Abstract
Objective
Although Essential Tremor is one of the most common movement disorders, we do not currently know which muscles are most responsible for tremor. Determining this requires multiple steps, one of which is characterizing the distribution of tremor among the degrees of freedom (DOF) of the upper limb.
Methods
Upper-limb motion was recorded while 22 subjects with ET performed postural and kinetic tasks involving a variety of limb configurations. We calculated the mean distribution of tremor among the seven DOF from the shoulder to the wrist, as well as the effect of limb configuration, repetition, and subject characteristics (sex, tremor onset, duration, and severity) on the distribution.
Results
On average, kinetic tremor was greatest in forearm pronation-supination and wrist flexion-extension, intermediate in shoulder internal-external rotation and wrist radial-ulnar deviation and then shoulder flexion-extension and elbow flexion-extension, and least in shoulder abduction-adduction. The average distribution of postural tremor was similar except for forearm pronation-supination, which played a smaller role than in kinetic tremor. Limb configuration and subject characteristics did significantly affect tremor, but practically only in forearm pronation-supination and wrist flexion-extension. There were no significant differences between repetitions, indicating that the distribution was consistent over the duration of the experiment.
Conclusions
This paper presents a thorough characterization of tremor distribution from the shoulder to the wrist.
Significance
Understanding which DOF exhibit the most tremor may lead to more targeted peripheral tremor suppression.
Keywords: Essential tremor, tremor distribution, upper limb, motion capture, inverse kinematics, power spectral density, degrees of freedom
1. Introduction
Essential Tremor (ET) is one of the most common movement disorders, affecting approximately 7 million people in the US (Louis and Ottman, 2014). Although treatments are available, many patients are left without satisfactory treatment options. The most efficacious medications, propranolol and primidone, are only effective in 50% of patients and, on average, only provide a 50% reduction in tremor (Elble and Deuschl, 2009). Botulinum toxin type A injections also provide some tremor reduction but are accompanied by dose-dependent hand weakness (Zesiewicz et al., 2013) unless guided by sensor-based measurements of tremor (Samotus et al., 2018, Samotus et al., 2017, Samotus et al., 2016). Surgical methods, such as deep brain stimulation and focused ultrasound, produce a greater reduction in tremor but are highly invasive; consequently, only about 1 in 30 ET patients undergoes DBS surgery (Kestenbaum et al., 2015). In a recent survey designed to discover gaps in their current care, ET patients listed “a treatment approach other than just medications and surgery” as one of the top items (Louis et al., 2015).
Peripheral tremor suppression, for example through low-pass filtering orthoses (Belda-Lois et al., 2007a, Belda-Lois et al., 2007b, Case et al., 2013, 2014, 2015, Fromme et al., 2019, Hashemi et al., 2004, Kotovsky and Rosen, 1998, Loureiro et al., 2005) or through electrical (Dosen et al., 2015, Freeman et al., 2015, Maneski et al., 2011, Prochazka et al., 1992) or vibratory stimulation (Feys et al., 2006, King et al., 2009) offers a potential alternative to medication and surgery, but we do not currently know where to intervene (which muscles or joints) to best suppress tremor. To optimally suppress tremor at the hand, we must understand which muscles contribute most to tremor.
Determining which muscles are most responsible for a patient’s tremor is a challenging problem that has, to the best of our knowledge, never been achieved in any type of tremor (pathological or physiological). The main difficulty arises from the fact that tremor propagates along the upper limb, so tremor at a joint may be caused by muscles throughout the upper limb, not just muscles acting on that joint. To understand, it is helpful to view tremor propagation as a multi-input multi-output system in which tremorogenic activity in the muscles of the upper limb are the inputs, tremulous displacements in the various degrees of freedom of the upper limb are the outputs, and the upper limb is the system that filters and mixes the inputs on their way to becoming outputs (Corie and Charles, 2019). Because the upper limb filters and mixes the inputs, it is not possible to determine which muscles contribute most to a patient’s tremor from measurements of the inputs or outputs alone, or even from a simple comparison of inputs and outputs. Instead, one must use a system-dynamics approach to establish the relationships between the inputs and outputs. Such an approach requires multiple steps, the first of which involve thoroughly characterizing the inputs and outputs. The purpose of this paper is to characterize the outputs (tremor).
A thorough characterization of tremor in the upper limb includes the distribution of tremor among the degrees of freedom (DOF) of the upper limb, i.e. determining which DOF has the most tremor, second-most tremor, and so on. Furthermore, if this information is to be useful in the future to develop tremor-suppressing devices for a variety of patients and conditions, we need to understand how this distribution varies across patients and time, and whether the distribution depends on conditions (such as task and limb configuration) or subject characteristics (e.g. sex; tremor severity, onset, and duration). Such a characterization does not currently exist, whether in a prior study or combination of prior studies. That said, a few studies have investigated tremor distribution in a subset of DOF, and some of these studies have determined the variability or effect of a limited subset of conditions or subject characteristics. Rocon and Pons measured tremor in 21 patients with ET while they performed a variety of tasks (Rocon and Pons, 2011). However, they measured tremor in only four DOF (in the elbow, forearm, and wrist), and only a high-level summary of their results is available, with virtually no detail regarding the mean or variability of tremor distribution. According to the high-level summary, they found in this subset of DOF that tremor amplitude increased proximal-distally, and that tremor frequency was similar between these DOF and independent of task. To guide injection of Botulinum toxin type A, Jog and colleagues used flexible goniometers to measure tremor in 6 DOF while 24 patients with ET performed postural tasks (Samotus et al., 2017, Samotus et al., 2016). However, since the goal of their study was to demonstrate how to target tremor-generating muscles at individual joints and in individual patients, they did not compare tremor across joints or patients, so their results did not compare tremor magnitude across more than 3 DOF or include the variability across patients or the effect of subject characteristics. We recently presented a method to characterize tremor in the 7 DOF from the shoulder to the wrist (Geiger et al., 2018) but only included preliminary results from a relatively small number of patients (all with mild ET), and without characterizing the variability across patients or the effect of task or subject characteristics. In summary, although subsets of DOF and settings have been studied, the distribution of tremor throughout the upper limb is unknown, as is the variability in this distribution across patients and time, and the effect of conditions and subject characteristics.
To address this gap, we measured tremor in all 7 main DOF from the shoulder to the wrist in 23 patients from a broad spectrum of ET severity during both postural and kinetic tasks in a variety of limb configurations. From these measurements we determined the mean distribution of postural and kinetic tremor from the shoulder to the wrist, as well as the effect of limb configuration, repetition, and subject characteristics. This characterization represents a necessary step toward unraveling the relationship between tremorogenic muscle activity and tremor and, eventually, determining which muscles are most responsible for a patient’s tremor.
2. Methods
2.1. Subjects
Twenty-five subjects with ET completed the study at the NIH Clinical Center in Bethesda, MD. Two subjects were later excluded because of technical difficulties during data collection, and one subject was excluded because she exhibited far more tremor than the rest of the subjects (almost 4 standard deviations above the mean), so results represent data from 22 subjects (Table 1). Prior to beginning the study each subject provided informed consent in accordance with NIH’s Institutional Review Board. Within one year prior to the experiment, each subject underwent a neurological exam performed by neurologists specializing in movement disorders. The neurologists assessed the subject’s tremor and determined if it was consistent with ET or other tremor disorders. Subjects were excluded from our study if their history included stroke, head trauma, seizures, movement disorders other than ET, psychotic disorders, cardiac pacemaker implantation, or DBS. In particular, if the subject’s tremor was found to include elements from other tremor disorders (e.g., Parkinson’s Disease or dystonia), the subject was excluded from the experiment. Before the experiment, each subject was evaluated using The Essential Tremor Rating Assessment Scale (TETRAS) (Elble et al., 2008, Elble et al., 2012a, 2012b), which was performed by one of two people: a neurologist (DH) specializing in tremor or a research assistant (ACP) trained by DH in administering the TETRAS. The TETRAS was only used to recruit a broad distribution of tremor severities.
