamb ML, Toskin I, Wi TE, Taylor MM. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sex Transm Infect 2017;93(S4):S3–S15. doi: 10.1136/sextrans-2016-053069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbst De Cortina S, Bristow CC, Joseph Davey D, Klausner JD. A systematic review of point of care testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infect Dis Obstet Gynecol 2016;2016:4386127. doi: 10.1155/2016/4386127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doernberg SB, Komarow L, Tran TTT, et al. Simultaneous evaluation of diagnostic assays for pharyngeal and rectal Neisseria gonorrhoeae and Chlamydia trachomatis using a master protocol. Clin Infect Dis 2019;ciz1105. doi: 10.1093/cid/ciz1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wingrove I, McOwan A, Nwokolo N, Whitlock G. Diagnostics within the clinic to test for gonorrhoea and chlamydia reduces the time to treatment: a service evaluation. Sex Transm Infect 2014;90(6):474. [DOI] [PubMed] [Google Scholar]
- 27.Keizur EM, Goldbeck C, Vavala G, et al. Safety and effectiveness of same-day Chlamydia trachomatis and Neisseria gonorrhoeae screening and treatment among gay, bisexual, transgender, and homeless youth in Los Angeles, California, and New Orleans, Louisiana. Sex Transm Dis 2020;47(1):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badman SG, Vallely LM, Toliman P, et al. A novel point-of-care testing strategy for sexually transmitted infections among pregnant women in high-burden settings: results of a feasibility study in Papua New Guinea. BMC Infect Dis 2016;16(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudau M, Peters RP, De Vos L, et al. High prevalence of asymptomatic sexually transmitted infections among human immunodeficiency virus-infected pregnant women in a low-income South African community. Int J STD AIDS 2018;29(4):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrett N, Mitchev N, Osman F, et al. Diagnostic accuracy of the Xpert CT/NG and OSOM Trichomonas Rapid assays for point-of-care STI testing among young women in South Africa: a cross-sectional study. BMJ Open. 2019;9(2):e026888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verwijs MC, Agaba SK, Sumanyi J-C, et al. Targeted point-of-care testing compared with syndromic management of urogenital infections in women (WISH): a cross-sectional screening and diagnostic accuracy study. Lancet Infect Dis 2019;19(6):658–669. [DOI] [PubMed] [Google Scholar]
- 32.Harding-Esch EM, Cousins EC, Chow SLC, et al. A 30-min nucleic acid amplification point-of-care test for genital chlamydia trachomatis infection in women: a prospective, multi-center study of diagnostic accuracy. EBioMedicine 2018;28:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widdice LE, Hsieh Y-H, Silver B, Barnes M, Barnes P, Gaydos CA. Performance of the Atlas Genetics rapid test for chlamydia trachomatis and womenʼs attitudes toward point-of-care testing. Sex Transm Dis 2018;45(11):723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin DJ, Athamanolap P, Chen L, et al. Mobile nucleic acid amplification testing (mobiNAAT) for Chlamydia trachomatis screening in hospital emergency department settings. Sci Rep 2017;7(1):4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harding-Esch EM, Fuller SS, Chow SLC, et al. Diagnostic accuracy of a prototype rapid chlamydia and gonorrhoea recombinase polymerase amplification assay: a multicentre cross-sectional preclinical evaluation. Clin Microbiol Infect 2019;25(3):380.e1–380.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly H, Coltart CEM, Pant Pai N, et al. Systematic reviews of point-of-care tests for the diagnosis of urogenital Chlamydia trachomatis infections. Sex Transm Infect 2017;93(S4):S22–S30. [DOI] [PubMed] [Google Scholar]
- 37.Samarawickrama A, Cheserem E, Graver M, Wade J, Alexander S, Ison C. Pilot study of use of the BioStar Optical ImmunoAssay GC point-of-care test for diagnosing gonorrhoea in men attending a genitourinary medicine clinic. J Med Microbiol 2014;63(pt 8):1111–1112. [DOI] [PubMed] [Google Scholar]
- 38.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gynecol 2009;200(2):188.e1–e7. [DOI] [PubMed] [Google Scholar]
- 39.Gaydos CA, Klausner JD, Pai NP, Kelly H, Coltart C, Peeling RW. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect 2017;93(S4):S31–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the Cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2017;56(2):e01091–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaydos C, Schwebke J, Dombrowski J, et al. Clinical performance of the Solana® point-of-care Trichomonas assay from clinician-collected vaginal swabs and urine specimens from symptomatic and asymptomatic women. Expert Rev Mol Diagn 2017;17(3):303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murtagh MM. The point-of-care diagnostic landscape for sexually transmitted infections (STIs). https://www.who.int/reproductivehealth/topics/rtis/Diagnostic-Landscape-for-STIs-2019.pdf. Accessed January 31, 2020.