Table 1:
Subject characteristics, sorted by the amount of power in the tremor band (from least to most severe). Dur., Hand., Ht, Wt, and Family Hist represent the duration of the disorder in years, handedness, height, weight, and family history of ET, respectively. The TETRAS score is divided into scores for activities of daily living, performance, and a total score. Power was calculated during the data analysis and represents the mean total power in acceleration in the tremor band (4–12 Hz), averaged over all trials (see Methods). NK stands for “not known.”
| Sex | Age | Age of Onset | Dur. | Hand. | Ht (cm) | Wt (kg) | Family Hist | TETRAS Scale | Measured | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADL | Perf. | Total | Power/103 (deg2/s4) | Severity | ||||||||
| M | 56 | 17 | 39 | R | 176 | 85 | Yes | 23 | 17.5 | 40.5 | 16 | Mild |
| M | 65 | 17 | 48 | L | 172 | 94 | Yes | 15 | 18 | 33 | 17 | Mild |
| M | 69 | 8 | 61 | R | 177 | 89 | Yes | 25 | 21.5 | 46.5 | 19 | Mild |
| M | 72 | 25 | 47 | R | 168 | 95 | No | 13 | 13.5 | 26.5 | 27 | Mild |
| F | 81 | 57 | 24 | R | 166 | 53 | Yes | 30 | 26.5 | 56.5 | 29 | Mild |
| F | 63 | 20 | 43 | R | 164 | 69 | Yes | 19 | 18 | 37 | 35 | Mild |
| M | 70 | 64 | 6 | R | 178 | 75 | Yes | 18 | 16.5 | 34.5 | 36 | Mild |
| M | 69 | 49 | 20 | R | 176 | 111 | Yes | 22 | 25.5 | 47.5 | 39 | Mild |
| M | 69 | 65 | 4 | R | 175 | 105 | Yes | 7 | 14 | 21 | 41 | Mod |
| M | 64 | 13 | 51 | R | 178 | 105 | Yes | 28 | 18.5 | 46.5 | 42 | Mod |
| F | 61 | 35 | 26 | R | 164 | 61 | Yes | 24 | 20 | 44 | 45 | Mod |
| F | 66 | 45 | 21 | R | 172 | 101 | Yes | 25 | 20 | 45 | 58 | Mod |
| F | 75 | 65 | 10 | R | 166 | 64 | Yes | 28 | 20 | 48 | 67 | Mod |
| M | 51 | 16 | 35 | R | 174 | 78 | Yes | 28 | 21 | 49 | 68 | Mod |
| F | 70 | 22 | 48 | R | 174 | 98 | NK | 15 | 22.5 | 37.5 | 85 | Mod |
| F | 52 | 28 | 24 | R | 164 | 82 | Yes | 16 | 18.5 | 34.5 | 134 | Severe |
| M | 63 | 16 | 47 | R | 183 | 99 | Yes | 24 | 20.5 | 44.5 | 156 | Severe |
| M | 48 | 5 | 43 | R | 180 | 141 | Yes | 14 | 20 | 34 | 174 | Severe |
| M | 58 | 25 | 33 | L | 187 | 111 | Yes | 29 | 35 | 64 | 250 | Severe |
| F | 76 | 55 | 21 | R | 168 | 67 | Yes | 26 | 28.5 | 54.5 | 422 | Severe |
| F | 20 | 5 | 15 | R | 168 | 57 | Yes | 30 | 32 | 62 | 444 | Severe |
| M | 69 | 17 | 52 | R | 187 | 118 | Yes | 29 | 37 | 66 | 520 | Severe |
2.2. Experimental Set-up
We followed some of the methodology presented in (Geiger et al., 2018) but also made several important improvements, so we present our methods in detail here. To measure tremor in the 7 main DOF of the upper limb (Figure 1A), we placed electromagnetic motion capture sensors (trakSTAR 3DGuidance by Ascension Technologies, Shelburne, VT) on the trunk and right arm of subjects in the following locations: sternum, inferior to the suprasternal notch; dorsal aspect of the distal upper arm, proximal to the elbow; dorsal aspect of the distal forearm, a few centimeters proximal to the wrist joint center; dorsum of the hand, bridging the third and fourth metacarpals (Figure 1B–C). Additional sensors, used only during calibration (see below), were placed on the acromion (straddling the acromial angle) and the end of a stylus. Each sensor is capable of measuring motion in 6 DOF with a static accuracy of 1.4 mm in translation and 0.5° in rotation. Data were collected at 360 samples/s. Subjects were also instrumented with wireless surface electromyography (EMG) sensors (Trigno IM by Delsys, Natick, MA) over 15 muscles of the upper limb (data not included in this paper). Since the motion capture system is susceptible to interference from ferromagnetic materials and electromagnetic noise, before data collection we minimized ferromagnetic materials in the immediate environment and tested the quality of the measurements, which was found to be satisfactory. Furthermore, our data processing methods (see below), which focused on the difference in orientation between neighboring sensors and included double differentiation, further mitigated the effects of interference.
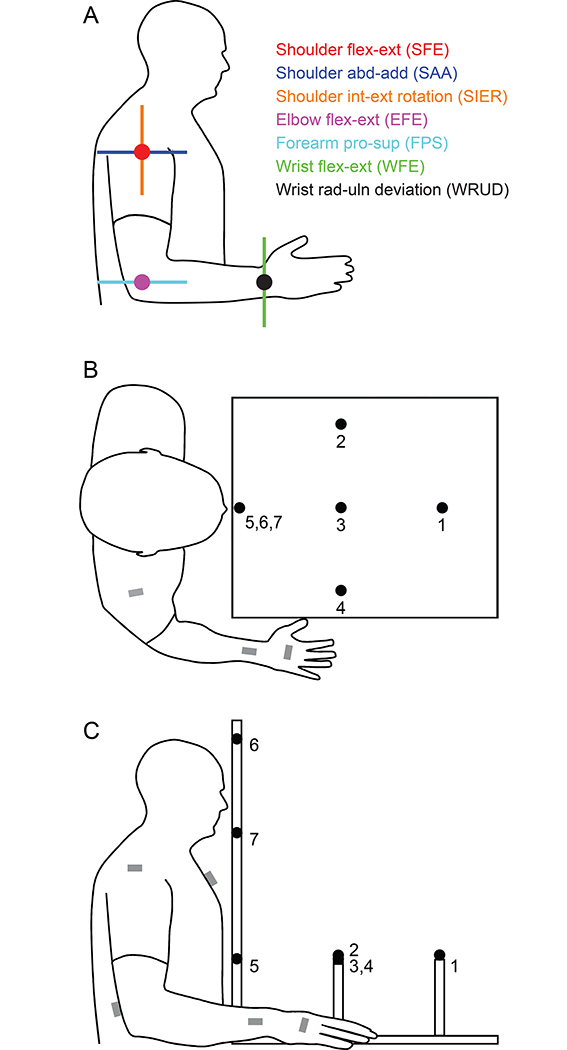
Figure 1:
Joint-angle definitions and experimental setup. A: We measured tremor in each of the 7 main degrees of freedom of the upper limb. Each degree of freedom is indicated by its axis of rotation, with dots representing axes that are perpendicular to the plane of the figure. B-C: Motion-capture sensors were placed at the locations marked by the gray rectangles. During postural tasks, subjects pointed at one of the seven targets, and during kinetic tasks, subjects moved their finger between target 5 and one of the other targets. Between trials, subjects rested their right hand on the table to the right of the targets, as shown.
To calibrate the motion capture setup, we used the landmark calibration method recommended by the International Society of Biomechanics (Wu et al., 2005), with a slight modification to the landmarks on the hand to enable in-vivo use, which is described in detail in (Clark et al., 2020). Briefly, the stylus was used to record the location of specific landmarks on the trunk and upper limb relative to the motion capture sensors. To locate the center of rotation of the glenohumeral joint, we found the instantaneous center of rotation from subjects’ movements in shoulder flexion, extension, and abduction recorded with the sensors on the upper arm and scapula.
During the experiment, subjects were seated comfortably in front of a table. On top of the table were 7 targets distributed throughout the workspace of the subject (Figure 1B–C). We measured tremor as subjects pointed at a target or moved between targets (see below). Targets were comprised of a thin piece of foam mounted on a dowel, as foam would bend easily and interfere minimally with subject’s tremor if the subject touched the target. The seven targets represented posture and movement locations common to activities of daily living. The location of these targets was adjusted for individual subjects as follows. Five targets were arranged roughly in the horizontal plane (targets 1–5 in Figure 1B–C). The closest target (target 5) was placed roughly 4 cm from the subject in the sagittal plane, approximately at the level of the xyphoid process. The target farthest from the subject (target 1) was also in the sagittal plane, placed so the subject could touch it with the tip of his/her index finger when the elbow was extended at 30 degrees. The three middle targets (targets 2–4) were placed halfway between the farthest and nearest target, with the left and right targets (targets 2 and 4) placed at 45° from the line connecting the closest and farthest targets. Two additional targets (targets 6 and 7) were placed directly above the closest target (target 5) at the level of the top of the subject’s head and the bottom of the subject’s chin, respectively (Figure 1B–C).
2.3. Experimental Protocol
The experiment included postural and kinetic trials to allow us to measure both postural and kinetic tremor. During postural trials, subjects were instructed to point at a given target with their index finger, getting close to the target without touching it, and to hold that position for 30 seconds. We asked subjects to avoid touching the targets to minimize sensory feedback, which might potentially affect the tremor. Subjects repeated this task for each of the seven targets, in pseudo-random order. During kinetic trials, subjects moved back and forth between target 5 and a given target for 30 seconds. We instructed subjects to touch the targets and to move at a speed that could be maintained comfortably for the duration of the test. Subjects repeated this task for each of the 6 targets (not counting target 5), also in pseudo-random order. Whether a given subject first performed the postural or kinetic trials was also randomized. At the end of each 30 second trial, subjects were asked to place their hand in a predefined area (Figure 1B–C) for 5 to 10 seconds; this allowed some rest and provided a marker for the beginning and end of each trial. After completing the postural and kinetic trials, subjects repeated the whole process two more times, resulting in 21 postural and 18 kinetic trials per subject. The total time to complete all trials was approximately 30–45 minutes.
2.4. Data Processing
We converted the motion capture sensor data into joint angles in the following DOF (positive direction listed first): shoulder flexion-extension (SFE), shoulder adduction-abduction (SAA), shoulder internal-external humeral rotation (SIER), elbow flexion-extension (EFE), forearm pronation-supination (FPS), wrist flexion-extension (WFE), and wrist ulnar-radial deviation (WRUD) (Figure 1A). The shoulder was defined as the thoracohumeral joint, and the carrying angle of the elbow and axial rotation angle of the wrist (about the long axis of the third metacarpal) were assumed to be zero. This process of converting motion capture sensor data into joint angles is referred to as inverse kinematics calculations and is explained in detail in (Clark et al., 2020). In performing our inverse-kinematics calculations, we adhered to the ISB recommendations (Wu et al., 2005) in defining all DOF except the three DOF in the shoulder. To explain, the ISB convention recommends for the shoulder a Y-X-Y sequence, which places anatomical shoulder position (zero flexion-extension, abduction-adduction, and humeral internal-external rotation) in gimbal lock, where joint angles are ill-defined. Since many of the postures and movements were close to anatomical shoulder position, we defined the shoulder DOF using a Z-X-Y rotation sequence, which moves gimbal lock far from anatomical shoulder position (in 90° of shoulder abduction). As mentioned above, our definition parses shoulder movement into shoulder flexion-extension, abduction-adduction, and internal-external humeral rotation.
After converting the motion sensor data to joint angles, we calculated angular acceleration in each DOF using numerical differentiation. Prior to each differentiation, the data were filtered using a 10th order Butterworth filter with cut-off frequency at 20 Hz. The power spectral density (PSD) of the acceleration data was estimated using Welch’s method, implemented via Matlab’s pwelch function, with 50% overlap between windows. To achieve a robust estimate of the PSD, we varied the number of windows between 8 and 38 to determine the optimal number of windows. We found that increasing the number of windows beyond 18 produced only slight improvements in noise reduction, so we used 18 windows instead of the default of 8 windows in Matlab’s pwelch function.
The amount of tremor in each DOF was defined as the power in the angular acceleration of that DOF contained in the tremor band (4–12 Hz), calculated by numerical integration of the PSD from 4 to 12 Hz. Characterizing the amount of tremor in terms of the power in the tremor band is more robust than relying on the amplitude of peaks in the tremor band; power takes advantage of all the data points in the tremor band, and the process of integrating the area under the PSD curve low-pass filters noise in the PSD curve. In contrast, peak amplitude relies on a single data point, and detecting peaks is heavily affected by noise and requires a peak detection algorithm with somewhat arbitrary parameters. As power in voluntary movements and postural instability is located below 4 Hz, these activities did not contribute to the power measure. Importantly, because the DOF were defined according to ISB recommendations (Wu et al., 2005) in terms of angular motion of distal limb segments relative to proximal segments, this power measure represents the amount of tremor in distal segments relative to proximal segments (similar to how an electrogoniometer would measure tremor at a joint). Thus, according to our definition of the amount of tremor in each DOF, distal DOF do not inherit tremor from proximal DOF the way distal segments inherent absolute motion from proximal segments. We chose this definition deliberately as it treats joints independently and is relevant to tremor suppression efforts that act on muscles and joints.
To investigate if different DOF exhibited the same tremor frequency, we also determined the frequency and amplitude of the greatest statistically significant peak in the tremor band. Statistically significant peaks were detected using a sliding-window constant-false-alarm-rate detection algorithm (McDonough and Whalen, 1995), with a 1.0 Hz window and 1.5 Hz sidebands. This method performed a statistical comparison between the point at the middle of the sliding window and the means of the sidebands. If the point at the middle of the window was a local maximum (i.e. greater than its two neighbors) and significantly greater than the sidebands (α = 0.05), it was classified as a peak. After identifying all peaks in the tremor band, we selected the largest peak.
2.5. Data Analysis
2.5.1. Distribution of tremor amount
The primary purpose of the analysis was to determine how the amount of tremor was distributed among the DOF of the upper limb, and how this distribution changed with tasks, targets, repetitions, and subject characteristics.
2.5.1.1. Effect of task, target, and repetition
In analyzing the data, we found that subjects exhibited far more kinetic tremor than postural tremor (see Results); when analyzing the distribution of postural and kinetic tremor together, results were dominated by kinetic tremor. Therefore, we analyzed the distribution of postural and kinetic tremor separately. The processes for analyzing postural and kinetic tremor were the same, so we describe here only the process for postural tremor. There was a very wide range in tremor power between subjects. Since we were interested in the distribution of postural tremor independent of tremor severity, we normalized each subject’s postural tremor by his/her mean postural tremor. The resulting postural tremor values were unitless and represented a subject’s postural tremor in a specific DOF, target, and repetition, compared to that subject’s average postural tremor. To identify statistically significant effects of target and repetition on the distribution of postural tremor between DOF, we ran an ANOVA of normalized postural tremor with the following factors: DOF (1–7), target (1–7 except 5), repetition (1–3), DOF*target, and DOF*repetition. Post-hoc analysis was performed using Tukey’s honest significance test using a significance level of 0.05. This process was repeated for kinetic tremor.
2.5.1.2. Effect of subject characteristics
We also determined the effect of subject characteristics on tremor distribution. Subject characteristics included sex (M vs. F), age of onset (early, middle, late), duration of disorder (short, medium, long), and tremor severity (mild, moderate, severe), and were defined as follows. For sex, subjects were divided into 13 males and 9 females. For age of onset, we determined three groups: early onset (≤20 years, 10 subjects), middle onset (>20 and ≤60 years, 9 subjects), and late onset (>60 years, 3 subjects). Disease duration was determined by subtracting the subject’s estimated age of onset from their current age and was grouped similarly into: short duration (≤20 years, 5 subjects), medium duration (>20 and ≤40 years, 8 subjects), and long duration (>40 years, 9 subjects). Tremor severity was determined by averaging a subject’s power over all DOF and trials (including both postural and kinetic trials) and dividing subjects into groups according to this average power: mild tremor (power < 4×104 deg2/sec4, 8 subjects), moderate tremor (4×104 ≥ power <105 deg2/sec4, 7 subjects), and severe tremor (power ≥ 105 deg2/sec4, 7 subjects).
To identify statistically significant effects of these subject characteristics on the distribution of postural tremor between DOF, we performed an ANOVA of normalized postural tremor with the following factors: DOF, sex, age of onset, duration of disorder, and tremor severity. The model included main effects and two-factor interactions with DOF. Post-hoc analysis was performed using Tukey’s honest significance test using a significance level of 0.05. This process was repeated for kinetic tremor.
2.5.2. Distribution of tremor frequency
A secondary purpose of the analysis was to investigate peak frequency. First, we determined if different DOF exhibited the same tremor frequency. To characterize the dominant frequencies in each DOF, we determined the frequency of the greatest statistically significant peak in each trial for each DOF. To test for differences between DOF, we performed an ANOVA of peak frequency with fixed factors DOF (1–7) and task (postural vs. kinetic) and random factor subject, but we only included the 8 subjects with severe tremor because they exhibited the most consistent peaks (explained in more detail in Results). Second, we used this same ANOVA to test for differences in peak frequency between postural and kinetic tasks.