- 43.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1. Published February 27, 2017. Accessed April 27, 2020.
- 44.Eyre DW, Sanderson ND, Lord E, et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Eurosurveillance. 2018;23(27):1800323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lahra MM, Martin I, Demczuk W, et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg Infect Dis 2018;24(4):735–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poncin T, Merimeche M, Braille A, et al. Two cases of multidrug-resistant Neisseria gonorrhoeae related to travel in south-eastern Asia, France, June 2019. Eurosurveillance. 2019;24(36). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unemo M, Bradshaw CS, Hocking JS, et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis 2017;17(8):e235–e279. [DOI] [PubMed] [Google Scholar]
- 48.Low N, Unemo M. Molecular tests for the detection of antimicrobial resistant Neisseria gonorrhoeae: when, where, and how to use? Curr Opin Infect Dis 2016;29(1):45–51. [DOI] [PubMed] [Google Scholar]
- 49.Chan CH, McCabe CJ, Fisman DN. Core groups, antimicrobial resistance and rebound in gonorrhoea in North America. Sex Transm Infect 2012;88(3):200–204. [DOI] [PubMed] [Google Scholar]
- 50.Turner KME, Round J, Horner P, et al. An early evaluation of clinical and economic costs and benefits of implementing point of care NAAT tests for Chlamydia trachomatis and Neisseria gonorrhoea in genitourinary medicine clinics in England. Sex Transm Infect 2014;90(2):104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fingerhuth SM, Bonhoeffer S, Low N, Althaus CL. Antibiotic-resistant Neisseria gonorrhoeae spread faster with more treatment, not more sexual partners. PLOS Pathog 2016;12(5):e1005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kenyon CR, Schwartz IS. Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in Neisseria gonorrhoeae. Emerg Infect Dis 2018;24(7):1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turner KM, Christensen H, Adams EJ, et al. Analysis of the potential for point-of-care test to enable individualised treatment of infections caused by antimicrobial-resistant and susceptible strains of Neisseria gonorrhoeae: a modelling study. BMJ Open. 2017;7(6):e015447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fingerhuth SM, Low N, Bonhoeffer S, Althaus CL. Detection of antibiotic resistance is essential for gonorrhoea point-of-care testing: a mathematical modelling study. BMC Med 2017;15(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuite AR, Gift TL, Chesson HW, Hsu K, Salomon JA, Grad YH. Impact of rapid susceptibility testing and antibiotic selection strategy on the emergence and spread of antibiotic resistance in gonorrhea. J Infect Dis 2017;216(9):1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadiq ST, Mazzaferri F, Unemo M. Rapid accurate point-of-care tests combining diagnostics and antimicrobial resistance prediction for Neisseria gonorrhoeae and Mycoplasma genitalium. Sex Transm Infect 2017;93(S4):S65–S68. [DOI] [PubMed] [Google Scholar]
- 57.Toskin I, Peeling RW, Mabey D, et al. Point-of-care tests for STIs: the way forward. Sex Transm Infect 2017;93(S4):S1–S2. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. Technical consultation on in vitro diagnostics for AMR, 27–28 March 2019, WHO Headquarters, Geneva: meeting report. Geneva, Switzerland: World Health Organization; 2019. https://apps.who.int/iris/handle/10665/326481. Accessed Feb 23, 2020. [Google Scholar]
- 59.Foundation for Innovative New Diagnostics (FIND). Antimicrobial resistance. https://www.finddx.org/amr/. Accessed February 9, 2020.
- 60.Drain PK, Hyle EP, Noubary F, et al. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 2014;14(3):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med 2012;9(9):e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization. Evidence brief: sexually transmitted infections. https://www.who.int/reproductivehealth/publications/stis-evidence-brief/en/. Published 2019. Accessed May 21, 2020.