3. Results
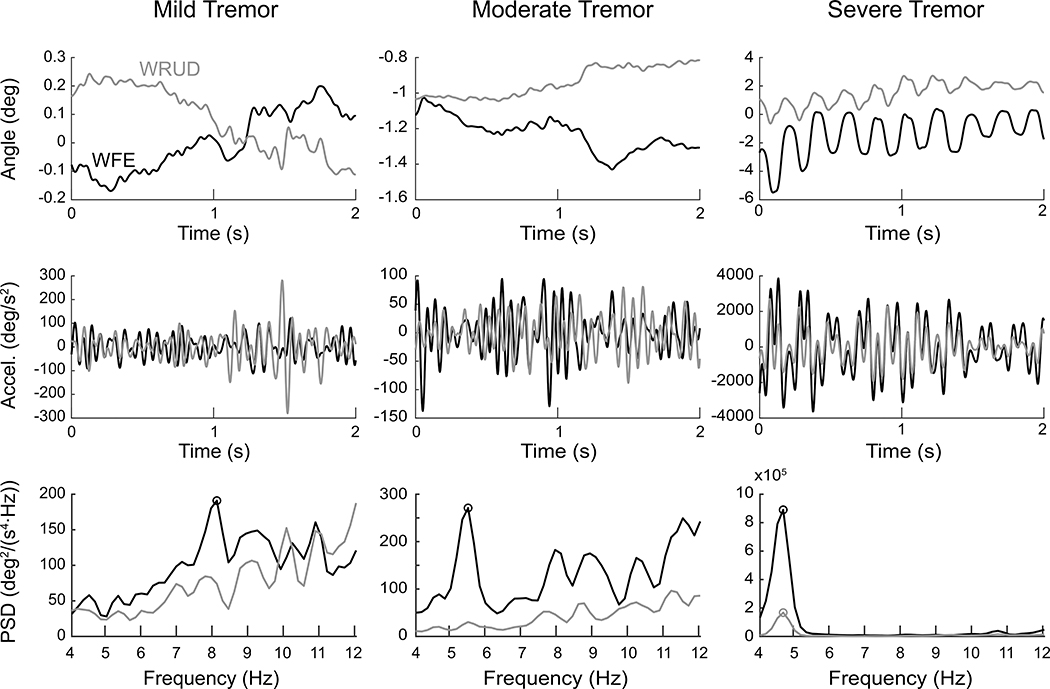
As described above, raw sensor data were transformed into joint angles, filtered joint accelerations, and finally PSD estimates (Figure 2). Subjects exhibited a wide range of tremor amplitudes; the subject with the most tremor had 33 times more tremor power than the subject with the least tremor (see Table 1). Individual subjects exhibited far more kinetic tremor than postural tremor. On average, kinetic tremor power was 76 times greater than postural tremor power (range 3–271). The difference between kinetic and postural tremor was especially large for the three distal-most DOF; for FPS, WFE, and WRUD, kinetic tremor power was 122 times greater than postural tremor power (compared to 41 times for the four proximal DOF). Note that the dominance of kinetic tremor is not due to the large, voluntary back-and-forth movements in the kinetic tasks; the power of the voluntary movements consisted of frequencies below 1 Hz and was therefore not included in the calculation of tremor power, which included frequencies between 4 and 12 Hz.
Figure 2:
Representative postural test data in wrist flexion-extension (WFE, in black) and radial-ulnar deviation (WRUD, in gray) for a mild (left), moderate (center), and severe (right) subject. The first and second rows focus on 2 seconds of a trial and show the angular displacement and filtered angular acceleration in each degree of freedom, calculated from raw sensor data using inverse kinematics. The third row shows the power spectral density (PSD) of acceleration calculated from the 30-second trial from which the 2-second portions in the top two rows were taken. The circles indicate statistically significant peaks identified by our peak-detection algorithm. Note the differences in scales between the mild, moderate, and severe cases.
3.1. Distribution of tremor amount
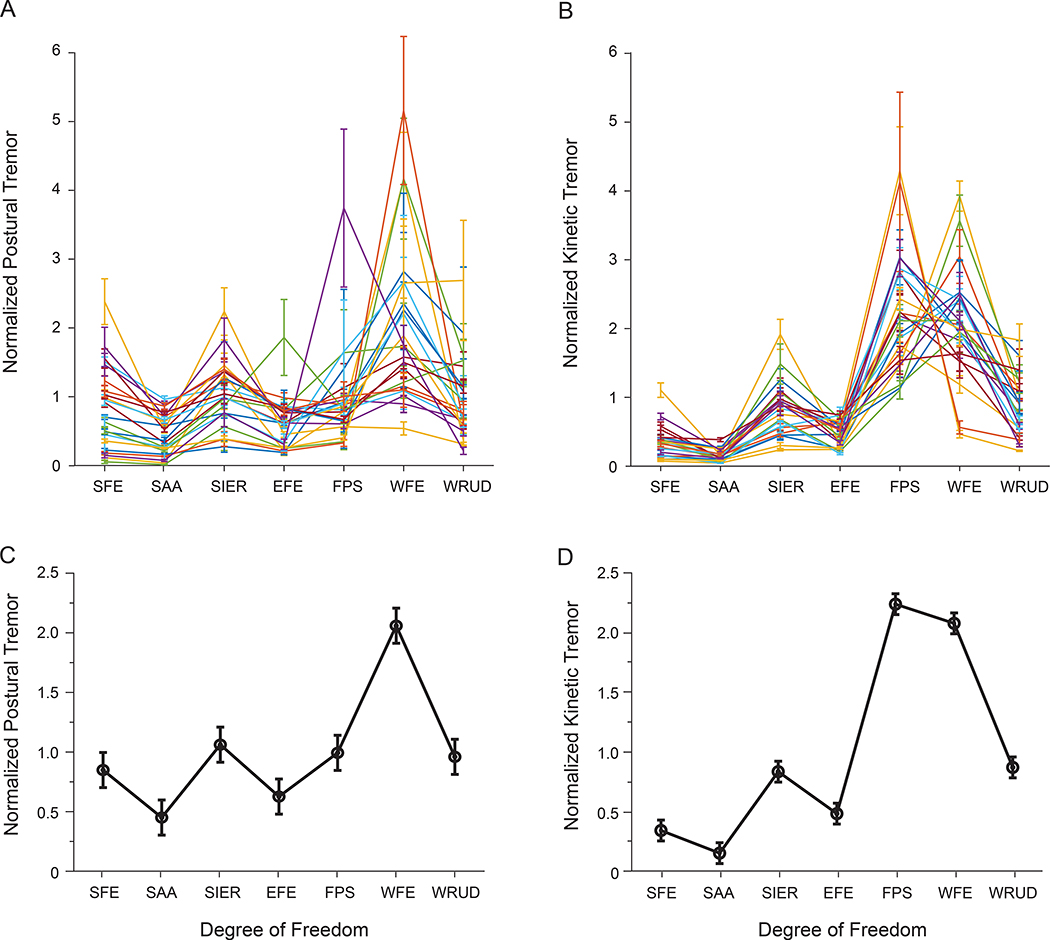
We found substantial variability in tremor distribution between subjects, especially for postural tremor (Figure 3A–B). Nevertheless, the ANOVA revealed significant differences in tremor between DOF (Table 2 and Table 3). Regarding postural tremor, WFE had significantly more power than all other DOF; SIER, FPS, WRUD had more power than EFE and SAA; and SFE had more power than SAA (Figure 3C). Regarding kinetic tremor, FPS and WFE exhibited more power than WRUD and SIER; WRUD and SIER showed more power than EFE and SFE; and EFE and SFE had more power than SAA (Figure 3D). Overall, the distribution of tremor was similar for postural and kinetic tremor except that tremor in FPS played a larger role in kinetic tremor compared to postural tremor (compare Figure 3C and Figure 3D).
Figure 3:
Normalized postural tremor (A, C) and kinetic tremor (B,D), presented separately for each subject (A-B) and averaged across subjects (C-D). Error bars indicate ±1 standard error. Degree-of-freedom abbreviations are defined in Figure 1A.
Table 2:
Effect of target and repetition on the distribution of normalized postural tremor among the degrees of freedom (DOF) of the upper limb. Nparm and DF represent the number of parameters and degrees of freedom associated with the ANOVA.
| Source | Nparm | DF | Sum of Squares | F Ratio | Prob > F |
|---|---|---|---|---|---|
| DOF | 6 | 6 | 735.64794 | 47.0464 | <0.0001 |
| Target | 6 | 6 | 123.18445 | 7.8779 | <0.0001 |
| Rep | 2 | 2 | 2.02159 | 0.3879 | 0.6785 |
| DOF*Target | 36 | 36 | 370.14026 | 3.9452 | <0.0001 |
| DOF*Rep | 12 | 12 | 7.20099 | 0.2303 | 0.9970 |
Table 3:
Effect of target and repetition on the distribution of normalized kinetic tremor among the degrees of freedom (DOF) of the upper limb. Nparm and DF represent the number of parameters and degrees of freedom associated with the ANOVA.
| Source | Nparm | DF | Sum of Squares | F Ratio | Prob > F |
|---|---|---|---|---|---|
| DOF | 6 | 6 | 1644.7895 | 349.2661 | <0.0001 |
| Target | 5 | 5 | 201.6467 | 51.3829 | <0.0001 |
| Rep | 2 | 2 | 1.8647 | 1.1879 | 0.3050 |
| DOF*Target | 30 | 30 | 274.8608 | 11.6732 | <0.0001 |
| DOF*Rep | 12 | 12 | 1.0138 | 0.1076 | 0.9999 |
3.1.1. Effect of target and repetition
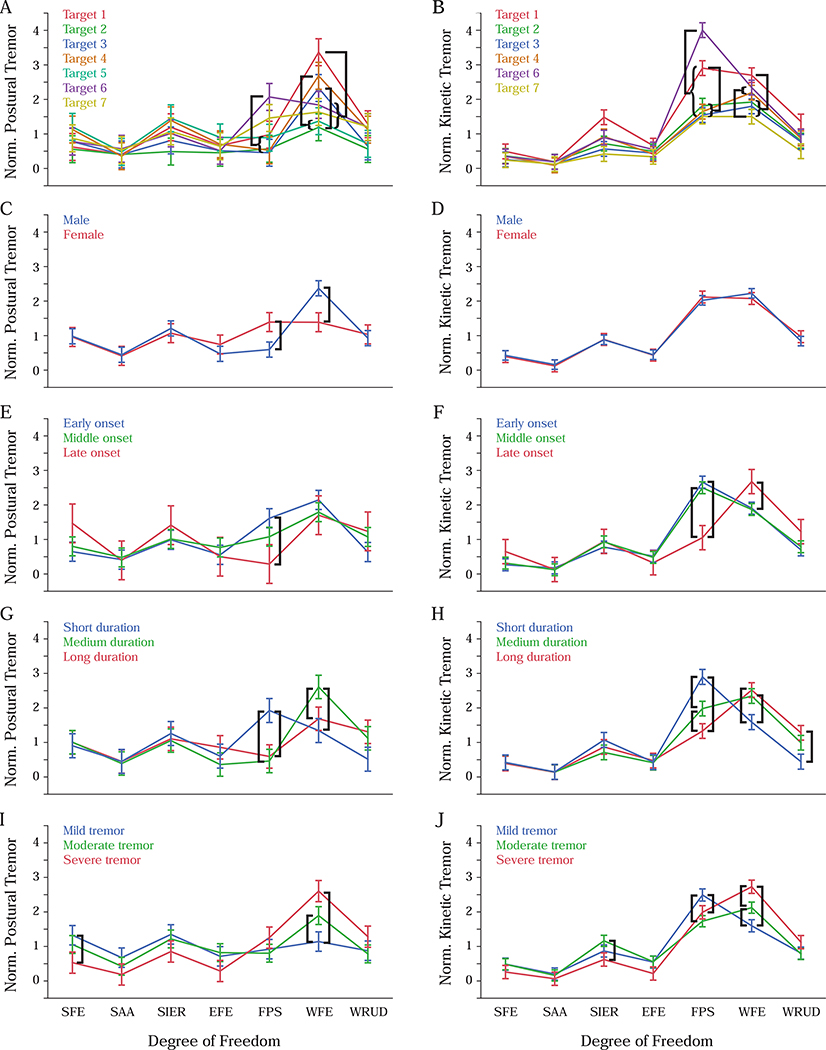
Target had a significant effect on the distribution of both postural and kinetic tremor (Table 2 and Table 3). We were only interested in differences in tremor in a given DOF at different targets (as opposed to between different DOF and targets); among these differences, the only significant differences occurred in FPS and WFE. Regarding postural tremor (Figure 4A): FPS had more tremor at target 6 than at targets 2–5; and WFE had more tremor at target 1 than at targets 2 and 5–7, more tremor at target 4 than targets 2 and 5, and more tremor at target 3 than target 2. Regarding kinetic tremor (Figure 4B): FPS had more tremor at target 6 than at target 1, and more tremor at target 1 than at all other targets; and WFE had more tremor at target 1 than at targets 2, 3, and 7, and more tremor at target 4 and 6 than at target 7.
Figure 4:
Effect of target (A-B) and subject characteristics (sex (C-D), tremor onset (E-F), tremor duration (G-H), and tremor severity (I-J)) on the distribution of normalized postural tremor (left column) and normalized kinetic tremor (right column). Error bars represent ±1 standard error, and black bars indicate significant differences within a given degree of freedom (p=0.05). Degree-of-freedom abbreviations are defined in Figure 1A.
In contrast, repetition did not have a significant effect on tremor distribution (Table 2 and Table 3), indicating that the distribution of tremor did not change significantly between repetitions under identical conditions (task and target), which were spaced 10–15 minutes apart (i.e. 20–30 minutes between first and last).
3.1.2. Effect of subject characteristics
Effects of subject characteristics on tremor distribution were almost entirely limited to FPS and WFE. None of the main effects of subject characteristics were significant (Table 4 and Table 5), but this simply reflects the fact that we normalized the tremor power within each subject. The important results lie in the interactions with DOF, which were all significant; however, only few of these effects represented differences in the same DOF. Of the 25 significant differences between subject characteristics in the same DOF (indicated in Figure 4C–J), all but three occurred in FPS and WFE. Some of these differences were shared across postural and kinetic tremor: persons with early-onset tremor had more postural and kinetic tremor in FPS than persons with late-onset tremor (Figure 4E–F), and persons with short-duration tremor had more postural and kinetic tremor in FPS than persons with medium- or long-duration tremor (Figure 4G–H); also, persons with medium-duration tremor had more postural and kinetic tremor in WFE than persons with short-duration tremor (Figure 4G–H), and persons with severe or moderate tremor had more postural and kinetic tremor in WFE than persons with mild tremor (Figure 4I–J).
Table 4:
Effect of subject characteristics on the distribution of normalized postural tremor among degrees of freedom (DOF) of the upper limb. Nparm and DF represent the number of parameters and degrees of freedom associated with the ANOVA.
| Source | Nparm | DF | Sum of Squares | F Ratio | Prob > F |
|---|---|---|---|---|---|
| DOF | 6 | 6 | 383.95947 | 25.5548 | <0.0001 |
| Sex | 1 | 1 | 1.99E-21 | 0 | 1 |
| Onset | 2 | 2 | 1.37E-19 | 0 | 1 |
| Duration | 2 | 2 | 2.38E-19 | 0 | 1 |
| Severity | 2 | 2 | 1.77E-19 | 0 | 1 |
| DOF*Sex | 6 | 6 | 137.97967 | 9.1834 | <0.0001 |
| DOF*Onset | 12 | 12 | 90.4714 | 3.0107 | 0.0003 |
| DOF*Duration | 12 | 12 | 184.51981 | 6.1405 | <0.0001 |
| DOF*Severity | 12 | 12 | 294.70591 | 9.8072 | <0.0001 |
Table 5:
Effect of subject characteristics on the distribution of normalized kinetic tremor among degrees of freedom (DOF) of the upper limb. Nparm and DF represent the number of parameters and degrees of freedom associated with the ANOVA.
| Source | Nparm | DF | Sum of Squares | F Ratio | Prob > F |
|---|---|---|---|---|---|
| DOF | 6 | 6 | 1004.8153 | 199.296 | <0.0001 |
| Sex | 1 | 1 | 1.47E-20 | 0 | 1 |
| Onset | 2 | 2 | 9.46E-19 | 0 | 1 |
| Duration | 2 | 2 | 1.41E-19 | 0 | 1 |
| Severity | 2 | 2 | 4.98E-19 | 0 | 1 |
| DOF*Sex | 6 | 6 | 3.5202 | 0.6982 | 0.6511 |
| DOF*Onset | 12 | 12 | 65.882 | 6.5336 | <0.0001 |
| DOF*Duration | 12 | 12 | 118.5904 | 11.7607 | <0.0001 |
| DOF*Severity | 12 | 12 | 153.9718 | 15.2695 | <0.0001 |
3.2. Distribution of tremor frequency
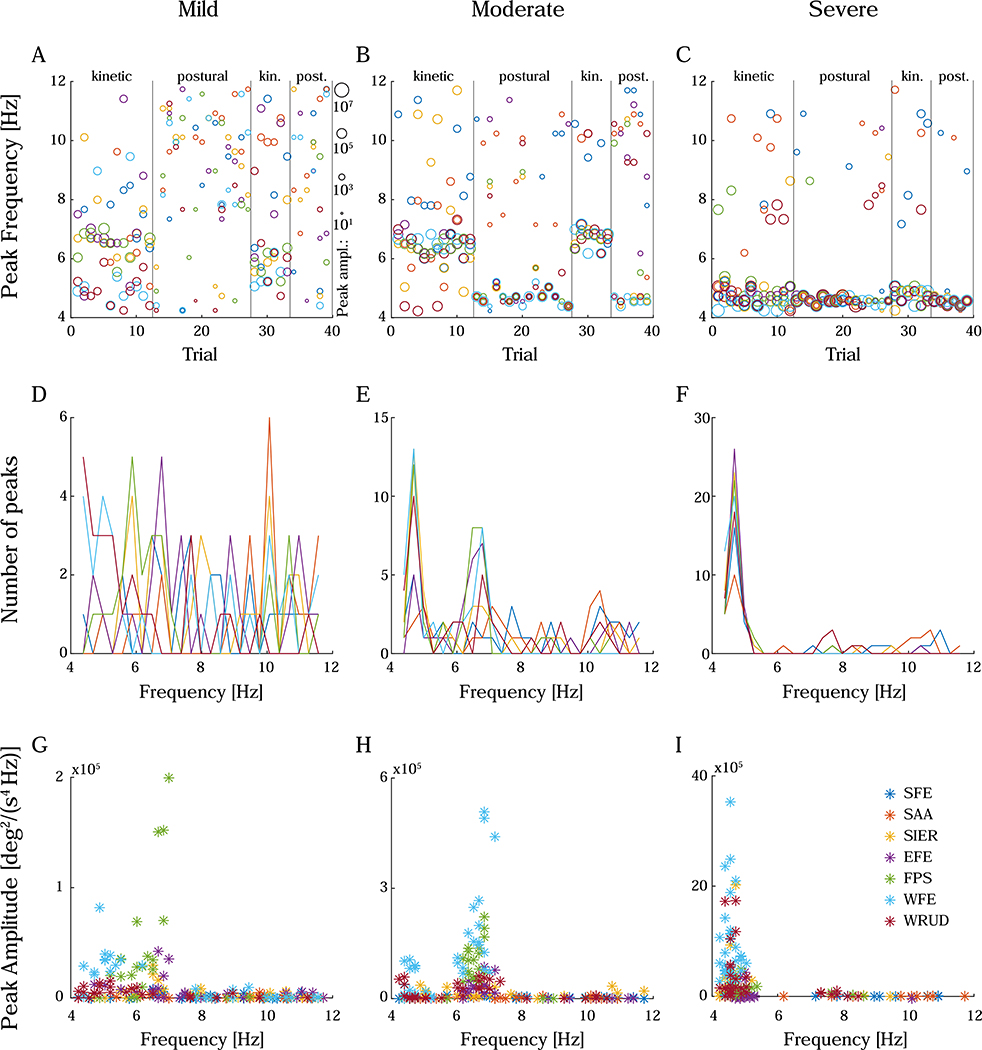
A secondary purpose of the analysis was to ascertain if different DOF tremored at the same frequency. Unfortunately, only 81% of trials (range among subjects: 70–90%) exhibited a statistically significant peak (see Methods), and some of these peaks were deemed spurious. To clarify, subjects with severe tremor generally exhibited a baseline of peak frequencies that were mostly consistent from one trial to the next (Figure 5C). Occasionally, trials exhibited different peak frequencies in one or multiple DOF, but on subsequent trials the peak frequency typically returned to the baseline. The departures from the baseline appeared random and, importantly, the peak frequencies that differed significantly from the baseline typically belonged to peaks that were smaller in amplitude (Figure 5F,I). Considering it unlikely that tremor frequency would shift by multiple Hz from one trial to the next, we assumed that the peaks that differed significantly from the baseline were caused by noise, which was amplified due to the double differentiation from position to acceleration. As the amplitude of peaks decreased, the proportion of spurious peaks increased; therefore, subjects with mild or moderate tremor exhibited more spurious peaks than subjects with severe tremor (Figure 5, left and middle columns), and postural tasks exhibited more spurious peaks than kinetic tasks, especially for subjects with mild or moderate tremor (e.g. Figure 5A). Therefore, in our analysis of peak frequency, we relied more heavily on the 8 subjects with severe tremor.
Figure 5:
Peak frequency and amplitude, displayed as peak frequency in chronologically ordered trials (A-C), histogram of peak frequencies (D-F), and peak amplitude vs. peak frequency (G-I). Examples include a subject with mild tremor (left column), a subject with moderate tremor (middle column), and a subject with severe tremor (right column). In severe tremor, peaks tended to group around a common frequency (C, F). Occasionally, peak frequency jumped significantly from one trial to the next, but these jumps appeared random, and the peak frequency returned to the common frequency on subsequent trials. Importantly, the peak frequencies that differed significantly from the common frequency typically belonged to peaks that were much smaller in amplitude (Figure 8F, I). Considering it unlikely that tremor frequency would shift by multiple Hz from one trial to the next, peaks that differed significantly from the baseline were assumed to be spurious (likely due to the increased noise caused by double differentiation). As the amplitude of peaks decreased, the proportion of spurious peaks increased; subjects with mild or moderate tremor (left and middle columns) exhibited more spurious peaks than subjects with severe tremor. In A-C, trials measuring kinetic or postural tremor are separated by vertical bars, and the size of the markers represents the peak amplitude (in deg2/(s4 Hz)) on the log scale shown to the right of subfigure A. The color legend for all subfigures is shown in subfigure I (for degree-of-freedom abbreviations, see Figure 1A).
Our analysis did not reveal any convincing evidence in favor of a difference in peak frequency between DOF. An ANOVA of peak frequency in severe subjects found that SFE and SAA had significantly higher peak frequency; however, given that 1) these two DOF had low amounts of tremor (Figure 3C–D), 2) the proportion of spurious peaks increased as tremor decreased, and 3) the frequency of most spurious peaks were above the baseline frequency, the increased mean peak frequency in SFE and SAA likely reflects the higher proportion of spurious peaks in these DOF.
We also investigated differences in peak frequency between postural and kinetic tasks. Approximately one fourth of all subjects exhibited a visually distinguishable decrease in peak frequency as trials transitioned from kinetic to postural tasks, similar to (Schuhmayer et al., 2017). The most striking example is shown in Figure 5B. However, most of these subjects had mild or moderate tremor; the other three fourths of the subjects, including all but one of the subjects with severe tremor (who exhibited the clearest, most consistent peaks) did not show a visibly discernable change in peak frequency between postural and kinetic tasks (e.g., see Figure 5C). In harmony with this observation, the ANOVA of peak frequency in subjects with severe tremor did not find any significant difference in peak frequency between postural and kinetic tasks (F(1)=0.17, p=0.69).
4. Discussion
Because the DOF of the musculoskeletal system are mechanically coupled, tremor spreads from a given muscle proximally and distally throughout the upper limb. Therefore, understanding tremor and how best to intervene requires, among others, studies involving multiple DOF. Here we have taken a step in this direction by characterizing how ET is distributed among the DOF of the upper limb.
4.1. Distribution of tremor amount
On average, we found the distribution of the amount of postural tremor (WFE > SIER, FPS, WRUD > EFE, SAA and SFE > SAA) and kinetic tremor (FPS, WFE > WRUD, SIER > EFE, SFE > SAA) to be similar except that tremor in FPS played a larger role in kinetic tremor than in postural tremor (Figure 3C–D). The distribution of postural tremor, which we measured in 23 patients covering a broad spectrum of ET severity (Table 1), is similar to our prior, preliminary investigation of postural tremor distribution in 10 patients with mild ET, in which we defined tremor amount in terms of angular displacement instead of acceleration (Geiger et al., 2018). In that study, WFE showed by far the most tremor, then four DOF (FPS, SIER, WRUD, and SFE) exhibited similar amounts of tremor, and EFE and SAA had the lowest tremor.1 We also compared the distribution of tremor observed by Rocon and Pons (Rocon and Pons, 2011) in the four distal DOF (EFE, FPS, WFE, and WRUD) during both postural and kinetic tasks to the distribution we found among those four DOF. Only a high-level summary of their results is available, but Rocon and Pons summarized the distribution among these four DOF as a distal-to-proximal decrease in tremor. Our results do not support a proximal-to-distal increase since tremor in WRUD was either similar to FPS (postural tasks) or smaller than FPS (kinetic tasks), and since kinetic tremor in FPS and WFE were similar (Figure 3C–D). Jog et al found postural tremor to be greater in WFE than in FPS and WRUD (Rahimi et al., 2015), which agrees with our findings. Jog and colleagues also measured tremor in the shoulder and elbow (Samotus et al., 2017, Samotus et al., 2016) but did not include shoulder internal-external rotation or compare across joints, so it was not possible to extract distribution across those DOF from their publications.
Despite substantial variability in tremor distribution between subjects (Figure 3A–B), there were some similarities across subjects, as confirmed by the ANOVA (Figure 3C–D). We explain this similarity as follows. The distribution of tremor can be viewed as the result of a multi-input multi-output filtering operation, with tremorogenic activity in various muscles as the inputs, the musculoskeletal system as the filter, and tremor in various DOF as the outputs (Corie and Charles, 2019, Davidson and Charles, 2017). The distribution of the output depends on the distribution of the input and how the filter filters the inputs on their way to becoming outputs. Therefore, the degree to which the distribution of the output is stereotyped (between subjects) generally depends on the degree to which the distribution of the input is stereotyped and the degree to which the filtering properties of the filter are stereotyped. The distribution of the input is unknown, so we do not know the extent to which it contributes to similarity in the distribution of the output. However, the filtering properties of the system have been shown to be quite similar between subjects (Davidson and Charles, 2017). Therefore, even if the distribution of tremorogenic activity varied randomly between subjects, we would still expect some similarity in the distribution of the output.
4.1.1. Effect of target and repetition
Clinical evaluations of tremor often include multiple postures because different limb configurations (postures) are known to elicit different amounts of tremor. Accordingly, we found that different targets elicited different amounts of tremor, but only in FPS and WFE (Figure 4A–B). Comparing this effect between postural and kinetic trials is challenging because postural trials focused on a single target, whereas kinetic trials required subjects to move between a given target and target 5. Nevertheless, there were several similarities between postural and kinetic tremor: for both types of tremor, FPS exhibited more tremor for target 6 than targets 2–4, and WFE had more tremor for target 1 than targets 2 and 7. Targets 1 and 6 require the limb to be farthest from neutral posture, suggesting a possible effect of movement effort on tremor, but more research is required to test this hypothesis.
In contrast, differences in tremor distribution between repetitions were not significant, indicating that tremor distribution was relatively constant over the 20–30 minutes between trials. This finding suggests that if peripheral tremor suppression is achievable, it may be possible to suppress tremor using a simple device with constant or “semi-constant” parameters (e.g. adjustable by hand), without the need for sensors and continuous feedback loops.
4.1.2. Effect of subject characteristics
Similar to the effect of target on tremor distribution, the effects of subject characteristics on tremor distribution were almost entirely limited to FPS and WFE. Combining trends across postural and kinetic tremor (Figure 4C–J), we found that patients with early-onset tremor, patients with short-duration tremor, and patients with mild tremor tended to have more tremor in FPS, whereas patients with late-onset tremor, patients with long-duration tremor, and patients with severe tremor tended to have more tremor in WFE. This was especially true for kinetic tremor, but a trend toward this pattern was also visible in postural tremor. Note that these statements should not be combined (e.g. “Patients with early-onset, short-duration, mild tremor...”) since our subject pool included only one patient with early-onset, short-duration tremor and no subjects with late-onset, long-duration tremor (for any tremor severity).
4.2. Distribution of tremor frequency
We did not find any evidence in favor of a difference in peak frequency between DOF (Figure 5), consistent with the study by Rocon and Pons on tremor in EFE, FPS, WFE, and WRUD (Rocon and Pons, 2011). Prior studies on tremorogenic muscle activity found high coherence in EMG between muscles of the same limb but low coherence between muscles of different limbs (Raethjen et al., 2000). A system (such as the arm) driven by periodic input (such as tremorogenic muscle activity) will generally respond with periodic output at the same frequency. Therefore, our finding that different DOF of the same limb had similar tremor frequencies is consistent with the high intra-limb EMG coherence found in previous studies.
4.3. Methodological Considerations
Great care was taken to include only patients with ET. Before participating in our study, each subject was evaluated by a neurologist specializing in movement disorders. The neurologist assessed the subject’s tremor and determined if it was consistent with ET. Subjects were excluded if their tremor was found to include elements from other tremor disorders (e.g. Parkinson’s Disease or Dystonia).
Past studies of tremor involving multiple DOF sometimes characterized tremor in terms of linear acceleration of limb segments (Morrison and Newell, 1996, 1999, 2000a, 2000b). This approach describes the absolute motion of a body segment, which includes the motion of more proximal segments as well. In contrast, performing inverse kinematics allows one to isolate tremor to individual DOF. Also, some past studies investigated tremor in postures close to the end of the range of motion of a DOF (e.g. with the elbow fully extended), effectively reducing the total number of DOF of the arm. We investigated upper-limb postures closer to the middle of the range of motion of the various DOF, which is more representative of postures in daily life.
Including both postures and movements enabled the identification of differences between postural and kinetic tremor. Similarly, measuring tremor in a relatively large number of limb configurations (7) and movement directions/distances (6) allowed us to test the effect of limb configuration. Finally, including multiple repetitions spaced in time enabled the determination of changes over time (20–30 minutes between first and third repetition). Despite the relatively number of trials (thirty-nine 30-second trials) lasting a total of 30–45 minutes, subjects did not show signs of fatigue.
We analyzed the distribution of the amount of tremor using a large variety of methods but robustly found similar results, so we included only one of the methods in this paper. Other methods included analyses of un-normalized tremor (treating postural and kinetic tremor separately or together) as well as non-parametric analysis of rankings. Similarly, we used a variety of measures, including peak amplitude2 instead of power and angular position or velocity instead of acceleration, and found similar distributions in all cases (not shown).
4.4. Limitations
Since the goal of this study was to characterize the distribution of tremor in ET patients, the larger the population, the better. Although a larger population would have been preferred, our population was large enough to observe stereotyped behavior across a wide range of tremor severities, age of onset, and disorder duration. Since the purpose of this paper was to characterize tremor distribution in ET patients, we did not include a control group; indeed, a normal control group would not have a tremor other than physiological tremor.
Ideally, we would have measured tremor as subjects performed their normal activities of daily living (ADL). However, subjects were instrumented with a total of 20 sensors (5 motion-capture sensors and 15 surface EMG sensors), some of which were tethered, making it difficult to perform ADL in a natural manner. That said, the postures and movements included in the experiment were chosen to approximate those required during ADL.
To characterize tremor, we used a motion capture system that measures position, performed inverse kinematics to obtain joint angles, differentiated twice to obtain joint accelerations, and calculated the PSD of joint acceleration. We chose to measure position (instead of directly measuring acceleration) to facilitate the inverse kinematics required to separate motion into individual DOF. Unfortunately, numerical differentiation amplifies high-frequency noise, creating a noisy estimate of acceleration. Instead of analyzing the PSD of joint angle, we chose to characterize tremor in terms of the PSD of acceleration because this is more common practice (and would therefore allow for comparison with future studies). We also chose the PSD of joint acceleration because, compared to the PSD of joint angle, the PSD of joint acceleration emphasizes higher-frequency movements, including tremor, over lower-frequency movements such as those required during the kinetic tasks. To minimize the effect of noise amplified during differentiation, we low-pass filtered before each differentiation and computed the PSD using a large number of windows to further average out noise. As mentioned above, for comparison we repeated our analysis with position or velocity instead of acceleration and found the distribution of power among DOF to be virtually identical whether the power was calculated in terms of the PSD of position, velocity, or acceleration. That said, the noise in acceleration made it more difficult to detect peaks than it would have been using direct measurements of acceleration, especially in patients with mild and moderate tremor. Consequently, we were unable to determine for mild and moderate subjects if different DOF tremored at different frequencies.
As mentioned above, we quantified tremor in terms of the angular motion of one DOF relative to another, not in terms of the absolute movement of limb segments. Because distal limb segments “inherit” some of their absolute movement directly from proximal limb segments, the tremor distribution among limb segments will likely be different from the tremor distribution among DOF.
4.5. Conclusion
According to our observations, three of the four DOF with the greatest tremor were in the wrist and forearm. Therefore, in the absence of more understanding about how tremor propagates (see below), efforts to suppress tremor should focus first on distal DOF. For example, a single orthosis targeting FPS, WFE, and WRUD could potentially suppress most of a patient’s tremor. Since tremor frequency was similar between DOF (at least for severe tremor), low-pass filtering with a single (perhaps patient-specific) cut-off frequency may be effective for all DOF. That said, as tremor can propagate from a given muscle both proximally and distally, developing devices that suppress tremor in an optimal manner requires additional research to understand which muscles are most responsible for a patient’s tremor. To this end, we are analyzing the EMG data gathered alongside the kinematic data to determine the distribution of tremorogenic activity among the muscles of the upper limb. In parallel, we are using models of upper-limb dynamics to simulate the propagation of tremor throughout the upper limb (Corie and Charles, 2019, Davidson and Charles, 2017). Together, knowing how the input (tremorogenic muscle activity) is distributed, how the output (tremulous joint displacement) is distributed, and how the limb propagates tremor (through simulations), we expect to determine where to intervene to suppress tremor in an optimal manner.
Highlights.
We characterized the amount of tremor in the joints from the shoulder to the wrist.
Kinetic tremor was greatest in forearm pronation-supination and wrist flexion-extension.
Limb configuration and subject characteristics showed significant effects on tremor distribution.
Acknowledgements
Adam C. Pigg and Steven K. Charles were supported by NIH NINDS grant R15NS087447.
Johanna Thompson-Westra, Karin Mente, Carine Maurer, Dietrich Haubenberger, and Mark Hallett were supported by the NINDS, NIH Intramural Program.
In addition, Karin Mente was also supported by the Dystonia Medical Research Foundation.
Footnotes
Disclosures
Adam C. Pigg: None.
Johanna Thompson-Westra: None.
Karin Mente received a research fellowship from the Dystonia Medical Research Foundation.
Carine W. Maurer: None.
Dr. Haubenberger is currently an employee of Neurocrine Biosciences and previously served on the Clinical Advisory Board for CADENT Pharmaceuticals.
Dr. Hallett may accrue revenue on US Patent #6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB-Ricin) for the treatment of focal movement disorders, and US Patent #7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same (H-coil); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the Medical Advisory Board of CALA Health, Brainsway, and Cadent Therapeutics. He is on the Editorial Board of approximately 20 journals, and received royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, and Elsevier. Research funds have been granted by Merz for treatment studies of focal hand dystonia, Allergan for studies of methods to inject botulinum toxins, Medtronic, Inc. for a study of DBS for dystonia, and CALA Health for studies of a device to suppress tremor.
Steven K. Charles: None.
In (Geiger et al. 2018), shoulder tremor was characterized in the glenohumeral joint, not the thoracohumeral joint as in this paper; however, to enable comparison, we approximated the DOF as equivalent.
We repeated the analysis including all subjects and including only subjects with severe tremor (because their peaks were more reliable) and found that the distributions were similar.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belda-Lois JM, Martinez-Reyero AI, Castillo A, Rocon E, Pons JL, Loureiro R, et al. Controllable mechanical tremor reduction. Assessment of two orthoses. Technol Disabil 2007a;19(4):169–78. [Google Scholar]
- Belda-Lois JM, Page A, Baydal-Bertomeu JM, Poveda R, Barbera R. Biomechanical constraints in the design of robotic systems for tremor suppression In: Kommu SS, editor. Rehabilitation Robotics. Vienna, Austria: Itech Education and Publishing; 2007b. [Google Scholar]
- Case D, Taheri B, Richer E. Design and Characterization of a Small-Scale Magnetorheological Damper for Tremor Suppression. IEEE-ASME T Mech 2013;18(1):96–103. [Google Scholar]
- Case D, Taheri B, Richer E. Dynamical Modeling and Experimental Study of a Small-Scale Magnetorheological Damper. IEEE-ASME T Mech 2014;19(3):1015–24. [Google Scholar]
- Case D, Taheri B, Richer E. A Lumped-Parameter Model for Adaptive Dynamic MR Damper Control. IEEE-ASME T Mech 2015;20(4):1689–96. [Google Scholar]
- Clark R, Dickinson T, Loaiza J, Geiger D, Charles S. Tracking Joint Angles During Whole-Arm Movements Using Electromagnetic Sensors. J Biomech Eng 2020; 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corie TH, Charles S. Simulated Tremor Propagation in the Upper Limb: From Muscle Activity to Joint Displacement. J Biomech Eng 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AD, Charles SK. Fundamental Principles of Tremor Propagation in the Upper Limb. Ann Biomed Eng 2017;45:1133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosen S, Muceli S, Dideriksen JL, Romero JP, Rocon E, Pons J, et al. Online Tremor Suppression Using Electromyography and Low-Level Electrical Stimulation. IEEE Trans Neural Syst Rehabil Eng 2015;23(3):385–95. [DOI] [PubMed] [Google Scholar]
- Elble R, Comella C, Fahn S, Hallett M, Jankovic J, Juncos J, et al. The essential tremor rating assessment scale (TETRAS). Mov Disord 2008;23(1):S357–S. [Google Scholar]
- Elble R, LeWitt P, Lyons K, Ondo W, Pahwa R, Sethi K, et al. Inter-Rater Reliability of the Essential Tremor Rating Assessment Scale (TETRAS). Neurology 2012a;78:3. [Google Scholar]
- Elble R, LeWitt P, Lyons K, Ondo W, Pahwa R, Sethi K, et al. Reliability of The Essential Tremor Rating Assessment Scale (TETRAS). Mov Disord 2012b;27:S409–S. [Google Scholar]
- Elble RJ, Deuschl G. An update on essential tremor. Curr Neurol Neurosci 2009;9(4):273–7. [DOI] [PubMed] [Google Scholar]
- Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R, et al. Tremor amplitude is logarithmically related to 4-and 5-point tremor rating scales. Brain 2006;129:2660–6. [DOI] [PubMed] [Google Scholar]
- Feys P, Helsen WF, Verschueren S, Swinnen SP, Klok I, Lavrysen A, et al. Online movement control in multiple sclerosis patients with tremor: Effects of tendon vibration. Mov Disord 2006;21(8):1148–53. [DOI] [PubMed] [Google Scholar]
- Freeman CT, Sampson P, Burridge JH, Hughes AM. Repetitive control of functional electrical stimulation for induced tremor suppression. Mechatronics 2015;32:79–87. [Google Scholar]
- Fromme NP, Camenzind M, Riener R, Rossi RM. Need for mechanically and ergonomically enhanced tremor-suppression orthoses for the upper limb: a systematic review. J Neuroeng Rehabil 2019;16(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger DW, Eggett DL, Charles SK. A method for characterizing essential tremor from the shoulder to the wrist. Clin Biomech 2018;52:117–23. [DOI] [PubMed] [Google Scholar]
- Hashemi SM, Golnaraghi MF, Patla AE. Tuned vibration absorber for suppression of rest tremor in Parkinson’s disease. Med Biol Eng Comput 2004;42(1):61–70. [DOI] [PubMed] [Google Scholar]
- Kestenbaum M, Ford B, Louis ED. Estimating the Proportion of Essential Tremor and Parkinson’s Disease Patients Undergoing Deep Brain Stimulation Surgery: Five-Year Data From Columbia University Medical Center (2009–2014). Mov Disord Clin Pract 2015;2(4):384–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LK, Almeida QJ, Ahonen H. Short-term effects of vibration therapy on motor impairments in Parkinson’s disease. Neurorehabilitation 2009;25(4):297–306. [DOI] [PubMed] [Google Scholar]
- Kotovsky J, Rosen MJ. A wearable tremor-suppression orthosis. J Rehabil Res Dev 1998;35(4):373–87. [PubMed] [Google Scholar]
- Louis E, Rohl B, Rice C. Defining the Treatment Gap: What Essential Tremor Patients Want That They Are Not Getting. Tremor Other Hyperkinet Mov 2015; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol 2013;20(4):725–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov 2014; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro RCV, Belda-Lois JM, Lima ER, Pons JL, Sanchez-Lacuesta JJ, Harwin WS. Upper limb tremor suppression in ADL via an orthosis incorporating a controllable double viscous beam actuator. ICORR 2005. p. 119–22. [Google Scholar]
- Maneski LP, Jorgovanovic N, Ilic V, Dosen S, Keller T, Popovic MB, et al. Electrical stimulation for the suppression of pathological tremor. Med Biol Eng Comput 2011;49(10):1187–93. [DOI] [PubMed] [Google Scholar]
- McDonough RN, Whalen AD. Detection of Signals in Noise 2nd ed. San Diego, CA: Academic Press, 1995. [Google Scholar]
- Morrison S, Newell KM. Inter- and intra-limb coordination in arm tremor. Exp Brain Res 1996;110(3):455–64. [DOI] [PubMed] [Google Scholar]
- Morrison S, Newell KM. Bilateral organization of physiological tremor in the upper limb. Eur J Appl Physiol Occup Physiol 1999;80(6):564–74. [DOI] [PubMed] [Google Scholar]
- Morrison S, Newell KM. Limb stiffness and postural tremor in the arm. Motor Control 2000a;4(3):293–315. [DOI] [PubMed] [Google Scholar]
- Morrison S, Newell KM. Postural and resting tremor in the upper limb. Clin Neurophysiol 2000b;111(4):651–63. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Elek J, Javidan M. Attenuation of pathological tremors by functional electrical-stimulation I. Method. Ann Biomed Eng 1992;20(2):205–24. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Lindemann M, Schmaljohann H, Wenzelburger R, Pfister G, Deuschl G. Multiple oscillators are causing parkinsonian and essential tremor. Mov Disord 2000;15(1):84–94. [DOI] [PubMed] [Google Scholar]
- Rahimi F, Debicki D, Roberts-South A, Bee C, Bapat P, Jog M. Dynamic Decomposition of Motion in Essential and Parkinsonian Tremor. Can J Neurol Sci 2015;42(2):116–24. [DOI] [PubMed] [Google Scholar]
- Rocon E, Pons J. Exoskeletons in rehabilitation robotics: Tremor suppression In: Siciliano B, Khatib O, Groen F, editors. Berlin: Springer-Verlag, 2011. [Google Scholar]
- Samotus O, Kumar N, Rizek P, Jog M. Botulinum Toxin Type A Injections as Monotherapy for Upper Limb Essential Tremor Using Kinematics. Can J Neurol Sci 2018;45(1):11–22. [DOI] [PubMed] [Google Scholar]
- Samotus O, Lee J, Jog M. Long-term tremor therapy for Parkinson and essential tremor with sensor-guided botulinum toxin type A injections. Plos One 2017;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samotus O, Rahimi F, Lee J, Jog M. Functional Ability Improved in Essential Tremor by IncobotulinumtoxinA Injections Using Kinematically Determined Biomechanical Patterns - A New Future. Plos One 2016;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmayer N, Weber C, Kieler M, Voller B, Pirker W, Auff E, et al. Task-dependent variability of Essential Tremor. Parkinsonism Relat Disord 2017;41:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, van der Helm FCT, Veeger HEJ, Makhsous M, Van Roy P, Anglin C, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion - Part II: shoulder, elbow, wrist and hand. J Biomech 2005;38(5):981–92. [DOI] [PubMed] [Google Scholar]
- Zesiewicz TA, Shaw JD, Allison KG, Staffetti JS, Okun MS, Sullivan KL. Update on Treatment of Essential Tremor. Curr Treat Options Neurol 2013;15(4):410–23. [DOI] [PubMed] [Google Scholar]